Abstract
While NK cells can be readily generated for adoptive therapy with current techniques, their optimal application to treat malignant diseases requires an appreciation of the dynamic balance between signals that either synergise with, or antagonise each other. Individuals display wide differences in NK function which determine their therapeutic efficacy. The ability of NK cells to kill target cells or produce cytokines depends on the balance between signals from activating and inhibitory cell-surface receptors. The selection of NK cells with a predominant activating profile is critical for delivering successful antitumor activity. This can be achieved through selection of KIR mismatched NK donors and by using blocking molecules against inhibitory pathways. Optimum NK cytotoxicity may require licensing or priming with tumor cells. Recent discoveries in the molecular and cellular biology of NK cells inform in the design of new strategies, including adjuvant therapies, to maximise the cytotoxic potential of NK cells for adoptive transfer to treat human malignancies.
Biology of natural killer (NK) cells
NK cells are characterised phenotypically by the expression of CD56 and lack of expression of CD3. Around 90% of circulating NK cells are CD56dim and this population plays a key role in mediating cytotoxicity in response to target cell stimulation (1, 2). The remaining NK cells are CD56bright and have a greater capability to secrete and be stimulated by cytokines (3, 4). Unlike B and T cells, NK cells do not undergo antigen-dependent, somatic rearrangement of their receptors and do not possess clonally distributed, antigen-specific receptors comparable to immunoglobulins or T-cell receptors (TCRs). This enables NK cells to respond rapidly to specific stress signals, without the need for prior sensitization and clonal expansion. Interestingly, recent data question this dogma and suggest that NK cells possess features of ‘memory’, with limited antigen specificity and the ability to provide anamnestic antigen-specific response upon subsequent antigenic challenge (5).
Although classified as innate immune cells, phylogenetically NK cells appear to have coevolved with T cells rather than antecedent to them (6–8). Resting NK cells share common killing mechanisms with mature CD8+ effector T cells; they induce target cell apoptosis through calcium dependent exocytosis of perforin and granzyme, as well as through the Fas and tumour necrosis factor–related apoptosis-inducing ligand (TRAIL) pathways (4, 9). In addition, NK cells secrete cytokines, such as interferon-gamma (IFNγ) and tumour necrosis factor alpha (TNFα), and are involved in regulating the function of other lymphocytes, macrophages, dendritic cells and endothelial cells (10).
Recently micro RNAs, such as miR-150 and miR-181 (11), have been shown to play a key role in the development of NK cells and miR-29 (12) and miR15/6 (13) have been found to modulate cytokine production.
NK recognition of tumor targets
The combination of activating (in particular the natural cytotoxicity receptors [NCR] NKp46, NKp30, NKp44 and the membrane protein NKG2D) and inhibitory cell-surface receptors (notably the killer Ig-like receptors [KIRs] and the heterodimeric C-type lectin receptor NKG2A) determines whether NK cells will or will not kill target cells and produce cytokines during their effector phase of activation (Figure 1 and Table 1) (14).
Figure 1. NK cell activation by a kinetic segregation model.
Activating NK receptors generally use adapter proteins such as Fcεγ, and DAP12, which contain ITAM consensus sequences. These trigger a rise in intracellular Ca2+, and degranulation following phosphorylation by tyrosine kinases such as Syk and ZAP70. Similarly the majority of inhibitory NK cell receptors contain an ITIM consensus motif, phosphorylation of which results in recruitment of tyrosine specific phosphatases such as SHP-1, SH2-containing protein tyrosine phosphatise-1 (SHP-1) and SH2-containing inositol pholyphosphate 5-phosphatase (SHIP). These act by dephosphorylating ITAM motifs and SHIP degrades phosphatidylionsitol-3,4,5-trisphospate, leading to inhibition of sustained calcium signalling. The kinetic segregation model of NK cell activation (19) proposes that the normal balance between phosphorylation and dephosphorylation of these receptors is disturbed by physical extrusion of large phosphatases such as CD45 and CD148 from areas of close contact between the NK cell and its target. This leads to phosphorylation by small kinases of both the activating and inhibitory receptors that diffuse into, and are held at the immune synapse, and allows NK cell activation to be dependent on the balance between the number of activating and inhibitory ligands on the target cell. Several important receptor subtypes are not depicted in this diagram (e.g. the activating receptor CD16, which uses the ITAM containing adapter protein Fcεγ, see Table 1). In addition non-ITAM mediated activation also occurs, particularly by the NKG2D-DAP10 complex (102).
Table 1.
NK cell receptors
| KIR | KIRs are part of the immunoglobulin superfamily. KIRs either possess two (KIR2D) or three (KIR3D) extracellular Ig-like domains (103–105) and can either have an activating short (S) cytoplasmic domain or inhibitory long (L) domain (16). KIR receptors are able to bind four of the six types of class I in the human MHC (HLA-A, HLA-B, HLA-C and HLA-G). The details of the interaction between KIR3DL1 and HLA were recently demonstrated by crystallography (106). The target antigen within the MHC and the degree of specificity vary depending on the KIR receptor. For example, KIR2DL4 binds to HLA-G with broad specificity, whilst KIR3DL1 has a very narrow specificity and only binds to HLA Bw4. NK receptors are expressed stochastically on the surface of NK cells and these complex expression patterns allow individual NK cells to recognise cells missing one or more MHC class I molecules (6). |
| NKG2 (NKG2A, - C, -E and – F) and CD94 receptors | These molecules are part of the C-type lectin-like family and tend to be expressed on the cell surface as heterodimers between CD94 and NKG2A or NKG2C, which act as inhibitory or activating receptors respectively (107). CD94 can also be expressed as a homodimer, although it is not believed to have a role in signalling because it lacks a cytoplasmic domain. Unlike KIR expression, CD94/NKG2 receptor expression can be induced by a number of cytokines including IL-15 (108) and IL-12 (109). Both the inhibitory receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind HLA-E, with high and low affinity respectively. Interestingly, the most abundant peptides presented by this MHC class Ib molecule are peptides derived from the leader segments of other class I molecules, allowing NK cells to monitor the MHC class I proteins in a cell. Peptides from heat shock protein 60 (hsp60) are also presented on HLA-E and there is evidence that the inhibitory CD94/NKG2A receptors fail to bind HLA-E molecules containing hsp60 peptides, providing a mechanism for NK cells to potentially eliminate “stressed” cells (110). |
| NKG2D | This is a key receptor in NK cell activation, although it is also found on virtually all γδ and CD8+ T cells. The NKG2D receptor is encoded by a single gene that has little sequence homology with the other NKG2 genes and it acts through DAP10, using an ITAM independent mechanism (102). Unlike many of the other NK cell receptor genes, there is very little polymorphism in NKG2D in humans (111, 112). It is a member of the lectin-like receptor family and recognises the MHC class I related molecules MICA and B (113, 114). Ligands of the NKG2D receptor may be upregulated as part of the DNA damage response induced by chemotherapy and radiotherapy and it is likely that NK cells play a key role in mediating tumour cell apoptosis following these cytotoxic treatments (115). Intriguingly, polymorphisms in the NKG2D target MICA are associated with T-cell large granular lymphocyte leukaemia (116). |
| Natural cytotoxicity receptors | The natural cytotoxicity receptors (NCRs) include NKp30, NKp44 (117) and NKp46 (118). Activation of these receptors generally results in strong enhancement of NK-mediated cytolytic activity. NKp46 can bind viral haemagglutinins and activate cell lysis (119) but is also involved in mediating tumour cell destruction in myeloma (120). To add a further layer of complexity, NKp30 has splice variants, which have different functions, (121) with NKp30a and NKp30b being stimulatory and NKp30c inhibitory. These are coexpressed but the balance of the expression is predominantly genetically determined, giving rise to either activating or inhibitory profiles. The expression pattern has prognostic implications in gastrointestinal stromal tumours, with the inhibitory profile (NKp30c predominance) conferring a shortened survival (121). |
| CD16 | NK cells are also important mediators of antibody dependent cell-mediated cytotoxicity (ADCC). The CD56dim subset in particular mediates this effect through antibody mediated binding of the low affinity Fc receptor (FcγRIII / CD16). This binds to the IgG1 and IgG3 subclasses and enables NK cells to mediate ADCC. Through this pathway, NK cells are involved in eliciting the effects of monoclonal antibody therapies such as rituximab and gemtuzumab (96, 97). |
| LAIR-1 (CD305) | Leukocyte associated immunoglobulin-like receptor 1 (LAIR-1) is another inhibitory receptor that is linked to the SHP pathway (122). |
|
SLAM 2B4 (CD244) NTB-A (CD352) |
The signalling lymphocyte activation molecules (SLAM) are a family of receptors that include the 2B4 receptor and the NK, T and B cell antigen-A (NTB-A) (CD150). 2B4 is interesting in that it can have both activating (123) and inhibitory (124) properties and it interacts with CD48, which is widely expressed on haematopoietic cells. |
| Others | There are a large number of other activating and inhibitory receptors also expressed by NK cells including:; leukocyte immunoglobulin-like receptor B1 (LILRB1, CD85j); CD2-like receptor activating cytokine cells (CRACC); and DNAX accessory molecule-1 (DNAM-1/CD266). |
One of the primary functions of NK cells is the detection and killing of cells under expressing MHC class I, thus preventing viruses and tumours from evading T cell surveillance and this is often termed the ‘missing-self hypothesis’ (15). In humans this phenomenon is predominantly mediated by inhibitory killer cell immunoglobulin-like receptors (KIRs) and CD94/NKG2A, which recognise MHC class I and prevent NK cell mediated killing of cells expressing MHC class I (16).
NK-target cell interactions involve clustering of receptors at the contact area of both cells, termed immune synapses (17). The majority of activating NK receptors share common signalling pathways with B and T cell receptors; using adapter proteins, which contain immunoreceptor tyrosine-based activation motifs (ITAMs). Phosphorylation of ITAMs results in target cell killing through NK cell degranulation in response to increases in intracellular calcium. The majority of inhibitory NK cell receptors also contain a consensus sequence termed the immunoreceptor tyrosine-based inhibitory motif (ITIM), also activated by phosphorylation, which in turn results in dephosphorylation of ITAM motifs and inhibition of calcium signalling.
The mechanism by which NK cells integrate multiple activating and inhibitory signals is not fully understood and it is likely that multiple mechanisms are involved in the control of NK cell triggering, as in T cells (18). Recent studies suggest that a kinetic segregation model may be involved in NK cell activation (19). In this model, large phosphatases such as CD45 are excluded from the areas of membrane held in close proximity between the NK cell and its target. This leads to phosphorylation by small kinases of the activating and inhibitory NK receptors that are held in the areas of close contact by ligands on the surface of the target cell. This allows NK cell activation to be dependent on the complex summation of multiple activating and inhibitory signals.
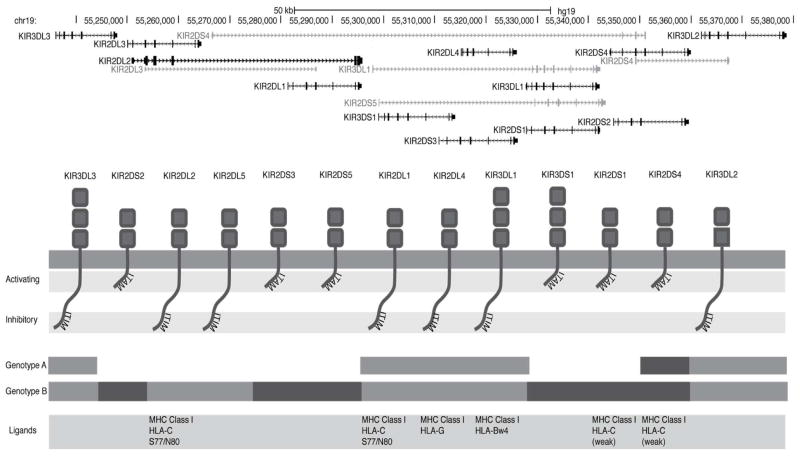
The KIR gene family on chromosome 19q13.4 encodes 15 highly polymorphic and rapidly evolving (20) activating and inhibitory receptors that are expressed principally by NK cells but also by subpopulations of T cells (Figure 2). These genes vary with respect to their presence or absence on different KIR haplotypes. They exhibit extensive allelic polymorphism, creating considerable diversity in the number of KIR genotypes observed in the population (21).
Figure 2. KIR genotype and receptor function.
Simplified schema of the KIR gene locus (modified from the UCSC genome browser http://genome.ucsc.edu/, hg19 (2009)). At least 37 haplotypes have been identified thus far; however, the KIR genotype can broadly be divided into two groups, depending on whether the person is homozygous for genotype A (AA), in which case they only express one activating KIR (KIR2DS4) or whether they have at least one B haplotype (Bx). The B haplotype is defined by the presence of a variable number of additional activating and inhibitory KIRs (KIR2DL5, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5 and KIR3DS1).
Relationship between KIR type and malignant cell targets
A number of solid tumors including melanoma (22), cervical cancer (23, 24) and nasopharyngeal cancer (25) have been associated with certain KIR genotypes. Haematological malignancies and response to therapy have also been linked to KIR genotype through a number of different mechanisms; however, the conclusions drawn from some of these studies should be interpreted with caution in view of the heterogeneity of the study populations and small study groups (see Table 2) (26–31). In the allogeneic stem cell transplant (allo-SCT) setting, receiving a graft from donors with the KIR2DS1 genotype was recently shown to be associated with reduced risk of relapse from acute myeloid leukaemia (AML) following both unrelated (32) and matched sibling donor allo-SCT (33). In addition, KIR genotype predicts response to treatment for haematological malignancy; for instance, the KIR3DS1 genotype predicts for increased relapse rate following autologous stem cell transplantation (auto-SCT) for myeloma, while patients who lack the activating receptor KIR3DS1 have a better outcome (28). The KIR genotype of the patient also appears to determine response to therapy with tyrosine kinase inhibitors (TKIs): in newly diagnosed patients with chronic myeloid leukaemia (CML) treated with imatinib, KIR2DS2 genotype conferred a lower probability of complete cytogenetic remission and reduced overall survival (34), which could be overcome by using dasatinib (35).
Table 2.
Association of KIR genotypes and haematological malignancies
| KIR genotype and associations with haematological malignancies | ||||
|---|---|---|---|---|
| KIR gene | Disease | Effect | No patients in study | References |
| Risk of disease | ||||
| KIR-2DS1 KIR-2DS5 |
Aplastic anaemia / Paroxysmal nocturnal haemoglobinuria | Reduced frequency in patient population | 55 | (125) |
| KIR2DS3 | AML/ALL | Reduced frequency in patient population | 40/32 | (126) |
| Activating KIR genes, especially KIR2DS2 |
Pre-B ALL | Reduced frequency in patient population | 102 | (127) |
| KIR3DS1 and KIR2DS1 | Hodgkin’s Lymphoma | Reduced frequency in patients | 84 families | (128) |
| KIR2DL2 and KIR2DS2 | CML | Reduced frequency in patients | 52 | (129) |
| KIR2DL2 and KIR2DL3 in combination with their HLA-Cw1 ligand | CML/AML | Increased frequency in patients | 19/29 | (31) |
| KIR2DL5a and 2DL5b | NK type lymphoma | Increased frequency in patients | 35 | (26) |
| KIR3DL1 and the HLA-Bw4-ligand | CLL | Increased frequency in patients in the presence of Bw4 and reduced risk when Bw4 absent. | 31 | (31) |
| Response to treatment | ||||
| KIR2DS1 | CML | Reduced response to imatinib and worse overall survival. Dasatinib therapy overcomes the poor prognostic impact of KIR2DS1. | 166 + validated a further 174 on SPIRIT2 | (34) (35) |
| KIR3DS1 | Myeloma | Worse DFS following autologous stem cell transplant. | 182 | (28) |
| Donor KIR B/B homozygotes | AML | Reduced risk of relapse following unrelated allogeneic transplant (relapse rate 15.4% B/B v 36.5% A/A) | 1086 | (130) |
| Donor KIR 2DS1 (plus coinherited 3DS1 and 2DL5a) | AML | Reduced relapse rates following HLA-identical sibling allogeneic stem cell transplant (relapse rate 13% with SD1+ve donor v 57% if −ve) | 248 patients: 68 with AML | (33) |
| Donor KIR 2DS1 | AML | Reduced relapse rates following HLA-identical unrelated donor allogeneic stem cell transplant (relapse rate 26.5% with 2DS1+ donor v 32.5% 2DS1−ve | 1277 | (32) |
| Donor KIR haplotype B | Myeloma | Improved progression free survival (30 v 14%) | 118 | (67) |
Licensing
An important concept in NK cell activation is that NK cells require ‘licencing’ by the appropriate class I ligand before they are able to become activated by the absence of MHC on other cells. A number of experiments in the mouse have established the role of major histocompatibility complex (MHC) class I in rendering NK cells functional (‘licensed’) (36). Conversely, failure to engage inhibitory receptors during development, due to lack of interaction with MHC class I, results in the generation of a subset of anergic or hyporesponsive peripheral NK cells (unlicensed KIRs) (37). Although the molecular mechanisms underlying the interactions between MHC class I and inhibitory receptors conferring NK cell responsiveness remain incompletely defined, the immunoreceptor tyrosine-based inhibitory motif (ITIM) of the inhibitory receptors is essential (37). Similarly, studies in humans have shown that the recognition of self HLA class I by inhibitory KIR is involved in the licensing of NK cells, endowing them with effector capacity during development and allowing the subsequent recognition of the absence of self MHC class I during interaction with target cells (missing self) (38). KIR2DL2 and KIR2DL3 recognise HLA-C allotypes characterized by Asn80 (grouped as HLA-C1); KIR2DL1, and to a weaker extent some KIR2DL2/3 allotypes, recognize HLA-C allotypes characterized by Lys80 (grouped as HLA-C2); KIR3DL1 recognises HLA-A and HLA-B allotypes with the Bw4 epitope (39). Thus NK licensing is an essential precursor to the acquisition of functionality. Upon encounter with cells with decreased expression of MHC class I molecules, i.e. “missing self” (15), licensed NK cells are no longer subject to inhibitory signals initiated by the engagement of MHC class I-specific receptors, promoting NK cell cytotoxicity and cytokine production. These findings have led to clinical studies that investigate the impact of selecting hematopoietic stem cell transplantation donors with an HLA and KIR type that favours NK-cell activation and thus, could be expected to promote an anti-leukemic effect (40–42). An interesting strategy for enhancing NK effector function is tumor-mediated NK priming (43, 44). Through the interaction of CD2 on NK cells with a ligand on the tumor, NK cells are rendered more cytotoxic, enabling them cells to lyse tumour cells that would normally be impervious to NK mediated destruction. A clinical trial of tumor-primed NK cells in AML was recently completed (43, 44).
Immune escape from NK cell control
A limitation to successful NK therapy is the capacity of a variety of malignancies to evade NK control. Several mechanisms have been identified:
Upregulation of MHC class I, Such a phenomenon has been described in myeloma where in early stage disease plasma cells express low levels of MHC class I and are recognised by, and susceptible to, NK cell-mediated killing. However, plasma cells derived from myelomatous pleural effusions, which only occur in advanced and aggressive disease, have high levels of MHC class I expression, allowing them to evade control by NK cells (45). Down regulation or release of NKG2D ligands, including MHC class I chain-related proteins A and B (MICA/B) and unique long 16 (UL16) binding proteins 1–6 (ULBP1-6)(46–48), have also been reported as potential immune evasion strategies in a number of haematological malignancies (49–53). In a c-myc transgenic mouse model of spontaneously arising lymphoma, NKG2D ligands were over-expressed on the surface of tumour cells at early stages of the disease, with progressive loss of expression, associated with escape from NK cell surveillance, in later stages of disease (54).
Abnormal overexpression of MICA/B has also been observed on CML CD34+ cells. However, in vivo, CML cells avoid NK-cell–mediated immune destruction by shedding MICA/B, thereby blocking NKG2D on CD56+ cells, thus preventing NK-cell activation by the target (55). Interestingly, different subpopulations of CML cells appear to exhibit different sensitivities to NK-mediated killing. In a recent report, quiescent CML CD34+ cells were found to be less susceptible to NK cell cytotoxicity than cycling CD34+ and CD34− cells (56, 57). The mechanisms behind the greater resistance of quiescent, primitive, leukemic cells to cell-mediated cytotoxicity are not well understood but may relate to the inherent metabolic inactivity of this population of cells. The observation that quiescent CD34+ cells express a higher surface level of the death receptors, TRAIL DR4 and DR5, suggests that TRAIL-enhancing agents such as bortezomib can be used to augment the sensitivity of quiescent CD34+ CML cells to NK cell mediated killing (56).
Down-regulation of activating receptors on the surface of NK cells. We showed that co-incubation with AML blasts impaired NK effector function. The mechanism leading to altered NK function is unclear. Several cytokines, including transforming growth factor-β (TGF-β), inhibit NK cell activation. A recent study suggests abnormal expression of the immunosuppressive tyrosine converting enzyme indoleamine 2, 3-dioxygenase (IDO) as a mechanism for reduced NK cytotoxicity in melanoma patients (58), as well as impaired T cell function in AML (59). Our own data support a role for IL-10 in AML-mediated NK cell dysfuntion (60).
Cell therapy with NK cells
Cellular therapy with NK cells has been accomplished either in isolation or in the context of an allogeneic transplant. A number of different strategies have been tested to provide pure populations of autologous or allogeneic NK cells, including purified NK cells from the peripheral blood of donors, unrelated cord blood units or in vitro propagated NK cell lines. Some of these strategies are discussed in more details below.
NK cell transfer and production by stem cell allografts
NK cells are usually the earliest blood lymphocytes to return after haematopoietic stem cell transplantation (61). The behaviour of NK cells following transplantation can be altered by the manipulation of the graft and post transplant immunosuppression. In particular, T cells in the graft are thought to impair NK responses post transplant, since KIR expression is augmented in T-cell depleted transplants (62).
The pioneering work of Velardi and coworkers (40) showing that donor-versus-recipient NK-cell alloreactivity could eliminate leukaemia relapse without increasing the risk of graft versus host disease (GVHD) in HLA mismatched recipients resulted in a new era in the exploitation of NK cells for cancer immunotherapy. In these studies the authors compared the outcome of patients based on the NK-alloreactivity of the donors (Figure 3). In patients with AML, transplantation from NK-alloreactive donors was associated with significantly lower relapse rate compared to non-NK-alloreactive donor (5 year relapse rate 0% vs. 75%; P=0.0008). These data led to several retrospective analyses of KIR ligand mismatch effect on outcome in the HLA-matched unrelated and matched sibling transplant settings. Although a number of large registry studies failed to show an increased graft-versus-leukaemia (GvL) effect (63–65), others showed a GvL effect associated with a survival advantage (41, 66–68). The conflicting data may be related to differences in the transplant conditioning protocols, patient populations, underlying diseases, graft composition, and post-transplant immunosuppressive regimens (69). Subsequent studies have shown that the accuracy of the prediction of relapse could be improved by taking into consideration the presence of inhibitory KIRs on the donor’s NK cells and the absence of corresponding KIR ligand in the recipient’s HLA repertoire (a receptor-ligand model) (70).
Figure 3. KIR ligand mismatch.
KIR Ligand mismatch is largely dictated by the ligands of the three inhibitory KIR receptors KIR2DL1, KIR3DL1 and KIR2DL2, which are highly prevalent (97%, 90% and 95% respectively). This model predicts that when when the transplant recipient is missing at least one of the three major classes of HLA ligands for inhibitory KIR, the donor NK cells will recognise the missing ligand in the host, resulting in an augmented graft versus leukaemia effect. Theoretically this can occur in the opposite direction (where the ligand is missing from the donor) but there is little evidence that this leads to worse rates of graft rejection.
More recently, specific activating KIR genes in donors were found to predict the risk of relapse following allogeneic transplantation for AML. Patients with AML who received allografts from donors who were positive for KIR2DS1 had a lower risk of relapse (26.5%) than those with allografts from donors who were negative for KIR2DS1 (32.5%); P=0.02 (32). The KIR2DS1-associated reduction in the rate of AML relapse was restricted to donors with HLA-C1/C1 or C1/C2, in whom KIR2DS1 expressing NK cells are presumed to be licensed, and the benefit was eliminated in transplants from donors with HLA-C2/C2, where KIR2DS1-expressing NK cells are expected to be tolerized in the setting of self HLA-C2 and unlikely to mediate leukemic cytotoxicity. Similar results have also been reported in the setting of matched sibling donor transplants; Patients with AML who received allografts from donors who were positive for KIR2DS1 had 4 times lower risk of relapse than those who received a graft from KIR2DS1 negative donors (33).
A limitation of many of these studies is that they do not take into account the effects of KIR licensing on NK function following allogeneic stem cell transplantation. Although it is presumed that the acquisition of NK cell function is influenced by the inhibitory input from the donor HLA, murine studies suggest a greater degree of plasticity and indicate that NK cell responsiveness can also be influenced by the inhibitory input from the recipient (71–73). Outcome following allogeneic stem cell transplantation may also be influenced in other ways by NK cells and KIR genotype, for example there is evidence that the rates of CMV reactivation are modulated by activating KIR receptor genotype (74, 75).
Adoptive NK cell therapy
Attempts to use adoptive NK cell therapy for the treatment of malignant disorders date back more than 25 years. In one of the pioneering studies by Rosenberg and colleagues, autologous ‘lymphokine activated killer cells’ combined with interleukin-2 (IL-2) were given to patients with metastatic renal cell cancer and melanoma (76). This resulted in a response in around 20% of patients. Disappointingly, in further studies, no consistent efficacy of autologous NK-cell therapy was found in cancer patients when compared with cohorts of matched controls treated with IL-2 alone (77).
The last decade has seen the development of a number of strategies for the ex vivo selection and expansion of clinical grade NK cells. These include magnetic selection using CD56 Miltenyi beads (+/− CD3 depletion) to directly select NK cells from apheresis products (Table 3). Protocols for ex vivo expansion of NK cells using EBV-LCL feeder cell lines, or artificial antigen presenting cells engineered to express co-stimulatory molecules and membrane-bound cytokines such as IL-15 or IL-21 (78, 79) have also been shown to yield large numbers of highly activated NK cells under good manufacturing practice (GMP) conditions.
Table 3.
Trials of Adoptive NK cell therapy
| Disease | Setting | Method of preparation | Cell number | Concomitant medication | n | Effect on patient outcome | |
|---|---|---|---|---|---|---|---|
| AML | NK-DLI for patients in first CR following chemotherapy | Apheresis followed by CD3 depletion and subsequent CD56 selection | 2.9 × 107/kg (range 0.5–8 × 107/kg) | Cyclophosphamide (day −7) Fludarabine (Day −6 to −2) |
10 | 100% 2 year event free survival | NKAML study (80) |
| AML | NK-DLI for poor prognosis AML | Apheresis followed by CD3 depletion and ex-vivo stimulation with IL2 | 1 ×105 – 2 ×107 | Low dose cyclophosphamide/melphalan vs. High dose cyclophosphamide and fludarabine. IL2 given to all patients |
19 | Complete haematological remission in 5 of 19 patients | (42) |
| Myeloma | Haploidentical KIR ligand mismatched NK- DLI followed by delayed autologous stem cell transplant | Apheresis followed by CD3 depletion | 2.4×107/kg | Melphalan and fludarabine conditioning | 10 | Near CR in 50% of patients | (81) |
| CD20+ NHL | NK-DLI for refractory disease (more than 2 salvage therapies) | Apheresis followed by CD3 depletion | 2.1 × 107/kg (range 0.2–40 × 107/kg) | Fludarabine (25mg/m2) Cyclophosphamide (60mg/m2) |
6 | 4 objective remissions 2 complete remissions |
(83) |
| Not stated | Allogeneic stem cell transplant | Apheresis followed by CD3 depletion and subsequent CD56 selection | 1.2×107/kg | Not stated | 14 | NK infusions well tolerated, aside one case of grade IV | (131) |
| High risk myeloid malignancies | Graft failure/mixed donor chimerism following allogeneic (haploidentical) stem cell transplant | Apheresis followed by CD3 depletion and subsequent CD56 selection | 1.61 × 107/kg (range 0.21–2.2) | None | 5 | Reversal of mixed donor chimerism in 2/5 | (132) |
| Not stated | Following 3-6/6 HLA matched T cell-depleted nonmyeloablative allogeneic transplant | Apheresis followed by CD3 depletion and CD56 selection | 1.06 × 107/kg | Infusion performed 6–8 weeks following fludarabine conditioned allogeneic transplant with alemtuzumab | 30 | Improved T cell recovery and duration of remission | (133) |
| Breast and ovarian carcinoma | NK DLI for patients with relapsed disease | Apheresis followed by CD3 depletion | 2.17 × 107/kg | Fludarabine and cyclophosphamide Total body irradiation in 7 patients IL2 x6 doses s.c. post DLI |
20 | TBI improved longevity of NK engraftment | (134) |
| Colorectal carcinoma, hepatocellular carcinoma, renal cell carcinoma and B-CLL | Previous allogeneic BMT with donor derived NK cells | Apheresis followed by ex vivo expansion with IL-2 | 0.1 × 107 followed by two doses mean 3.3 × 107 | Variable; some patients received sc IL-2 | 5 | Minor responses in 2 patients | (135) |
| Advanced non-small cell lung cancer | NK-DLI after chemotherapy | CD56 selection followed by culture with IL-15 | 0.46 × 107/kg (range 0.2–2.9) | 2–4 doses of NK DLIs given 2 days after chemotherapy (carboplatin-paclitaxel most commonly used) | 16 | Trend to better OS in subgroup receiving 4 doses of NK cells | (136) |
| Advanced renal cell carcinoma and malignant melanoma | NK-92 cell line infusion | Expansion of NK lymphoma cell line | Up to 3 × 109/m2 | Cell infusion alone | 12 | Infusion well tolerated. Possible response in 2 patients | (86) |
A number of studies have reported encouraging results of NK adoptive infusion in patients with AML. NK-donor lymphocyte infusions (NK-DLI) are generally well tolerated and GVHD has not been a major problem in the trials to date. In the NKAML pilot study, children with high risk AML who achieved in first complete remission following conventional chemotherapy received infusions of haploidentical NK cells, preceded by conditioning with cyclophosphamide and fludarabine. This was found to be both feasible and safe, and at 2 years there was 100% event free survival (80). In another study 5 of 19 adults with advanced AML achieved a complete haematological response following haploidentical NK-DLI and conditioning with low-dose total body irradiation or a combination of fludarabine and cyclophosphamide (42). Haploidentical NK cells have also been used with some success in concert with autologous transplantation for multiple myeloma (81, 82) and non Hodgkins lymphoma (83). Generally, without prior conditioning, allogeneic NK cells are only transiently detectable. Attempts have been made to augment NK responses and persistence using a variety of agents such as IL-2 (84) and bortezomib (85) with some success. A number of investigators have also studied the role of adoptive immunotherapy with the NK lymphoma derived cell line NK-92 (86). Although reported to be safe, limited data exist on the efficacy of this approach. Our group is exploring the use of off-the-shelf cord blood derived NK cells in the treatment of haematological malignancies.
Augmenting function of NK cells by adjuvant therapies
Modulation of the host immune system represents a promising therapeutic approach against cancer. NK cells are also thought to be involved in mediating the effects of many therapies used to treat haematological malignancies through a wide variety of mechanisms. In myeloma, for example, treatment with drugs such as melphalan, etoposide and doxorubicin activates DNA damage responses, resulting in up-regulation of the NK activating receptors, DNAX accessory molecule-1 (DNAM-1) and NKG2D ligands, leading to increased NK-mediated cell cytotoxicity against tumour cells (87). Similarly heat shock 90 inhibitors upregulate the expression of the NKG2D receptor ligands MICA and MICB, rendering myeloma cells more susceptible to NK cell degranulation (88). The histone deacetylase (HDAC) inhibitor valproic acid has also been shown to upregulate the expression of NKG2D ligands on the surface of AML blasts, thereby increasing their sensitivity to NK cell mediated destruction (89).
Novel drugs with immunomodulatory properties are increasingly being used to treat hematological malignancies. Most appear to impact NK cell activity or target susceptibility to NK lysis through a number of mechanisms (summarised in Table 2). Thalidomide increases the number of peripheral blood NK cells with enhanced in vitro cytotoxicity against patient myeloma cells (90). Lenalidomide activates NK function by promoting IL-2 release from bystander CD4+ helper T-cells (91–93), downregulating the expression of the programmed death receptor-1 ligand (PD-L1), the ligand for the inhibitory receptor programmed death receptor-1 (PD-1) expressed on both T and NK cells (94), and upregulation of Fas expression and costimulatory molecules on myeloma cells leading to greater Fas-mediated apoptosis. The proteasome inhibitor bortezomib has also been shown to modulate NK function by reducing the amount of peptide available to bind to MHC class I and consequently, expression of MHC class I on the cell surface, thereby increasing NK mediated lysis of myeloma cells (95) and CML progenitors (56).
NK cell effector functions can be exploited for the treatment of a number of malignancies through their ability to mediate antibody-dependent cellular cytotoxicity (ADCC) (23). A number of monoclonal antibodies such as rituximab bind to CD16 (the FCRγIII receptor) on the surface of NK cells; NK cell recognition of an antibody-coated target cell in turn results in rapid NK cell activation and degranulation (96, 97). Polymorphisms in the FCRγIII receptor have been shown to determine clinical response to rituximab (96); homozygosity for FcγRIIIa-158V is associated with higher FcγRIIIa membrane expression on NK cells (98) and increased affinity of binding to human IgG1 and IgG3 (99).
Future directions
The therapeutic potential of NK cells is likely to be harnessed in a number different ways in the future; ranging from improved donor selection in the context of allogeneic transplant, to novel strategies to augment NK cell tumour killing (100) and prevent tumour escape. As has been the case with T-cells (101), genetic engineering of NKs might also become an important method by which their therapeutic potential is manipulated and enhanced. Strategies to combine the adoptive infusion of ex vivo activated autologous or allogeneic NK cells with conventional chemotherapy or with novel immunomodulatory agents, are being tested in a number of clinical trials worldwide.
Acknowledgments
This research is supported in part by the MD Anderson Cancer Center Leukemia SPORE Grant CA100632, Leuka registered charity (286231).
Footnotes
Author contribution: JOD, KS, AJB and KR wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–6. [PubMed] [Google Scholar]
- 2.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–91. [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001 May 15;97(10):3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001 Jun 1;166(11):6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 5.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009 Jan 29;457(7229):557–61. doi: 10.1038/nature07665. Epub 2009/01/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. Epub 2005/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 7.Fischer U, Utke K, Somamoto T, Kollner B, Ototake M, Nakanishi T. Cytotoxic activities of fish leucocytes. Fish Shellfish Immunol. 2006 Feb;20(2):209–26. doi: 10.1016/j.fsi.2005.03.013. Epub 2005/06/09. eng. [DOI] [PubMed] [Google Scholar]
- 8.Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, et al. Global diversity and evidence for coevolution of KIR and HLA. Nat Genet. 2007 Sep;39(9):1114–9. doi: 10.1038/ng2077. Epub 2007/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 9.Lowin B, Peitsch MC, Tschopp J. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr Top Microbiol Immunol. 1995;198:1–24. doi: 10.1007/978-3-642-79414-8_1. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008 May;9(5):503–10. doi: 10.1038/ni1582. Epub 2008/04/22. eng. [DOI] [PubMed] [Google Scholar]
- 11.Bezman NA, Cedars E, Steiner DF, Blelloch R, Hesslein DG, Lanier LL. Distinct requirements of microRNAs in NK cell activation, survival, and function. J Immunol. 2010 Oct 1;185(7):3835–46. doi: 10.4049/jimmunol.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011 Sep;12(9):861–9. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan RP, Leong JW, Schneider SE, Keppel CR, Germino E, French AR, et al. MicroRNA-deficient NK cells exhibit decreased survival but enhanced function. J Immunol. 2012 Apr 1;188(7):3019–30. doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–44. doi: 10.1016/0167-5699(90)90097-s. Epub 1990/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Parham P. Taking license with natural killer cell maturation and repertoire development. Immunol Rev. 2006 Dec;214:155–60. doi: 10.1111/j.1600-065X.2006.00462.x. Epub 2006/11/15. eng. [DOI] [PubMed] [Google Scholar]
- 17.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):15062–7. doi: 10.1073/pnas.96.26.15062. Epub 1999/12/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. Jan;11(1):47–55. doi: 10.1038/nri2887. Epub 2010/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 19.Kohler K, Xiong S, Brzostek J, Mehrabi M, Eissmann P, Harrison A, et al. Matched sizes of activating and inhibitory receptor/ligand pairs are required for optimal signal integration by human natural killer cells. PLoS One. 2010;5(11):e15374. doi: 10.1371/journal.pone.0015374. Epub 2010/12/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khakoo SI, Rajalingam R, Shum BP, Weidenbach K, Flodin L, Muir DG, et al. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity. 2000 Jun;12(6):687–98. doi: 10.1016/s1074-7613(00)80219-8. Epub 2000/07/14. eng. [DOI] [PubMed] [Google Scholar]
- 21.Parham P. Immunogenetics of killer-cell immunoglobulin-like receptors. Tissue antigens. 2003 Sep;62(3):194–200. doi: 10.1034/j.1399-0039.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 22.Naumova E, Mihaylova A, Stoitchkov K, Ivanova M, Quin L, Toneva M. Prevalence of inhibitory over activating KIR signals in melanoma patients could contribute to the tumor escape of the immunosurveillance. Genes Immun. 2004 May;5:S2-S. [Google Scholar]
- 23.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010 Apr;47(2):115–23. doi: 10.1053/j.seminhematol.2010.01.011. Epub 2010/03/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrington M, Wang S, Martin MP, Gao XJ, Schiffman M, Cheng J, et al. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. Journal of Experimental Medicine. 2005 Apr 4;201(7):1069–75. doi: 10.1084/jem.20042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacic MB, Martin M, Gao XJ, Fuksenko T, Chen CJ, Cheng YJ, et al. Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidem Biomar. 2005 Nov;14(11):2673–7. doi: 10.1158/1055-9965.EPI-05-0229. [DOI] [PubMed] [Google Scholar]
- 26.Scquizzato E, Teramo A, Miorin M, Facco M, Piazza F, Noventa F, et al. Genotypic evaluation of killer immunoglobulin-like receptors in NK-type lymphoproliferative disease of granular lymphocytes. Leukemia. 2007 May;21(5):1060–9. doi: 10.1038/sj.leu.2404634. Epub 2007/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 27.Park IK, Giovenzana C, Hughes TL, Yu J, Trotta R, Caligiuri MA. The Axl/Gas6 pathway is required for optimal cytokine signaling during human natural killer cell development. Blood. 2009 Mar 12;113(11):2470–7. doi: 10.1182/blood-2008-05-157073. Epub 2008/10/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabriel IH, Sergeant R, Szydlo R, Apperley JF, DeLavallade H, Alsuliman A, et al. Interaction between KIR3DS1 and HLA-Bw4 predicts for progression-free survival after autologous stem cell transplantation in patients with multiple myeloma. Blood. 2010 Sep 23;116(12):2033–9. doi: 10.1182/blood-2010-03-273706. Epub 2010/06/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caligiuri MA. Human natural killer cells. Blood. 2008 Aug 1;112(3):461–9. doi: 10.1182/blood-2007-09-077438. Epub 2008/07/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamy T, Loughran TP., Jr How I treat LGL leukemia. Blood. 2011 Mar 10;117(10):2764–74. doi: 10.1182/blood-2010-07-296962. Epub 2010/12/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheyden S, Demanet C. Susceptibility to myeloid and lymphoid leukemia is mediated by distinct inhibitory KIR-HLA ligand interactions. Leukemia. 2006 Aug;20(8):1437–8. doi: 10.1038/sj.leu.2404279. Epub 2006/06/09. eng. [DOI] [PubMed] [Google Scholar]
- 32.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. The New England journal of medicine. 2012 Aug 30;367(9):805–16. doi: 10.1056/NEJMoa1200503. Epub 2012/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010 Sep;16(9):1257–64. doi: 10.1016/j.bbmt.2010.03.004. Epub 2010/03/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin D, Gabriel IH, Ahmad S, Foroni L, de Lavallade H, Clark R, et al. KIR2DS1 genotype predicts for complete cytogenetic response and survival in newly diagnosed chronic myeloid leukemia patients treated with imatinib. Leukemia. 2012 Feb;26(2):296–302. doi: 10.1038/leu.2011.180. Epub 2011/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 35.Ali S, Sergeant R, O’Brien SG, Foroni L, Hedgley C, Gerrard G, et al. Dasatinib may overcome the negative prognostic impact of KIR2DS1 in newly diagnosed patients with chronic myeloid leukemia. Blood. 2012 Jul 19;120(3):697–8. doi: 10.1182/blood-2012-04-421016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorfman JR, Raulet DH. Major histocompatibility complex genes determine natural killer cell tolerance. Eur J Immunol. 1996 Jan;26(1):151–5. doi: 10.1002/eji.1830260123. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005 Aug 4;436(7051):709–13. doi: 10.1038/nature03847. Epub 2005/08/05. eng. [DOI] [PubMed] [Google Scholar]
- 38.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006 Aug;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. Epub 2006/08/12. eng. [DOI] [PubMed] [Google Scholar]
- 39.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013 Feb;13(2):133–44. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002 Mar 15;295(5562):2097–100. doi: 10.1126/science.1068440. Epub 2002/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 41.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003 Aug 1;102(3):814–9. doi: 10.1182/blood-2003-01-0091. Epub 2003/04/12. eng. [DOI] [PubMed] [Google Scholar]
- 42.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005 Apr 15;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. Epub 2005/01/06. eng. [DOI] [PubMed] [Google Scholar]
- 43.North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C, et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. 2007 Jan 1;178(1):85–94. doi: 10.4049/jimmunol.178.1.85. [DOI] [PubMed] [Google Scholar]
- 44.Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, et al. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol. 2011 Dec 15;187(12):6227–34. doi: 10.4049/jimmunol.1101640. [DOI] [PubMed] [Google Scholar]
- 45.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005 Jan 1;105(1):251–8. doi: 10.1182/blood-2004-04-1422. Epub 2004/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 46.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002 Oct 15;169(8):4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 47.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002 Oct 17;419(6908):734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 48.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006 Mar 1;66(5):2520–6. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 49.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003 Aug 15;102(4):1389–96. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 50.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003 Dec 15;171(12):6891–9. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 51.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. The Journal of clinical investigation. 2004 Aug;114(4):560–8. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003 Apr 10;104(3):354–61. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 53.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004 Sep-Oct;6(5):558–68. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenner CD, King S, Przewoznik M, Wolters I, Adam C, Bornkamm GW, et al. Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol. 2010 Feb;40(2):494–504. doi: 10.1002/eji.200939937. Epub 2009/12/02. eng. [DOI] [PubMed] [Google Scholar]
- 55.Sconocchia G, Lau M, Provenzano M, Rezvani K, Wongsena W, Fujiwara H, et al. The antileukemia effect of HLA-matched NK and NK-T cells in chronic myelogenous leukemia involves NKG2D-target-cell interactions. Blood. 2005 Nov 15;106(10):3666–72. doi: 10.1182/blood-2005-02-0479. Epub 2005/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yong AS, Keyvanfar K, Hensel N, Eniafe R, Savani BN, Berg M, et al. Primitive quiescent CD34+ cells in chronic myeloid leukemia are targeted by in vitro expanded natural killer cells, which are functionally enhanced by bortezomib. Blood. 2009 Jan 22;113(4):875–82. doi: 10.1182/blood-2008-05-158253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004 Apr 1;103(7):2677–82. doi: 10.1182/blood-2003-06-2070. [DOI] [PubMed] [Google Scholar]
- 58.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012 Mar 15;72(6):1407–15. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 59.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007 Apr 1;109(7):2871–7. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 60.Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, et al. Leukemia-induced phenotypic and functional defects in natural killercells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014 Jan 31; doi: 10.3324/haematol.2013.087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamb LS, Jr, Gee AP, Henslee-Downey PJ, Geier SS, Hazlett L, Pati AR, et al. Phenotypic and functional reconstitution of peripheral blood lymphocytes following T cell-depleted bone marrow transplantation from partially mismatched related donors. Bone Marrow Transplant. 1998 Mar;21(5):461–71. doi: 10.1038/sj.bmt.1701110. Epub 1998/04/16. eng. [DOI] [PubMed] [Google Scholar]
- 62.Cooley S, McCullar V, Wangen R, Bergemann TL, Spellman S, Weisdorf DJ, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005 Dec 15;106(13):4370–6. doi: 10.1182/blood-2005-04-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morishima Y, Yabe T, Matsuo K, Kashiwase K, Inoko H, Saji H, et al. Effects of HLA allele and killer immunoglobulin-like receptor ligand matching on clinical outcome in leukemia patients undergoing transplantation with T-cell-replete marrow from an unrelated donor. Biol Blood Marrow Transplant. 2007 Mar;13(3):315–28. doi: 10.1016/j.bbmt.2006.10.027. Epub 2007/02/24. eng. [DOI] [PubMed] [Google Scholar]
- 64.Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002 Nov 15;100(10):3825–7. doi: 10.1182/blood-2002-04-1197. Epub 2002/10/24. eng. [DOI] [PubMed] [Google Scholar]
- 65.Farag SS, Bacigalupo A, Eapen M, Hurley C, Dupont B, Caligiuri MA, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006 Aug;12(8):876–84. doi: 10.1016/j.bbmt.2006.05.007. Epub 2006/07/26. eng. [DOI] [PubMed] [Google Scholar]
- 66.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007 Jul 1;110(1):433–40. doi: 10.1182/blood-2006-07-038687. Epub 2007/03/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A, et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol. 2005 Jun;129(5):631–43. doi: 10.1111/j.1365-2141.2005.05513.x. Epub 2005/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 68.Clausen J, Wolf D, Petzer AL, Gunsilius E, Schumacher P, Kircher B, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin Exp Immunol. 2007 Jun;148(3):520–8. doi: 10.1111/j.1365-2249.2007.03360.x. Epub 2007/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Penack O, Fischer L, Gentilini C, Nogai A, Muessig A, Rieger K, et al. The type of ATG matters -- natural killer cells are influenced differentially by Thymoglobulin, Lymphoglobulin and ATG-Fresenius. Transpl Immunol. 2007 Nov;18(2):85–7. doi: 10.1016/j.trim.2007.05.001. Epub 2007/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 70.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004 Jan 1;172(1):644–50. doi: 10.4049/jimmunol.172.1.644. Epub 2003/12/23. eng. [DOI] [PubMed] [Google Scholar]
- 71.Grzywacz B, Miller JS, Verneris MR. Use of natural killer cells as immunotherapy for leukaemia. Best Pract Res Clin Haematol. 2008 Sep;21(3):467–83. doi: 10.1016/j.beha.2008.07.008. Epub 2008/09/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller JS, Blazar BR. Control of acute myeloid leukemia relapse--dance between KIRs and HLA. The New England journal of medicine. Aug 30;367(9):866–8. doi: 10.1056/NEJMe1205900. Epub 2012/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 73.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010 Sep 27;207(10):2065–72. doi: 10.1084/jem.20100570. Epub 2010/09/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cook M, Briggs D, Craddock C, Mahendra P, Milligan D, Fegan C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006 Feb 1;107(3):1230–2. doi: 10.1182/blood-2005-03-1039. Epub 2005/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 75.Chen C, Busson M, Rocha V, Appert ML, Lepage V, Dulphy N, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006 Sep;38(6):437–44. doi: 10.1038/sj.bmt.1705468. Epub 2006/08/08. eng. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. The New England journal of medicine. 1985 Dec 5;313(23):1485–92. doi: 10.1056/NEJM198512053132327. Epub 1985/12/05. eng. [DOI] [PubMed] [Google Scholar]
- 77.Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O’Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995 Sep 1;76(5):824–32. doi: 10.1002/1097-0142(19950901)76:5<824::aid-cncr2820760517>3.0.co;2-n. Epub 1995/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 78.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJN, et al. Antigen Presenting Cell-Mediated Expansion of Human Umbilical Cord Blood Yields Log-Scale Expansion of Natural Killer Cells with Anti-Myeloma Activity. PLoS ONE. 2013;8(10):e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010 Feb 20;28(6):955–9. doi: 10.1200/JCO.2009.24.4590. Epub 2010/01/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008 Dec;143(5):641–53. doi: 10.1111/j.1365-2141.2008.07340.x. Epub 2008/10/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein AK, et al. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013 Feb;53(2):412–8. doi: 10.1111/j.1537-2995.2012.03764.x. quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010 Nov;59(11):1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brehm C, Huenecke S, Quaiser A, Esser R, Bremm M, Kloess S, et al. IL-2 stimulated but not unstimulated NK cells induce selective disappearance of peripheral blood cells: concomitant results to a phase I/II study. PLoS One. 2011;6(11):e27351. doi: 10.1371/journal.pone.0027351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lundqvist A, Berg M, Smith A, Childs RW. Bortezomib Treatment to Potentiate the Anti-tumor Immunity of Ex-vivo Expanded Adoptively Infused Autologous Natural Killer Cells. Journal of Cancer. 2011;2:383–5. doi: 10.7150/jca.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10(6):625–32. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 87.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009 Apr 9;113(15):3503–11. doi: 10.1182/blood-2008-08-173914. Epub 2008/12/23. eng. [DOI] [PubMed] [Google Scholar]
- 88.Fionda C, Soriani A, Malgarini G, Iannitto ML, Santoni A, Cippitelli M. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J Immunol. 2009 Oct 1;183(7):4385–94. doi: 10.4049/jimmunol.0901797. Epub 2009/09/15. eng. [DOI] [PubMed] [Google Scholar]
- 89.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008 Feb 1;111(3):1428–36. doi: 10.1182/blood-2007-07-101311. Epub 2007/11/13. eng. [DOI] [PubMed] [Google Scholar]
- 90.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001 Jul 1;98(1):210–6. doi: 10.1182/blood.v98.1.210. Epub 2001/06/22. eng. [DOI] [PubMed] [Google Scholar]
- 91.Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005 Dec 15;65(24):11712–20. doi: 10.1158/0008-5472.CAN-05-1657. Epub 2005/12/17. eng. [DOI] [PubMed] [Google Scholar]
- 92.Hsu AK, Quach H, Tai T, Prince HM, Harrison SJ, Trapani JA, et al. The immunostimulatory effect of lenalidomide on NK-cell function is profoundly inhibited by concurrent dexamethasone therapy. Blood. 2011 Feb 3;117(5):1605–13. doi: 10.1182/blood-2010-04-278432. Epub 2010/10/28. eng. [DOI] [PubMed] [Google Scholar]
- 93.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005 Jan;128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x. Epub 2005/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 94.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010 Sep 30;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. Epub 2010/05/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008 Feb 1;111(3):1309–17. doi: 10.1182/blood-2007-03-078535. Epub 2007/10/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dall’Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004 Jul 1;64(13):4664–9. doi: 10.1158/0008-5472.CAN-03-2862. Epub 2004/07/03. eng. [DOI] [PubMed] [Google Scholar]
- 97.Levy R, Weng WK. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. Journal of Clinical Oncology. 2003 Nov 1;21(21):3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 98.Vance BA, Huizinga TW, Wardwell K, Guyre PM. Binding of monomeric human IgG defines an expression polymorphism of Fc gamma RIII on large granular lymphocyte/natural killer cells. J Immunol. 1993 Dec 1;151(11):6429–39. [PubMed] [Google Scholar]
- 99.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. The Journal of clinical investigation. 1997 Sep 1;100(5):1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014 Feb 19; doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011 Aug 25;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003 Jun;4(6):557–64. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 103.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995 Apr 21;268(5209):405–8. doi: 10.1126/science.7716543. Epub 1995/04/21. eng. [DOI] [PubMed] [Google Scholar]
- 104.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati MS, Vitale M, et al. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995 May;2(5):439–49. doi: 10.1016/1074-7613(95)90025-x. Epub 1995/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 105.D’Andrea A, Chang C, Franz-Bacon K, McClanahan T, Phillips JH, Lanier LL. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J Immunol. 1995 Sep 1;155(5):2306–10. Epub 1995/09/01. eng. [PubMed] [Google Scholar]
- 106.Vivian JP, Duncan RC, Berry R, O’Connor GM, Reid HH, Beddoe T, et al. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011 Nov 17;479(7373):401–5. doi: 10.1038/nature10517. Epub 2011/10/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol. 1997 Apr 15;158(8):3603–9. Epub 1997/04/15. eng. [PubMed] [Google Scholar]
- 108.Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, et al. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci U S A. 1998 Feb 3;95(3):1172–7. doi: 10.1073/pnas.95.3.1172. Epub 1998/03/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Derre L, Corvaisier M, Pandolfino MC, Diez E, Jotereau F, Gervois N. Expression of CD94/NKG2-A on human T lymphocytes is induced by IL-12: implications for adoptive immunotherapy. J Immunol. 2002 May 15;168(10):4864–70. doi: 10.4049/jimmunol.168.10.4864. Epub 2002/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 110.Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002 Dec 2;196(11):1403–14. doi: 10.1084/jem.20020797. Epub 2002/12/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991 Apr 1;173(4):1017–20. doi: 10.1084/jem.173.4.1017. Epub 1991/04/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shum BP, Flodin LR, Muir DG, Rajalingam R, Khakoo SI, Cleland S, et al. Conservation and variation in human and common chimpanzee CD94 and NKG2 genes. J Immunol. 2002 Jan 1;168(1):240–52. doi: 10.4049/jimmunol.168.1.240. Epub 2001/12/26. eng. [DOI] [PubMed] [Google Scholar]
- 113.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999 Jul 30;285(5428):727–9. doi: 10.1126/science.285.5428.727. Epub 1999/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 114.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007 Sep;7(9):737–44. doi: 10.1038/nri2144. Epub 2007/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 115.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005 Aug 25;436(7054):1186–90. doi: 10.1038/nature03884. Epub 2005/07/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Viny AD, Clemente MJ, Jasek M, Askar M, Ishwaran H, Nowacki A, et al. MICA polymorphism identified by whole genome array associated with NKG2D-mediated cytotoxicity in T-cell large granular lymphocyte leukemia. Haematologica. 2010 Oct;95(10):1713–21. doi: 10.3324/haematol.2010.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998 Jun 15;187(12):2065–72. doi: 10.1084/jem.187.12.2065. Epub 1998/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, et al. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998 Sep 7;188(5):953–60. doi: 10.1084/jem.188.5.953. Epub 1998/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001 Feb 22;409(6823):1055–60. doi: 10.1038/35059110. Epub 2001/03/10. eng. [DOI] [PubMed] [Google Scholar]
- 120.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007 Sep 15;67(18):8444–9. doi: 10.1158/0008-5472.CAN-06-4230. Epub 2007/09/19. eng. [DOI] [PubMed] [Google Scholar]
- 121.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011 Jun;17(6):700–7. doi: 10.1038/nm.2366. Epub 2011/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 122.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997 Aug;7(2):283–90. doi: 10.1016/s1074-7613(00)80530-0. Epub 1997/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 123.Tangye SG, Cherwinski H, Lanier LL, Phillips JH. 2B4-mediated activation of human natural killer cells. Mol Immunol. 2000 Jun;37(9):493–501. doi: 10.1016/s0161-5890(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 124.Vaidya SV, Stepp SE, McNerney ME, Lee JK, Bennett M, Lee KM, et al. Targeted disruption of the 2B4 gene in mice reveals an in vivo role of 2B4 (CD244) in the rejection of B16 melanoma cells. J Immunol. 2005 Jan 15;174(2):800–7. doi: 10.4049/jimmunol.174.2.800. [DOI] [PubMed] [Google Scholar]
- 125.Howe EC, Wlodarski M, Ball EJ, Rybicki L, Maciejewski JP. Killer immunoglobulin-like receptor genotype in immune-mediated bone marrow failure syndromes. Exp Hematol. 2005 Nov;33(11):1357–62. doi: 10.1016/j.exphem.2005.07.005. Epub 2005/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 126.Shahsavar F, Tajik N, Entezami KZ, Fallah Radjabzadeh M, Asadifar B, Alimoghaddam K, et al. KIR2DS3 is associated with protection against acute myeloid leukemia. Iranian journal of immunology: IJI. 2010 Mar;7(1):8–17. Epub 2010/04/08. eng. [PubMed] [Google Scholar]
- 127.Almalte Z, Samarani S, Iannello A, Debbeche O, Duval M, Infante-Rivard C, et al. Novel associations between activating killer-cell immunoglobulin-like receptor genes and childhood leukemia. Blood. 2011 Aug 4;118(5):1323–8. doi: 10.1182/blood-2010-10-313791. Epub 2011/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 128.Besson C, Roetynck S, Williams F, Orsi L, Amiel C, Lependeven C, et al. Association of killer cell immunoglobulin-like receptor genes with Hodgkin’s lymphoma in a familial study. PLoS One. 2007;2(5):e406. doi: 10.1371/journal.pone.0000406. Epub 2007/05/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Middleton D, Diler AS, Meenagh A, Sleator C, Gourraud PA. Killer immunoglobulin-like receptors (KIR2DL2 and/or KIR2DS2) in presence of their ligand (HLA-C1 group) protect against chronic myeloid leukaemia. Tissue antigens. 2009 Jun;73(6):553–60. doi: 10.1111/j.1399-0039.2009.01235.x. Epub 2009/06/06. eng. [DOI] [PubMed] [Google Scholar]
- 130.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010 Oct 7;116(14):2411–9. doi: 10.1182/blood-2010-05-283051. Epub 2010/06/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meyer-Monard S, Passweg J, Siegler U, Kalberer C, Koehl U, Rovo A, et al. Clinical-grade purification of natural killer cells in haploidentical hematopoietic stem cell transplantation. Transfusion. 2009 Feb;49(2):362–71. doi: 10.1111/j.1537-2995.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- 132.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004 Nov;18(11):1835–8. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 133.Rizzieri DA, Storms R, Chen DF, Long G, Yang Y, Nikcevich DA, et al. Natural killer cell-enriched donor lymphocyte infusions from A 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010 Aug;16(8):1107–14. doi: 10.1016/j.bbmt.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011 Jan;13(1):98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009 Sep;1(5):753–64. doi: 10.2217/imt.09.47. [DOI] [PubMed] [Google Scholar]
- 136.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010 Dec;59(12):1781–9. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]