Abstract
Hemocytes are a key component of the mosquito immune system that kill pathogens via phagocytic, lytic and melanization pathways. Individual mosquitoes contain between 500 and 4,000 hemocytes, which are divided into three populations named granulocytes, oenocytoids and prohemocytes. Hemocytes can also be divided by their anatomical location with 75% of hemocytes circulating in the hemocoel (circulating hemocytes) and 25% of hemocytes attaching themselves to tissues (sessile hemocytes). Greater than 85% of the hemocytes in adult mosquitoes are granulocytes, which primarily kill pathogens by phagocytosis or lysis. Oenocytoids, on the other hand, are the major producers of the enzymes required for melanization while prohemocytes are small cells that participate in phagocytosis. Both circulating and sessile hemocytes engage in defense against pathogens. The circulatory system of mosquitoes also interacts with hemocytes and facilitates elimination of potential pathogens that enter the hemocoel.
Keywords: Mosquito, immunity, hemocyte, phagocytosis, melanization, lysis
1. Introduction
Like other insects, mosquitoes (family Culicidae) are continuously exposed to different microbes including some that cause disease. The juvenile stages of mosquitoes are aquatic while adults are terrestrial. Potential pathogens infect juvenile and adult mosquitoes by either oral ingestion or entry through the cuticle. The vast majority of mosquito species are also anautogenous, which means that adult females must ingest a blood meal from a vertebrate host to obtain nutrients necessary for the production of eggs. Blood feeding exposes mosquitoes to microorganisms that cause disease in the vertebrate host, and in some cases mosquitoes transmit these organisms when they blood feed on a new host. In the case of humans, mosquitoes transmit several major disease-causing organisms including the causative agents of malaria, lymphatic filariasis and dengue fever [1].
The innate immune system of mosquitoes is divided into cellular and humoral components that provide defense against a range of microbes including species that mosquitoes transmit to vertebrates. The cellular arm of the immune system consists of immune cells called hemocytes [2,3], while humoral components refer to soluble molecules in hemolymph such as pattern recognition receptors, antimicrobial peptides, and components of the phenoloxidase cascade [2,4-6]. The line between cellular and humoral immunity is also somewhat subjective given that hemocytes produce many humoral immune molecules, while many humoral molecules play important roles in regulating cellular immune defenses [7-12]. In this review, we summarize our understanding of the biology of hemocytes in mosquitoes including their classification, functions, and interactions with the circulatory system.
2. Hemocyte types
Hemocytes in mosquitoes and other insects form distinct populations that are classified using two non-exclusive criteria. The first classifies hemocytes by morphological, enzymatic, cytochemical and functional characteristics [13,14], while the second divides hemocytes by their anatomical location, with cells in hemolymph being called circulating hemocytes and cells attached to tissues being called sessile hemocytes [15,16]. Recent studies also clearly show that circulating hemocytes can become sessile and vice versa [17,18].
On the basis of light microscopic morphological analyses, studies have divided mosquito hemocytes into populations named plasmatocytes, oenocytoids, granulocytes, granular cells, and adipohemocytes [14,19-25]. Hillyer and Christensen [26] used light microscopy, electron microscopy, enzyme activity assays, and lectin binding assays to classify mosquito hemocytes into granulocytes, oenocytoids, adipohemocytes and thrombocytoids. Functional and comparative studies thereafter concluded that adipohemocytes and thrombocytoids are not hemocytes, but instead are fat body and pericardial cells, respectively, that were inadvertently collected during the hemolymph extraction process [2,27,28]. Castillo et al. [27] then concluded that mosquito hemocytes consist of granulocytes and oenocytoids plus a population of smaller cells called prohemocytes. This latter study also showed that mosquito hemocytes previously referred to as plasmatocytes are not a distinct cell type, but are instead granulocytes. Thus, it is now generally accepted that mosquitoes contain three populations of hemocytes: granulocytes, oenocytoids, and prohemocytes (Figs. 1 and 2).
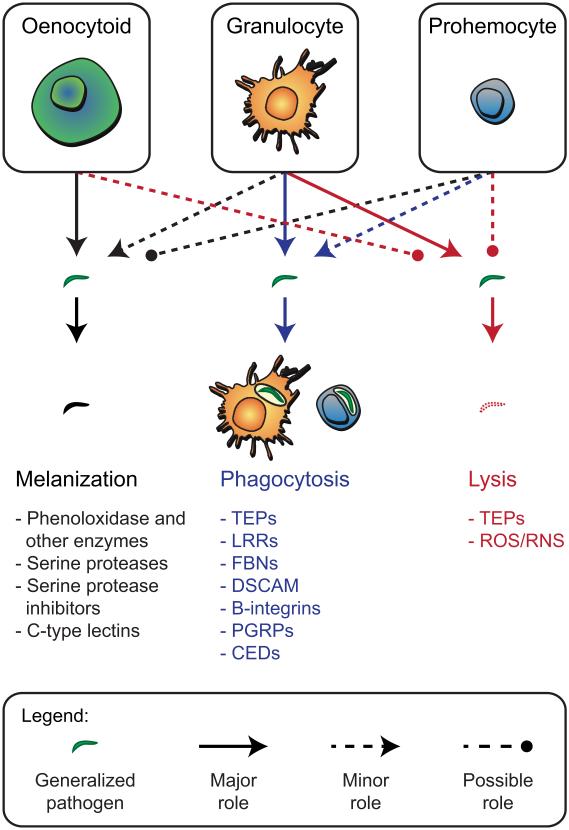
Figure 1.
Morphological and functional classification of circulating hemocytes. The upper portion of the figure shows granulocytes, oenocytoids and prohemocytes, while the lower portion lists gene products identified from mosquitoes with roles in three immune defense functions: melanization, phagocytosis, or lysis of pathogens. Solid arrows indicate hemocyte types with major roles in each of these defenses, dotted arrows indicate minor involvement, and dotted lines with circles indicate possible involvement.
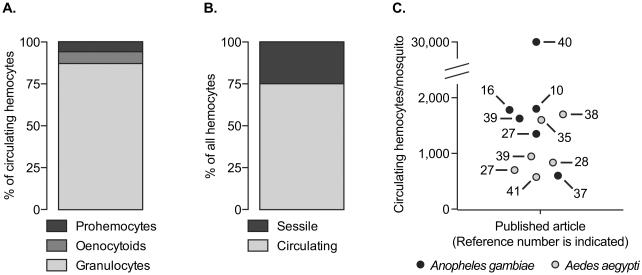
Figure 2.
The abundance of different hemocyte types in mosquitoes. A. Proportional distribution of granulocytes, oenocytoids and prohemocytes in the circulating hemocyte population. The data used for this graph originates primarily from Castillo et. al [27] and Baton et. al [10]. B. Proportional distribution of circulating and sessile hemocytes in the body of 6-day-old A. gambiae. These data were adapted from King and Hillyer [16]. C. Average number of total circulating hemocytes in mosquitoes as reported in different published articles. The numbers inside the graph represent the article number in the reference list, and black and grey circles denote A. gambiae and A. aegypti, respectively. When the article reported data on multiple treatment groups, the hemocyte numbers denote naïve mosquitoes at 3-7 days post-emergence.
Granulocytes are the most abundant hemocyte type, comprising 80-95% of the circulating hemocyte population. These cells are polymorphic and their cytoplasm contains varying amounts of membrane-delimited vesicles [26,29]. Granulocytes in circulation measure approximately 9 μm in diameter, but ex vivo these cells readily attach to foreign surfaces such as glass and spread to diameters that may exceed 35 μm. Granulocytes are distinguished from oenocytoids by their strong acid phosphatase activity, the presence of the chitin binding serine protease SP22D, and the strong binding of the lectins wheat germ agglutinin, Helix pomatia agglutinin and Galnathus nivales lectin [26,27,29]. Granulocytes are also highly phagocytic, whereas oenocytoids are not [28-32].
Oenocytoids comprise ≤10% of the circulating hemocyte population. They are round cells that measure approximately 9 μm in diameter but do not readily spread on foreign surfaces. They contain an eccentric nucleus and a homogenous cytoplasm, but their defining characteristic is that they are the major producers of phenoloxidase [26,27,29], which is the rate-limiting enzyme in the melanization immune pathway [4].
Prohemocytes comprise ≤10% of the circulating hemocyte population and are spherical cells that measure 4-6 μm in diameter. They are characterized by a high nuclear to cytoplasm ratio, and have been hypothesized to function as progenitor cells [27]. However, a recent study showed that these small hemocytes are phagocytic, and that they may arise from the asymmetric division of granulocytes [16]. This suggests that prohemocytes are fate restricted and not multipotent stem cells.
From an anatomical perspective, approximately 75% of the hemocytes in adult mosquitoes are in circulation while 25% are sessile (Fig. 2) [16]. Sessile hemocytes are distributed throughout the abdominal wall, the thoracic wall, the head, the maxillary palps, the legs, the midgut and the Malpighian tubules [16,17,33,34]. Of these locations, the vast majority of sessile hemocytes (65-78%) are present on the abdominal wall, including the tracheoles and the outer surface of the heart [16,17]. Similar to the hemocytes in circulation the vast majority of sessile hemocytes are also granulocytes.
3. Circulating hemocyte numbers
The number of circulating hemocytes in adult mosquitoes decreases with age [27,28,35,36], increases after blood feeding [37,38], and depending on the mosquito-pathogen combination, may increase, decrease or remain the same following infection [10,16,28,35,36,39,40]. While these trends are clear, the total number of hemocytes in circulation has recently received a significant amount of attention (Fig. 2). Studies conducted by multiple laboratories over a period of three decades have all shown that adult mosquitoes contain between 500 and 4,000 circulating hemocytes [10,16,27,28,35,37-39,41], with Anopheles gambiae containing more hemocytes than Aedes aegypti [27,39]. However, a recent paper on A. gambiae reported that mosquitoes contain between 25,000 and 40,000 circulating hemocytes [40], but a different research group using identical methodology was unable to replicate this finding [37]. A range of 500-4,000 circulating hemocytes in adult mosquitoes is also in agreement with the density of circulating hemocytes reported in other insect species. For example, adult D. melanogaster contain between 1000 and 2000 circulating hemocytes, adult Glossina morsitans contain 500-900 hemocytes per μl of hemolymph, and several other dipteran, lepidopteran and orthopteran insects contain hemocyte densities in this same range [42-47].
Studies reporting that mosquitoes contain between 500 and 4,000 circulating hemocytes roughly divide them into 80-95% granulocytes, ≤10% oenocytoids and ≤10% prohemocytes [26,27,29]. Studies that have recognized additional hemocytes types in mosquitoes also report that oenocytoids and prohemocytes comprise only a small proportion of the total hemocytes in circulation [23-25,36]. Collectively, these data agree with data from D. melanogaster, several species of Lepidoptera and other arthropods, where consistently only a small proportion of circulating hemocytes are identified as oenocytoids (called crystal cells in Drosophila) and prohemocytes [15,43,48]. However, these patterns differ strongly from Rodrigues et al. [40], which in addition to reporting that A. gambiae adults contain between 25,000 and 40,000 circulating hemocytes, also divide them into 2% granulocytes, 38% oenocytoids and 60% prohemocytes. Given these differences, it is likely the number of circulating hemocytes in mosquitoes will continue to be scrutinized.
4. Hematopoiesis
Hemocytes in mosquitoes have mostly been studied in adults, whereas studies in D. melanogaster and Lepidoptera have examined the origin, proliferation and maintenance of hemocyte populations in all life stages. In D. melanogaster, hemocytes originate during embryogenesis from head mesoderm [49]. During the larval stages of Drosophila and Lepidoptera, these embryonic hemocytes, both in circulation and in sessile form, replicate by autonomous cell division [48,50-52]. Additional hemocytes in larvae are produced from hematopoietic organs called lymph glands that derive from thoracic mesoderm, with the majority of these hemocytes being released into the hemocoel during the larva to pupa transition [3,53]. The lymph gland degenerates in the pupa, which leaves circulating and sessile hemocytes as the only possible sources for the production of new hemocytes. In adult Drosophila, hemocytes are not thought to replicate, resulting in an age-dependent decrease in hemocyte numbers [15].
No hematopoietic organs have been identified in adult mosquitoes, but hemocyte numbers clearly increase in response to infection by certain organisms and following a blood meal [10,16,35,37-39]. This increase in hemocyte numbers is primarily due to mitosis by circulating hemocytes and not the release of sessile hemocytes into circulation [16]. Furthermore, at least two pathways drive hemocyte replication: insulin-like signaling and Ras-MAPK signaling [37,38].
5. Phagocytosis by hemocytes
In insects, hemocyte-mediated immune responses include phagocytosis, encapsulation and nodulation. Phagocytosis is an evolutionarily conserved process that hemocytes exhibit in response to bacteria and other small foreign entities (Fig. 1). Here, the foreign body is recognized by humoral pattern recognition receptors (PRRs) that function as opsonins, and/or PRRs on the surface of hemocytes. The foreign body is then internalized into a membrane-delimited phagosome, the phagosome fuses with a lysosome, and the microbe is hydrolytically digested. Encapsulation and nodulation, in contrast, involve the irreversible binding of multiple hemocytes to the surface of large foreign objects or bacterial aggregates, respectively, to form a multicellular sheath [54]. Hemocytes from Lepidoptera and many other insects readily phagocytose a diversity of single cell microbes like bacteria and protozoa, while also forming capsules around multicellular parasites like nematodes and parasitoid wasps.
Mosquito hemocytes also phagocytose a range of bacteria and other small foreign entities yet have rarely been reported to form cellular capsules. Mosquito granulocytes, and to a lesser extent prohemocytes, are highly phagocytic and initiate this process within 5 min of pathogen exposure [16,29,32]. Up to 95% of circulating hemocytes are phagocytic, and it has been estimated that some hemocytes can phagocytose hundreds of bacteria within 24 h of infection [28,30]. This immune process is effective in the sequestration of bacteria, yeast, Plasmodium and small inanimate particles [25,29-32,55], and is carried out by both the circulating and sessile populations [16,17]. The strong effectiveness of the phagocytic response in adult mosquitoes has also been linked to their small body size [56].
Several mosquito PRRs are involved in regulating phagocytosis. The most studied of these factors is the complement-like protein, thioester containing protein 1 (TEP1). Following proteolytic activation, TEP1 opsonizes bacteria and targets them for phagocytosis [57]. Other PRRs with roles in phagocytosis include additional members of the TEP protein family (TEP3, TEP4), leucine rich repeat containing proteins (LRRs; LRIM1), fibrinogen-related proteins (FBN8), and DSCAM, which is a hypervariable immunoglobulin that can be transpliced into over 31,000 variants [58-60]. Although some of these proteins opsonize pathogens, others likely act upstream of the opsonization response (e.g., LRIM1 [61]). For some pathogens, deposition of melanin onto the surface of the foreign entity is also required prior to the initiation of phagocytosis [29,31,32].
Transmembrane receptors that are expressed on the surface of hemocytes and are implicated as PRRs include a β integrin (BINT2), a peptidoglycan recognition protein (PGRPLC), a low-density lipoprotein receptor-related protein (LRP1), and a protein containing both zinc finger and LITAF domains [59,60]. These factors may recognize pathogens directly or recognize pathogens after they have been opsonized by humoral factors. Finally, the intracellular proteins CED2, CED5 and CED6 regulate the internalization of pathogens. TEP1-, TEP3-, LRIM1- and LRP1-mediated phagocytosis occurs through the CED6 pathway, whereas TEP4- and BINT2-mediated phagocytosis occurs through the CED2/CED5 pathway [60].
6. Hemocyte-mediated humoral responses: melanization and lysis
Hemocytes produce factors with roles in humoral immune responses (Fig 1). These include components of the melanization response, which is primarily regulated by a network of proteases referred to as the phenoloxidase (PO) cascade. Activation of the PO cascade results in conversion of tyrosine to melanin [4]. Associated cross-linking of proteins also results in the deposition of melanin on the surface of pathogens, which in the mosquito literature is often referred to as melanotic encapsulation [4,62]. Mosquito hemocytes produce several factors implicated in the melanization response including multiple phenoloxidases, phenylalanine hydroxylase, dopachrome conversion enzyme, serine proteases, serine protease inhibitors and C-type lectins [7-12]. These enzymes together with PRRs are also considered essential in formation of melanotic capsules [4,26,27,29,63-68]. In some insects, oenocytoids lyse upon immune challenge to release PO and other components of the PO cascade that lack signal peptides for secretion. In contrast, it remains unclear how PO is released from mosquito hemocytes.
Mosquito hemocytes also produce cytotoxic effector molecules. For example, hemocyte-produced Tep1 binds to Plasmodium parasites and targets them for killing [57,61,69,70]. Tep1 exists in hemolymph as part of a protein complex composed of Tep1 and the leucine-rich repeat containing proteins, LRIM1 and APL1. Plasmodium infection causes dissociation of the complex, freeing Tep1 to bind and lyse pathogens. Other lytic factors, such as antimicrobial peptides and reactive oxygen and nitrogen intermediates are also produced by hemocytes [7-12,68]. Production of nitric oxide, for example, increases in hemocytes following infection, and is involved in the killing of bacteria and malaria parasites [71,72]. Lastly, cytotoxic intermediates generated by the PO cascade directly kill viruses in mosquitoes and other insects [73,74].
7. Wound healing
Some insects close external wounds by means of a clotting reaction called coagulation [75]. In mosquitoes, the coagulation response includes: (1) the lysis of granulocytes at the wound site, (2) the deposition of melanin, (3) the binding of additional hemocytes, and (4) the regeneration of the epidermis [76]. However, other than detecting hemocyte-produced prophenoloxidase at wound sites [77], nothing is known about other molecules mosquito hemocytes produce during the wound healing response.
8. Hemocytes and hemolymph circulation
Hemocytes are propelled throughout the hemocoel by the mosquito’s circulatory system (Fig. 3). Anatomically, the mosquito circulatory system consists of hemolymph (hemocytes plus plasma), the hemocoel, and a series of muscular pumps. The primary pump is the dorsal vessel, which is a tube-like structure that extends along the dorsal midline of the insect and is divided into a thoracic aorta and an abdominal heart [78]. Hemolymph enters the dorsal vessel through paired valves, called ostia, which are located at the thoraco-abdominal junction and at the anterior portion of each abdominal segment. Once inside the dorsal vessel, hemolymph is sequentially propelled in both the anterograde (toward the head) and retrograde (toward the posterior abdomen) directions until it is discharged into the hemocoel through excurrent openings located at the distal ends of the insect. Hemolymph then flows back toward the ostia, at which point it re-enters the heart and completes its circulatory cycle [78,79].
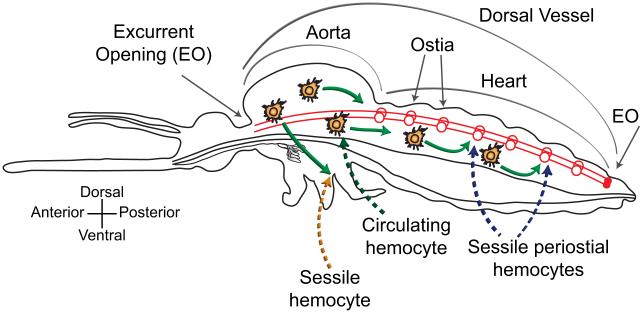
Figure 3.
Hemolymph circulation and hemocyte-based immunity. Hemolymph enters the dorsal vessel (heart and aorta; red lines) through ostia (red circles) and is propelled in anterograde and retrograde directions. Hemocytes either circulate (green arrows showing extracardiac movement during anterograde heart flow) or are sessile. In response to infection, hemocytes aggregate in the regions surround the ostia, thus becoming periostial hemocytes. This diagram is based on the heart physiology study of Glenn et. al [78], and the immunity work of King and Hillyer [16,17].
Pathogens that enter the hemocoel can be encountered by either hemocytes in circulation or by sessile hemocytes surveying the hemolymph as it flows by them. Sessile hemocytes have the highest probability of encountering pathogens when they are located in areas of high hemolymph flow. While sessile hemocytes are scattered throughout the hemocoel, the only location they consistently aggregate in mosquitoes is the surface of the heart [16,17]. Sessile hemocytes flanking regions surrounding the ostia are called periostial hemocytes, and rapidly phagocytose bacteria and malarial parasites within seconds of entry into the hemocoel. As an infection progresses, circulating hemocytes also move into periostial regions, where they bind the cardiac musculature and each other, and amplify the phagocytosis response. Periostial hemocyte aggregation occurs in a time- and infection dose-dependent manner, and once this process is triggered, the number of periostial hemocytes remains elevated for the mosquito’s lifetime [17]. The periostial regions are the only locations of the hemocoel where circulating pathogens accumulate, and are also the only locations where hemocytes aggregate in response to infection [16,17]. This immune response represents a co-adaptation of the mosquito circulatory and immune systems.
9. Concluding remarks
The current literature clearly indicates that hemocytes are an essential component of the mosquito immune system. However, several aspects of hemocyte biology in mosquitoes remain unclear. First, the vast majority of what we know about hemocyte function derives from only three species: A. gambiae, A. aegypti, and Armigeres subalbatus. However, with an estimated 3,500 species of mosquitoes, it is uncertain how representative these data are for the Culicidae as a whole. Second, while recent papers clearly show that hemocytes are capable of proliferating in adults [16,37,38], virtually nothing is known about the developmental origin of these cells in embryos and/or larvae, nor the regulatory pathways that regulate the differentiation of hemocytes into morphologically and functionally distinct populations. In addition, almost no literature exists at present on the function of mosquito hemocytes during the immature stages. Third, although we have begun to understand the molecular basis of hemocyte-mediated immunity [7-12], these studies have ignored the sessile population of hemocytes, and have failed to distinguish the differential role that granulocytes, oenocytoids and prohemocytes play in the infection response. Fourth, hemocyte-mediated immunity has so far been studied in the context of bacterial, malarial, or filarial nematode infection. However, understanding of how mosquito hemocytes recognize these entities as foreign, the signaling pathways activated in hemocytes, and effector responses produced remain incompletely understood. In addition, while some viruses infect mosquito hemocytes [80], what role if any hemocytes play in antiviral defense is unknown. Emerging molecular and imaging methodologies will undoubtedly continue to illuminate the important role these cells play in controlling infection.
Highlights.
A major component of the mosquito immune system are hemocytes
Hemocytes are anatomically divided into circulating and sessile hemocytes
Hemocytes are functionally divided into granulocytes, oenocytoids and prohemocytes
Hemocytes kill pathogens via phagocytosis, melanization and lysis
10. Acknowledgments
This work was supported by NSF grants IOS-1051636 and IOS-1257936 to JFH, and NIH grant R01AI033108 to MRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
11. References
- 1.Becker N, Petric D, Zgomba M, Boase C, Dahl C, Madon M, Kaiser A. Mosquitoes and their control. 2nd Springer-Verlag; New York: 2010. [Google Scholar]
- 2.Hillyer JF. Mosquito immunity. Adv Exp Med Biol. 2010;708:218–238. doi: 10.1007/978-1-4419-8059-5_12. [DOI] [PubMed] [Google Scholar]
- 3.Strand MR. The insect cellular immune response. Insect Science. 2008;15:1–14. [Google Scholar]
- 4.Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–199. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Levashina EA. Immune responses in Anopheles gambiae. Insect Biochem Mol Biol. 2004;34:673–678. doi: 10.1016/j.ibmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Dong Y, Garver L, Dimopoulos G. Specificity of the innate immune system: a closer look at the mosquito pattern-recognition receptor repertoire. In: Rolff J, Reynolds SE, editors. Insect infection and immunity: evolution, ecology, and mechanisms. Oxford University Press; 2009. pp. 69–85. [Google Scholar]
- 7.Aliota MT, Fuchs JF, Mayhew GF, Chen CC, Christensen BM. Mosquito transcriptome changes and filarial worm resistance in Armigeres subalbatus. BMC Genomics. 2007;8:463. doi: 10.1186/1471-2164-8-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartholomay LC, Cho WL, Rocheleau TA, Boyle JP, Beck ET, Fuchs JF, Liss P, Rusch M, Butler KM, Wu RC, et al. Description of the transcriptomes of immune response-activated hemocytes from the mosquito vectors Aedes aegypti and Armigeres subalbatus. Infect Immun. 2004;72:4114–4126. doi: 10.1128/IAI.72.7.4114-4126.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * First high throughput transcriptomic analysis of mosquito hemocytes.
- 9.Bartholomay LC, Mayhew GF, Fuchs JF, Rocheleau TA, Erickson SM, Aliota MT, Christensen BM. Profiling infection responses in the haemocytes of the mosquito, Aedes aegypti. Insect Mol Biol. 2007;16:761–776. doi: 10.1111/j.1365-2583.2007.00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Baton LA, Robertson A, Warr E, Strand MR, Dimopoulos G. Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics. 2009;10:257. doi: 10.1186/1471-2164-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Transcriptomic analysis of the hemocyte response to Plasmodium infection.
- 11.Choi YJ, Fuchs JF, Mayhew GF, Yu HE, Christensen BM. Tissue-enriched expression profiles in Aedes aegypti identify hemocyte-specific transcriptome responses to infection. Insect Biochem Mol Biol. 2012;42:729–738. doi: 10.1016/j.ibmb.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto SB, Lombardo F, Koutsos AC, Waterhouse RM, McKay K, An C, Ramakrishnan C, Kafatos FC, Michel K. Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc Natl Acad Sci U S A. 2009;106:21270–21275. doi: 10.1073/pnas.0909463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Transcriptomic analysis of the hemocyte response to Plasmodium infection.
- 13.Jones JC. Current concepts concerning insect hemocytes. American Zoologist. 1962;2:209–246. [Google Scholar]
- 14.Kaaya GP, Ratcliffe NA. Comparative study of hemocytes and associated cells of some medically important dipterans. J Morphol. 1982;173:351–365. doi: 10.1002/jmor.1051730310. [DOI] [PubMed] [Google Scholar]
- 15.Honti V, Csordas G, Kurucz E, Markus R, Ando I. The cell-mediated immunity of Drosophila melanogaster: hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol. 2014;42:47–56. doi: 10.1016/j.dci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 16.King JG, Hillyer JF. Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: hemocyte mitosis following infection. BMC Biol. 2013;11:55. doi: 10.1186/1741-7007-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** Comprehensive map of circulating and sessile hemocytes in naïve and infected mosquitoes, and description of mitosis by circulating hemocytes.
- 17.King JG, Hillyer JF. Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog. 2012;8:e1003058. doi: 10.1371/journal.ppat.1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** Characterization of hemocyte-mediated immune responses on the surface of the heart.
- 18.Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreadis TG, Hall DW. Neoaplectana carpocapsae: encapsulation in Aedes aegypti and changes in host hemocytes and hemolymph proteins. Exp Parasitol. 1976;39:252–261. doi: 10.1016/0014-4894(76)90125-9. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez S, Lanz H, Rodriguez MH, Torres JA, Martinez-Palomo A, Tsutsumi V. Morphological and cytochemical characterization of female Anopheles albimanus (Diptera: Culicidae) hemocytes. J Med Entomol. 1999;36:426–434. doi: 10.1093/jmedent/36.4.426. [DOI] [PubMed] [Google Scholar]
- 21.Drif L, Brehelin M. The circulating hemocytes of Culex pipiens and Aedes aegypti: cytology, histochemistry, hemograms and functions. Developmental and Comparative Immunology. 1983;7:687–690. [Google Scholar]
- 22.Wang Z, Lu A, Li X, Shao Q, Beerntsen BT, Liu C, Ma Y, Huang Y, Zhu H, Ling E. A systematic study on hemocyte identification and plasma prophenoloxidase from Culex pipiens quinquefasciatus at different developmental stages. Exp Parasitol. 2011;127:135–141. doi: 10.1016/j.exppara.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Araujo HC, Cavalcanti MG, Santos SS, Alves LC, Brayner FA. Hemocytes ultrastructure of Aedes aegypti (Diptera: Culicidae) Micron. 2008;39:184–189. doi: 10.1016/j.micron.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Brayner FA, Araujo HR, Cavalcanti MG, Alves LC, Peixoto CA. Ultrastructural characterization of the hemocytes of Culex quinquefasciatus (Diptera: Culicidae) Micron. 2005;36:359–367. doi: 10.1016/j.micron.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Da Silva JB, De Albuquerque CM, De Araujo EC, Peixoto CA, Hurd H. Immune defense mechanisms of Culex quinquefasciatus (Diptera: Culicidae) against Candida albicans infection. J Invertebr Pathol. 2000;76:257–262. doi: 10.1006/jipa.2000.4980. [DOI] [PubMed] [Google Scholar]
- 26.Hillyer JF, Christensen BM. Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti. Histochem Cell Biol. 2002;117:431–440. doi: 10.1007/s00418-002-0408-0. [DOI] [PubMed] [Google Scholar]
- 27.Castillo JC, Robertson AE, Strand MR. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem Mol Biol. 2006;36:891–903. doi: 10.1016/j.ibmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** Classified A. gambiae and A. aegypti circulating hemocytes into a scheme that has largely been adopted by the mosquito research community.
- 28.Hillyer JF, Schmidt SL, Fuchs JF, Boyle JP, Christensen BM. Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell Microbiol. 2005;7:39–51. doi: 10.1111/j.1462-5822.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 29.Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- ** Functional characterization of A. subalbatus circulating hemocytes.
- 30.Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int J Parasitol. 2007;37:673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillyer JF, Schmidt SL, Christensen BM. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004;6:448–459. doi: 10.1016/j.micinf.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Hillyer JF, Schmidt SL, Christensen BM. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J Parasitol. 2003;89:62–69. doi: 10.1645/0022-3395(2003)089[0062:RPAMOB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Barillas-Mury C, Han YS, Seeley D, Kafatos FC. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999;18:959–967. doi: 10.1093/emboj/18.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danielli A, Kafatos FC, Loukeris TG. Cloning and characterization of four Anopheles gambiae serpin isoforms, differentially induced in the midgut by Plasmodium berghei invasion. J Biol Chem. 2003;278:4184–4193. doi: 10.1074/jbc.M208187200. [DOI] [PubMed] [Google Scholar]
- 35.Christensen BM, Huff BM, Miranpuri GS, Harris KL, Christensen LA. Hemocyte population changes during the immune response of Aedes aegypti to inoculated microfilariae of Dirofilaria immitis. J Parasitol. 1989;75:119–123. [PubMed] [Google Scholar]
- 36.Brayner FA, Araujo HR, Santos SS, Cavalcanti MG, Alves LC, Souza JR, Peixoto CA. Haemocyte population and ultrastructural changes during the immune response of the mosquito Culex quinquefasciatus to microfilariae of Wuchereria bancrofti. Med Vet Entomol. 2007;21:112–120. doi: 10.1111/j.1365-2915.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 37.Bryant WB, Michel K. Blood feeding induces hemocyte proliferation and activation in the African malaria mosquito, Anopheles gambiae Giles. J Exp Biol. 2014;217:1238–1245. doi: 10.1242/jeb.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Description of blood feeding induced hemocyte proliferation in A. gambiae.
- 38.Castillo J, Brown MR, Strand MR. Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 2011;7:e1002274. doi: 10.1371/journal.ppat.1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** First demonstration of blood-feeding induced hemocyte proliferation in A. aegypti.
- 39.Coggins SA, Estevez-Lao TY, Hillyer JF. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Dev Comp Immunol. 2012;37:390–401. doi: 10.1016/j.dci.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti) Med Vet Entomol. 2012;26:271–281. doi: 10.1111/j.1365-2915.2011.00993.x. [DOI] [PubMed] [Google Scholar]
- 42.Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 43.Stoepler TM, Castillo JC, Lill JT, Eleftherianos I. Hemocyte density increases with developmental stage in an immune-challenged forest caterpillar. PLoS One. 2013;8:e70978. doi: 10.1371/journal.pone.0070978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva JE, Boleli IC, Simoes ZL. Hemocyte types and total and differential counts in unparasitized and parasitized Anastrepha obliqua (Diptera, Tephritidae) larvae. Braz J Biol. 2002;62:689–699. doi: 10.1590/s1519-69842002000400017. [DOI] [PubMed] [Google Scholar]
- 46.Ademolu KO, Idowu AB, Olatunde G. Hemocyte populations in Zonocerus variegatus (L.) (Orthoptera: Pyrgomorphidae) during post-embryonic development. Acta Entomologica Sinica. 2010;53:470–473. [Google Scholar]
- 47.Eleftherianos I, Xu M, Yadi H, Ffrench-Constant RH, Reynolds SE. Plasmatocyte-spreading peptide (PSP) plays a central role in insect cellular immune defenses against bacterial infection. J Exp Biol. 2009;212:1840–1848. doi: 10.1242/jeb.026278. [DOI] [PubMed] [Google Scholar]
- 48.Gardiner EM, Strand MR. Hematopoiesis in larval Pseudoplusia includens and Spodoptera frugiperda. Arch Insect Biochem Physiol. 2000;43:147–164. doi: 10.1002/(SICI)1520-6327(200004)43:4<147::AID-ARCH1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 49.Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- 50.Markus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, Somogyi K, Kronhamn J, Hultmark D, Ando I. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106:4805–4809. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan J, Xu M, Zhang K, Wang X, Chen S, Li T, Xiang Z, Cui H. Characterization of hemocytes proliferation in larval silkworm, Bombyx mori. J Insect Physiol. 2013;59:595–603. doi: 10.1016/j.jinsphys.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol. 2011;221:121–131. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satyavathi VV, Minz A, Nagaraju J. Nodulation: An unexplored cellular defense mechanism in insects. Cell Signal. 2014;26 doi: 10.1016/j.cellsig.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez-Martinez S, Lanz H, Rodriguez MH, Gonzalez-Ceron L, Tsutsumi V. Cellular-mediated reactions to foreign organisms inoculated into the hemocoel of Anopheles albimanus (Diptera: Culicidae) J Med Entomol. 2002;39:61–69. doi: 10.1603/0022-2585-39.1.61. [DOI] [PubMed] [Google Scholar]
- 56.Oliver JD, Dusty Loy J, Parikh G, Bartholomay L. Comparative analysis of hemocyte phagocytosis between six species of arthropods as measured by flow cytometry. J Invertebr Pathol. 2011;108:126–130. doi: 10.1016/j.jip.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- * Discovery of Tep1 as a pattern recognition receptor.
- 58.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domaincontaining receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lombardo F, Ghani Y, Kafatos FC, Christophides GK. Comprehensive genetic dissection of the hemocyte immune response in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2013;9:e1003145. doi: 10.1371/journal.ppat.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moita LF, Wang-Sattler R, Michel K, Zimmermann T, Blandin S, Levashina EA, Kafatos FC. In vivo identification of novel regulators and conserved pathways of phagocytosis in A. gambiae. Immunity. 2005;23:65–73. doi: 10.1016/j.immuni.2005.05.006. [DOI] [PubMed] [Google Scholar]
- * Identification of genes involved in the phagocytosis response.
- 61.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Bartholomay LC. Infection barriers and responses in mosquito-filarial worm interactions. Current Opinion in Insect Science. 2014 doi: 10.1016/j.cois.2014.08.006. THIS ISSUE. [DOI] [PubMed] [Google Scholar]
- 63.Barillas-Mury C. CLIP proteases and Plasmodium melanization in Anopheles gambiae. Trends Parasitol. 2007;23:297–299. doi: 10.1016/j.pt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs S, Behrends V, Bundy JG, Crisanti A, Nolan T. Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PLoS One. 2014;9:e84865. doi: 10.1371/journal.pone.0084865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulley MM, Zhang X, Michel K. The roles of serpins in mosquito immunology and physiology. J Insect Physiol. 2013;59:138–147. doi: 10.1016/j.jinsphys.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hillyer JF, Christensen BM. Mosquito phenoloxidase and defensin colocalize in melanization innate immune responses. J Histochem Cytochem. 2005;53:689–698. doi: 10.1369/jhc.4A6564.2005. [DOI] [PubMed] [Google Scholar]
- 67.Johnson JK, Rocheleau TA, Hillyer JF, Chen CC, Li J, Christensen BM. A potential role for phenylalanine hydroxylase in mosquito immune responses. Insect Biochem Mol Biol. 2003;33:345–354. doi: 10.1016/s0965-1748(02)00257-6. [DOI] [PubMed] [Google Scholar]
- 68.Yassine H, Kamareddine L, Osta MA. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 2012;8:e1003029. doi: 10.1371/journal.ppat.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Severo M, Levashina EA. Mosquito complement-like system in anti-Plasmodium responses. Current Opinion in Insect Science. 2014 doi: 10.1016/j.cois.2014.07.007. THIS ISSUE. [DOI] [PubMed] [Google Scholar]
- 71.Hillyer JF, Estevez-Lao TY. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev Comp Immunol. 2010;34:141–149. doi: 10.1016/j.dci.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci U S A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Andres J, Rani S, Varjak M, Chase-Topping ME, Beck MH, Ferguson MC, Schnettler E, Fragkoudis R, Barry G, Merits A, et al. Phenoloxidase activity acts as a mosquito innate immune response against infection with Semliki Forest virus. PLoS Pathog. 2012;8:e1002977. doi: 10.1371/journal.ppat.1002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao P, Lu Z, Strand MR, Jiang H. Antiviral, anti-parasitic, and cytotoxic effects of 5,6-dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response. Insect Biochem Mol Biol. 2011;41:645–652. doi: 10.1016/j.ibmb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theopold U, Schmidt O, Soderhall K, Dushay MS. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 2004;25:289–294. doi: 10.1016/j.it.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Lai SC, Chen CC, Hou RF. Electron microscopic observations on wound-healing in larvae of the mosquito Armigeres subalbatus (Diptera: Culicidae) J Med Entomol. 2001;38:836–843. doi: 10.1603/0022-2585-38.6.836. [DOI] [PubMed] [Google Scholar]
- 77.Lai SC, Chen CC, Hou RF. Immunolocalization of prophenoloxidase in the process of wound healing in the mosquito Armigeres subalbatus (Diptera: Culicidae) J Med Entomol. 2002;39:266–274. doi: 10.1603/0022-2585-39.2.266. [DOI] [PubMed] [Google Scholar]
- 78.Glenn JD, King JG, Hillyer JF. Structural mechanics of the mosquito heart and its function in bidirectional hemolymph transport. J Exp Biol. 2010;213:541–550. doi: 10.1242/jeb.035014. [DOI] [PubMed] [Google Scholar]
- 79.Andereck JW, King JG, Hillyer JF. Contraction of the ventral abdomen potentiates extracardiac retrograde hemolymph propulsion in the mosquito hemocoel. PLoS One. 2010;5:e12943. doi: 10.1371/journal.pone.0012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parikh GR, Oliver JD, Bartholomay LC. A haemocyte tropism for an arbovirus. J Gen Virol. 2009;90:292–296. doi: 10.1099/vir.0.005116-0. [DOI] [PubMed] [Google Scholar]