Abstract
Glycosylation affects many essential T cell processes and is intrinsically controlled throughout the lifetime of a T cell. CD43 and CD45 are the two most abundant glycoproteins on the T cell surface and are decorated with O- and N-glycans. Global T cell glycosylation and specific glycosylation of CD43 and CD45 are modulated during thymocyte development and T cell activation; T cells control the type and abundance of glycans decorating CD43 and CD45 by regulating expression of glycosyltransferases and glycosidases. Additionally, T cells regulate glycosylation of CD45 by expressing alternatively spliced isoforms of CD45 that have different glycan attachment sites. The glycophenotype of CD43 and CD45 on T cells influences how T cells interact with the extracellular environment, including how T cells interact with endogenous lectins. This review focuses on changes in glycosylation of CD43 and CD45 occurring throughout T cell development and activation and the role that glycosylation plays in regulating T cell processes, such as migration, T cell receptor signaling, and apoptosis.
Keywords: glycosylation, T cell, CD43, CD45
Introduction
Glycosylation of cell surface proteins on lymphocytes is a dynamic process that affects a multitude of cellular processes including pathogen recognition by the immune system,1 leukocyte migration,2 and tumor immunology.3 The addition of glycans to proteins is modulated at several levels, including cell type-specific, developmental and differentiation state-specific, and functional stimulus-specific glycosylation (reviewed in Refs. 4–9). Genes encoding glycan-modifying proteins—glycosyltransferases and glycosidases—account for 1–2% of the protein-coding genes in eukaryotic genomes,10, 11 and the expression and activity of these proteins depends on the cell type and environmental context. However, simply determining expression and activity levels of glycan-modifying enzymes in a particular cell subset will not definitively reveal whether and how specific glycan structures on specific glycoproteins are modified, because factors, such as glycosyltransferase competition for substrates in the Golgi and expression of alternatively spliced isoforms of proteins, also determine whether and how a protein will be glycosylated. Thus, to understand the range of glycans that can decorate a specific protein, it is important to consider which T cell subset expresses this glycoprotein, which isoform of this glycoprotein is expressed, and which glycan-modifying enzymes are concurrently expressed.
T cells undergo major changes in glycoprotein glycosylation during development in the thymus and activation in the periphery. Glycans found on CD43 and CD45, two abundant glycoproteins on the T cell surface, are thus dynamically modulated throughout the life of a T cell. These changes in glycosylation are crucial for proper T cell responses. In this review, we will discuss changes in glycosylation on CD43 and CD45 that occur during T cell development, activation, and survival.
Polypeptide structures of CD43 and CD45
CD43 and CD45 are two of the most abundant transmembrane glycoproteins on the T cell surface. These two proteins are expressed throughout all stages of T cell development, from double negative thymocytes to memory T cells,12 and play vital roles in T cell development, activation, and survival.13–15 CD43 and CD45 are recognized by a variety of counter-receptors, many of which are endogenous lectins produced by other immune cells, endothelial cells, and tumor cells.16 Thus, glycosylation of CD43 and CD45 is critical in regulating how T cells interact with their environment.
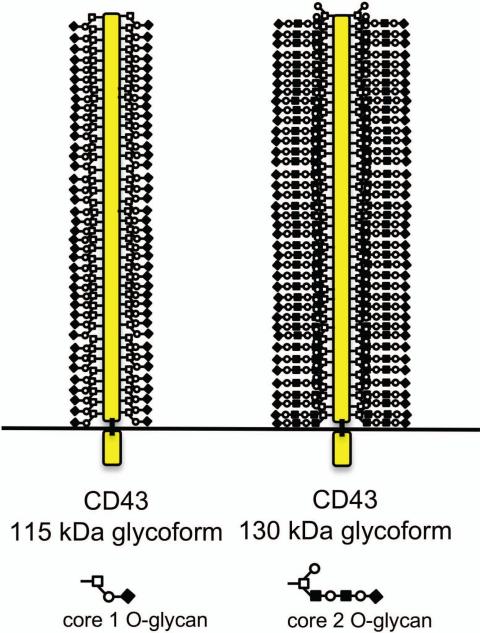
CD43 is a large, mucin-type glycoprotein with a small, globular cytoplasmic domain. The gene that codes for CD43 has only one exon, encoding a 400 amino acid polypeptide.17 The cytoplasmic domain of CD43 interacts with members of the ezrin– radixin–moesin family of cytoskeleton linker proteins to regulate T cell migration,18 activation,19, 20 and survival.21 The extracellular region of CD43 extends ~45 nm from the T cell surface and is decorated by ~80 O-linked glycans dispersed over the entire extracellular domain (Figs. 1 and 2).22 Human CD43 also has one N-glycan site, the significance of which has not been determined.23, 24 T cells express two glycoforms of CD43, with molecular weights of 115 kDa and 130 kDa due to decoration with either core 1 or core 2 O-glycans9 (Fig. 2), respectively. Thus, given that the predicted molecular weight of the CD43 polypeptide backbone is ~ 44 kDa, compared to the observed molecular weight of 115–130 kDa, ~62-66% of the mass of CD43 is constituted of glycans.
Figure 1.
Thymocytes and T cells express two glycoforms of CD43. The CD43 polypeptide backbone extends ~45 nm from the T cell surface and is decorated by ~80 O-linked glycans. Human CD43 also has one N-glycan site, which is not depicted in this schematic, as the significance of this glycan has not been determined. CD43 can be decorated by either sialylated core 1 O-glycans or core 2 O-glycans, generating the 115 kDa and the 130 kDa glycoforms of CD43, respectively. The 115 kDa glycoform of CD43 is the primary glycoform expressed by immature double negative thymocytes, mature CD4 or CD8 single positive thymocytes, and naïve peripheral T cells. The 130 kDa glycoform of CD43 is upregulated by immature double positive thymocytes and by activated peripheral T cells.
Figure 2.
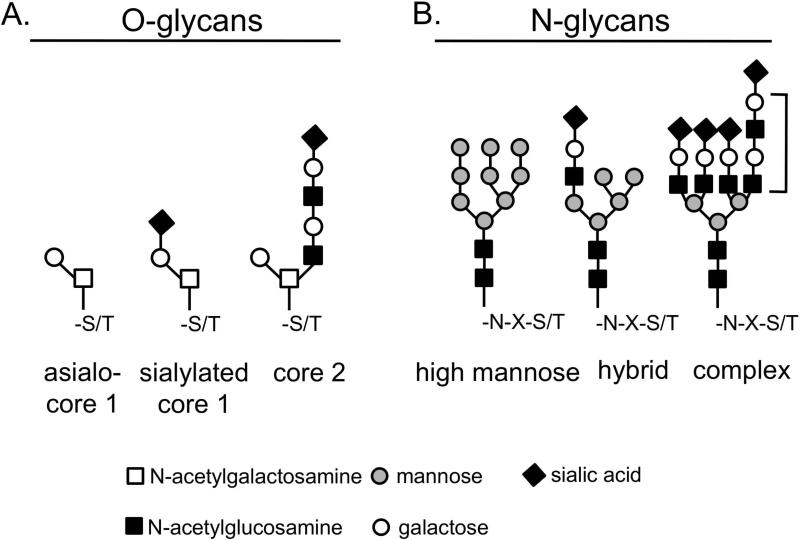
T cells decorate their glycoproteins with asialo-core 1, sialylated core 1, and core 2 O-glycans and high mannose-, hybrid-, and complex-type N-glycans. (A) Core 1 structures can be uncapped (asialo) or α2,3-sialylated by the enzyme ST3Gal1. Core 1 O-glycans can also be extended into core 2 O-glycans by the enzyme C2GnT. Core 2 O-glycans contain lactosamine (Gal-GlcNAc) sequences and may or may not also be capped by ST3Gal1 (this schematic depicts uncapped core 2 O-glycans). (B) High mannose N-glycans are precursors for hybrid-type and complex-type N-glycans. Complex N-glycans on T cells are elaborated by the action of the enzyme GnT-V, which adds a β1,6-linked GlcNAc residue that can be further extended into a polylactosamine sequence (bracket). Complex N-glycans can be capped by α2,3- or α2,6-linked sialic acid.
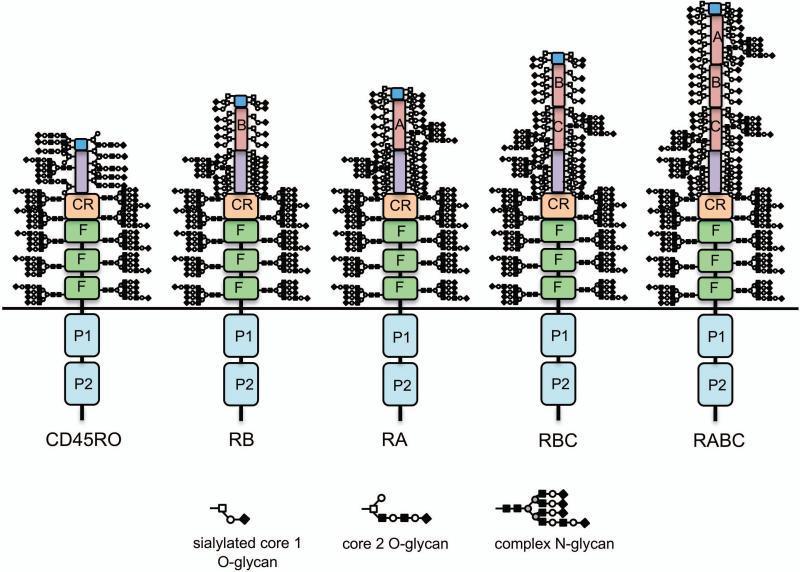
There are five isoforms of CD45 found on human lymphocytes that extend between ~28–52 nm from the T cell surface (Fig. 3).25-27 The intracellular region of CD45 is common to all CD45 isoforms and is composed of tandem phosphatase domains that are crucial for T cell receptor signaling.28, 29 Extracellularly, all five CD45 isoforms include three membrane proximal fibronectin type III repeats and a cysteine-rich region; these domains are the primary sites of N-linked glycosylation.30 The extracellular region of CD45 may contain up to three domains termed A, B, and C, resulting from alternative splicing of exons 4, 5, and 6, respectively, which are the primary sites of O-linked glycosylation.25 While there are at least eight possible isoforms of CD45, only RO (containing no alternatively spliced domains), RA, RB, RBC, and RABC polypeptides are found on human and murine lymphocytes.25, 31 Expression of alternatively spliced isoforms of CD45 is modulated throughout T cell development and activation; double negative thymocytes express RA, RB, and RBC, double positive thymocytes express primarily RO, mature single positive thymocytes express RB and RBC, naïve peripheral T cells express RB, and activated and memory T cells express RO.31–34
Figure 3.
There are five isoforms of CD45 on human lymphocytes due to alternative splicing. Intracellularly, all isoforms of CD45 have tandem phosphatase domains (P1 and P2; only P1 is enzymatically active). Extracellularly, all isoforms have three fibronectin type III repeats (F), a cysteine-rich region (CR), and a terminal region. The F, CR, and terminal regions are the primary sites of N-linked glycosylation. Alternative splicing of exons 4, 5, and 6 generates up to three additional extracellular domains—termed A, B, and C, respectively—and a total of five isoforms of CD45: RO (with no alternatively spliced domains), RB (exon 5), RA (exon 4), RBC (exons 5 and 6), and RABC (exons 4-6). The extended region and alternatively spliced regions are the primary sites of O-linked glycosylation, although RA and RC also have canonical sites of N-linked glycosylation. The schematics of the five isoforms of CD45 depict glycans based on the following assumptions: 1) The molecular weights of CD45RO, RB, RA, RBC, and RABC are 180, 190, 210, and 230, respectively; 2) Each N-glycan is complex and contributes ~4 kDa to the overall molecular weight; RO has core 2 O-glycans, each contributing ~1.5 kDa to the overall molecular weight; RB, RA, and RBC have sialylated core 1 O-glycans, each contributing ~0.7 kDa to the overall molecular weight; 3) All N-glycan sites in the F, CR, and extended regions are occupied in all isoforms; 4) Some O-glycan sites may overlap between isoforms, but the total number of O-glycans should be higher for larger isoforms (i.e., RO should have the least number of O-glycans, while RABC should have the most O-glycans); 5) Domains with more serine and threonine residues should have more potential O-glycosylation sites (i.e., RB has the least and RA has the most); and 6) The alternatively spliced domains A, B, and C should have O-glycan occupancy approximately proportional to the number of serine and threonine residues contained in the polypeptide sequences.
Glycans also contribute substantially to the overall mass of each CD45 isoform. Given that the predicted molecular weight of the CD45 polypeptide ranges between ~123 kDa and ~141kDa,35 depending on the isoform, and the observed molecular weight of CD45 ranges between 180 kDa and 230 kDa, ~32–36% of the mass of CD45 is made up of glycans. Specifically, all isoforms of CD45 are decorated with up to 11 N-glycans in the membrane proximal region, each contributing ~3–4 kDa to the overall molecular weight of CD45. The membrane distal regions of CD45 are decorated with ~8–47 O-glycans, depending on the expression of the alternatively spliced domains, each contributing ~1 kDa to the overall molecular weight of CD45.
Changes in glycosylation during development, activation, and differentiation into effector cells
O-glycans
Eight core O-glycan structures have been described.36 Thymocytes and T cells display primarily core 1 and core 2 O-glycan structures (Fig. 2 and Table 1). Core 1 O-glycans can be capped by sialic acid added by β-galactoside α2,3-sialyltransferase 1 (ST3Gal1) and/or extended into core 2 O-glycans by core 2 β-1,6-N-acetylglucosaminyltransferase (C2GnT). C2GnT adds a β1,6-linked N-acetylglucosamine (β1,6-GlcNAc) to the core 1 structure that can be further extended into a polylactosamine sequence. In the thymus, sialylated core 1 structures are found on double negative immature thymocytes and on mature CD4 or CD8 single positive thymocytes, while asialo-core 1 and core 2 structures are found on immature double positive thymocytes.16, 37–39 Peripheral naive T cells in general express sialylated core 1 O-glycans. Activation of CD4+ and CD8+ T cells results in a general loss of sialylation and an increase in core 2 O-glycans; this appears to be due primarily to de novo synthesis of hyposialylated CD4315 and CD4540 (see below and Table 1), 33, 36 although there may be microheterogeneity in sialylation at specific sites.37, 41, 42 Additionally, Grabie et al. showed that ST3Gal1 is differentially expressed during differentiation of CD4+ T cells into Th1 and Th2 effector populations following an activation stimulus.43 Specifically, Th2 cells express ST3Gal1 that can sialylate core 1 O-glycans, while Th1 cells do not express ST3Gal1 and have asialo-core 1 O-glycans.43 Both Th1 and Th2 cells express C2GnT and can bear glycoproteins decorated with core 2 O-glycans.44
Table 1.
Global, CD43-, and CD45-specific glycosylation during T cell development
| Thymus Cell type: | Double negative | Double positive | CD4 or CD8 single positive |
|---|---|---|---|
| Global O-glycans: | Sialylated core 1 | Asialo-core 1; core 2 | Sialylated core 1 |
| Global N-glycans: | High mannose; hybrid | High mannose; hybrid | α2,6-sialylated complex |
| CD43 O-glycans: | Core 1 | Core 1; core 2 | Core 1 |
| CD45 isoform: | RA, RBC, RB | RO | RB, RBC |
| CD45 O-glycans: | nda | Sialo-core 1; core 2 | nd |
| CD45 N-glycans: | High mannose; hybrid | High mannose; hybrid | α2,6-sialylated complex |
| Periphery Cell type: | Naive CD4+ or CD8+ | Activated CD8+ | Activated CD4+ | Th1 | Th2 |
|---|---|---|---|---|---|
| Global O-glycans: | Sialylated core 1 | Asialo-core 1; core 2 | Asialo-core 1; core 2 | Asialo-core 1; core 2 | Sialylated core 1 |
| Global N-glycans: | α2,6-sialylated complex | Complex | Complex | Complex | α2,6-sialylated complex |
| CD43 O-glycans: | Core 1 | Core 1; core 2 | Core 1; core 2 | Core 1; core 2 | nd |
| CD45 isoform: | RB | RO | RO | RO | RO |
| CD45 O-glycans: | Sialylated core 1 | Asialo-core 1 | asialo-core 1 | nd | nd |
| CD45 N-glycans: | α2,6-sialylated complex | Complex | Complex | Complex | nd |
Nd: not determined
As previously mentioned, CD43 is decorated with ~80 O-glycans, and on T cells, CD43 is found primarily in two glycoforms, displaying either core 1 or core 2 O-glycans. Two monoclonal antibodies, S7 and 1B11, that recognize murine CD43 decorated with core 1 or core 2 O-glycans, respectively, have been used to characterize glycoform expression of CD43 throughout thymocyte development and T cell activation. In the thymus, both immature and mature thymocytes are reactive with the S7 antibody and thus express CD43 with core 1 O-glycans, while immature double positive thymocytes are also reactive with the 1B11 antibody, and therefore also express CD43 decorated with core 2 O-glycans, consistent with the global glycosylation profile of these thymocyte subsets described above.45, 46 In the periphery, both naïve and activated T cells express core 1 O-glycosylated CD43, while activated T cells additionally express core 2 O-glycosylated CD43.37, 45, 47, 48
The alternatively spliced extracellular domains of CD45 are also decorated with core 1 and core 2 O-glycans.24, 49 Different isoforms of CD45 differ in the extent of potential O-glycosylation. Unlike S7 and 1B11 monoclonal antibodies for CD43, few CD45 antibodies have been evaluated for specific CD45 glycoform binding,41, 50, 51 and most studies use changes in global T cell glycosylation to infer how glycosylation of CD45 changes throughout T cell development and activation. Thus, immature double negative thymocytes and mature CD4 or CD8 single positive thymocytes express CD45RA/RBC/RB and CD45RB/RBC, respectively, with sialylated core 1 O-glycans, while double positive thymocytes express CD45RO decorated with asialo-core 1 and core 2 O-glycans. In the periphery, naïve T cells express CD45RB predicted to be decorated with sialylated core 1 O-glycans, while activated T cells, like double positive thymocytes, express CD45RO decorated with asialo-core 1 and core 2 O-glycans.40
N-glycans
N-glycan structures are classified as high-mannose, hybrid-type, and complex-type (Fig. 2). Thymocytes and T cells can express all three types of N-glycans (Table 1).38, 52 High-mannose N-glycan structures can be trimmed by mannosidases to create substrates for glycosyltransferases that create hybrid and complex N-glycan structures. Complex N-glycans found on T cells can be tri- or tetra-antennary structures created by the action of N-acetylglucosaminyltransferase V (GnT-V). GnT-V adds a β1,6-linked GlcNAc residue to the mannose core, which can be further extended into a polylactosamine sequence and capped by terminal sialic acid residues in either α2,3- or α2,6-linkages. In the thymus, α2,3- and α2,6-linked sialic acid is added to N-glycans by ST3Gal4 and ST6Gal1, respectively.53 Addition of sialic acid is modulated throughout thymocyte development: both cortical and medullary thymocytes bear α2,3-linked sialic acids on N-glycans, while mature medullary thymocytes also bear α2,6-linked sialic acids on N-glycans.53 Like mature thymocytes, naïve T cells that leave the thymus also express N-glycans capped with α2,6-linked sialic acid. Once activated, peripheral T cells upregulate N-glycan processing enzymes and thus increase complex N-glycans on the cell surface, while simultaneously decreasing levels of α2,6-linked sialic acid.54–56
As mentioned previously, the membrane proximal domains of CD45 that are common to all isoforms are the primary sites of N-linked glycosylation. Human CD45RO has 11 potential sites of N-glycosylation.25 Early analyses of N-glycans on CD45 revealed that roughly half of the N-glycans on CD45 on thymocytes are high mannose or hybrid type, while the majority of N-glycans on CD45 on peripheral T cells are complex type.57–60 As mentioned in the previous paragraph, the sialyltransferase ST6Gal1 modifies complex N-glycans.61, 62 CD45 is an established acceptor substrate for this enzyme.53, 63 Thus, both mature medullary thymocytes and naive peripheral T cells express CD45 with N-glycans capped with α2,6-linked sialic acid, while CD45 on activated T cells express N-glycans that are not capped with α2,6-linked sialic acid.
Glycan-dependent functions of CD43 and CD45
CD43 and CD45 interact with endogenous lectins in a glycan-dependent manner to regulate several T cell functions (Table 2). Thus, as glycans on CD43 and CD45 are modulated throughout T cell development and activation as described previously, so are the interactions with endogenous lectins and potential functional outcomes.
Table 2.
Lectin coreceptors of CD43 and CD45 on thymocytes and T cells
| CD43 coreceptor | Glycan | Function | Reference |
|---|---|---|---|
| E-selectin | Sialyl Lewis x structure on core 2 O-glycans | T cell migration | 47, 48,66 |
| Galectin-1 | Lactosaminea | T cell apoptosis | 79, 84 |
| Galectin-3 | Lactosamine | Unknown | 80 |
| Macrophage galactose-type lectin | Terminal GalNAc | Unknown | 75 |
| Mannose receptord | Mannoseb | Unknown | 60 |
| CD45 coreceptor | Glycan | Function | Reference |
|---|---|---|---|
| Galectin-1 | Lactosaminea | T cell apoptosis | 78, 79 |
| Galectin-3 | Lactosamine | T cell apoptosis | 80 |
| Placental protein 14 | Lactosaminec | T cell apoptosis | 73, 74 |
| CD22/Siglec-2 | a2,6-linked sialic acidd | T cell signaling | 67, 68 |
| Macrophage galactose-type lectin | Terminal GalNAc | T cell apoptosis | 75 |
| Serum mannan-binding protein | Mannose; GlcNAc | Unknown | 57 |
| Mannose receptor | Mannoseb | Unknown | 60 |
Binding is inhibited by a2,6-linked sialic acid
Interaction was determined for the cysteine-rich region of the mannose receptor only60
T cell migration
T cells migrate to sites of inflammation in order to generate effective immune responses. E-selectin is an adhesion protein expressed by activated endothelial cells that are important in recruiting T cells to sites of inflammation. CD43 has been identified as an endogenous coreceptor for E-selectin.64 Specifically, several groups have found that, on T cells, E-selectin binds to the 130 kDa glycoform of CD43 bearing sialyl Lewis × tetrasaccharide (SLex) on core 2 O-glycans.47, 48, 65, 66 As activated T cells upregulate CD43 bearing core 2 O-glycans, and thus the SLex glycan ligands for E-selectin, these cells can preferentially bind to E-selectin and migrate to sites of inflammation to generate an immune response.
T cell receptor signaling
T cell receptor signaling is vital for proper positive and negative selection of thymocytes and activation of peripheral T cells. Activity of the CD45 intracellular phosphatase domain is critical for regulating signaling thresholds required for signaling through the T cell receptor.29, 67, 68 There is intriguing evidence to suggest a role for glycans in regulating the activity of the CD45 intracellular phosphatase domains. Briefly, bulky N-and O-glycans and negatively charged sialic acid residues on the extracellular domains of CD45 can keep individual CD45 molecules physically separated on the plasma membrane, resulting in increased T cell receptor signaling, most likely due to activation of the intracellular phosphatase domains of CD45.69 Conversely, reduction in sialic acid content and/or binding of multivalent lectins to the extracellular domain of CD45 results in clustering or oligomerization of CD45 and decreased T cell receptor signaling;69–72 oligomerization of CD45 also has been shown to decrease activity of the intracellular phosphatase domains of CD45 in cell extracts.70 Thus, when CD45 is bound by lectins such as placental protein 14 (PP-14),73, 74 macrophage galactose-type lectin (MGL),75 or galectin-1,70 clustered CD45 molecules would be predicted to have inactive phosphatase domains and thus negatively regulate T cell receptor signaling (Table 2).
The role of CD43 in T cell receptor signaling is less clear than that of CD45. Historically, it was thought that CD43 negatively regulates T cell activation by sterically hindering interactions of T cells with antigen presenting cells via CD43's large extracellular domain.76 Recent studies have also explored the participation of the cytoplasmic domain of CD43 in T cell receptor signaling events.20, 77 However, few studies exploring the role of CD43 in T cell receptor signaling have examined the specific role of glycans on CD43 in this process.
T cell apoptosis
Specific thymocyte subsets and activated peripheral T cells undergo apoptosis induced by galectin-1, the prototypic member of the family of β-galactoside-binding lectins.78 Three glycoprotein counter-receptors, CD43, CD45, and CD7 are involved in T cell death induced by galectin-1.79–82 Expression of CD7 on human T cells is required for galectin-1-induced apoptosis.81 Although neither CD43 nor CD45 is required for galectin-1-induced apoptosis, expression of both of these glycoproteins enhances apoptosis induced by galectin-1.83, 84 Galectin-1 binding to CD43 and CD45 is dependent on the relative amount and type of glycans present on these glycoproteins. Although galectin-1 preferentially binds to lactosamine sequences that are not capped by α2,6-linked sialic acid,63 such as those found on core 2 O-glycans and complex N-glycans, galectin-1 may also bind to low affinity, yet highly abundant glycans such as core 1 O-glycans if presented in sufficient density on a polypeptide backbone (i.e., a mucin, such as CD43).70, 84 Core 2 O-glycans on CD45 appear to be required for galectin-1-induced T cell apoptosis,83, 85 while either core 1 or core 2 O-glycans on CD43 are sufficient for galectin-1 binding and apoptotic signaling.84
In the thymus, galectin-1 induces apoptosis of immature thymocytes undergoing negative selection.86 CD43 and CD45 on immature thymocytes are decorated with core 2 O-glycans and can bind galectin-1. In contrast, CD45 on mature thymocytes bears core 1 O-glycans as well as N-glycans capped with α2,6-linked sialic acid. Thus, this “glycophenotype” inhibits galectin-1 binding, due both to loss of core 2 O-glycans and increased α2,6-sialylation. Moreover, this glycosylation renders mature thymocytes resistant to galectin-1-induced apoptosis.63, 70 In the periphery, naïve T cells also express CD45 with core 1 O-glycans and α2,6-linked sialic acid, and are likewise resistant to galectin-1-induced apoptosis. Upon activation, CD4+ and CD8+ T cells express CD43 and CD45 bearing core 2 O-glycans and complex N-glycans with reduced α2,6-linked sialic acid, thus rendering activated T cells susceptible to galectin-1-induced apoptosis.70 However, CD4+ Th2 effector cells express sialylated core 1 O-glycans and α2,6-linked sialic acid on N-glycans, which reduces galectin-1 binding and decreases Th2 cell susceptibility to galectin-1-induced apoptosis.87 Thus, although thymocytes and T cells are universally exposed to galectin-1 in the thymus and peripheral tissues, specific T cell subsets are resistant to galectin-1-induced apoptosis due to differential glycosylation of CD43 and CD45.
Concluding remarks and remaining questions
T cells intrinsically regulate interactions with the extracellular environment, including those with endogenous lectins, by regulating the type and abundance of glycans on the cell surface glycoproteins CD43 and CD45. Thus, processes such as migration, T cell receptor signaling, and apoptosis, which are all affected by glycans on CD43 and CD45, are “decided” at the level of the T cell. It is therefore critical to analyze glycans on CD43 and CD45 at specific points during T cell development and activation in order to infer how a T cell might function at that point.
Currently, our understanding of thymocyte and T cell glycosylation during development and activation is largely limited to analyses of global cellular glycosylation patterns, with few analyses of glycoprotein-specific glycosylation patterns. Many questions regarding the glycosylation of CD43 and CD45 during the lifetime of a T cell remain unanswered. For instance, how do changes in the glycosylation of the extracellular domain of CD43 affect the intracellular functions of CD43? Unlike CD45, for which a role for glycans in regulating the activity of the intracellular phosphatase domains of CD45 has been suggested, few studies have evaluated the functional role of the glycans on CD43. Secondly, how are each of the individual ~80 O-glycans on CD43 and the ~11 N-glycans and ~8-47 O-glycans on CD45 modulated throughout the lifetime of a T cell? What is the role of microheterogeneity of glycans on these glycoproteins?41 Finally, how does glycosylation of CD43 and CD45 on thymocytes and T cells compare to that of CD43 and CD45 expressed by other leukocytes, such as B cells or dendritic cells? Answering these questions will bring us closer to understanding how the intricate nature of glycosylation of CD43 and CD45 regulates T cell development and function.
Acknowledgments
The authors would like to thank Drs. Sandra Thiemann, Jenny Davies, and Sam Strom for critical reading of the manuscript and Brian McMorran for assistance with the figures. This work was supported by NIH Grant HL102989 (to LGB) and NIH T32 Grant CA009056:35 (to MCC).
References
- 1.Doores KJ, et al. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci U S A. 2010;107:13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi S, et al. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T cell migration and HIV entry. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coelho V, et al. Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci U S A. 2010;107:18587–18592. doi: 10.1073/pnas.1009388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baum LG, Crocker PR. Glycoimmunology: ignore at your peril! Immunol Rev. 2009;230:5–8. doi: 10.1111/j.1600-065X.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 5.Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley P. Golgi glycosylation. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin A, Imperiali B. The expanding horizons of asparagine-linked glycosylation. Biochemistry. 2011;50:4411–4426. doi: 10.1021/bi200346n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis JW, et al. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 9.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto K, et al. Comprehensive analysis of glycosyltransferases in eukaryotic genomes for structural and functional characterization of glycans. Carbohydr Res. 2009;344:881–887. doi: 10.1016/j.carres.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K, et al. Functional states of homooligomers: insights from the evolution of glycosyltransferases. J Mol Biol. 2010;399:196–206. doi: 10.1016/j.jmb.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Jeremiah Bell J, Bhandoola A. T cell lineage determination. Immunol Rev. 2010;238:12–22. doi: 10.1111/j.1600-065X.2010.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perillo NL, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 14.Lai JC, et al. CD45 regulates migration, proliferation, and progression of double negative 1 thymocytes. J Immunol. 2010;185:2059–2070. doi: 10.4049/jimmunol.0902693. [DOI] [PubMed] [Google Scholar]
- 15.Onami TM, et al. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 16.Earl LA, Baum LG. CD45 glycosylation controls T cell life and death. Immunol Cell Biol. 2008;86:608–615. doi: 10.1038/icb.2008.46. [DOI] [PubMed] [Google Scholar]
- 17.de Laurentiis A, et al. Mass spectrometry-based identification of the tumor antigen UN1 as the transmembrane CD43 sialoglycoprotein. Mol Cell Proteomics. 2011;10:M111.007898. doi: 10.1074/mcp.M111.007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon JL, et al. CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T cell trafficking and CD43 phosphorylation. Mol Biol Cell. 2011;22:954–963. doi: 10.1091/mbc.E10-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allenspach EJ, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 20.Tong J, et al. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med. 2004;199:1277–1283. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JB, Chang J. CD43 Expression Regulated by IL-12 Signaling Is Associated with Survival of CD8 T Cells. Immune Netw. 2010;10:153–163. doi: 10.4110/in.2010.10.5.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991;1:347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- 24.Barran P, et al. Modification of CD43 and other lymphocyte O-glycoproteins by core 2 N-acetylglucosaminyltransferase. Glycobiology. 1997;7:129–136. doi: 10.1093/glycob/7.1.129. [DOI] [PubMed] [Google Scholar]
- 25.Streuli M, et al. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987;166:1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woollett GR, Williams AF, Shotton DM. Visualisation by low-angle shadowing of the leucocyte-common antigen. A major cell surface glycoprotein of lymphocytes. EMBO J. 1985;4:2827–2830. doi: 10.1002/j.1460-2075.1985.tb04010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall MN, Shotton DM, Barclay AN. Expression of soluble isoforms of rat CD45. Analysis by electron microscopy and use in epitope mapping of anti-CD45R monoclonal antibodies. Immunology. 1992;76:310–317. [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver CT, et al. CD8+ T cell clones deficient in the expression of the CD45 protein tyrosine phosphatase have impaired responses to T cell receptor stimuli. Mol Cell Biol. 1991;11:4415–4422. doi: 10.1128/mcb.11.9.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders A, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010;22:339–348. doi: 10.1016/j.cellsig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Okumura M, et al. Comparison of CD45 extracellular domain sequences from divergent vertebrate species suggests the conservation of three fibronectin type III domains. J Immunol. 1996;157:1569–1575. [PubMed] [Google Scholar]
- 31.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 32.Fujii Y, et al. CD45 isoform expression during T cell development in the thymus. Eur J Immunol. 1992;22:1843–1850. doi: 10.1002/eji.1830220725. [DOI] [PubMed] [Google Scholar]
- 33.McNeill L, et al. CD45 isoforms in T cell signalling and development. Immunol Lett. 2004;92:125–134. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Fukuhara K, et al. A study on CD45 isoform expression during T cell development and selection events in the human thymus. Hum Immunol. 2002;63:394–404. doi: 10.1016/s0198-8859(02)00379-8. [DOI] [PubMed] [Google Scholar]
- 35.Nam HJ, et al. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. J Exp Med. 2005;201:441–452. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill DJ, Clausen H, Bard F. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011;21:149–158. doi: 10.1016/j.tcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Piller F, et al. Human T-lymphocyte activation is associated with changes in O-glycan biosynthesis. J Biol Chem. 1988;263:15146–15150. [PubMed] [Google Scholar]
- 38.Balcan E, Gümüş A, Sahin M. The glycosylation status of murine [corrected] postnatal thymus: a study by histochemistry and lectin blotting. J Mol Histol. 2008;39:417–426. doi: 10.1007/s10735-008-9180-3. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie W, et al. Regulation of alpha 2,3-sialyltransferase expression correlates with conversion of peanut agglutinin (PNA)+ to PNA- phenotype in developing thymocytes. J Biol Chem. 1993;268:3801–3804. [PubMed] [Google Scholar]
- 40.Amado M, et al. Peanut agglutinin high phenotype of activated CD8+ T cells results from de novo synthesis of CD45 glycans. J Biol Chem. 2004;279:36689–36697. doi: 10.1074/jbc.M405629200. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez JD, et al. T cell activation results in microheterogeneous changes in glycosylation of CD45. Int Immunol. 2007;19:847–856. doi: 10.1093/intimm/dxm053. [DOI] [PubMed] [Google Scholar]
- 42.Chervenak R, Cohen JJ. Peanut lectin binding as a marker for activated T-lineage lymphocytes. Thymus. 1982;4:61–67. [PubMed] [Google Scholar]
- 43.Grabie N, et al. Beta-galactoside alpha2,3-sialyltransferase-I gene expression during Th2 but not Th1 differentiation: implications for core2-glycan formation on cell surface proteins. Eur J Immunol. 2002;32:2766–2772. doi: 10.1002/1521-4141(2002010)32:10<2766::AID-IMMU2766>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 44.Lim YC, et al. IL-12, STAT4-dependent up-regulation of CD4(+) T cell core 2 beta-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol. 2001;167:4476–4484. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- 45.Jones AT, et al. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 46.Ellies LG, et al. The CD43 130-kD peripheral T cell activation antigen is downregulated in thymic positive selection. Blood. 1996;88:1725–1732. [PubMed] [Google Scholar]
- 47.Matsumoto M, et al. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 48.Alcaide P, et al. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127:1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 49.Furukawa K, et al. Structural study of the O-linked sugar chains of human leukocyte tyrosine phosphatase CD45. Eur J Biochem. 1998;251:288–294. doi: 10.1046/j.1432-1327.1998.2510288.x. [DOI] [PubMed] [Google Scholar]
- 50.Pulido R, et al. Identification of amino acids at the junction of exons 3 and 7 that are used for the generation of glycosylation-related human CD45RO and CD45RO-like antigen specificities. J Exp Med. 1994;179:1035–1040. doi: 10.1084/jem.179.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cyster JG, Fowell D, Barclay AN. Antigenic determinants encoded by alternatively spliced exons of CD45 are determined by the polypeptide but influenced by glycosylation. Int Immunol. 1994;6:1875–1881. doi: 10.1093/intimm/6.12.1875. [DOI] [PubMed] [Google Scholar]
- 52.Paessens LC, et al. The glycosylation of thymic microenvironments. A microscopic study using plant lectins. Immunol Lett. 2007;110:65–73. doi: 10.1016/j.imlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Baum LG, et al. Characterization of terminal sialic acid linkages on human thymocytes. Correlation between lectin-binding phenotype and sialyltransferase expression. J Biol Chem. 1996;271:10793–10799. doi: 10.1074/jbc.271.18.10793. [DOI] [PubMed] [Google Scholar]
- 54.Chen HL, et al. T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J Biol Chem. 2009;284:32454–32461. doi: 10.1074/jbc.M109.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comelli EM, et al. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J Immunol. 2006;177:2431–2440. doi: 10.4049/jimmunol.177.4.2431. [DOI] [PubMed] [Google Scholar]
- 56.Kaech SM, et al. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 57.Uemura K, et al. A unique CD45 glycoform recognized by the serum mannan-binding protein in immature thymocytes. J Biol Chem. 1996;271:4581–4584. doi: 10.1074/jbc.271.9.4581. [DOI] [PubMed] [Google Scholar]
- 58.Sato T, et al. Structural study of the sugar chains of human leukocyte common antigen CD45. Biochemistry. 1993;32:12694–12704. doi: 10.1021/bi00210a019. [DOI] [PubMed] [Google Scholar]
- 59.Baldwin TA, Ostergaard HL. Developmentally regulated changes in glucosidase II association with, and carbohydrate content of, the protein tyrosine phosphatase CD45. J Immunol. 2001;167:3829–3835. doi: 10.4049/jimmunol.167.7.3829. [DOI] [PubMed] [Google Scholar]
- 60.Martínez-Pomares L, et al. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J Biol Chem. 1999;274:35211–35218. doi: 10.1074/jbc.274.49.35211. [DOI] [PubMed] [Google Scholar]
- 61.Joziasse DH, et al. Branch specificity of bovine colostrum CMP-sialic acid: Gal beta 1----4GlcNAc-R alpha 2----6-sialyltransferase. Sialylation of bi-, tri-, and tetraantennary oligosaccharides and glycopeptides of the N-acetyllactosamine type. J Biol Chem. 1987;262:2025–2033. [PubMed] [Google Scholar]
- 62.Weinstein J, de Souza-e-Silva U, Paulson JC. Purification of a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase and a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase to homogeneity from rat liver. J Biol Chem. 1982;257:13835–13844. [PubMed] [Google Scholar]
- 63.Amano M, et al. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 64.Merzaban JS, et al. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood. 2011 doi: 10.1182/blood-2010-11-320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuhlbrigge RC, et al. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto M, et al. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J Immunol. 2007;178:2499–2506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- 67.Stamenkovic I, et al. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 68.Sgroi D, Koretzky GA, Stamenkovic I. Regulation of CD45 engagement by the B-cell receptor CD22. Proc Natl Acad Sci U S A. 1995;92:4026–4030. doi: 10.1073/pnas.92.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 70.Earl LA, Bi S, Baum LG. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J Biol Chem. 2010;285:2232–2244. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desai DM, et al. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 72.Majeti R, et al. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279:88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 73.Rachmilewitz J, et al. Negative regulation of T cell activation by placental protein 14 is mediated by the tyrosine phosphatase receptor CD45. J Biol Chem. 2003;278:14059–14065. doi: 10.1074/jbc.M211716200. [DOI] [PubMed] [Google Scholar]
- 74.Ish-Shalom E, et al. alpha2,6-Sialylation promotes binding of placental protein 14 via its Ca2+-dependent lectin activity: insights into differential effects on CD45RO and CD45RA T cells. Glycobiology. 2006;16:173–183. doi: 10.1093/glycob/cwj053. [DOI] [PubMed] [Google Scholar]
- 75.van Vliet SJ, et al. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006;7:1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 76.Thurman EC, et al. Regulation of in vitro and in vivo T cell activation by CD43. Int Immunol. 1998;10:691–701. doi: 10.1093/intimm/10.5.691. [DOI] [PubMed] [Google Scholar]
- 77.Mattioli I, et al. Comparative analysis of T cell costimulation and CD43 activation reveals novel signaling pathways and target genes. Blood. 2004;104:3302–3304. doi: 10.1182/blood-2004-04-1536. [DOI] [PubMed] [Google Scholar]
- 78.Perillo N, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 79.Pace KE, et al. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 80.Stillman BN, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 81.Pace KE, et al. CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J Immunol. 2000;165:2331–2334. doi: 10.4049/jimmunol.165.5.2331. [DOI] [PubMed] [Google Scholar]
- 82.Bi S, et al. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283:12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen JT, et al. CD45 modulates galectin-1-induced T cell death: regulation by expression of core 2 O-glycans. J Immunol. 2001;167:5697–5707. doi: 10.4049/jimmunol.167.10.5697. [DOI] [PubMed] [Google Scholar]
- 84.Hernandez JD, et al. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol. 2006;177:5328–5336. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- 85.Cabrera PV, et al. Haploinsufficiency of C2GnT-I glycosyltransferase renders T lymphoma cells resistant to cell death. Blood. 2006;108:2399–2406. doi: 10.1182/blood-2006-04-018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perillo NL, et al. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]