Abstract
Object
Approximately 20% of patients with an intracranial saccular aneurysm report a family history of intracranial aneurysm (IA) or subarachnoid hemorrhage. A better understanding of predictors of aneurysm detection in familial IA may allow more targeted aneurysm screening strategies.
Methods
The Familial Intracranial Aneurysm (FIA) study is a multicenter study, in which the primary objective is to define the susceptibility genes related to the formation of IA. First-degree relatives (FDRs) of those affected with IA are offered screening with magnetic resonance (MR) angiography if they were previously unaffected, are ≥ 30 years of age, and have a history of smoking and/or hypertension. Independent predictors of aneurysm detection on MR angiography were determined using the generalized estimating equation version of logistic regression.
Results
Among the first 303 patients screened with MR angiography, 58 (19.1%) had at least 1 IA, including 24% of women and 11.7% of men. Ten (17.2%) of 58 affected patients had multiple aneurysms. Independent predictors of aneurysm detection included female sex (odds ratio [OR] 2.46, p = 0.001), pack-years of cigarette smoking (OR 3.24 for 20 pack-years of cigarette smoking compared with never having smoked, p < 0.001), and duration of hypertension (OR 1.26 comparing those with 10 years of hypertension to those with no hypertension, p = 0.006).
Conclusions
In the FIA study, among the affected patients’ FDRs who are > 30 years of age, those who are women or who have a history of smoking or hypertension are at increased risk of suffering an IA and should be strongly considered for screening.
Keywords: familial aneurysm, genetics, intracranial aneurysm, magnetic resonance angiography, screening study
Intracranial saccular aneurysms are acquired lesions, accounting for ~ 80% of all nontraumatic SAHs. Several uncommon heritable disorders are associated with brain aneurysms, including autosomal-dominant polycystic kidney disease,3 Marfan syndrome,17 Ehlers–Danlos syndrome Type IV,4 hereditary hemorrhagic telangiectasia,11 pseudoxanthoma elasticum, multiple endocrine neoplasia Type I, and neurofibromatosis Type 1.16 Outside of these rare heritable disorders, population-based8,18 and nonpopulation-based10,12–14 data suggest that there is an increased occurrence of IA and SAH in first- and second-degree relatives of those with SAH, with the highest risk being noted in siblings. Selected factors such as older age, female sex, history of hypertension, higher lipid levels, and elevated blood glucose have been suggested to increase the possibility of aneurysm detection in this setting.10,12
To clarify further the occurrence of IA detection on brain MR angiography studies in families with several members affected, we report the initial results of MR angiography screening of FDRs—siblings, parents, and children—of those affected with an IA in a multicenter study of familial IA. We also report the predictors of IA detection on brain MR angiography among these FDRs.
Clinical Materials and Methods
The FIA study is an international, multicenter study including 26 clinical centers, which represents 41 recruitment sites in North America, New Zealand, and Australia.2 The study was approved by the institutional review board/ethics committee at each of the study centers and recruitment sites.
Families with multiple members in whom IAs were diagnosed were enrolled to identify the chromosomal regions associated with an increased risk of IA and to determine the effects of environmental factors on the expression of genes within these regions. Individuals with either ruptured or unruptured IAs were considered to have the phenotype. Patients were excluded if they had a fusiform IA; an intranidal aneurysm with an arteriovenous malformation; a family history of polycystic kidney disease, Ehlers–Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, or moyamoya syndrome; or if informed consent could not be obtained from the patient or family members.
Detailed medical records and the information acquired by telephone screening of probands and family members with a reported history of IA or SAH were reviewed by the Verification Committee. Two neurologists independently reviewed the records and determined if the participant met all the inclusion and exclusion criteria. In cases of disagreement, a third neurologist was consulted.
Definite, probable, and possible aneurysms were defined as follows. 1) Definite: medical records document IA on cerebral angiogram, operative report, or autopsy, or a non-invasive imaging report (MR or CT angiography) demonstrates an IA measuring ≥ 7 mm in diameter. 2) Probable: death certificate notes an IA without supporting documentation or autopsy, or notes an SAH without mention of aneurysm, and data obtained during telephone screening are consistent with ruptured IA (severe headache or altered level of consciousness) rapidly leading to death. A non-invasive imaging study documents an IA that is < 7 mm but > 3 mm in diameter. 3) Possible: noninvasive imaging report documents an IA measuring between 2 and 3 mm in diameter. Death certificate notes an SAH without supporting documentation, autopsy, or recording of headache or altered level of consciousness based on information acquired during telephone screening. Death certificate lists “aneurysm” without specifying cerebral location or accompanying SAH.
Data regarding important environmental risk factors were collected. Hypertension was defined as a history of hypertension prior to the diagnosis of IA. The date of diagnosis of hypertension was recorded. History of a diagnosis of diabetes or hypercholesterolemia was recorded. Smoking history was recorded as ever/never/current smoker, and if the patient was a current or former cigarette smoker, the number of pack-years prior to diagnosis of IA was calculated. The number of alcoholic beverages consumed per day, cups of caffeinated coffee per day, highest academic grade completed, and marital status were also noted.
The FDRs of those affected with IA were offered screening with MR angiography if they were previously unaffected, ≥ 30 years of age, and had a history of smoking and/or hypertension. The FIA Imaging Center at Mayo Clinic, Rochester, Minnesota, ensured that high-quality MR angiography screening studies were performed at the imaging centers and interpreted all imaging examinations that were performed as part of the FIA study. All FIA enrolling and clinical centers were eligible to be imaging centers (see Appendix). Prior to the imaging sessions for the individuals in this study, the centers underwent an accreditation process, beginning with the center performing an MR angiography study as close to a standardized sequence as appropriate, given the vendor and field strength of the MR unit. Generally accepted parameters for intracranial MR angiography imaging have been developed over time. To assist the imaging centers with the optimization of their MR angiograms, an MR physicist and MR technologist were available for consultation at the imaging center. Accredited imaging centers included General Electric, Philips, and Siemens MR systems at 1.5-, 3.0-, and 4.0-T field strengths.
The imaging parameters for the standard protocol included a scout image of choice, a 3D time-of-flight MR angiogram, and an axial T2-weighted fast spin echo sequence. The 3D time-of-flight MR angiogram typically included 3 axial slabs of 32 sections per slab with 1.4-mm-thick sections, resulting in an imaging volume that included the posterior inferior cerebellar arteries to the bifurcation of the pericallosal and callosal marginal arteries. The axial fast spin echo T2-weighted sequence was performed with 4-mm-thick sections with zero skip and included the same imaging volume as the MR angiography sequence. The fast spin echo sequence was performed to exclude giant aneurysms with such slow flow that, due to saturation effects, these lesions might not be depicted with standard MR angiography imaging. Also, this allowed evaluation of the total size of partially thrombosed aneurysms. On completing a trial of the standard protocol, the other imaging centers forwarded the study in a DICOM format to the central FIA Imaging Center. Central imaging center review of the qualifying MR angiogram was performed to determine if changes to the imaging technique were required prior to certification.
Accredited centers were eligible to obtain images in the patients and forwarded the completed studies in a blinded manner to the imaging center. When received, the DICOM–formatted studies were postprocessed by the imaging coordinator. Maximum intensity projections of the MR angiogram were created to include the right carotid, left carotid, and posterior circulations, with 10° rotations per image. Hard-copy films of the collapsed image, maximum intensity projections, and fast spin echo images were created for archival purposes.
Two experienced neuroradiologists at the imaging center reviewed all screening MR angiograms, and cases were adjudicated to define the level of certainty of aneurysm presence as outlined. Some patients had independently undergone MR or CT angiography for their standard clinical care. These studies were also reviewed in a blinded manner by the 2 neuroradiologists.
After the imaging findings were interpreted by the 2 readers as concordant, the results were forwarded to the Coordinating Center in Cincinnati. Cases requiring adjudication included those for which the first reader detected an aneurysm that was not identified by the second reader, aneurysms detected by both readers but at different anatomical sites, or aneurysms identified by both readers in which the maximal measurement differed by > 1 mm. In these cases, a consensus interpretation by the 2 readers was completed. If a consensus adjudication could not be successfully accomplished, then an additional blinded interpretation by a third experienced neuroradiologist was performed.
Statistical Analysis
An aneurysm was considered suitable as a case for the current analysis if the diameter was ≥ 2 mm based on MR angiography findings; that is, was definite, probable, or possible. A generalized estimating equation version of multiple logistic regression was used in these analyses, with aneurysm case status as the binary outcome variable.9 The generalized estimating equation model takes into account the lack of independence of outcomes within a family by considering the members of each family as a cluster of correlated events. Several potential risk factors were considered for possible inclusion in the model, as follows: patient age, sex, history and duration of hypertension, smoking history and pack-years of cigarette smoking, marijuana use, history and amount of alcohol use, history and amount of caffeine consumption, estrogen replacement therapy, body mass index, history of diabetes or of increased cholesterol, marital status, and educational level completed. Regression diagnostics was used to assess the final model for collinearity.
Results
The MR angiography screening was performed in 303 patients, and of these 58 (19.1%) had at least 1 aneurysm (Fig. 1). Overall, 71 aneurysms were detected, with multiple lesions being detected in 10 (17.2%) of 58 patients: 1 had 4 IAs, 1 had 3 lesions, and 8 had 2. The characteristics of those with and without an IA detected on MR angiography are summarized in Table 1. Women had an IA on MR angiography in 44 (24%) of 183 screening studies, compared with 14 (11.7%) of 120 studies obtained in men. Those in whom an IA was detected had a mean age of 53.7 years compared with 50.4 years for those with negative findings on MR angiography. A history of current or prior cigarette smoking was present in 90% of those with IAs compared with 72% without lesions. The mean number of pack-years of cigarette smoking was 27.4 in those with an IA, compared with 17.8 in those without an IA. The duration of hypertension was 6.5 years in those with an IA, compared with 4.4 years in those without an IA.
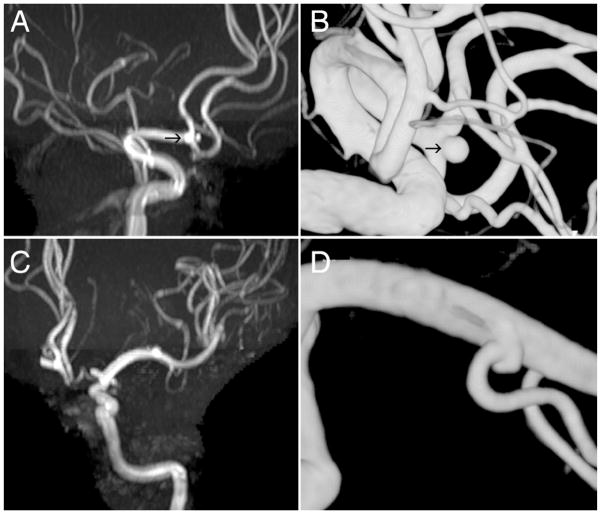
Fig. 1.
Preoperative MR angiogram (A) and DS angiogram (B) demonstrating a 2-mm ACoA aneurysm (arrows), which was successfully treated with coil embolization. Magnetic resonance angiogram (C) and DS angiogram (D) obtained in another patient of a wide-necked 2-mm aneurysm on the left M1 branch.
TABLE 1.
Risk factors in 58 patients with aneurysms on MR angiography screening compared with 246 patients with negative results
| Risk Factor | Aneurysm on MR Angiogram
|
No Aneurysm on MR Angiogram
|
||||
|---|---|---|---|---|---|---|
| Value | SD | Range | Value | SD | Range | |
| mean value | ||||||
| age (yrs) | 53.7 | 10.8 | 32–77 | 50.4 | 12.8 | 19–77 |
| alcohol-containing drinks/day | 0.47 | 0.88 | 0–4 | 0.81 | 1.68 | 0–10 |
| caffeinated coffee, cups/day | 4.94 | 4.72 | 0.5–30 | 4.43 | 3.96 | 0–27 |
| cigarette smoking, pack-years | 27.4 | 24.1 | 0–112.5 | 17.8 | 21.0 | 0–134 |
| highest grade completed | 12.2 | 2.37 | 7–20 | 13.3 | 2.84 | 5–24 |
| body mass index | 27.2 | 5.05 | 17–41 | 27.7 | 4.98 | 18–48 |
| duration of hypertension (yrs) | 6.52 | 9.84 | 0–34 | 4.43 | 8.00 | 0–35 |
| no. (%) | ||||||
| female sex | 44 (76) | 139 (57) | ||||
| ever smoked cigarettes | 52 (90) | 176 (72) | ||||
| ever smoked cigars | 1 (2) | 26 (11) | ||||
| history of diabetes | 5 (9) | 11 (5) | ||||
| history of elevated cholesterol | 18 (31) | 88 (36) | ||||
| history of hypertension | 29 (50) | 119 (48) | ||||
| marijuana use | 6 (10) | 22 (9) | ||||
SD = standard deviation.
Numerous factors were evaluated as potential predictors of IA detection on MR angiography screening. In the univariate analysis, history of cigarette smoking and pack-years of cigarette smoking were predictors of IA detection, and a higher level of education and having graduated from high school were associated with a decreased risk (Table 2). In a multivariate analysis, independent predictors of detection of IA included female sex (OR 2.46), pack-years of cigarette smoking (OR 3.24 for 20 pack-years of cigarette smoking compared with never having smoked), and duration of hypertension (OR 1.26 when comparing those with 10 years of hypertension to those with no hypertension; Table 3).
TABLE 2.
Analysis of selected risk factors as potential predictors of aneurysm detection on MR angiography screening*
| Risk Factor | OR† | SE | p Value |
|---|---|---|---|
| age at interview (per yr) | 1.02 | 0.010 | 0.097 |
| age | |||
| age (40–60 yrs) compared to <40 yrs | 1.14 | 0.259 | 0.606 |
| age (>60 yrs) compared to <40 yrs | 1.09 | 0.280 | 0.763 |
| history of hypertension | 1.02 | 0.242 | 0.845 |
| duration of hypertension (per yr) | 1.02 | 0.012 | 0.093 |
| ever smoked cigarettes | 2.85 | 0.401 | 0.009 |
| cigarette smoking pack-years (per yr) | 1.01 | 0.004 | 0.007 |
| ever smoked cigars | 0.18 | 0.992 | 0.083 |
| ever used alcohol | 0.77 | 0.257 | 0.317 |
| alcohol (drinks/day) | 0.85 | 0.099 | 0.107 |
| caffeine (cups/day) | 1.02 | 0.025 | 0.380 |
| excessive caffeine (>4 cups/day) | 1.07 | 0.270 | 0.808 |
| marijuana use | 1.13 | 0.370 | 0.735 |
| diabetes | 1.70 | 0.400 | 0.187 |
| high cholesterol | 0.83 | 0.247 | 0.457 |
| body mass index | 0.98 | 0.027 | 0.545 |
| estrogen replacement therapy‡ | 1.30 | 0.258 | 0.313 |
| currently married | 1.25 | 0.296 | 0.448 |
| highest grade completed (per grade completed) | 0.89 | 0.043 | 0.006 |
| high-school graduate | 0.57 | 0.269 | 0.039 |
| sex (F) | 2.07 | 0.280 | 0.009 |
SE = standard error.
Odds ratios per unit (or, when applicable, analysis is of risk factor history in comparison to those without a history of the factor).
Restricted to female participants.
TABLE 3.
Multivariate analysis of selected factors as potential predictors of aneurysms detected on MR angiography screening*
| Risk Factor | OR (95% CI) | SE | p Value |
|---|---|---|---|
| log (pack-years of cigarette smoking) | 3.24† (1.81–5.79) | 0.072 | <0.001 |
| sex | 2.46‡ (1.46–4.54) | 0.290 | 0.001 |
| duration of hypertension | 1.26§ (1.07–1.49) | 0.009 | 0.006 |
CI = confidence interval.
Odds ratio for 20 pack-years of cigarette smoking compared to never having smoked.
Odds ratio for females compared to males.
Odds ratio for 10 years of hypertension compared to no hypertension.
The characteristics of the lesions detected (71 IAs among 58 patients) are summarized in Table 4. Most of the aneurysms were small: 2 IAs were ≥7 mm in maximal diameter; 19 were 4–6 mm; and 50 were 2–3 mm. Aneurysms arose most commonly on the ICA, with 31 such lesions (8 OphA, and 23 other ICA), followed in frequency by the MCA (20), PCoA (8), anterior cerebral artery (7), ACoA (3), and other locations (2).
TABLE 4.
Characteristics of aneurysms detected*
| Characteristic | No. of Patients (%) | No. of Aneurysms (%) |
|---|---|---|
| size (diameter of largest aneurysm; 58 patients) | ||
| max diameter (mm) | ||
| ≥7 | 2 (3) | |
| 4–6 | 17 (29) | |
| 2–3 | 39 (67) | |
| size (all aneurysms; 71 lesions) | ||
| max diameter (mm) | ||
| ≥7 | 2 (3) | |
| 4–6 | 19 (27) | |
| 2–3 | 50 (70) | |
| location (all aneurysms; 71 lesions) | ||
| artery of origin | ||
| ICA | 31 (44) | |
| OphA | 8 (11) | |
| other ICA | 23 (32) | |
| MCA | 20 (28) | |
| PCoA | 8 (11) | |
| ACA | 7 (10) | |
| ACoA | 3 (4) | |
| other | 2 (3) | |
ACA = anterior cerebral artery.
During a mean follow-up period of 10 months (through September 1, 2006), of the 58 patients in whom aneurysms were detected, 10 patients (17%) underwent some form of surgical or endovascular intervention for 11 aneurysms (Table 5). Overall, 5 aneurysms were treated with clip ligation, 4 were treated with coils, and in 1 coil insertion was attempted unsuccessfully and surgery is planned. In addition, a 2-mm OphA aneurysm was wrapped in a patient with a 3-mm ACoA aneurysm that ruptured during follow-up.
TABLE 5.
Aneurysms detected on MR angiography screening: short-term management and outcome*
| Case No. | Aneurysm Site | Size (mm) | Treatment |
|---|---|---|---|
| 1 | rt ICA | 7 | clip ligation |
| 2 | lt OphA | 8 | coil insertion |
| 3 | rt MCA | 4 | aneurysm growth on later MR angio, clip ligation |
| 4 | ACoA | 3 | ruptured & then clip ligation |
| rt OphA | 2 | wrapped | |
| 5 | ACoA | 2 | coil-treated |
| 6 | SCA | 2 | coil-treated |
| 7 | lt MCA | 5 | attempted coil insertion unsuccessful; op planned |
| 8 | rt PCoA | 4 | coil-treated |
| 9 | lt MCA | 5 | clip ligation |
| 10 | lt MCA | 2 | clip ligation |
Eleven lesions in 10 patients were treated surgically, and 60 aneurysms in 48 patients were managed conservatively. Abbreviations: angio = angiography; SCA = superior cerebellar artery.
Both of the aneurysms that were ≥ 7 mm in maximal diameter were treated. In the 17 people with 4- to 6-mm aneurysms, in addition to the 4 with successful or attempted treatment, 4 others had confirmation of the IA on CT angiography and in 1 the lesion was confirmed on MR angiography. In the 39 patients with 2- to 3-mm aneurysms, 4 lesions were treated, 7 were also noted on a CT angiogram, and 1 was noted on a repeated MR angiogram.
Overall, among the 58 patients followed for a mean of 10 months, through September 1, 2006, 1 rupture occurred in a 3-mm ACoA aneurysm 15 months after enrollment. Given the single rupture among the 56 patients with aneurysms < 7 mm in diameter, the rupture risk was 2% per year.
Discussion
It is apparent that there is an increased risk of IA detection among FDRs of patients presenting with an IA. The degree of this increased risk, and in particular, predictors of an increased possibility of IA detection, are not clear. The results of our study suggest that, in the FIA cohort among families with at least 2 members suffering from a brain aneurysm, in the FDRs of those affected there is a relatively high risk of aneurysm detection on MR angiography screening. Overall, aneurysms were detected in 19.1% of these “high-risk” FDRs, a risk that was even higher in women than in men. This compares to a frequency of detection in the general population of 1–2%.1 It is noteworthy that, in this research study, to increase the cost-effectiveness of the MR angiography screening, only FDRs who were ≥ 30 years of age and who had a history of either hypertension or cigarette smoking were screened with MR angiography. In a multivariate model, both duration of hypertension and pack-years of cigarette smoking increased the risk of IA detection. For each 20 pack-years of cigarette smoking compared to never having smoked, there was a >3-fold increased risk of harboring an aneurysm (OR 3.24), and duration of hypertension also increased the risk, with an OR of 1.26 when comparing those with 10 years of hypertension to those with no hypertension. Sex was also important, with women being more than twice as likely to harbor an aneurysm (OR 2.46).
These data can be compared with previously reported familial screening studies. In one study of 438 individuals from 85 families without polycystic kidney disease, 38 (8.7%) had an IA. Patients were screened if > 30 years of age and if they were an FDR of someone with an IA.13 As was the case in the current study, most of the aneurysms detected were small. In the earlier study, 41 (66.1%) of 62 aneurysms confirmed on DS angiography were ≤ 6 mm in diameter, and 17 were > 7 mm in size. In the current study, only 2 (3%) of 58 aneurysms were ≥ 7 mm in diameter. In the earlier study, aneurysms for which location was reported were most commonly located on the MCA (33 [57%] of 58), a higher number than would typically be expected. In the current study, 28% were MCA aneurysms, and 44% were ICA aneurysms, which is typical of the distribution of unruptured IAs.7,19 Large screening studies have also been performed in patients with sporadic SAH. Among 626 FDRs of 160 patients with sporadic SAH, 4% of them had aneurysms (25 of 626).10,12
Another feature of the aneurysms noted in the current study, as suggested previously, was the frequency of multiple lesions, which were noted in 10 (17.2%) of 58 patients in the current study. In a previous study of characteristics of IA in patients with familial SAH, the authors noted that 26% of those with familial SAH had multiple aneurysms, compared with 10% of those with sporadic SAH.15 That study also noted that familial aneurysms were generally larger at the time of the rupture, and the patients affected were more likely to have multiple aneurysms than people with sporadic lesions. The size of the aneurysms associated with hemorrhage cannot be defined from the current study because only 1, a small ACoA aneurysm, hemorrhaged during the short course of follow-up.
Magnetic resonance angiography was the screening tool used for the current study. Previous data support the suggestion that this is a valid screening tool for IA. The inter-observer agreement in defining IA in asymptomatic patients is excellent, and more recent advances in MR angiography have further improved this interobserver agreement, even among small aneurysms.5,6 Intraarterial cerebral arteriography remains the gold standard for aneurysm detection, but does entail a low risk of stroke and other complications. Arteriographic confirmation of the aneurysm’s presence was not required for the current study. Nevertheless, 26 of the patients did have confirmation of the aneurysm, either during coil embolization, at the time of surgical intervention, or via confirmatory arteriography or CT angiography.
The available follow-up data for the current cohort reveal 1 aneurysm rupture during a short period of follow-up (mean follow-up ~ 10 months). Considering that 1 patient among 56 with a < 7-mm aneurysm had a rupture during follow-up, this would lead to an annualized rupture rate of 2%. This is higher than the 0.1% rupture risk among all unruptured aneurysms 2–6 mm in diameter in the International Study of Unruptured Intracranial Aneurysms.19 It is also noteworthy that another patient from a family with familial IA had an aneurysm rupture prior to MR angiography screening.
There are several limitations of the current study. Because the patients selected for screening were required to have either a history of hypertension or of cigarette smoking, these particular data arise from a group of relatively “high-risk” FDRs. Cerebral arteriography was not performed to confirm all aneurysms. It is recognized that there is an occurrence of false-positive and false-negative aneurysm detection on MR angiograms, especially for very small aneurysms. This is particularly important given that most of the aneurysms in this study were 2–3 mm in diameter. In the current study, 8 of these small aneurysms were confirmed at the time of either coil embolization (2 lesions), clip ligation (2), or arteriography (4). The overall frequency of false positives (or false negatives) in this study is unknown because arteriographic confirmation was not completed. In another familial IA screening study,13 of the 43 DS angiograms performed to confirm the MR angiography findings, 7 (16%) of 43 were negative, with the suspected IA later found to be a vessel loop. It is noteworthy that the prior study was performed > 10 years ago and that current MR angiography screening probably has a higher specificity and sensitivity,5,6 even for very small aneurysms.
Healthcare providers are faced with the need to recommend optimum screening strategies for patients who have familial IAs. Questions that follow in this circumstance include the possibility of detecting an aneurysm in the affected relatives if 2 or more members of the family are affected, and whether there are risk factors that increase the likelihood of abnormal results on the screening study. In our study the findings support the suggestion that among the FDRs of those affected, the risk of having an IA is considerable. The risk is accentuated by a history of hypertension, of cigarette smoking, and in female FDRs. These data further confirm that once an FDR of an affected patient reaches the age of 30 years, the risk of harboring a brain aneurysm is high and screening should be strongly considered, especially among women or those who either smoke or have hypertension. A key question that remains unanswered is whether the familial IAs behave differently from nonfamilial IAs. The short period of follow-up in the current study precludes definitive comment regarding long-term natural history, and that will be clarified with ongoing follow-up.
Conclusions
In familial IAs, among the affected patients’ FDRs who are > 30 years of age, those who are women or who have a history of smoking or hypertension are at increased risk of having a brain aneurysm revealed on screening MR angiography. These data further suggest that these FDRs are at particularly high risk and should be strongly considered for aneurysm screening.
Acknowledgments
This study was funded by grants from the National Institute of Neurological Disorders and Stroke (Grant No. R-01 NS39512).
Abbreviations used in this paper
- ACoA
anterior communicating artery
- CT
computed tomography
- DICOM
Digital Information and Communications in Medicine
- DS
digital subtraction
- FDR
first-degree relative
- FIA
Familial Intracranial Aneurysm
- IA
intracranial aneurysm
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MR
magnetic resonance
- OphA
ophthalmic artery
- OR
odds ratio
- PCoA
posterior communicating artery
- SAH
subarachnoid hemorrhage
Appendix
Clinical Centers—University of Alabama at Birmingham: W. Fisher (principal investigator [PI]); H. Forson, coordinator
Clinical Trials Research Unit, University of Auckland and Auckland City Hospital, New Zealand: C. Anderson (PI); E. Mee (PI); C. Howe, coordinator; S. Vos, coordinator
Royal Perth Hospital, Sir Charles Gairdner Hospital, Royal Adelaide Hospital, Royal Melbourne Hospital, Alfred Hospital, West-mead Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, Australia: C. Anderson (PI); G. Hankey (PI); N. Knuckey (PI); J. Laidlaw (PI); P. Reilly (PI); N. Dorsch (PI); M. Morgan (PI); M. Besser (PI); J. Rosenfeld (PI); K. Athanasiadis, coordinator; A. Claxton, coordinator; V. Dunne, coordinator; J. Griffith, coordinator; J. Davidson, coordinator; S. Pope, coordinator; Amanda Froelich, coordinator
Brigham and Women’s Hospital: A. Day (PI); R. Brach, coordinator
University of Cincinnati: D. Woo (co-PI); M. Zuccarello (co-PI); A. Ringer (co-PI); H. Yeh (co-PI); K. Franklin, coordinator
Cleveland Clinic Foundation: P. Ramussen (PI); D. Andrews-Hinders, coordinator; T. Wheeler, coordinator
Columbia University: E. S. Connolly (PI); R. Sacco (co-PI); D. LaMonica, coordinator
University of Florida: S. B. Lewis (PI); A. Royster, coordinator
Indianapolis Neurosurgical Group: T. Payner (PI); N. Miracle, coordinator
Johns Hopkins University: K. Murphy (PI); B. Kohler, coordinator
Massachusetts General Hospital: C. Ogilvy (PI); D. Buckley, coordinator; J. Manansala, coordinator
London Health Science Center Research, Inc.: G. Ferguson (PI); C. Mayer, coordinator; J. Peacock, coordinator
Notre Dame Hospital: G. Rouleau (PI); A. Desjarlais, coordinator
University of Maryland: E. F. Aldrich (PI); C. Aldrich, coordinator; C. Byard, coordinator
Mayo Clinic: R. D. Brown (PI); L. Jaeger, coordinator
University of Michigan: L. Morgenstern (PI); M. Concannon, coordinator
New Jersey Medical School: A. I. Qureshi (PI); P. Harris-Lane, coordinator
Northwestern University: H. Batjer (PI); G. Joven, S. Thompson, coordinators
University of Ottawa: M. T. Richard (PI); A. Hopper (PI)
University of Pittsburgh: A. B. Kassam (PI); K. Lee, coordinator
University of California, San Francisco: C. Johnston (PI); K. Katsura, coordinator
University of Southern California: S. Giannotta (PI); D. Fishback, coordinator
Stanford University Medical Center: G. Steinberg (PI); D. Luu, coordinator; M. Coburn, coordinator
University of Texas at Houston: M. Malkoff (PI); A. Wojner, coordinator
University of Virginia: N. Kassell (PI); B. Worrall (co-PI); G. Radakovic, coordinator
University of Washington: D. Tirschwell (PI); P. Tanzi, coordinator
Washington University: C. Derdeyn (PI); M. Catanzaro, coordinator
University of Manitoba (Winnipeg): A. Kaufmann (PI); D. Gladish, coordinator
References
- 1.Atkinson JL, Sundt TM, Jr, Houser OW, Whisnant JP. Angiographic frequency of anterior circulation intracranial aneurysms. J Neurosurg. 1989;70:551–555. doi: 10.3171/jns.1989.70.4.0551. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Sauerbeck LR, Foroud T, Huston J, III, Pankratz N, Meissner I, et al. The Familial Intracranial Aneurysm (FIA) study protocol. BMC Med Genet. 2005;6:17. doi: 10.1186/1471-2350-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman AB, Rubinstein D, Hughes R, Stears JC, Earnest MP, Johnson AM, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 4.Edwards A, Taylor GW. Ehlers-Danlos syndrome with vertebral artery aneurysm. Proc R Soc Med. 1969;62:734–735. [PubMed] [Google Scholar]
- 5.Gibbs GF, Huston J, III, Bernstein MA, Riederer SJ, Brown RD., Jr Improved image quality of intracranial aneurysms: 3.0-T versus 1.5-T time-of-flight MR angiography. AJNR Am J Neuroradiol. 2004;25:84–87. [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs GF, Huston J, III, Bernstein MA, Riederer SJ, Brown RD., Jr 3.0-Tesla MR angiography of intracranial aneurysms: comparison of time-of-flight and contrast-enhanced techniques. J Magn Reson Imaging. 2005;21:97–102. doi: 10.1002/jmri.20247. [DOI] [PubMed] [Google Scholar]
- 7.International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 8.Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. 2002;33:1321–1326. doi: 10.1161/01.str.0000014773.57733.3e. [DOI] [PubMed] [Google Scholar]
- 9.Liang KY, Zeger SL. Longitudinal data analysis using general linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 10.Magnetic Resonance Angiography in Relatives of Patients with Subarachnoid Hemorrhage Study Group. Risks and benefits of screening for intracranial aneurysms in first-degree relatives of patients with sporadic subarachnoid hemorrhage. N Engl J Med. 1999;341:1344–1350. doi: 10.1056/NEJM199910283411803. [DOI] [PubMed] [Google Scholar]
- 11.Maher CO, Piepgras DG, Brown RD, Jr, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32:877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 12.Raaymakers TW. Aneurysms in relatives of patients with sub-arachnoid hemorrhage: frequency and risk factors. MARS Study Group. Magnetic Resonance Angiography in Relatives of patients with Subarachnoid hemorrhage. Neurology. 1999;53:982–988. doi: 10.1212/wnl.53.5.982. [DOI] [PubMed] [Google Scholar]
- 13.Ronkainen A, Hernesniemi J, Puranen M, Niemitukia L, Vanninen R, Ryynanen M, et al. Familial intracranial aneurysms. Lancet. 1997;349:380–384. doi: 10.1016/S0140-6736(97)80009-8. [DOI] [PubMed] [Google Scholar]
- 14.Ronkainen A, Miettinen H, Karkola K, Papinaho S, Vanninen R, Puranen M, et al. Risk of harboring an unruptured intracranial aneurysm. Stroke. 1998;29:359–362. doi: 10.1161/01.str.29.2.359. [DOI] [PubMed] [Google Scholar]
- 15.Ruigrok YM, Rinkel GJ, Algra A, Raaymakers TW, Van Gijn J. Characteristics of intracranial aneurysms in patients with familial subarachnoid hemorrhage. Neurology. 2004;62:891–894. doi: 10.1212/01.wnl.0000115104.19787.8e. [DOI] [PubMed] [Google Scholar]
- 16.Schievink WI. Genetics and aneurysm formation. Neurosurg Clin N Am. 1998;9:485–495. [PubMed] [Google Scholar]
- 17.Schievink WI. Marfan syndrome and intracranial aneurysms. Stroke. 1999;30:2767–2768. doi: 10.1161/01.str.30.12.2759-g. [DOI] [PubMed] [Google Scholar]
- 18.Schievink WI, Schaid DJ, Michels VV, Piepgras DG. Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg. 1995;83:426–429. doi: 10.3171/jns.1995.83.3.0426. [DOI] [PubMed] [Google Scholar]
- 19.Wiebers DO, Whisnant JP, Huston J, III, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]