Abstract
Background
The presence of moderate to severe bone marrow (BM) fibrosis has been shown to be an adverse feature in patients with primary myelodysplastic syndromes (MDS). However, the clinical importance of BM fibrosis is not clear in therapy-related MDS.
Methods
We retrieved all t-MDS cases (n=266) diagnosed at our hospital over a 10-year period (2003–2012). Reticulin and trichromestains were performed in cases in which BM fibrosis was suspected on initial evaluation of hematoxylin & eosin stained slide. BM fibrosis was graded according to European consensus guidelines, and a score of MF2/MF3 was defined as moderate/severe fibrosis.
Result
Moderate/severe BM fibrosis was found in 47 (17%) patients. Compared to 219 patients with no/mild BM fibrosis, the patients with moderate/severe fibrosis presented with severer thrombocytopenia (p=0.039), higher numbers of circulating blasts (p=0.051) but similar degrees of anemia and neutropenia, transfusion requirements, and similar incidences of hepatosplenomegaly and constitutional symptoms. Histological examination revealed a comparable BM cellularity and BM blast percentage, but markedly increased megakaryocytes (p<0.001) in the fibrotic group. Although the risk distribution of cytogenetic data was similar according to the New Comprehensive Cytogenetic Scoring criteria, −5 and −17 were more frequently observed in t-MDS with moderate/severe BM fibrosis (p=0.031 and p=0.043 respectively). With a median follow-up of 11.5 months, patients with moderate/severe BM fibrosis showed a similar risk of acute myeloid leukemia transformation, and a comparable overall survival in univariate and multivariate analyses.
Conclusions
Moderate/severe BM fibrosis in patients with t-MDS is associated with certain clinicopathological and genetic features. However, unlike the situation in patients with primary MDS, moderate/severe BM fibrosis does not add additional risk to patients with therapy-related MDS.
Keywords: therapy-related myelodysplastic syndromes, bone marrow fibrosis, cytogenetics, overall survival, AML transformation
Background
Assessment of bone marrow (BM) fibrosis has been shown to have clinical and prognostic implications in various hematological neoplasms. BM fibrosis adversely affects therapeutic efficacy and outcome in patients with chronic myelogenous leukemia and plasma cell myeloma. [1,2] In primary myelofibrosis (PMF), collagenous BM fibrosis has been reported to predict an adverse outcome [3–5] even within the poor risk patient group defined by the International Prognostic Scoring System (IPSS). [6] In myelodysplastic syndromes (MDS), the clinical relevance of BM fibrosis was not well recognized in the past and this histological feature was not incorporated into the 2008 World Health Organization (WHO) classification. [7] Recently, several studies have been conducted in patients with primary (de novo) MDS patients. These studies show that moderate to severe BM fibrosis occurs in about 10–20% of primary MDS patients; and BM fibrosis is closely associated with multilineage dysplasia, profound cytopenia(s), and red cell/platelet transfusion dependence. [8–10] Additionally, primary (de novo) MDS patients with moderate/severe BM fibrosis have an inferior overall survival, either attributable to profound BM failure or to an increased rate of leukemic evolution. [11] In MDS patients who received hematopoietic stem cell transplantation (HSCT), a delayed BM engraftment was observed in patients with any degree of BM fibrosis, and additionally, moderate/severe BM fibrosis was found to be an independent risk for an inferior event free survival post HSCT. [12] Therapy-related MDS (t-MDS) occurs in patients who have received cytotoxic therapies for prior malignancy, or rarely, for non-malignant diseases. [7] In general, t-MDS is clinically aggressive and these neoplasms respond poorly to conventional therapy used for patients with primary (de novo) MDS. [13–16] Due to a general poor outcome of patients with t-MDS, in the 2008 WHO classification t-MDS and therapy-related acute myeloid leukemia (t-AML) are not considered as separate entities, but are classified together as therapy-related myeloid neoplasms (t-MN). [17,18] However, heterogeneity in survival has been observed in t-MDS patients. Bacher et al reported that patients with t-MDS and t-AML share genetic features but can be separated into prognostically relevant subgroups by using blast count and cytogenetic risk profiles. [19] Recently, Quintas-Cardama and colleagues proposed a prognostic model that can separate t-MDS patients into three groups that have different overall survival and risk of transformation to AML. [20] In this study, our aim is to assess the prognostic importance of moderate/severe BM fibrosis as a risk factor for t-MDS patients and to determine if this BM histological feature should be used in the development of risk-adapted therapeutic strategies.
Material and Methods
Patients
Over a 10-year period (2003–2012), we identified 440 t-MDS patients diagnosed and treated at M.D. Anderson Cancer Center. After a retrospective review of clinical charts and pathology reports, 266 patients were included in this study based on the following criteria: (1) Confirmed history of chemotherapy and/or radiation therapy prior to the diagnosis of t-MDS; (2) The bone marrow biopsyspecimen obtained at time of initial diagnosis of t-MDS was available for assessment of fibrosis; and (3) BM fibrosis was not due to primary cancer or other concomitant disease. For patients who received only radiation therapy, we excluded patients treated with brachy therapy only, radioisotopes, and those patients whose treatment fields did not include hematopoietic bone marrow. A total of 174 patients were excluded due to the following reasons: 1). the initial diagnosis was made at the referring centers, and there was no clear documentation of the presence or absence of BM fibrosis; and there were no material available for review at the time of this study; 2). BM biopsy in adequate for fibrosis assessment. 3). BM had concomitant tumor/lymphoma infiltrate. 4). Ambiguous clinical history, could not confirm causative relationship between cytotoxic exposure and MDS. 5). Follow-up information was not available. The study was conducted in accordance with the Declaration of Helsinki and the regulations of the Ethical Committee of M.D. Anderson Cancer Center.
Bone Marrow Assessment and Laboratory Data
The diagnosis of a t-MDS was established using the criteria described by the WHO 2008. [7] All diagnoses were confirmed in conjunction with clinical follow-up. Reticulin and trichromestains were performed in cases in which fibrosis was suspected on initial hematoxylin & eosin evaluation of BM biopsy specimens based on any of the following features: increased stromal cells, cellular streaming/crushing, scarring/scleredema, dilated sinuses and osteosclerosis. BM fibrosis was graded according to European Myelofibrosis Network criteria [21] as MF 0, 1, 2 and 3. An MF score of 2 or 3 was considered to be moderate or severe BM fibrosis. Figure 1 illustrates a case of t-MDS with MF-2 fibrosis. A 500-cell count or a 200 cell count in hemodiluted specimens was performed based on examination of multiple fields of BM aspirate smears. Myeloblasts were enumerated as a percentage of total BM nucleated cells. Due to significant BM fibrosis, some patients had a “dry tap” and in these cases, CD34 immunohistochemical study was performed on the BM biopsy specimen to better assess the number of BM blasts. Peripheral blood (PB) blasts were <20% in all the cases. The complete blood count (CBC) data at the time of diagnosis including white blood cell count (WBC), absolute neutrophil count (ANC), hemoglobin level (Hb), and platelet count were recorded. Other clinical findings relevant to BM fibrosis, including hepatosplenomegaly and constitutional symptoms were recorded.
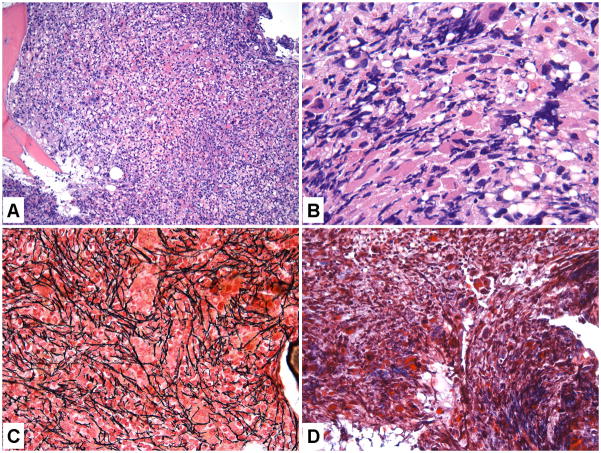
Figure 1.
Bone marrow (BM) features of a therapy-related myelodysplastic syndromes (t-MDS) case with MF-2 BM fibrosis. A. Hematoxylin and eosin (H&E magnification 200×) showing a hypercellular BM; B. Markedly increased dysplastic megakaryocytes (H&E 1000×); C, Reticulin stain (400×) showing markedly increased reticulin fibrosis; D. Trichrome stain (400×) showing focal small bundles of fibrosis.
Cytogenetic Classifications
Conventional cytogenetic analysis was performed using standard methods as previously described. [22] Twenty metaphases were analyzed, if available, and the results were reported using the International System for Human Cytogenetic Nomenclature. In some cases a lesser number of metaphases were available and fluorescence in situ hybridization (FISH) was performed in order to confirm clonal cytogenetic abnormalities. Overall, we only included karyotype information with adequate metaphases for clonality analysis. The cytogenetic risk score was assigned to each case following the New Comprehensive Cytogenetic Scoring System for primary MDS and oligoblastic AML as well as the International Prognostic Scoring System-Revised (IPSS-R) grouping criteria. [23,24]
Molecular Analysis
NPM1 mutations were assessed by using primers designed to amplify mutational hotspots spanning codons 956–971 of exon 12, followed by capillary electrophoresis as described previously. [25] The FLT3 internal tandem duplication (FLT3-ITD) and tyrosine kinase domain codon 835/836 point mutations (FLT3-D835) were detected by a fluorescent-based multiplex PCR assay followed by capillary electrophoresis. [25] For FLT3-D835 point mutation analysis, PCR products were digested with Eco RV before capillary electrophoresis. JAK2 V617, K-RAS and N-RAS mutations at codons 12, 13 and 61 were tested using PCR followed by pyrosequencing as described previously. [26] Mutations in exons 8 and 17 of the KIT gene were detected using Sanger sequencing. [27] Mutation studies were performed as a part of routine MDS/AML work up at our hospital and no additional testing for this study was conducted.
Treatment for Therapy-Related MDS and Follow-up Evaluation
Therapies received by patients with t-MDS were grouped as follows: growth factor and/or supportive care; standard cytotoxic chemotherapy; hypomethylating agents, immunomodulatory therapy (thalidomide/lenalidomide, investigational drugs, and immunosuppressive agents); and allogeneic hematopoietic stem cell transplantation (HSCT). If a patient had received more than one treatment, the patient was ascribed to the category corresponding to the more intensive treatment. In patients with moderate/severe BM fibrosis at the time of diagnosis, BM fibrosis was reassessed in some patients with follow-up BM biopsy available.
Statistical Analyses
For continuous variables, data were reported as medians and ranges and were compared by Mann-Whitney U test. For numerical variables, data were reported as the number of patients if not specified otherwise. Fisher’s exact test or χ2 was used for category comparison. Overall survival was calculated from the day of t-MDS diagnosis to death from any cause or to the last follow-up date. Distribution of OS was estimated by the Kaplan-Meier method; and comparisons between subgroups were performed using the log-rank test. Multivariate prognostic analysis was performed using the Cox regression model with categorical variables. All p values were two-tailed and were considered significant when <0.05.
RESULTS
Patients Characteristics
The demographic and hematologic features of the t-MDS patients are listed in Table 1. The prior diseases were grouped as hematological malignancies, solid tumors and non-hematological diseases (autoimmune diseases). The details of these prior malignancies are shown in the supplement Table 1. Of the 266 patients with t-MDS, moderate/severe BM fibrosis was present in 47 patients (17%), including 28 men and 19 women with a median age of 64 years. The age and gender were comparable between t-MDS patients with or without moderate/severe BM fibrosis. Hepatosplenomegaly, consumptive symptoms, and transfusion dependence were not statistically different either. Notably, while white cell count (WBC), absolute neutrophil count (ANC), and hemoglobin (Hb) levels were comparable, t-MDS patients with significant BM fibrosis had severer thrombocytopenia (42 × 109/L vs. 62 × 109/L, p=0.039). With a comparable BM cellularity, cases with moderate/severe fibrosis showed significantly increased megakaryocytes (p<0.001). The BM blasts were comparable between cases with or without significant fibrosis; however, t-MDS patients with significant BM fibrosis had a higher number of peripheral blood circulating blasts (p=0.051).
Table 1.
Demographic and Clinicopathologic Comparison of the Patients with t-MDS with or without Moderate/Severe Bone Marrow Fibrosis
| Bone Marrow Fibrosis
|
p | ||
|---|---|---|---|
| (MF 0–1) (n=219) |
(MF 2–3) (n=47) |
||
| Age (Years) | 64 (22–88) | 64 (18–79) | 0.603 |
| Gender (Male/Female) | 124/95 | 28/19 | 0.419 |
| Cancer history | |||
| Hematopoietic malignance | 152 (69.4%) | 23 (48.9%) | 0.027 |
| Solid tumor | 62 (28.3%) | 22 (46.8%) | |
| Non malignant disease | 5 (2.3%) | 2 (4.3%) | |
| Prior Treatment for cancer | |||
| Radiation only | 5 (2.3%) | 6 (12.8%) | 0.004 |
| Chemotherapy only | 155 (70.8%) | 28 (59.6%) | |
| Chemotherapy and radiation | 59 (26.9%) | 13 (27.7%) | |
| Peripheral blood | |||
| • White blood cell count (×10 9/L) | 3.0 | 2.9 | 0.872 |
| • Absolute neutrophil count (×109/L) | 1.4 | 1.3 | 0.872 |
| • Hemoglobin (g/dL) | 9.8 (6.8–16.0) | 9.3 (5.5–13.8) | 0.260 |
| • Platelets (×109/L) | 62 (6–388) | 42 (7–434) | 0.039 |
| • Peripheral myeloblasts (%) | 0 (0–18%) | 0 (0–15%) | 0.051 |
| Bone marrow (BM) findings | |||
| • Myeloblasts (%) | 3 (0–19%) | 4 (0–13%) | 0.144 |
| • Cellularity (%) | 50 (5–100%) | 50 (10–100%) | 0.190 |
| • Megakaryocyte numbers | |||
| Decreased (≤1/HPF) | 41 (37.6%) | 4 (9.8%) | <0.001 |
| Normal (2–5/HPF) | 28 (25.7%) | 8 (19.5%) | |
| Increased (≥6/HPF) | 40 (36.7%) | 29 (70.7%) | |
| Hepatosplenomegaly | 5 (2.3%) | 2 (4.4%) | 0.343 |
| Constitutional symptoms | 24 (11.0%) | 9 (20.0%) | 0.084 |
| Transfusion dependency | 64 (29.0%) | 16 (35.6%) | 0.243 |
Moderate/severe BM fibrosis was observed more frequently in t-MDS patients with a history of solid tumor versus hematological malignancy (46.8% vs. 28.3%, p=0.027). As for treatment modalities, there seemed more patients with moderate/severe BM fibrosis received radiation therapy alone (n=6, 12.8%) for prior malignancies, compared with patients with no or only mild BM fibrosis (n=5, 2.3%). However, when we combined all patients who received radiation, either radiation alone or radiation as an adjuvant to chemotherapy, we could not find any correlation of BM fibrosis with radiation therapy (p=0.908) (Table 1).
Cytogenetic Features in Patients with Significant BM Fibrosis
The median metaphases obtained for karyotyic analysis was 20 (range, 3–30) in the group with moderate/severe BM fibrosis and also 20 (range, 2–40) in the non-fibrotic group (not significant). Overall cytogenetic analysis was available/successful in 262 of 266 (98.5%) patients (Table. 2), including 45/47 (95.7%) in the group with moderate/severe BM fibrosis and 217/219 (99.1%) patients in the non-fibrotic group. Cases of t-MDS with or without moderate/severe BM fibrosis had a similar frequency of an abnormal karyotype at the time of diagnosis (80.0% versus 82%); a comparable cytogenetic risk distribution by the New Comprehensive Cytogenetic Scoring System for primary MDS and oligoblastic AML; [24] a comparable rate of a complex karyotype (more than 3 abnormalities) as well as amonosomal karyotype (at least one monosomatic chromosome and at least one structural abnormality). In both groups, abnormalities involving chromosomes 5 (−5 or −5q) and 7 (−7 or −7q) were the most frequently observed cytogenetic abnormalities; and −5, −7 and −17 were closely related with complex and monosomal karyotypes (Table 2). Additionally, +8, del (5q), del (7q) and −7 were observed at similar frequencies (11.1% vs 9.7%, p=0.785; 15.6% vs. 24%, p=0.246; 2.2% vs. 9.2%, p=0.141 and 42.2% vs. 33.6%, p=0.305) in t-MDS with or without moderate/severe BM fibrosis. However, t-MDS cases with moderate/severe BM fibrosis had a higher frequency of −5 and −17 (24.4% vs. 11.5%, p=0.031; 17% vs. 7.3%, p=0.043, respectively).
Table 2.
Cytogenetic Comparison of the Patients with t-MDS with or without Moderate/Severe Bone Marrow Fibrosis
| Bone Marrow Fibrosis | P | ||
|---|---|---|---|
| MF0–1 | MF2–3 | ||
| Patients | 217/219 (99%) | 45/47 (96%) | |
| Normal karyotype | 39 (18.0%) | 9 (20.0%) | 0.832 |
| Complex Karyotype | 98 (45.2%) | 24 (53.3%) | 0.330 |
| Monosomal | 96 (44.2%) | 24 (53.3%) | 0.324 |
| Karyotype | |||
| Cytogenetic Categories by IPSS-R | |||
| Very good risk | 2 (0.9%) | 1 (2.2%) | |
| Good risk | 52 (24.0%) | 11 (24.4%) | 0.462 |
| Intermediate risk | 26 (12.0%) | 6 (13.3%) | |
| Poor risk | 49 (22.6%) | 5 (11.1%) | |
| Very poor risk | 88 (40.6%) | 22 (48.9%) | |
| Trisomy 8 | 21 (9.7%) | 5 (11.1%) | 0.785 |
| Del (5)q | 52 (24.0%) | 7 (15.6%) | 0.246 |
| Monosomy 5 | 25 (11.5%) | 11 (24.4%) | 0.031 |
| Del (7)q | 20 (9.2%) | 1 (2.2%) | 0.141 |
| Monosomy 7 | 73 (33.6%) | 19 (42.2%) | 0.305 |
| Monosomy 17 | 16 (7.3%) | 8 (17.8%) | 0.043 |
Abbreviation: IPSS-R: revised international prognosis scoring system for myelodysplastic syndromes.
Mutations Studies
RAS, FLT3, KIT, NPM1 and JAK2 V617 mutations were performed in variable subsets of patients as a part of the routine work-up. These mutations were infrequent in t-MDS patients. In patients with moderate/severe BM fibrosis, 1 of 32 (3.1%) had a RAS mutation, 1 of 33 (3%) had FLT3 ITD, 0 of 22 (0%) had KIT mutation, 1 of 12 (8.3%) had NPM1 mutation, and 2 of 11 (18.2%) had JAK2 V617mutation. Of t-MDS patients with no/mild BM fibrosis, RAS mutation was present in 2/92 (2.2%), FLT3 ITD in 2/103 (1.9%), KIT mutation in 0/38 (0%), NPM1 mutation in 1/35 (2.9%), and JAK2 V617 in 1/9 (11.1%) cases. There were no statistically significant differences in the frequency of these mutations between two groups.
Bone Marrow Fibrosis Post Treatment for t-MDS
The presence or absence of moderate/severe BM fibrosis did not affect therapeutic decisions and the two groups of patients received comparable treatment modalities including best supportive care (12/47 vs. 66/219), immunomodulatory agents (2/47 vs. 5/219), hypomethylating drugs (23/47 vs. 88/219), induction chemotherapy (3/47 vs. 32/219) and allogeneic hematopoietic stem cell transplantation (HSCT) (7/47 vs. 28/219) (p=0.501). At our hospital, for MDS follow-up assessment, BM biopsy is only required for patients who either have inadequate BM aspirate material obtained or who are status post HSCT. Overall, a total of 28 patients with moderate/severe BM fibrosis had follow-up BM biopsy specimens available. Sixteen of these patients received hypomethylating agent treatment, and of these patients, 3 patients had complete hematological response (CR), 1 patient had stable disease and 9 patients had disease progression. None of these 16 patients (including 3 patients with CR) showed improvement of BM fibrosis, including the three responders. Eight (8) of 28 patients received best supportive care only and 3 patients received induction chemotherapy, and none of the patients showed improvement of BM fibrosis. Seven (7) of 28 patients received HSCT, of them, 5 patients achieved CR or BM CR after HSCT, and two of these five patients showed complete resolution of BM fibrosis.
Bone Marrow Fibrosis and Patient Outcome Analysis
Outcomes were assessed by using AML progression and overall survival (OS) after excluding patients treated with HSCT. With a median follow-up of 11.5 months, t-MDS with and without moderate/severe BM fibrosis showed similar rates of AML progression (9/39 vs. 48/191, p=0.482) and time to progression (8 months vs. 9 months, log rank, p=0.926) (Figure 2A) by Kaplan-Meier estimate. Moderate/severe BM fibrosis was not a significant hazard for AML progression in multivariate analysis when age, gender, BM blasts, ANC, Hb, platelet count and cytogenetic risk were co-analyzed (p=0.558). Moderate/severe BM fibrosis was also not a hazard for an inferior overall survival (median 10 months vs. 12 months in non-fibrotic group, log rank, p=0.972) by Kaplan-Meier estimate (Figure 2B), or by Cox Regression analysis (p=0.772). The effects of moderate/severe BM fibrosis on outcomes were compared in t-MDS patients with less than 5% BM blasts, as well as in patients with ≥ 5% BM blasts; moderate/severe BM fibrosis did not correlate significantly with AML progression or median OS in neither of these groups. (Figure 3)
Figure 2.

In patients with therapy-related myelodysplastic syndromes who did not receive hematopoietic stem cell transplant, the presence or absence of significant bone marrow fibrosis has no impact on cumulative incidence of transformation to acute myeloid leukemia (A) (p=0.926, log-rank test) or overall survival (P = 0.972, log-rank test) (B).
Figure 3.

The presence of moderate/sever bone marrow (BM) fibrosis is not a significant predictor for cumulative incidence of acute myeloid leukemia evolution (A & B) or overall survival (C & D) in patients with<5% (A & C) or ≥ 5% BM blasts (B & D).
DISCUSSION
In this study, we show that moderate/severe BM fibrosis is present in about 17% of t-MDS patients. This frequency is similar to that reported in primary (de novo) MDS patients [11]. Patients with primary MDS who have moderate/severe BM fibrosis have been shown to be a subgroup of patients who experience severer anemia, transfusion dependency, a high probability of AML progression and an inferior overall survival, [11] In contrast, in the therapy-related setting, moderate/severe BM fibrosis was not associated with severer anemia, or transfusion dependency; importantly, not a hazard for leukemia transformation or an inferior outcome.
The differences are likely attributable to clinical and biological differences between t-MDS and primary MDS. Compared with primary MDS, t-MDS cases show a higher frequency of cytogenetic abnormalities and these cytogenetic abnormalities are skewed towards higher risk groups (our unpublished data) using the New Comprehensive Cytogenetic Scoring System for primary MDS and oligoblastic AML criteria. [24] In additional, prior cytotoxic exposure often cause additional damage at the cellular and DNA level of hematopoietic cells; and these underlying genetic/epigenetic alterations likely explain chemo-resistance and poor responses to conventional therapy in t-MDS. [14,13,15,16] It has been known that in t-MDS, many of the well-known risk factors for primary MDS, such as morphological dysplasia and a precise division of BM blasts, may not be so important. [17] Rather, the clinical and biological behavior of this group of diseases may largely depend on underlying genetic/epigenetic alterations. In t-MDS patients, we did observe that moderate/severe BM fibrosis was accompanied by significantly increased megakaryocytes and severer thrombocytopenia. It has been shown that in many diseases, BM stromal fibrosis is associated with abnormalities of the number and/or function of megakaryocytes and platelets. [28] Our data indicate that similarly, megakaryocytic proliferation and their pathologic interaction with BM stroma likely play a central role in BM fibrosis occurring in therapy-related MDS. We also looked into specific cytogenetic alterations and found that monosomy 5 and 17 (often in the context of a complex karyotype) were significantly more frequent in the BM fibrotic group. Our previous study showed that myeloid neoplasms with isolated monosomy 17 frequently present with both myelodysplastic and myeloproliferative features, associated with significant BM fibrosis and osteosclerosis. [29] In PMF and post polycythemia vera/essential thrombocythemia fibrosis, chromosomal abnormalities are observed in less than one third of patients, and the most frequent karyotypic abnormalities are del (20q), del (13q), trisomy 8 and 9, and duplication 1q. [30] However, during disease progression to AML or MDS, cytogenetic abnormalities are observed in >90% of these patients, and are often of a complex karyotype with frequent involvement of chromosomes 5 and 7. [31] These findings indicate that these chromosomal aberrations, at least in part, may be responsible for the concurrence of dysplasia and BM fibrosis in various myeloid neoplasms, through promoting proliferation of hematopoietic stem cells, megakaryocytes, stromal cells and interrupting their interactions at multiple levels. Interestingly, the cytogenetic criteria used in primary myelofibrosis scoring systems increasingly resemble those used in MDS; these similarities likely reflect the overlapping molecular genetic alterations underpinnings of these two heterogeneous disorders. JAK2 V617 mutations are positive in approximately 50–60% PMF cases, and predict the risk of major clinical events including leukemic transformation. [32] JAK2 V617 mutations were low in primary MDS, and were detected in only 1 of 24(4%) patients in the study conducted by Della Porta and colleagues. [11] In our t-MDS patients, JAK2 V617 mutation frequency was similarly low (2/11, 18%) and did not distinguish cases with or without moderate/severe BM fibrosis. However, we only tested JAK2 V617 mutation in a small subset of cases; and a clear conclusion may require further validation in larger numbers of cases. The frequencies of other gene mutations (RAS, FLT3, KIT and NPM1) were similarly low in patients with t-MDS, and showed no difference between patients with or without moderate/severe BM fibrosis.
Hypomethylating agents and HSCT showed encouraging results in patients with t-MDS. [33,34] Our study showed that the presence moderate/severe BM fibrosis did not affect overall responses to hypomethylating agents and/or HSCT; however, substantial BM fibrosis did not regress in patients who received hypomethylating agents, even in those patients who achieved complete hematological responses. Similar findings have been reported in myeloproliferative neoplasms that moderate/severe BM fibrosis is not significantly altered by supportive or conventional chemotherapy. [35,36] In contrast, 2 of 5 patients who achieved CR or BM CR after HSCT, showed complete resolution of BM fibrosis at the time of last follow-up, likely attributable to graft-anti-fibrosis effect. We also show that moderate/severe BM fibrosis is more frequently observed in patients with t-MDS secondary to solid tumors as opposed to hematological malignancies. It is not yet clear if BM fibrosisis more prone to occur after certain types of therapies that are more frequently administered to solid tumor patients.
In conclusion, moderate/severe BM fibrosis can be seen in a subset of t-MDS patients and is associated with lower platelet counts, and a higher frequency of −5 and −17chromosomal abnormalities. However, moderate/severe BM fibrosis is not an adverse risk factor for survival and AML progression in patients with t-MDS. We believe that that clinical aggressiveness of t-MDS is mostly attributable to genetic/epigenetic alterations. These findings not only further illustrate the differences of MDS occurring in the de novo versus therapy-related setting, but also provide useful information for future risk models for t-MDS patients.
Supplementary Material
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SW designed and guided the experiments, BF, CO, MG, WX, JJ, CB, SV, TM and GG conducted the experiments, BF, SW and JM analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Informed consent was obtained from all patients for being included in the study.
References
- 1.Kvasnicka HM, Thiele J, Schmitt-Graeff A, Diehl V, Zankovich R, Niederle N, Leder LD, Schaefer HE. Bone marrow features improve prognostic efficiency in multivariate risk classification of chronic-phase Ph (1+) chronic myelogenous leukemia: a multicenter trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19 (12):2994–3009. doi: 10.1200/JCO.2001.19.12.2994. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian R, Basu D, Dutta TK. Significance of bone marrow fibrosis in multiple myeloma. Pathology. 2007;39 (5):512–515. doi: 10.1080/00313020701570038. [DOI] [PubMed] [Google Scholar]
- 3.Thiele J, Kvasnicka HM. Hematopathologic findings in chronic idiopathic myelofibrosis. Seminars in oncology. 2005;32 (4):380–394. doi: 10.1053/j.seminoncol.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Thiele J, Kvasnicka HM. Grade of bone marrow fibrosis is associated with relevant hematological findings-a clinicopathological study on 865 patients with chronic idiopathic myelofibrosis. Annals of hematology. 2006;85 (4):226–232. doi: 10.1007/s00277-005-0042-8. [DOI] [PubMed] [Google Scholar]
- 5.Vener C, Fracchiolla NS, Gianelli U, Calori R, Radaelli F, Iurlo A, Caberlon S, Gerli G, Boiocchi L, Deliliers GL. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood. 2008;111 (4):1862–1865. doi: 10.1182/blood-2007-09-112953. [DOI] [PubMed] [Google Scholar]
- 6.Gianelli U, Vener C, Bossi A, Cortinovis I, Iurlo A, Fracchiolla NS, Savi F, Moro A, Grifoni F, De Philippis C, Radice T, Bosari S, Lambertenghi Deliliers G, Cortelezzi A. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25 (9):1193–1202. doi: 10.1038/modpathol.2012.87. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114 (5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Verhoef GE, De Wolf-Peeters C, Ferrant A, Deprez S, Meeus P, Stul M, Zachee P, Cassiman JJ, Van den Berghe H, Boogaerts MA. Myelodysplastic syndromes with bone marrow fibrosis: a myelodysplastic disorder with proliferative features. Annals of hematology. 1991;63 (5):235–241. doi: 10.1007/BF01698371. [DOI] [PubMed] [Google Scholar]
- 9.Della Porta MG, Malcovati L. Myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2011;96 (2):180–183. doi: 10.3324/haematol.2010.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buesche G, Teoman H, Wilczak W, Ganser A, Hecker H, Wilkens L, Gohring G, Schlegelberger B, Bock O, Georgii A, Kreipe H. Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22 (2):313–322. doi: 10.1038/sj.leu.2405030. [DOI] [PubMed] [Google Scholar]
- 11.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, Passamonti F, Invernizzi R, Castello A, Magrini U, Lazzarino M, Cazzola M. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27 (5):754–762. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 12.Kroger N, Zabelina T, van Biezen A, Brand R, Niederwieser D, Martino R, Lim ZY, Onida F, Schmid C, Garderet L, Robin M, van Gelder M, Marks R, Symeonidis A, Kobbe G, de Witte T. Allogeneic stem cell transplantation for myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2011;96 (2):291–297. doi: 10.3324/haematol.2010.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voso MT, D’Alo F, Greco M, Fabiani E, Criscuolo M, Migliara G, Pagano L, Fianchi L, Guidi F, Hohaus S, Leone G. Epigenetic changes in therapy-related MDS/AML. Chemico-biological interactions. 2010;184 (1–2):46–49. doi: 10.1016/j.cbi.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Greco M, D’Alo F, Scardocci A, Criscuolo M, Fabiani E, Guidi F, Di Ruscio A, Migliara G, Pagano L, Fianchi L, Chiusolo P, Hohaus S, Leone G, Voso MT. Promoter methylation of DAPK1, E-cadherin and thrombospondin-1 in de novo and therapy-related myeloid neoplasms. Blood cells, molecules & diseases. 2010;45 (3):181–185. doi: 10.1016/j.bcmd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Li M, Sun C, Francisco L, Chakraborty S, Sabado M, McDonald T, Gyorffy J, Chang K, Wang S, Fan W, Li J, Zhao LP, Radich J, Forman S, Bhatia S, Bhatia R. Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer cell. 2011;20 (5):591–605. doi: 10.1016/j.ccr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2005;19 (12):2232–2240. doi: 10.1038/sj.leu.2404009. [DOI] [PubMed] [Google Scholar]
- 17.Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, Larson RA, Vardiman JW. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. American journal of clinical pathology. 2007;127 (2):197–205. doi: 10.1309/NQ3PMV4U8YV39JWJ. [DOI] [PubMed] [Google Scholar]
- 18.Vardiman JW, Arber DA, Brunning RD, Larson RA, Matutes E, Baumann I, Thiele J, editors. Therapy-related myeloid neoplasms. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. International Agency for Research on Cancer (IARC); Lyon: 2008. [Google Scholar]
- 19.Klyuchnikov E, Holler E, Bornhauser M, Kobbe G, Nagler A, Shimoni A, Konecke C, Wolschke C, Bacher U, Zander AR, Kroger N. Donor lymphocyte infusions and second transplantation as salvage treatment for relapsed myelofibrosis after reduced-intensity allografting. British journal of haematology. 2012 doi: 10.1111/bjh.12013. [DOI] [PubMed] [Google Scholar]
- 20.Quintas-Cardama AKH, Shan J, Jabbour E, Faderl S, Wierda WG, Ravandi F, Kadia T, Wang SA, Pierce S, Kantarjian HM, Garcia-Manero G. A prognostic model of therapy-related myelodysplastic syndrome for predicting survival and transformation to acute myeloid leukemia. Blood. 2012;118(Suppl 1):967. doi: 10.1016/j.clml.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90 (8):1128–1132. [PubMed] [Google Scholar]
- 22.Khoury JD, Sen F, Abruzzo LV, Hayes K, Glassman A, Medeiros LJ. Cytogenetic findings in blastoid mantle cell lymphoma. Hum Pathol. 2003;34 (10):1022–1029. doi: 10.1053/s0046-8177(03)00412-x. S004681770300412X [pii] [DOI] [PubMed] [Google Scholar]
- 23.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120 (12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schanz J, Tuchler H, Sole F, Mallo M, Luno E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki K, Steidl C, Fonatsch C, Pfeilstocker M, Nosslinger T, Valent P, Giagounidis A, Aul C, Lubbert M, Stauder R, Krieger O, Garcia-Manero G, Faderl S, Pierce S, Le Beau MM, Bennett JM, Greenberg P, Germing U, Haase D. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30 (8):820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Konoplev S, Medeiros LJ, Koeppen H, Leventaki V, Vadhan-Raj S, Jones D, Kantarjian HM, Falini B, Bueso-Ramos CE. Cuplike nuclei (prominent nuclear invaginations) in acute myeloid leukemia are highly associated with FLT3 internal tandem duplication and NPM1 mutation. Cancer. 2009;115 (23):5481–5489. doi: 10.1002/cncr.24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millecker L, Lennon PA, Verstovsek S, Barkoh B, Galbincea J, Hu P, Chen SS, Jones D. Distinct patterns of cytogenetic and clinical progression in chronic myeloproliferative neoplasms with or without JAK2 or MPL mutations. Cancer genetics and cytogenetics. 2010;197 (1):1–7. doi: 10.1016/j.cancergencyto.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson SA, Lin P, Chen SS, Chen L, Abruzzo LV, Luthra R, Medeiros LJ, Wang SA. Therapy-related acute myeloid leukemia with t(8;21) (q22;q22) shares many features with de novo acute myeloid leukemia with t(8;21)(q22;q22) but does not have a favorable outcome. American journal of clinical pathology. 2009;131 (5):647–655. doi: 10.1309/AJCP5ETHDXO6NCGZ. [DOI] [PubMed] [Google Scholar]
- 28.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23 (33):8520–8530. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 29.Kanagal-Shamanna R, Bueso-Ramos CE, Barkoh B, Lu G, Wang S, Garcia-Manero G, Vadhan-Raj S, Hoehn D, Medeiros LJ, Yin CC. Myeloid neoplasms with isolated isochromosome 17q represent a clinicopathologic entity associated with myelodysplastic/myeloproliferative features, a high risk of leukemic transformation, and wild-type TP53. Cancer. 2012;118 (11):2879–2888. doi: 10.1002/cncr.26537. [DOI] [PubMed] [Google Scholar]
- 30.Hussein K, Van Dyke DL, Tefferi A. Conventional cytogenetics in myelofibrosis: literature review and discussion. European journal of haematology. 2009;82 (5):329–338. doi: 10.1111/j.1600-0609.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 31.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105 (3):973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 32.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, Massa M, Rosti V, Campanelli R, Villani L, Viarengo G, Gattoni E, Gerli G, Specchia G, Tinelli C, Rambaldi A, Barbui T. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110 (12):4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 33.Klimek VM, Dolezal EK, Tees MT, Devlin SM, Stein K, Romero A, Nimer SD. Efficacy of hypomethylating agents in therapy-related myelodysplastic syndromes. Leukemia research. 2012;36 (9):1093–1097. doi: 10.1016/j.leukres.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Breccia M, Salaroli A, Loglisci G, Martelli M, D’Elia GM, Nanni M, Mauro FR, Alimena G. 5′-Azacitidine for therapy-related myelodysplastic syndromes after non-Hodgkin lymphoma treatment. Leukemia research. 2011;35 (10):1409–1411. doi: 10.1016/j.leukres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Buhr T, Busche G, Choritz H, Langer F, Kreipe H. Evolution of myelofibrosis in chronic idiopathic myelofibrosis as evidenced in sequential bone marrow biopsy specimens. American journal of clinical pathology. 2003;119 (1):152–158. doi: 10.1309/PTVG-B3DX-B8A8-M7KD. [DOI] [PubMed] [Google Scholar]
- 36.Morel F, Le Bris MJ, Herry A, Morice P, De Braekeleer M. Trisomy 15 as the sole abnormality in myelodysplastic syndromes: case report and review of the literature. Leukemia & lymphoma. 2003;44 (3):549–551. doi: 10.1080/1042819021000055084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.