Abstract
Purpose
Most prostate, colon and breast cancer cells are resistant to growth inhibitory effects of suberoylanilide hydroxamic acid (SAHA). We have examined whether the high oxidative stress in these cells causes a loss of SAHA activity and if so, whether pretreatment with an anti-oxidant can sensitize these cells to SAHA.
Methods
A DNA-Hoechst dye fluorescence measured cell growth and dichlorfluorescein-diacetate (DCF-DA) dye fluorescence measured reactive oxygen species (ROS). Growth inhibitory and ROS-generating activities of SAHA in androgen-treated or untreated LNCaP cells and PC-3 prostate cancer cells, HT-29 and HCT-115 colon cancer cells, MDA-MB231 breast cancer cells and A549 and NCI-H460 lung cancer cells with or without pretreatment with an anti-oxidant Vitamin E was determined. SAHA activity against LNCaP cells treated with another anti-oxidant N-acetyl cysteine (NAC) was also determined. Liquid chromatography–mass spectrometry (LC–MS) was used to determine intracellular SAHA level.
Results
SAHA treatment markedly inhibits LNCaP cell growth, when the cells are at a low ROS level. SAHA is, however, inactive against the same cell line, when the cells are at a high ROS level. A significant decrease in SAHA level was observed in LNCaP cells with high ROS after 24-and 72-h treatment when compared to cells with low ROS. Vitamin E pretreatment that reduces cellular ROS, synergistically sensitizes oxidatively stressed LNCaP, PC-3, HT-29, HCT-115 and MDA-MB231 cells, but not the A-549 and NCI-H460 cells with low ROS to SAHA. NAC treatment also sensitized androgen-treated LNCaP cells to the growth inhibitory effects of SAHA.
Conclusion
Response to SAHA could be improved by combining anti-oxidants such as Vitamin E with SAHA for the treatment of oxidatively stressed human malignancies that are otherwise resistant to SAHA.
Keywords: Histone deacetylase, Oxidative stress, Prostate cancer, Drug metabolism
Introduction
Histone deacetylase (HDAC) is a class of enzymes present primarily in the nucleus that de-acetylates histones H3 and H4 (reviewed in [1]). HDAC activity prevents expression of genes that are required for cell cycle arrest and to induce apoptosis (reviewed in [2]). Therefore, HDAC inhibition arrests cell proliferation and causes apoptosis, cellular differentiation and/or senescence (reviewed in [3]). Suberoylanilide hydroxamic acid (SAHA, Vorinostat [Zolinza], Merck) is a HDAC inhibitor that causes arrest of cell proliferation and cell death ([4, 5] and related references therein). It has undergone advanced clinical trials against lymphoma [6], and recently, it has been approved for the treatment of cutaneous T-cell lymphoma (CTCL). SAHA, however, is inactive against human prostate, breast, colon and other cancers.
A popular model of human prostate cancer (CaP) is the LNCaP cell line. LNCaP is an androgen-responsive human CaP cell line that was established in the early 1980s from a metastatic lesion in the lymph node of a patient with CaP [7, 8]. In 1997, Ripple et al. [9] first reported that, in LNCaP cells, treatment with graded concentrations of R1881, an androgen analog, generates varying levels of reactive oxygen species (ROS) such as superoxide, hydroxyl radical and hydrogen peroxide as determined by DCF dye oxidation assay. When treated with R1881 concentrations less than 0.1 nM, “low androgen,” LNCaP cells showed significantly lower cellular ROS when compared to treatment with 1–10 nM R1881, “normal to high androgen.” However, within the 1–10 nM R1881 concentration range, no significant difference was observed in the amount of LNCaP cell growth or ROS generation. In addition to LNCaP cells, other human prostate, colon and some breast cancer cells also have high ROS levels; whereas, human lung cancer cells are remarkably low in cellular ROS (see “Results”).
Although SAHA has been successful in the treatment of lung, lymphoma and several other types of cancer, multiple clinical trials have failed to show efficacy of SAHA against prostate, colon, breast and other types of human malignancies [10, 11]. There can be several reasons for cellular resistance to SAHA: (i) SAHA may kill cells by inducing oxidative stress (reviewed in [12]). Compared to cells with low oxidative stress such as lung cancer and lymphoma cells, cells with high oxidative stress may be immune to drugs that can induce cell kill by inducing oxidative stress; (ii) high superoxide dismutase (SOD) enzyme activity in these cells may neutralize oxidative stress produced by SAHA and thus, inhibit its activity [13]; or (iii) SAHA may be oxidized by the high levels of ROS produced in the prostate, colon or breast cancer cells [14] and thereby, requires high drug concentrations that are not clinically achievable.
Here, we demonstrate that the inactivity of SAHA against CaP cells with high ROS is not due to changes in SOD activity or due to intrinsic cellular resistance to ROS, but due to a rapid decrease in intracellular SAHA concentrations in cells with high ROS levels. Reduction in ROS levels by silencing a major enzyme in ROS-producing pathway activates SAHA against CaP cells. Reducing cellular ROS by pretreatment with an anti-oxidant such as Vitamin E or N-acetyl cysteine (NAC) also synergistically increases SAHA sensitivity of CaP, colon and breast cancer cells, but not that of the lung cancer cells that have low intrinsic ROS. These data can be useful for designing clinical trials for SAHA in combination with Vitamin E for cancers with high oxidative stress that are unresponsive to SAHA as a single agent.
Methods
Cell culture
Human CaP cells LNCaP and PC-3, colon cancer cells HT-29 and HCT-115, lung cancer cells A549 and NCI-H460 and breast cancer cell MDA-MB231 were purchased from the American Type Culture Collection (Manassas, VA). The cells were maintained in humidified air containing 5% CO2 at 37°C in 10-cm-diameter tissue culture plates in either Dulbecco’s modified Eagle medium (DMEM) or RPMI-1640 medium supplemented with 5% heat-inactivated fetal bovine serum (FBS) and 1% 100× antibiotic, antimycotic solution (F5 medium). The cell lines are routinely tested every month for morphology, growth characteristics and hormone responsiveness (when applicable).
Androgen deprivation and SAHA treatment
Androgen-deprived growth conditions for LNCaP cells were simulated using DMEM containing 4% charcoal stripped FBS (CSS) plus 1% non-stripped FBS (F1/C4 medium) following previously published protocol [9]. Two days after transfer, cells were trypsinized, counted and seeded in F1/C4. The day after seeding, cells were treated with specific concentrations of an androgen analog R1881, which is widely used as a surrogate for androgen in cell culture conditions [9]. Treated cells were incubated for another 24 h in humidified air containing 5% CO2 at 37°C before the addition of SAHA. Depending on the experiment, SAHA was added by serial dilution to 96-well tissue culture plates or at calculated concentrations to 10-cm tissue culture plates. After SAHA addition, cells were incubated for 3 days in humidified air containing 5% CO2 at 37°C before analysis.
DCF assay
At the end of incubation, cells in 96-well plates were assayed for total ROS production in live cells with 2′, 7′-dichloro-fluorescein diacetate (DCF) dye (Molecular Probes, Inc., Eugene, OR) following a published protocol [9].
DNA assay
Cells seeded in 96-well tissue culture plates that were previously used in the DCF assay were thawed at room temperature. Hoechst dye (33258) was prepared in 0.05 M Tris (pH 7.5), 2 M NaCl, 1 mM ethylenediamine-tetra-acetate (high salt TNE) to make a final stock dye concentration of 10 μg/ml following a published procedure [9].
Cellular SAHA estimation by LC–MS
Sample preparation
Cells were trypsinized, counted, pelleted, washed once with PBS, dried and pellets were stored below −70°C. The day of the experiment, pellets were incubated in ice for 5 min in 100 μL lysis buffer (0.25 M sucrose, 0.06 M KCl, 0.05 M NaCl, 0.01 M 2-(N-morpholino) ethanesulfonic acid (MES), 0.01 M MgCl2, 0.001 M CaCl2, 0.0001 M phenyl-methyl-sulfonyl-fluoride (PMSF), 1 mM EDTA and 0.2% Triton X-100 (pH 6.5). Ten volumes chilled 99.5% acetonitrile, 0.5% acetic acid was added to all lysates, vortexed vigorously and incubated in ice for another 5 min for SAHA to be extracted into the organic solvent. Tubes were centrifuged at 5,000g for 5 min, and a calculated volume of the organic layer (generally 80% of the total organic solvent added) was aspirated carefully from the top. The organic solvent was dehydrated under a flow of nitrogen, redissolved in 50 μL 99.5% acetonitrile, 0.5% acetic acid. About 10 μL of each extract was used for LC–MS analysis, and the assay was repeated three times. All data were normalized to the total volume of cell extract and expressed as ng SAHA/106 cells.
Chromatography
SAHA level in LNCaP cells was determined by a modification of a published LC–MS method of determining SAHA in patient serum [15]. The LC–MS system consists of an Agilent (Palo Alto, CA) 1100 auto sampler and binary pump, Agilent 1100 column thermostat and an Agilent Zorbax 300SB—C18 column (3.5 μM, 2.1 × 100 mm). The mobile phase solvent A was acetonitrile and acetic acid (99.5%:0.5% v/v), and solvent B was water and acetic acid (99.5%:0.5% v/v). The solvent gradient and the flow rates were adjusted as shown in Table 1. A 5 min post-run column wash at 10% solvent A, 90% solvent B was maintained at 0.2 mL/min. The column thermostat was maintained at 25°C for the complete run.
Table 1.
LC–MS solvent gradient and flow rates for SAHA
| Time (min) | Solvent A (%) | Solvent B (%) | Flow rate (ml/min) |
|---|---|---|---|
| 0–3 | 10 | 90 | 0.2 |
| 3–4 | 10 | 90 | 0.3 |
| 4–7 | 85 | 15 | 0.3 |
| 7–10 | 90 | 10 | 0.3 |
| 10–14 | 90 | 10 | 0.3 |
| 14–15 | 10 | 90 | 0.3 |
| 15–17 | 10 | 90 | 0.2 |
| 17–20 | 10 | 90 | 0.2 |
Mass detector
Mass detection was carried out with Agilent 1100 quadruple moment bench-top mass spectrometer with electrospray ionization in the positive ion mode at 3,000 V. For both the single ion MS and scanning MS/MS mode, the desolvation temperature was 340°C with the drying gas flow rate of 12 l/min at a nebular pressure of 40 psig. The scan mode was between 150 and 300 m+/z, and the single ion detection (SIM) modes were set at 265.2, 232.2 and 172.2 m+/z. All data were collected, stored and analyzed using Agilent software for data collection, peak detection and integration.
Construction of LNCaP clones stably transfected with siSSAT
The clones were created following a procedure previously reported from our laboratory [16]. The clones were tested once every month for androgen responsiveness following published protocol [16].
HDAC assay
A high throughput HDAC assay was standardized using a Biomol (Plymouth Meeting, PA) HDAC assay kit with minor modifications of the manufacturer supplied protocol. Briefly, at the end of the drug treatment, media in the 96-well assay plates were discarded, and cells were washed once with 25% PBS and then allowed to swell in 30 μL deionized double distilled water for 1 h at room temperature. Plates were then frozen at or below −70°C. The day of the experiment, the plates were thawed at 4°C for 30 min. About 15 μL of the cell lysates were transferred to 96-well white round bottom plates, mixed thoroughly with 10 μL HDAC assay buffer (50 mM Tris–HCl, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, pH 8.0) and 25 μL manufacturer supplied fluorescence tagged HDAC substrate (KI-104, Biomol Inc.) appropriately diluted in the same HDAC assay buffer. The plates were incubated at 37°C for 30 min. The reaction was stopped with a manufacturer supplied Developer solution (Developer I, 20x, Biomol Inc.) containing 200 μM trichostatin A (TSA), and the plates were read within an hour at 360 nm excitation/ 460 nm emission in a Saphire2 (Tecan US, Inc., Durham, NC) multimode plate reader using 150 mV Photomultiplier voltage setting.
The remaining 15 μL of the cell lysates were used for DNA assay using 85 μL deionized double distilled water and 200 μL Hoechst 33258 dye following DNA assay protocol described earlier. All DNA fluorescence data were multiplied by a factor of two in order to determine the DNA reading of the total cell lysates.
Western blot analysis of acetylated histones
Total cellular histones were isolated following a published procedure [18]. Prior to gel loading, pH of the histone extracts was adjusted to 7.2 with 1 M NaOH. A 10-μL aliquot from each sample was set aside for protein estimation. The rest of the samples were run in SDS–PAGE, and western blot analysis was carried out following a procedure previously published from our laboratory [16] using anti-acetyl H4 antibody (Millipore, Temecula, CA). β-actin was used as control for protein loading. The acetyl histone H4 band intensities were calculated and normalized to β-actin intensities.
Results
SAHA inhibits growth of prostate cancer cells only at low oxidative stress
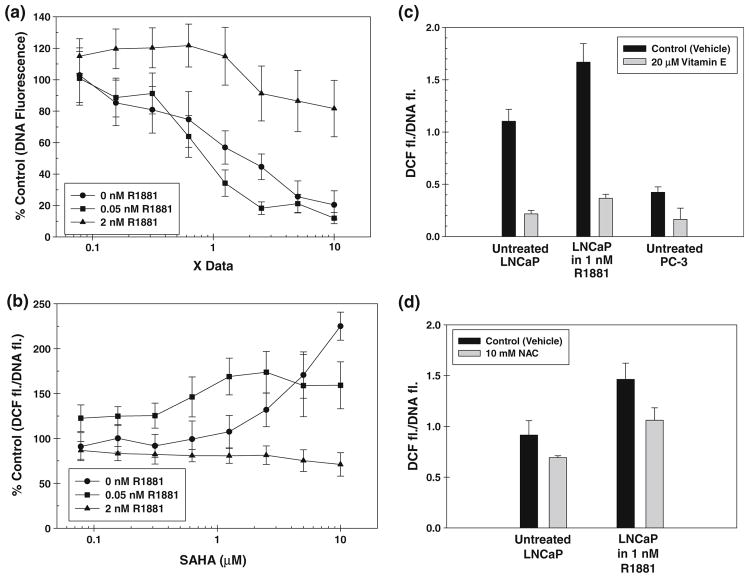
Fluorescence readings of Hoechst dye (Hoechst 33258) complex with DNA in the nuclei of cancer cell lines are proportional to the number of cells present in each well [9]. DNA fluorescence of LNCaP cells after pretreatment with R1881 followed by increasing concentration of SAHA from 0 to 10 μM expressed as percent of control untreated cells is shown in Fig. 1a. In LNCaP cells pretreated with no R1881 and 0.05 nM R1881, cell growth was inhibited almost linearly with a logarithmic increase in SAHA concentration. In LNCaP cells pretreated with 2 nM R1881, however, SAHA has negligible effect on cell growth at all concentrations tested. These data suggest that LNCaP cells exposed to normal serum androgen (2 nM) are relatively resistant to growth inhibitory effect of SAHA when compared to cells growing at low or no androgen.
Fig. 1.
a Relative DNA-Hoechst dye fluorescence as a measure of cell growth in SAHA-treated LNCaP cells expressed as percent of DNA fluorescence in cells not treated with SAHA is plotted against SAHA concentration in (A) cells treated with no R1881 (filled circle); (B) cells treated with 0.05 nM R1881 (filled square); and (C) cells treated with 2 nM R1881 (filled triangle). b Cellular ROS levels measured as a ratio of DCF fluorescence:DNA fluorescence are plotted vs. SAHA concentration in (A) cells treated with no R1881 (filled circle); (B) cells treated with 0.05 nM R1881 (filled square); and (C) cells treated with 2 nM R1881 (filled triangle). c Cellular ROS levels measured as a ratio of DCF fluorescence:DNA fluorescence in androgen-treated and androgen-untreated LNCaP cells with (
 ) or without Vitamin E pretreatment (
) or without Vitamin E pretreatment (
 ) and in androgen-independent PC-3 cells. d Cellular ROS levels measured as a ratio of DCF fluorescence:DNA fluorescence in androgen-treated and androgen-untreated LNCaP cells with (
) and in androgen-independent PC-3 cells. d Cellular ROS levels measured as a ratio of DCF fluorescence:DNA fluorescence in androgen-treated and androgen-untreated LNCaP cells with (
 ) or without NAC pretreatment (
) or without NAC pretreatment (
 )
)
Growth inhibitory effect of SAHA is not dependent on cellular oxidative stress in prostate cancer cells
Fluorescence of oxidized DCF dye is proportional to the total cellular ROS. When DCF fluorescence is normalized with the DNA fluorescence from the same well of the 96-well plate, the ratio DCF fluorescence: DNA fluorescence is proportional to the ROS generated per cell. The plots of the ratio of DCF/DNA fluorescence of LNCaP cells with or without pretreatment with various R1881 concentrations versus increasing SAHA concentrations are presented in Fig. 1b. In LNCaP cells pretreated with no R1881 or low concentration of R1881 (0.05 nM), ROS levels increase with an increase in SAHA concentration. In LNCaP cells pretreated with 2 nM R1881, however, increase in SAHA concentration has negligible effect on total cellular ROS levels. Total cellular ROS levels at all SAHA concentrations are higher in cells treated with 2 nM R1881 than in cells treated with 0.05 nM R1881. The ROS levels in both androgen-treated and androgen-untreated LNCaP cells with or without anti-oxidant Vitamin E or NAC pre-treatment are shown in Fig. 1c and d along with that in Vitamin E pretreated and untreated androgen-independent PC-3 cells. Both Vitamin E and NAC pretreatment reduced cellular ROS. Under our treatment conditions, Vitamin E was somewhat more effective in reducing cellular ROS than was NAC.
Effect of SAHA against siSSAT LNCaP cells
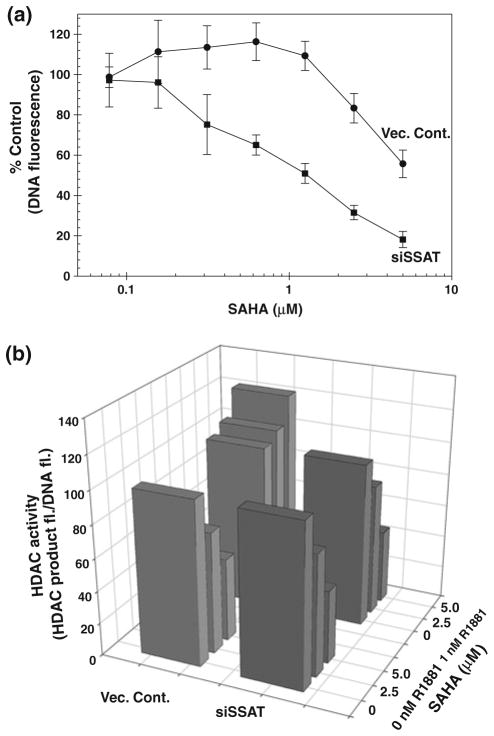
Next, we tested if the loss of SAHA activity in R1881 treated cells is indeed due to high ROS levels in these cells and not due to other effects of androgen on these cells. We have previously established that spermidine/spermine acetyl transferase (SSAT) activity [17] is a major contributor in androgen-induced ROS production in LNCaP cells [16]. We have constructed a LNCaP cell clone stably transfected with siRNA against SSAT (siSSAT) that reduces SSAT expression by >90%. R1881 treatment has no significant effect on ROS production in siSSAT clone when compared to a marked increase in LNCaP cells transfected with the control vector (Vec. cont.) containing scrambled sequence [16]. Growth inhibitory effects of SAHA on 2 nM R1881 pretreated Vec. cont. and siSSAT cells are expressed as % control of DNA fluorescence of corresponding cells treated with appropriate concentrations of R1881, but not treated with SAHA. The plot of % control of DNA fluorescence vs. SAHA concentrations are shown in Fig. 2a. The growth inhibitory effect of SAHA is significantly pronounced in 2 nM R1881 siSSAT cells (that do not produce ROS after androgen treatment) when compared to what observed for the Vec. cont. cells.
Fig. 2.
a Relative DNA-Hoechst dye fluorescence as a measure of cell growth in SAHA-treated Vec. Cont. (filled circle) and siSSAT (filled square) clones of LNCaP cells expressed as percent of DNA fluorescence in corresponding cells not treated with SAHA is plotted against SAHA concentration. b Relative cellular HDAC activities in terms of the ratio of HDAC product fluorescence: DNA-Hoechst dye fluorescence expressed as percent of that ratio in cells not treated with SAHA are plotted against both SAHA concentration and R1881 treatment. Vec. Cont. (
 ) and siSSAT (
) and siSSAT (
 ) clones of LNCaP cells
) clones of LNCaP cells
Effect of SAHA on HDAC activity in the siSSAT clone
Next, we determined the effect of graded concentrations of SAHA on the HDAC activities in Vec. cont. and siSSAT cell lines. The HDAC activity is expressed as a ratio of HDAC product fluorescence/DNA fluorescence in relative fluorescence unit (FU). The results are shown in Fig. 2b. All data were normalized to the same ratio in corresponding cells growing under identical conditions (with or without R1881), but without SAHA treatment. In cells not treated with androgen (in the front), SAHA has nearly similar efficiency in inhibiting HDAC activity in both Vec. cont. (light gray) and siSSAT (dark gray) cell lines. At concentrations > 1 μM, however, SAHA does not inhibit HDAC activity in R1881 pretreated Vec. cont. cells (light gray), while inhibits HDAC activity in R1881 treated cells to similar extent as in R1881 untreated siSSAT cells (dark gray). Thus, the HDAC inhibitory effects of SAHA parallel the ability of SAHA in arresting growth of androgen-treated siSSAT cells and not the growth of androgen-treated Vec. cont. cells (Fig. 2a). Note that the SAHA concentrations used for HDAC assay are somewhat less than that in the growth curves shown in Fig. 1 as very little DNA can be obtained from siSSAT cells treated with SAHA concentrations > 5 μM for reliable HDAC assay results.
Effect of SAHA in Vitamin E and NAC pretreated cells
Based on these results, we hypothesize that the high cellular ROS is responsible for the deactivation of SAHA in prostate cancer cells. Therefore, we propose that pretreatment of cells with an anti-oxidant that is known to reduce cellular ROS levels should sensitize the cells to SAHA. We pretreated prostate cancer cells LNCaP (both treated and untreated with R1881) and PC-3, colon cancer cells HT-29, breast cancer cells MDA-MB231 and lung cancer cells A549 and NCI-H460 cells with α-Tocopherol succinate (Vitamin E). For LNCaP cells treated with R1881, Vitamin E was added right before R1881 addition to neutralize any excess ROS production due to androgen treatment. The DCF/DNA fluorescence values representing cellular ROS for each cell line used in this study are listed in Table 2. The ROS levels of immortalized normal prostate epithelial cells are also included in the Table for comparison. Effect of 96 h treatment with graded concentrations of Vitamin E on cell growth has been determined separately (data not shown). From that study, Vitamin E and NAC concentrations that are non-toxic to each cell line have been selected for pretreatment. Both Vitamin E and NAC treatment markedly reduce the ROS levels in LNCaP and PC-3 cells. Similar reduction in cellular ROS by Vitamin E has been reported in breast and colon cancer cells as well [19]. Due to the very low level of oxidative stress in lung cancer cells, the effect of Vitamin E treatment on the ROS levels in these cells cannot be accurately determined.
Table 2.
ROS levels of different types of human cancer cells as determined by DCF assay
| DCF fluorescence/DNA fluorescence
|
||
|---|---|---|
| Mean | SD | |
| LNCaP (low ROS) | 1.13 | 0.02 |
| LNCaP (high ROS) | 1.85 | 0.03 |
| PC-3 | 0.42 | 0.03 |
| HT-29 | 0.49 | 0.04 |
| MDA-MB231 | 0.19 | 0.03 |
| A549 | 0.09 | 0.08 |
| PrEC-E6 | 0.11 | 0.07 |
Results are expressed as the ratio of DCF fluorescence/DNA fluorescence
Each data point is a mean of at least 6 separate wells run in triplicate
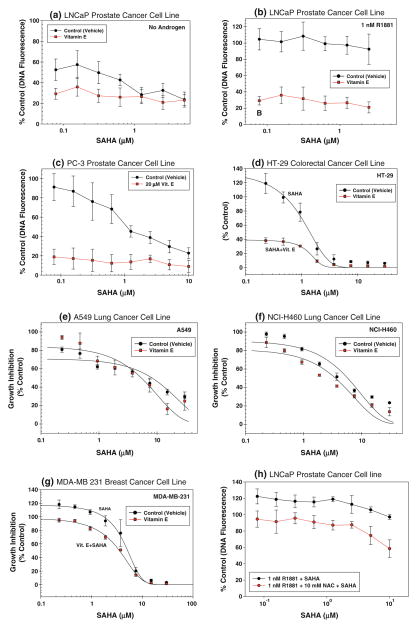
The effects of SAHA on the growth of Vitamin E pre-treated and untreated human cancer cells and NAC pre-treated and untreated LNCaP cells are shown in Fig. 3. All data are normalized as % control of DNA fluorescence of corresponding cells treated with Vitamin E or NAC alone. Both androgen-untreated and androgen-treated LNCaP cells (Fig. 3a, b, and h, respectively) as well as PC-3 cells (Fig. 3c) become sensitive to growth inhibition by SAHA after pretreatment with a non-toxic dose of 20 μM Vitamin E or 10 mM NAC (see Fig. 3h). The effect of Vitamin E pretreatment is more pronounced than that of NAC pre-treatment as under these conditions, Vitamin E is more effective in reducing cellular ROS levels than in NAC (Fig. 1c and d). SAHA sensitivity of HT-29 and MDA-MB231 (Fig. 3d and g) cells are also relatively higher in Vitamin E pretreated cells, when compared to Vitamin E untreated cells. Using the formalism developed by Chou and Talalay [20], it was determined that Vitamin E pre-treatment synergistically enhanced the SAHA sensitivity. It is noted that there is a marked difference in growth inhibitory effect of SAHA against these cell lines at clinically achievable SAHA dose of 1 μM. The lung cancer cells A549 and NCI-H460 with low ROS levels, however, do not show any appreciable increase in SAHA sensitivity after Vitamin E pretreatment at any concentration of SAHA (Fig. 3e and f).
Fig. 3.
Growth inhibitory effect of SAHA with (filled circle) without c (filled square) pretreatment with a non-toxic concentration of Vitamin E expressed as DNA fluorescence percent of corresponding SAHA untreated cells plotted against SAHA concentrations in a LNCaP prostate cancer cells growing without androgen with or without 20 μM Vitamin E, b LNCaP cells growing in the presence of 1 nM R1881 with or without 20 μM Vitamin E, c PC-3 prostate cancer cells with or without 20 μM Vitamin E, d HT-29 colorectal cancer cells with or without 6 μM Vitamin E, e A549 lung cancer cells with or without 20 μM Vitamin E, f NCI-H460 lung cancer cells with or without 20 μM Vitamin E, g MDA-MB-231 breast cancer cells with or without 3 μM Vitamin E and h LNCaP cells growing in the presence of 1 nM R1881 with or without 10 mM NAC
Effect of Vitamin E pretreatment on SAHA-induced changes in acetyl histone levels
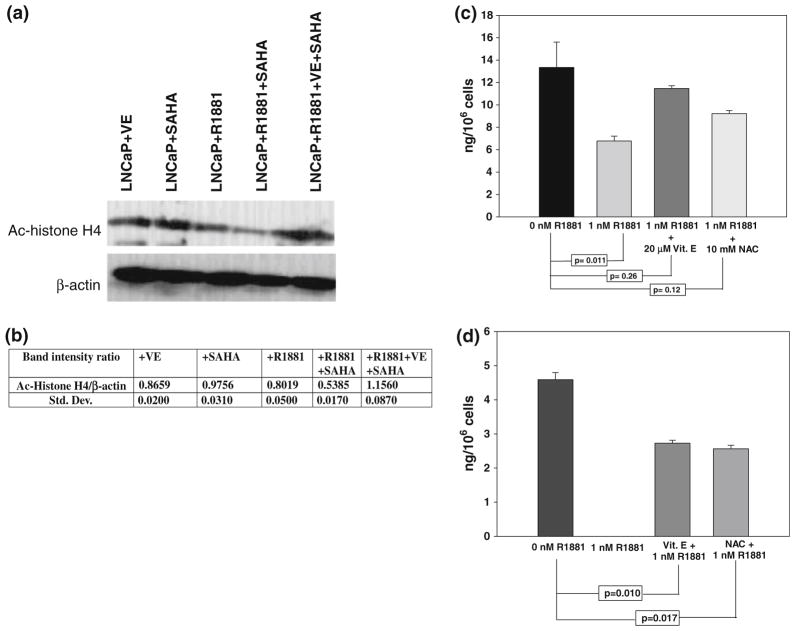
Western blot analysis of acetyl histone H4 levels in LNCaP cells treated with 20 μM Vitamin E alone, 1 nM R1881 alone and 2 μM SAHA alone, along with a combination of R1881 + SAHA and Vitamin E + R1881 + SAHA, using antibody targeted toward anti-acetyl histone H4 has been performed. Western blot of β-actin is used to control for protein loading. A representative western blot is shown in Fig. 4a. The corresponding mean of the ratio of band intensities of acetyl histone H4: β-actin and the standard deviation of three independent runs are shown in Fig. 4b. Vitamin E or R1881 alone has little effect on the acetyl histone H4 level. SAHA treatment causes a small, but significant (P < 0.03, using Student’s t test) increase in the acetyl histone level that shows that SAHA inhibits HDAC activity in LNCaP cells growing in the absence of androgen. There is a marked decrease in acetyl histone H4 level in R1881 pretreated cells, suggesting an appreciable loss of HDAC inhibitory activity of SAHA in the presence of R1881. Pretreatment with Vitamin E almost completely restores the acetyl histone H4 level in R1881-treated cells, showing HDAC inhibitory activity of SAHA is not lost in R1881-treated cells that have been pretreated with Vitamin E.
Fig. 4.
a Representative western blot of acetyl histone H4 (Ac-histone H4) and corresponding β-actin protein from: LNCaP cells treated with 20 μM Vitamin E (Lane #1), LNCaP cells treated with 2 μM SAHA (Lane #2), LNCaP cells treated with 1 nM R1881 (Lane #3), LNCaP cells treated with 1 nM R1881 and 2 μM SAHA (Lane #4), and LNCaP cells treated with 1 nM R1881, 20 μM Vitamin E and 2 μM SAHA (Lane #5). b Mean and standard deviations of the ratios of intensities of Ac-histone H4 band:β-actin bands from the corresponding lanes. c Intracellular SAHA levels in LNCaP cells treated with 2 μM SAHA (
 ), pretreated with 1 nM R1881 followed by 2 μM SAHA (
), pretreated with 1 nM R1881 followed by 2 μM SAHA (
 ), treated with 20 μM Vitamin E + 1 nM R1881 followed by 2 μM SAHA (
), treated with 20 μM Vitamin E + 1 nM R1881 followed by 2 μM SAHA (
 ) or treated with 10 mM NAC + 1 nM R1881 followed by 2 μM SAHA (
) or treated with 10 mM NAC + 1 nM R1881 followed by 2 μM SAHA (
 ) for 24 h as determined by LC–MS and calculated from a SAHA standard curve (see text). d Intracellular SAHA levels in LNCaP cells treated with 2 μM SAHA (
) for 24 h as determined by LC–MS and calculated from a SAHA standard curve (see text). d Intracellular SAHA levels in LNCaP cells treated with 2 μM SAHA (
 ), pretreated with 1 nM R1881 followed by 2 μM SAHA (not detected), treated with 20 μM Vitamin E + 1 nM R1881 followed by 2 μM SAHA (
), pretreated with 1 nM R1881 followed by 2 μM SAHA (not detected), treated with 20 μM Vitamin E + 1 nM R1881 followed by 2 μM SAHA (
 ) or treated with 10 mM NAC + 1 nM R1881 followed by 2 μM SAHA (
) or treated with 10 mM NAC + 1 nM R1881 followed by 2 μM SAHA (
 ) for 72 h
) for 72 h
LC–MS estimation of intracellular SAHA concentration
Using the procedure standardized during this study (see “Methods” for detail), SAHA is detected as a single peak in LNCaP cell extracts. Cellular SAHA concentrations in LNCaP cells are expressed as ng SAHA/106 cells using a standard curve for SAHA generated using LNCaP cell extracts spiked with calculated amounts of SAHA. SAHA concentrations in cells treated with 5 μM SAHA for 24 h either untreated or pretreated with 1 nM R1881 are shown in Fig. 4b. Within 24 h of treatment, the SAHA level in LNCaP cells pretreated with R1881 is less than half of that in R1881 untreated cells. In Vitamin E and NAC pretreated cells, however, there is no significant decrease in intra-cellular SAHA level, at least in the first 24 h of treatment as shown in Fig. 4c. This effect becomes more pronounced after 72-h SAHA treatment as shown in Fig. 4d. In general, SAHA levels are lower in all cells after 72 h treatment as compared to that after 24 h treatment. After 72 h treatment, in cells treated with both Vitamin E and R1881 or NAC and R1881, SAHA levels are significantly lower than that in control R1881-untreated cells. In cells treated with R1881 alone, however, there was no detectable level of SAHA under our assay conditions. These data clearly suggest that in LNCaP cells, SAHA levels go down considerably faster in R1881-treated cells when compared to that in R1881-untreated cells or cells that are treated with R1881 along with an anti-oxidant such as Vitamin E or NAC.
Discussion
The data presented earlier show that SAHA is inactive specifically against cancer cells with high oxidative stress probably due to oxidative degradation of SAHA in these cells. A reduction of oxidative stress in these cells by Vitamin E or NAC pretreatment sensitizes the otherwise SAHA resistant cancer cells with high oxidative stress to the growth inhibitory activity of SAHA.
In LNCaP cells treated with no androgen (F1/C4 medium) or low androgen (0.05 nM R1881), DNA fluorescence, which is a measure for cell growth, decreases almost linearly with a logarithmic increase in SAHA concentration (Fig. 1a). Thus, SAHA inhibits LNCaP prostate cancer cell growth, when functioning at low androgen conditions (≤0.05 nM R1881) with IC50 < 1 μM. In LNCaP cells growing in normal androgen level (2 nM R1881), however, there is little effect on cell growth even at 10 μM SAHA (Fig. 1a). The changes in relative DNA fluorescence with increasing SAHA concentrations clearly demonstrate that SAHA inhibits growth of LNCaP cells in a medium with low androgen (0 and 0.05 nM R1881), but not in a medium with high androgen (1 nM R1881).
To test if changes in ROS have effects on the growth inhibitory activities of SAHA, cellular ROS levels are compared with cell growth under low and high androgen conditions. In LNCaP cells growing in the absence of androgen (F1/C4 medium), cellular ROS levels increase (Fig. 1b) as cell growth decreases (Fig. 1a) supporting the published observation that SAHA treatment increases cellular ROS levels, which was hypothesized to be one of the reasons for the cell growth inhibition by SAHA [21]. In LNCaP cells, growing at 0.05 nM R1881, however, very similar growth inhibition has been observed (Fig. 1a) without any appreciable increase in ROS levels (Fig. 1b). On the other hand, LNCaP cells with high intrinsic ROS levels (Fig. 1b) growing in the presence of normal androgen conditions (2 nM R1881) are resistant to SAHA (Fig. 1a). These data suggest that the growth inhibitory effects of SAHA are most likely not due to an increase in cellular ROS levels in SAHA-treated cells. The results also show that LNCaP cells are not intrinsically resistant to the growth inhibitory effects of SAHA and exhibit SAHA resistance only when grown at normal serum androgen levels producing high ROS. As androgen-dependent cells are mainly found in patients with normal serum androgen levels at an early stage of CaP recurrence, it is likely that most early stage patients with prostate cancer will not respond to SAHA at the serum SAHA level of ~349 ng/ mL (~1.3 μM) for patients given clinically approved oral SAHA dose of 400 mg qd [22]. On the other hand, androgen-resistant CaP cells such as PC-3 are intrinsically resistant to SAHA below 10 μM (Fig. 3c). Thus, advanced prostate cancer in patients with low serum androgen levels will also not likely to respond to SAHA. Therefore, if the reason for the loss of SAHA activity against oxidatively stressed CaP cells can be determined, it may be possible to treat patients with CaP with SAHA either at an early or at a late stage of the disease under conditions where SAHA activity will not be reduced.
SAHA may affect superoxide dismutase (SOD) enzyme activity differently in the presence of androgen, causing changes in the amount of ROS [13] and thereby, indirectly affecting cytoplasmic ROS levels at high androgen conditions. However, the SOD assay data show that there is no significant difference in the SOD activity of LNCaP cells that have been pretreated with 0.05 or 1 nM R1881 prior to treatment with 10 μM SAHA (data not shown). These results rule out the possibility that androgen-induced changes in SOD activity are responsible for altering cellular oxidative stress and therefore, SAHA sensitivity of cells growing at different androgen concentrations.
In the siSSAT LNCaP cell clones that are unable to produce ROS upon androgen treatment [16], SAHA has marked growth inhibitory effect in cells treated with 2 nM R1881 (Fig. 2a). The effect is similar to that of SAHA against LNCaP cells growing at low androgen concentration (see Fig. 1a). We have also determined that the cellular HDAC activity is very similar in LNCaP cells either transfected with the siSSAT vector or a control vector with scrambled sequence (data not shown). HDAC activity in vector control cells pretreated with 1 nM R1881 and then treated with increasing concentrations of SAHA increases after an initial decrease (Fig. 2b, light gray bars). HDAC activity in R1881 untreated vector control cells (Fig. 2b, light gray bars) as well as androgen-treated and untreated siSSAT cells (Fig. 2b, dark gray bars) decreases in a similar fashion. Since both these cell lines are derived from the same parental LNCaP cells, effect on SAHA uptake, excretion, changes in chromatin structure, etc. is expected to remain the same in both cell lines and therefore, are unlikely as possibilities for the differential activity of SAHA in these two cell lines. Therefore, the growth inhibitory activity of SAHA against R1881-treated siSSAT clones of LNCaP cells and the anomalous increase in HDAC activity in R1881-treated vector control LNCaP cell clones (Fig. 2b, light gray bars) is possibly due to a loss of SAHA activity in these cells. Thus, we propose that an oxidation of intracellular SAHA in high ROS containing R1881-treated LNCaP cells is likely to be a major reason for the loss of SAHA activity against human CaP cells.
A mechanism other than HDAC inhibition for the growth inhibitory activity of SAHA has been considered. The possibility of changes in cellular polyamine levels in siSSAT cells altering the chromatin structure and thus, modifying SAHA activity is a possibility. There are, however, only minor changes in cellular polyamine levels between Vec. cont. and siSSAT cell lines [16]. Thus, the possibility of cellular polyamines that may affect chromatin structure and thus, altering SAHA sensitivity of the siSSAT cells is also unlikely.
Based on these results, we hypothesize that cancer cells with high ROS levels may be insensitive to SAHA due to a loss of SAHA level due to cellular oxidation. Therefore, we propose that a treatment with an anti-oxidant such as Vitamin E should increase SAHA activity against these cells.
Based on this hypothesis, we have studied the growth inhibitory effect of SAHA on human prostate, colon and breast cancer cells with high oxidative stress and lung cancer cells with low oxidative stress with or without pretreatment with Vitamin E. Although the ROS levels are relatively less in PC-3 cells when compared to LNCaP cells, they are both higher than those in normal prostatic epithelial cells (Table 2). Under conditions when Vitamin E treatment lowers the ROS levels to similar extent in prostate, colon and breast cancer cell lines, all cell lines show similar sensitivity to growth inhibitory effects of SAHA (Fig. 3b). The ROS levels of human lung cancer cell lines and immortalized normal prostatic epithelial cell line tested under all culture conditions are relatively lower than that in all other cancer cell lines studied (Table 2). Lung cancer cells that are already sensitive to SAHA, however, do not show any appreciable increase in SAHA sensitivity after Vitamin E pretreatment. Thus, with the exception of lung cancer cells, all cancer cell lines tested showed a synergistic increase in SAHA sensitivity after Vitamin E pretreatment. This increase was also observed, albeit at to a lesser degree, in androgen-treated LNCaP cells pretreated with another anti-oxidant NAC.
Our LC–MS data show that within 24 h after treatment, SAHA level in LNCaP cells pretreated with 1 nM R1881 is half of that in R1881 untreated cells (Fig. 4c) and is reduced to undetectable level after 72 h treatment. This could be due either to oxidation of SAHA by the high ROS level present in androgen-treated LNCaP cells, or to an uptake inhibition or an increased excretion of SAHA in androgen-treated cells or a combination of all. Since SAHA activity is higher against siSSAT clones of LNCaP cells than against Vec. cont. clones, the changes in uptake/excretion of SAHA in LNCaP cells due to R1881 treatment that may affect SAHA activity is unlikely. From these observations, we have concluded that oxidative degradation of SAHA in highly oxidatively stressed cancer cell lines is a likely reason for SAHA insensitivity of prostate, colon and breast cancer cells. Reducing oxidative stress by pretreatment with an anti-oxidant Vitamin E sensitizes these cells to SAHA and can be clinically translated.
Conclusion
The data shown in Fig. 3 clearly demonstrate that SAHA at clinically achievable serum level (~1.3 μM) [22] is inactive against all cell lines that are not pretreated with Vitamin E. Both androgen-dependent and androgen-independent prostate cancer cells growing in the absence of androgen in addition to breast and colon cancer cells are highly sensitive to SAHA at a concentration much below the clinically achievable serum level, when pretreated with anti-oxidants such as Vitamin E that lowers the cellular oxidative stress. Therefore, we propose that highly oxidatively stressed tumors that are resistant to SAHA will become sensitive to the growth inhibitory effect of SAHA, if SAHA is given in combination with Vitamin E.
Thus, in prostate, colon and breast cancer cells:
SAHA-induced increase in cellular ROS is not the cause of growth inhibitory effects of SAHA;
SAHA is probably oxidized by high ROS present in these cells.
Lowering of cellular oxidative stress by Vitamin E pretreatment sensitizes both androgen-dependent as well as androgen-independent CaP cells as well as colon and breast cancer cells to growth inhibitory effects of SAHA.
We believe these results will help design successful clinical trials using a combination treatment of SAHA with anti-oxidants such as Vitamin E in human malignancies that are otherwise unresponsive to SAHA.
Supplementary Material
Acknowledgments
Prof. F. Michael Hoffman for helpful suggestion for drug synergism determination and Merck Inc. for financial support and generous supply of SAHA.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-010-1364-3) contains supplementary material, which is available to authorized users.
Conflict of interest statement This work was partially supported by a research grant by Merck Inc., which is responsible for commercialization of SAHA.
References
- 1.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 2.Mehnert JM, Kelly WK. Histone deacetylase inhibitors: biology and mechanism of action. Cancer J. 2007;13:23–29. doi: 10.1097/PPO.0b013e31803c72ba. [DOI] [PubMed] [Google Scholar]
- 3.Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009;280:125–133. doi: 10.1016/j.canlet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Richon VM, Zhou X, Rifkind RA, Marks PA. Histone deacetylase inhibitors: development of suberoylanilide hydroxamic acid (SAHA) for the treatment of cancers. Blood Cells Mol Dis. 2001;27:260–264. doi: 10.1006/bcmd.2000.0376. [DOI] [PubMed] [Google Scholar]
- 5.Seo SK, Jin HO, Lee HC, et al. Combined effects of sulindac and suberoylanilide hydroxamic acid on apoptosis induction in human lung cancer cells. Mol Pharm. 2008;73:1005–1012. doi: 10.1124/mol.107.041293. [DOI] [PubMed] [Google Scholar]
- 6.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral SAHA (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horoszewicz JS, Leong SS, Kawinski E, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 8.Horoszewicz JS, Leong SS, Chu TM, et al. The LNCaP cell line-a new model for studies on human prostatic carcinoma. Models for CaP. 1980;37:115–132. [PubMed] [Google Scholar]
- 9.Ripple MO, Henry WF, Rago RP, Wilding G. Pro-oxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Nat Cancer Inst. 1997;89:40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 10.Luu TH, Morgan RJ, Leong L, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California cancer consortium study. Clin Cancer Res. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vansteenkiste J, Van Cutsem E, Dumez H, et al. Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal or non-small cell lung cancer. Invest New Drugs. 2008;26:483–488. doi: 10.1007/s10637-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 12.Rosato RR, Grant S. Histone deacetylase inhibitors: insights into mechanisms of lethality. Exp Opin Ther Targ. 2005;9:809–824. doi: 10.1517/14728222.9.4.809. [DOI] [PubMed] [Google Scholar]
- 13.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 14.Samuni Y, Flores-Santana W, Krishna MC, Mitchell JB, Wink DA. The inhibitors of histone deacetylase suberoylanilide hydroxamate and trichostatin A release nitric oxide upon oxidation. Free Radic Biol Med. 2009;47:419–423. doi: 10.1016/j.freeradbiomed.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parise RA, Holleran JL, Beumer JH, Ramalingam S, Egorin MJ. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:108–115. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Basu HS, Thompson TA, Church DR, et al. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69:7689–7695. doi: 10.1158/0008-5472.CAN-08-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Celano P, Mank AR, et al. Structure of the human spermidine/spermine N1-acetyltransferase gene (exon/intron gene organization and localization to Xp22.1) Biochem Biophys Res Commun. 1992;187:1493–1502. doi: 10.1016/0006-291x(92)90471-v. [DOI] [PubMed] [Google Scholar]
- 18.Thomas CE, Kellher NL, Mizzen CA. Mass spectrometric characterization of human histone H3: a bird’s eye view. J Proteome Res. 2006;5:240–247. doi: 10.1021/pr050266a. [DOI] [PubMed] [Google Scholar]
- 19.Borek C. Dietary antioxidants and human cancer. Integr Cancer Ther. 2004;3:333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci (USA) 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.