Abstract
Homologous recombination is the most complex of all recombination events that shape genomes and produce material for evolution. Homologous recombination events are exchanges between DNA molecules in the lengthy regions of shared identity, catalyzed by a group of dedicated enzymes. There is a variety of experimental systems in E. coli and Salmonella to detect homologous recombination events of several different kinds. Genetic analysis of homologous recombination reveals three separate phases of this process: pre-synapsis (the early phase), synapsis (homologous strand exchange) and post-synapsis (the late phase). In E. coli, there are at least two independent pathway of the early phase and at least two independent pathways of the late phase. All this complexity is incongruent with the originally ascribed role of homologous recombination as accelerator of genome evolution: there is simply not enough duplication and repetition in enterobacterial genomes for homologous recombination to have a detectable evolutionary role, and therefore not enough selection to maintain such a complexity. At the same time, the mechanisms of homologous recombination are uniquely suited for repair of complex DNA lesions called chromosomal lesions. In fact, the two major classes of chromosomal lesions are recognized and processed by the two individual pathways at the early phase of homologous recombination. It follows, therefore, that homologous recombination events are occasional reflections of the continual recombinational repair, made possible in cases of natural or artificial genome redundancy.
Keywords: Homologous chromosomes, repeats, homologous alignment, strand exchange, recombination intermediate, Holliday junction, crossing-over, conversion, chromosomal lesion, double-strand break, inter-strand crosslink, disintegrated, replication fork, replication fork restart, RecA, RecBCD, RecFOR, RecG, RuvABC
1. Introduction into Homologous Recombination
1.1. Definition of HR and its place among other recombination mechanisms
Changes in DNA are called mutations. Mutations can be changes in one base, in several bases, in many bases. Recombination is also a change in DNA. How is it different from mutation? Generally speaking, recombination is a change in DNA that is not a point mutation, — for example, change or deletion or addition of several nucleotides. In other words, recombination can be defines as a DNA rearrangement — a change in the order of DNA elements. There are four basic types of enzymatic mechanisms for DNA rearrangements (also see (202) for a similar classification) (Table 1).
Table 1.
Comparison of various types of genetic recombination in respect of frequency, DNA requirements and the enzymes that catalyze these events.
| Recombination type | Frequency | Requirements | Catalyzed by |
|---|---|---|---|
| 1. Illegitimate | extremely low | Micro- or no homology | various DNA-processing enzymes |
| 2. Transposition | very low (regulated) | Ends of the jumping element | Transposase |
| 3. Homologous | low (when DNA damage is low) | Extensive homology | RecA, RecBC, RecFOR,... |
| 4. Site-specifichigh | A pair of short sites | Site-specific recombinase (integrase, invertase) | |
This chapter will concentrate on Homologous Recombination (HR). In Biology, homology means “fundamental similarity”. Applied to DNA, homology in strict sense means “sequence identity”. Thus, “homologous recombination” most frequently represents exchange between two homologous chromosomes — long DNA molecules with essentially the same DNA sequence, containing a few differences.
How is homologous recombination detected? One needs to bring two homologous chromosomes, residing initially in different cells, together in a single cell. In order to be able to detect exchange between the two homologous chromosomes genetically, one also needs a way to separate the two chromosomes (products of HR) into individual cells, to be able to score the phenotypes of individual chromosomes. Finally, to distinguish the two recombinant chromosomes from one another, as well as from the parental chromosomes, one needs scorable traits associated with these chromosomes that results in distinguishing phenotypes at the organismal level. In short, one needs chromosomes carrying phenotype-changing alleles of a pair of genes. Such scorable alleles are called “markers” (Fig. 1A).
Fig. 1.
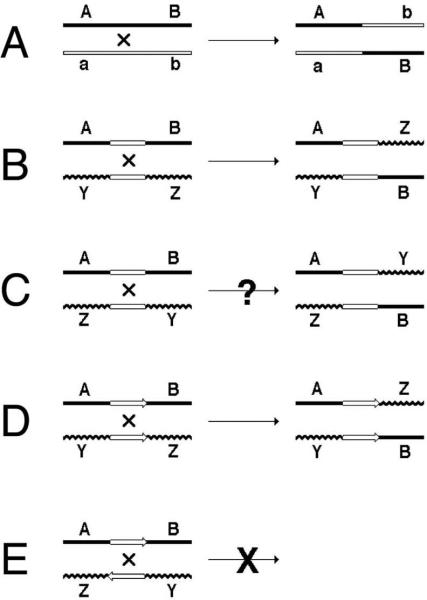
Illustrations to the search for definition of homologous recombination. DNA duplex is shown as a single line. The straight lines (whether open or filled) represent homologous DNA, the wavy and straight lines represent heterologous DNAs. A, B, a, b, Y and Z represent “markers” — DNA sequences that confer distinct phenotypes. “A” and “a” represent alleles of the same gene. Explanations are in the text.
From this diagram of the simplest AB x ab cross it is evident why homologous recombination is thought to have a prominent role in evolution: by combining alleles that have arisen in different lineages of the same species, homologous recombination accelerates evolution via generating additional diversity. Under the most prevalent case of stabilizing selection, homologous recombination will enhance elimination of mildly deleterious alleles from the population by combining several of them into organisms that will then have difficulty leaving progeny. However, this role of homologous recombination in evolution of most bacteria in general and E. coli in particular is questionable, mostly for two reasons: 1) most bacteria are clonal, with little means of recombinational cross-talk between lineages; 2) the size of typical bacterial populations is such that, with the relatively small bacterial genomes, all combinations of independently-arisen alleles can be generated by mutagenesis alone.
Returning to our definition of homologous recombination, the two chromosomes do not need to be homologous over their entire length for this exchange, as a rather limited region of homology is enough (Fig. 1B). Therefore, homologous recombination is “exchange between two DNA sequences in the region of shared homology”. The minimal length of such a shared region of homology fluctuates from one recombination system to another, contributing to confusion about what to consider homologous recombination and what not. The confusion is exacerbated by the fact that short identical sequences are frequently involved in illegitimate recombination events, while the sites by which site-specific recombination events are catalyzed can also be viewed as short regions of homology. Homologous recombination is distinguished from other recombination events not only by the requirement for extended length of homology, but also by the enzymatic systems catalyzing the event. Homologous recombination is catalyzed by specialized and complex enzymatic machinery, at the core of which lies the still mysterious ability of the homologous recombinase protein (RecA in bacteria, RadA in archaea, Rad51 in eukaryotes) to recognize homology between two DNAs independently of their actual sequence (57). According to this logic, if recombination between 20 bp homologies in E. coli is completely prevented in a recA mutant, it is considered a homologous recombination event (4). And vice versa, even if the length of homology mediating the exchange is a significant 500 bp, but such a recombination occurs with a good frequency in a recA mutant, this RecA-independent recombination is “homology-driven illegitimate recombination” event (reviewed in (31)). To provide some kind of a reference at this point, RecA-dependent recombination, although quite low, is already detectable between 12 base pair-long identical sequences in some experimental systems (290) and becomes the predominant mode of exchange between shared homologies 100 bp or longer in most experimental systems (76, 139, 200, 222, 295).
Finally, an important clarification, illustrated by the trick question: if we flip one of the chromosomes 180° in Fig. 1B, can we still perform the exchange (Fig. 1C)? After all, this would be still an “exchange in the region of homology”. The answer is, of course, “no”, because DNA sequences have polarity (unless they are inverted palindromes), and the regions of homology have to be not only aligned, but also co-oriented to promote recombination (Fig. 1D and E). Therefore, the final definition of homologous recombination is “exchange between two DNA sequences in the region of aligned homology, catalyzed by dedicated homology-recognition systems”.
1.2. The principle of HR. The Holliday junction generation and resolution
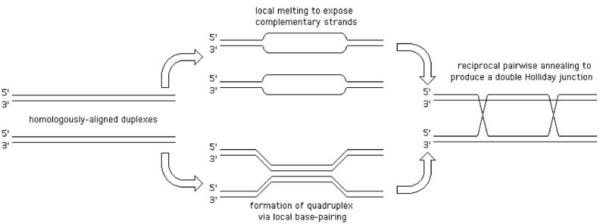
The exchange in homologous recombination goes through formation of branched DNA molecules, which are then “resolved”. There are several types of branched DNA that can all yield exchange upon resolution; as a convenient example, we will consider the formation of, perhaps, the most complex of these, but whose resolution is the most straightforward. Aligned homologous DNA duplexes (Fig. 2, left) could be imagined to locally melt (Fig. 2, center top) to facilitate formation of the recombination intermediate, in which the strands of the two duplexes are separated and exchanged in alternative pairing to form two hybrid duplexes (Fig. 2, right). Alternatively, the aligned homologous duplexes could be imagined to first form a local quadruplex via the major or minor grove interactions (79, 227, 347) (Fig. 2, center bottom) that then evolves into the intermediate on the right. It should be stressed that neither of the two transient events (Fig. 2, center) was ever detected, — they are shown here to facilitate understanding the nature of the recombination intermediate. This homologous alignment of two DNA duplexes with subsequent strand exchange is the critical step in homologous recombination reaction, catalyzed by dedicated recombinases (the RecA proteins in bacteria). The places in the recombination intermediate (Fig. 2, right) where the two original duplexes end, while the hybrid duplexes begin, are called Holliday junctions, after Robin Holliday who first proposed their existence and explained their importance (121). The formation of Holliday junctions in recombining molecules was subsequently confirmed in electron microscopy studies (30, 259, 260). Specifically, Holliday proposed that DNA junctions are “resolved” by single-strand cleavages into either one or the other pair of opposite strands. The two alternative ways to resolve a Holliday junction can be better imagined if we isomerize the junction by rotating two adjacent arms by 180° (260) (Fig. 3). This operation makes the junction four-fold symmetrical, whereas before it was two-fold symmetrical. Such a fourfold symmetrical junction can be resolved by two-strand cleavage in either one diagonal direction or the other (Fig. 3). It should be stressed that the cleavage affects only two opposite strands at the junction out of the total four. Both ways resolve the junction, freeing the participating duplexes from each other, while the resulting nicks at identical positions in the participating chromosomes are later sealed by DNA ligase (Fig. 3).
Fig. 2.
Theoretically-conceivable pathways of formation of homologous recombination intermediate with two Holliday junctions.
Fig. 3.
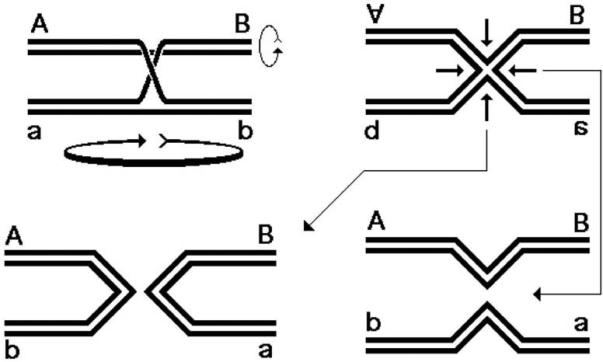
Resolution of a single Holliday junction.
Now, where does the exchange of shoulders between participating chromosomes come from? It turns out that cutting the Holliday junction in one direction returns the situation to the parental chromosomes, whereas cutting in the perpendicular direction results in chromosomes that have their shoulders exchanged. Since there are two junctions in our original recombination intermediate (Fig. 2, right), resolution of the second junction also yields either the parental or recombinant arrangement of markers, and the combination of the two becomes a matrix of 2 × 2 = 4 possibilities (Fig. 4). Two of these possibilities give parental combinations of shoulders, while the other two give recombinants. Formation of these recombinants is detectable genetically if the parental chromosomes have marked shoulders.
Fig. 4.
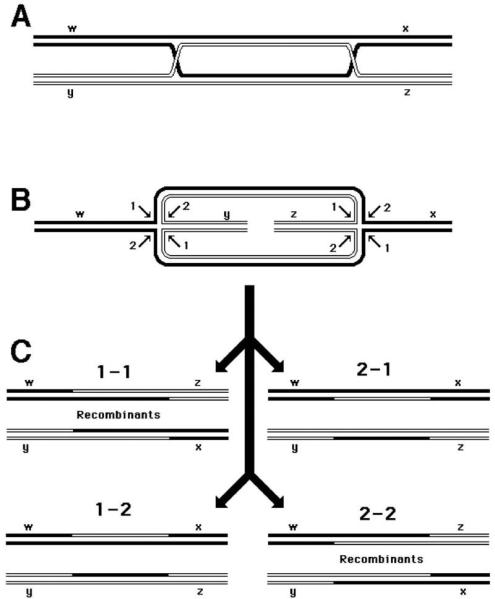
Resolution of the double Holliday junction recombination intermediate. Each diagonal pair of resolution cuts is numbered either “1” or “2” for each junction. If the two junctions freely isomerize and are resolved independently of each other, four outcomes of the resolution are expected. In two of the outcomes, the chromosome arms will be exchanged, resulting in recombinant chromosomes. A. A joint molecule with two junctions as shown in Fig. 2, right. B. The same joint molecule isomerized so as to show both junctions in the open planar configuration (258). C. The four resolution outcomes, numbered according to the resolution options realized at the left and the right junctions. Note that two outcomes (1-1and 2-2) produce chromosomes with recombinant shoulders, while the other two outcomes (1-2 and 2-1) produce chromosomes with parental shoulders.
It should be again stressed that Holliday junctions represent only one type of branched DNA molecules, and we considered them mostly for the reason that their resolution is understood best.
1.3. The basic types — HR between homologs, HR between unrelated chromosomes sharing short regions of identity, HR by repeats: deletions, inversions and unequal sister chromatid exchange
We began with defining “recombination” as DNA rearrangements, but eventually spoke of homologous recombination as of something that occurs most often between almost identical chromosomes, which, intuitively, precludes rearrangements. In the above examples, homologous recombination generates chromosomes with new combinations of alleles, originally present on separate chromosomes (Fig. 1A and 4C). Note that the order of genes on the chromosomes is not changed in this process. Exchange between mostly homologous chromosomes is the first basic type of HR and the only one that does not rearrange chromosomes.
Rearrangement is a change in the order of genes on the chromosomes (268). Homologous recombination changes the order of genes on the chromosomes, for example, as a result of the discussed above (Fig. 1D) exchange between mostly unrelated chromosomes in the region of shared identity. This can take the form of “insertion by homology”, if one of the chromosomes was a circular one (Fig. 5A). Recombination can also change the order of genes within the chromosome if the chromosome carries repeats: identical DNA sequences in either direct or inverted orientation. In a reaction that can be viewed as a direct reversal of “insertion by homology”, looping-out at direct repeats mediates formation of a deletion (Fig. 5B). The resulting circles are detectable either genetically (208) or, when the number of tandem repetitions is significant, even physically (282). If the repeats are in the inverted orientation, the product of recombination will be an inversion (Fig. 6A). Finally, unequal exchange between two identical sister chromosomes carrying direct repeats leads to the simultaneous formation of a duplication and a deletion (Fig. 6B), while such an “alternative” exchange between sister chromatids carrying inverted repeats leads to formation of dysfunctional chromosomes and to sure cell death (Fig. 6C) — this is why this latter rearrangement is only detectable in specialized systems (2.3.2.).
Fig. 5.
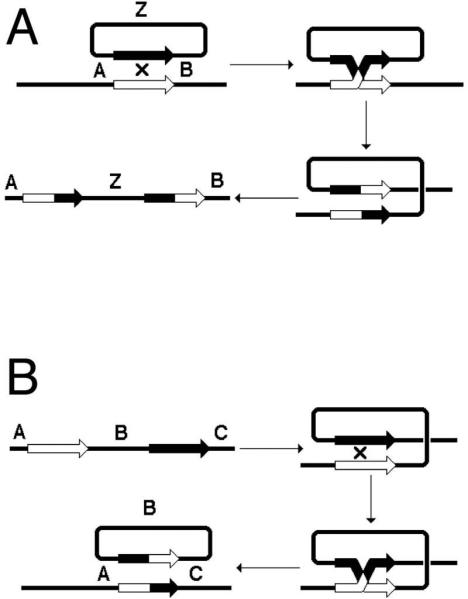
Homologous recombination can generate insertions or deletions. A. Recombination between a circular and a linear chromosomes by the region of shared homology inserts the circular chromosome into the linear one. B. Recombination between two directly-oriented regions of homology on the same chromosome leads to the loss of genetic information (deletion) in the form of a circle.
Fig. 6.
Homologous recombination can generate inversions, unequal sister chromatid exchange, as well as dysfunctional chromosomes. A. Recombination between inverted repeats within the same chromosome generates an inversion. B. Recombination between sister chromatids carrying direct repeats can lead to unequal sister chromatid exchange (deletion in one chromatid, duplication in the other). C. Recombination between sister chromatids carrying inverted repeats generates dysfunctional chromosomes and most of the times will result in cell death.
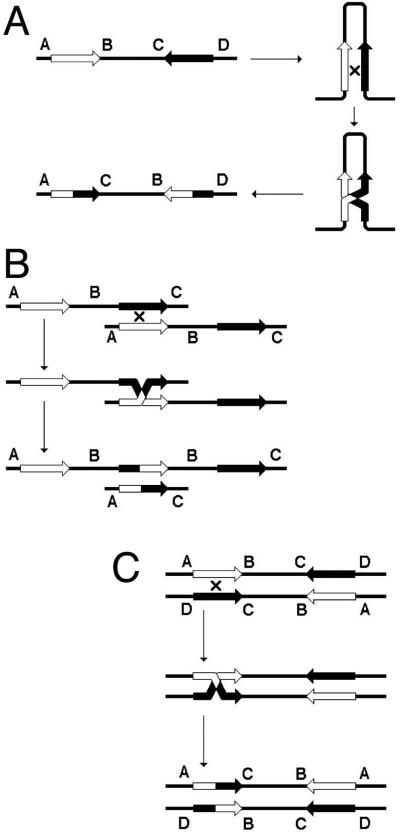
The important point to remember is that a chromosome with repeats is susceptible to rearrangements. Another important point to remember is that the frequency of these rearrangements is still very low (10-3 - 10-4), so the gross lineage viability is not affected, and one needs a selection to detect these rearrangements. Finally, repeats can recombine without rearranging the chromosome — either by a close double crossover or by gene conversion (Fig. 7) — these recombination events become “visible” if scorable genetic markers are placed within the repeats. “Gene conversion” is a homology-guided non-reciprocal transfer of information from one chromosome to another.
Fig. 7.
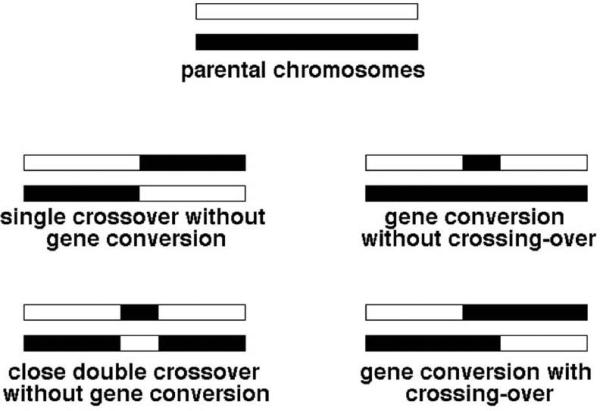
Crossing-over versus gene conversion: classification of detectable events. The two parental chromosomes are shown at the top. Alternatively, these could be two repeated sequences within the same chromosome. Although the two chromosomes (or repeats) are essentially homologous over their entire length, they are assumed to have enough markers (easily scorable disagreements in their primary DNA sequences) to facilitate fine mapping of the recombination events and conversion tracts. The four major types of recombination events, all results of alternative processing of a single recombination intermediate as in Fig. 2 (right) and Fig. 4A, are presented.
1.4. Single crossover versus double crossover in mapping chromosomal genes
HR can be used to map mutations. There are two peculiar properties of HR that allow one to do this. Property #1 is that HR is an infrequent event. In the AB x ab cross, most of the outcomes will be parental chromosomes, and only a few recombinants (Ab and aB) will be obtained. Property #2 is that the probability that an exchange would happen between any two base pairs in DNA is minuscule, but not zero. When there is a significant length of homology between the two markers on the chromosomes, all these tiny probabilities are multiplied thousands and millions of times to result in a detectable recombination. Therefore, the frequency of HR between the two close markers is proportional to the physical distance between the two markers. For a pair of more distant markers, this frequency becomes a complex function of the physical distance. Properties #1 and #2 combined allow one to map markers along the chromosomes, measuring the frequency of their segregation from each other (Fig. 8A). For example, if in the cross a1b1c1 × a2b2c2 the frequency of segregation of “a” from “b” is 10%, b from c is 5%, whereas a from c is 13%, and if we know that the chromosomes in this organism are linear, we arrive at the gene order: a -10% - b - 5% - c (Fig. 8A).
Fig. 8.
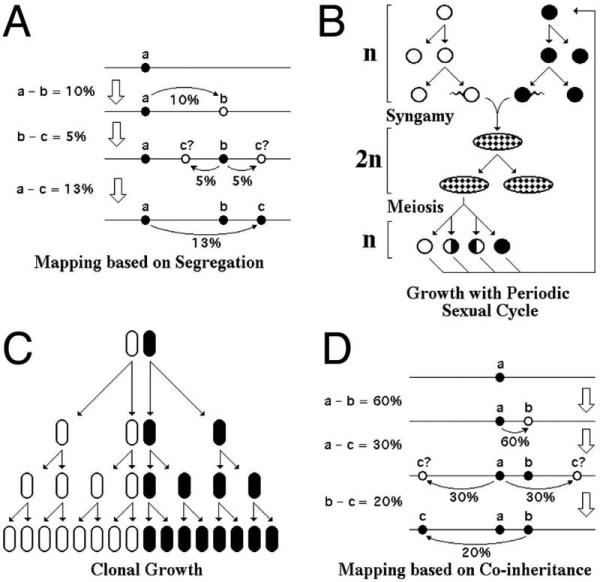
Chromosomal mapping in eukaryotes and in bacteria: peculiarities of the life cycles. Explanations in the text. A. Mapping based on segregation frequencies in eukaryotes. B. Growth of a unicellular eukaryote with periodic sexual events: either syngamy or meiosis. Circles denote gametes (1n cells), ovals denote zygotes (2n cells). C. Strictly clonal growth in the majority of bacteria. D. Mapping based on co-inheritance frequencies in bacteria.
However, this familiar algorithm belongs to the eukaryotic genetics. The eukaryotic life cycle facilitates HR via syngamy and meiosis (Fig. 8B). Syngamy is a life stage when two entire genome complements are brought together in a single nucleus of a zygote in preparation for meiosis. In zygote, every chromosome has its homolog, — an essentially identical chromosome, with a few differences (our “markers”, for example). Zygote can multiply mitotically for some time (in higher multicellular eukaryotes this zygotic clonal multiplication becomes synonymous with the vegetative life stage itself) (Fig. 8B). Meiosis is a special division of a zygote, distinct from mitosis, that regenerates gametes, or cells with single chromosomal sets. During meiosis, each pair of homologous chromosomes undergoes from one to several exchanges, so there are no non-recombinant chromosomes in gametes. Finally, all natural eukaryotic chromosomes are linear. Any number of exchanges between two homologous linear chromosomes always regenerate monomeric chromosomes.
The nature of viable chromosomal recombinants is quite different in bacteria, because the vast majority of bacteria grow as clones without detectable exchange of genetic information (Fig. 8C), forcing a completely different metric for mapping (Fig. 8D and see below). In the absence of syngamy as a life stage, it is close to impossible to bring in an entire chromosome from another cell. Even if such a chromosome could have been introduced in a bacterial cell, in the absence of the mechanisms to maintain diploidy, such a zygote would be unstable and would immediately segregate the two chromosomes into their own cells. Instead, what is achievable in E. coli is to bring an unstable linear piece of another chromosome into the cell. However, such an exchange of a whole chromosome with a subchromosomal fragment creates a problem: the chromosome becomes fragmented and, therefore, unstable itself (degradation, inability to complete replication or to finish segregation) (Fig. 9A). To preserve the functionality of the chromosome during recombination with a linear chromosomal piece, two exchanges are required in bacteria (Fig. 9B).
Fig. 9.
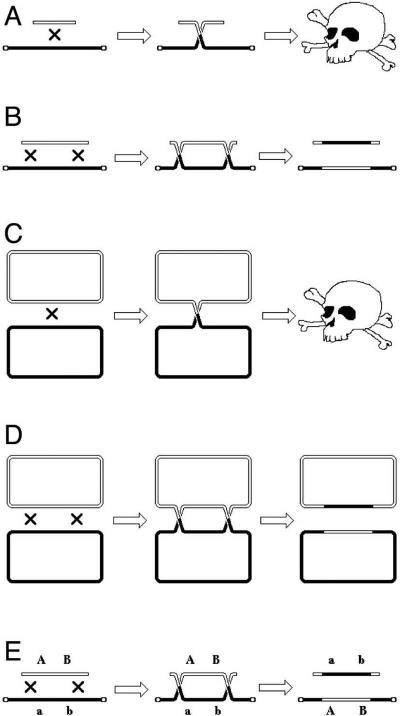
Detriment of single exchanges in bacterial chromosomes and permissibility of double exchanges. A. A single exchange with a linear subchromosomal fragment introduces unprotected ends into the chromosome leading to chromosome degradation. The chromosome is shown linear, with telomere-protected ends, which does not change the argument. B. The same situation as in “A”, but with two exchanges, which leave the chromosome physically intact, even though genetically different. C. A single exchange between two circular chromosomes creates a chromosomal dimer, which will kill the cell if left unresolved. D. The same situation as in “C”, but again with two exchanges, that keep the two recombining chromosomes monomeric and, therefore, functional. E. The double exchange from “B” is shown with markers to illustrate a better applicability of coinheritance, rather than segregation, to quantify crosses in systems in which only double exchanges survive.
Another common trait of bacterial chromosomes that complicates bacterial genetics is their circularity. Even if there were a way to create a bacterial zygote, — a cell that would contain two whole, genetically marked chromosomes, a single exchange between two circular chromosomes would create a dimer chromosome, which is equally non-functional (Fig. 9C). The solution to this complication again is provided by the double exchange, which preserves the original, monomeric state of the chromosome (Fig. 9D):
Therefore, in bacteria with their mostly circular chromosomes and with the majority of recombinational events involving exchanges with linear subchromosomal fragments, recombinants between chromosomes are always the result of two (or any even number of) exchanges. In these conditions, it is more convenient to look for the simultaneous incorporation of two markers from the subchromosomal fragment, — the so-called co-inheritance of the two markers (Fig. 9E). The higher the frequency of co-inheritance in bacteria, the closer the two genes are on the chromosome, opposite to what is surmised from recombination frequencies in eukaryotes. For example, if among the progeny that inherited the “a” gene, 60% inherited “b” and 30% “c”, while among those that inherited “b” only 20% inherited “c”, one concludes that the order of genes is c-a-b (Fig. 8D). Parenthetically, since in E. coli most of the fine mapping is done with P1 transduction (2.1.2.), co-inheritance also reflects the probability of two loci to be co-packaged in the P1 capsid, which takes ~100 kbp of DNA. The important concept to remember is that, if a genetic cross involves one complete and one incomplete chromosome, or two complete circular chromosomes, it will require two (or any even number of) exchanges to generate viable progeny.
1.5. Viable single crossover and half-crossover events
It should be noted that not all homologous recombination product formation in bacteria requires double crossovers, — only events between two chromosomes do (even if one of them is only a piece of the chromosome, as long as it is linear). Final recombination products can be obtained by single crossover events when recombination happens between closely-spaced repeats on the same chromosome or between the chromosome and a low-copy-number plasmid. Bacteriophage chromosomes are physically linear during replication and homologous recombination (even though genetically they may be circular), so single crossovers predominate in products of phage-by-phage recombination. Single crossovers are also OK in phage-by-plasmid recombination, when the plasmid is small, while the phage has space to accommodate this extra DNA.
Remarkably, phage-by-phage and phage-by-plasmid crosses reveal yet another type of crossovers, which can be called “half-crossovers” (Fig. 10), representing the “break-copy” principle of homologous recombination (167). In fact, it is currently thought that a significant fraction, perhaps even a majority, of homologous recombination events in the chromosome are of the half-crossover, break-copy type (60, 163), but they are genetically silent because they happen within the same chromosome and reflect recombinational repair of disintegrated replication forks (5.2.).
Fig. 10.
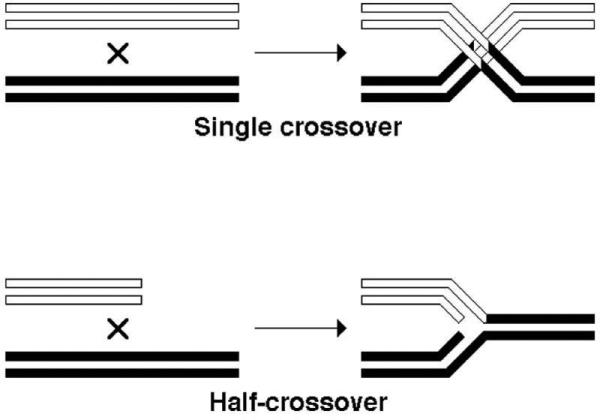
The difference between a full single crossover event and a half-crossover event. In this case, both strands of participating DNA duplexes are shown. One homologous duplex has open strands, the other homolog has filled strands.
1.6. Detection of homologous recombination
Specific approaches to detect homologous recombination can be roughly subdivided onto genetic, physical and metabolic ones. Genetic approaches include selections, when cells carrying non-recombinant parental markers cannot grow (since this is the overwhelmingly predominant approach, no citations are necessary), and screens — when recombinants look different from non-recombinants, like in papillation or sectoring assays (90, 147). Physical detection looks for 1) the formation of intermediate density species when the two parents have distinct densities (88, 232, 308); 2) change in the overall chromosomal size — for example, multimerization or monomerization of a circular plasmid (87, 145); 3) change in the chromosome configuration — for example, turning circular plasmid into linear concatemeric form (356); 4) change in size of specific fragments (256, 309); 5) stimulation of the experimental chromosome replication — for example, stimulation of plasmid replication by an infecting phage with shared homology (155, 220). Polymerase chain reaction allows one to detect even the elusive recombination intermediates, especially during synchronized reactions in eukaryotes (314); however, due to the extremely fast rate of recombination in E. coli and Salmonella, this method is less applicable in these organisms. Finally, metabolic detection relies on transcription of the recombinant DNA to yield detectable product of the restored gene (24, 193).
2. Specific recombination systems
Three basic formats of recombination reactions in relation to the topology of the participating substrates are possible: 1) circular-by-circular; 2) circular-by-linear; 3) linear-by-linear (Fig. 11). Recombination in E. coli can be detected between two chromosomal DNA sequences, or between the chromosome and an extrachromosomal genetic element (phage, plasmid), or between two extrachromosomal elements. When the chromosome is involved, the cell is a merozygote (= partial zygote) by definition, but when both recombining DNAs are extrachromosomal, the E. coli cell is most of the time a complete zygote for those DNAs, even if a transient one in crosses involving phages.
Fig. 11.
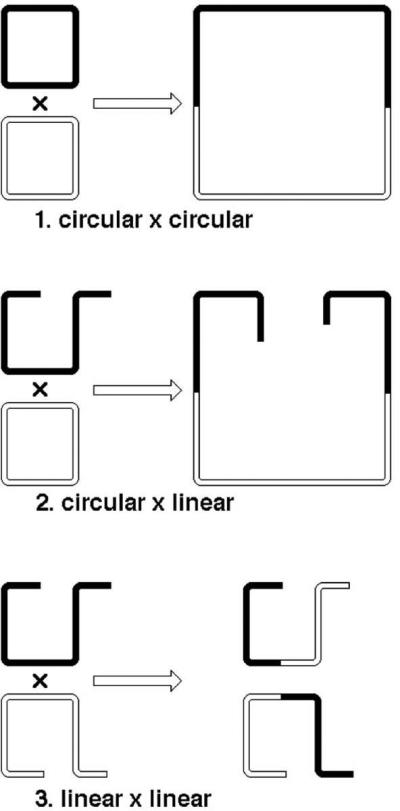
The three basic topological formats of recombination reaction. In spite of the argument of Fig. 9, for the sake of clarity a single crossover is shown in all cases.
2.1. Chromosome-based unstable merozygotes
When a linear piece of a different chromosome comes to a recipient cell via conjugation with an Hfr strain, or as a result of transduction or transformation, the resulting merozygotes are unstable because the introduced piece does not have its ends protected and will be eventually degraded by the cellular exonucleases, if not incorporated into the resident chromosome. The entering linear piece may even have a replication origin — but the ensuing replication, revealed by production of multiple recombinants from a single exconjugant cell (196, 251, 349), does not matter as long as the piece stays linear, because it is unstable. The topological format of this recombination is circular-by-linear, and the formation of viable products always requires double crossovers.
2.1.1. Conjugative recombination
Bacterial cells are frequently infected by big plasmids that spread to uninfected cells of related bacteria by building conjugation bridges between two cells, spooling through the bridge one DNA strand starting from a specific “transfer” origin and replicating it in the recipient cell into a duplex (101, 335). In E. coli, such a plasmid is called “F”, from “fertility factor” (127, 141, 171, 343). When the F-plasmid is integrated into the host chromosome (usually via homologous recombination (44, 62) at IS elements (70), but sometimes as a result of transposition of these elements itself (327)), it still tries to conjugate into a susceptible bacterium, now carrying the whole host chromosome with it (133). The strain that has F-plasmid in the chromosome is called Hfr (for “high frequency of recombination”), or “male”, or “donor” strain, while the susceptible strain without F-plasmid is called the F-, or “female”, or “recipient” strain (64). The mating bridge between the male and female strains is built to support transfer of DNA much longer than the 100 kbp F-plasmid; however the DNA transfer itself is quite vulnerable to unknown chromosomal factors (348), — as a result, even under optimal conditions, the probabilities of transferring one-quarter, one-half and the entire chromosome can be calculated as only 0.2, 0.039 and 0.0015 (302). Transfer of an incomplete chromosome, a sub-chromosomal fragment, turns the recipient cell into a merozygote, in which the circular resident chromosome can recombine with the sometimes quite long linear piece of the Hfr (“donor”) chromosome. In 80% of the cases, such recombination leads to incorporation of the bulk of the linear DNA as a single “chunk” into the recipient chromosome; in the rest 20%, shorter segments of the donor subchromosomal piece are incorporated into the donor chromosome independently of each other (302).
The frequency of such recombination after conjugation depends on several factors, the major ones being: 1) the frequency of conjugational transfer itself, which may vary from one donor-recipient pair to another; 2) the DNA replication proficiency of both the donor and the recipient strains; 3) the recombination proficiency of the recipient; 4) the level of DNA damage in the donor strain. For example, if the donor cells are treated with DNA-damaging agents before transfer, much shorter pieces of their chromosome are incorporated into the recipient genome; if the recipient cells are treated instead, the frequency of recombinants (relative to the survivors) is increased (342, 350).
Sometimes after integration into the donor chromosome, F-plasmid breaks free, picking a piece of the chromosomal DNA with it; such F-plasmids carrying segments of the host chromosome are called F’ (201). When an F’ conjugates into a recipient cell, it is stably maintained there, making the cell diploid for the chromosomal region the plasmid carries. In these stable merodiploids, two types of recombinants are detected: F’-plasmid integration into the chromosome by homology and the exchange between chromosomal and F’-plasmid-carrying markers, frequently a non-reciprocal one (201) (and also see 2.2.1.). Even if no recombination is detected, F’ transfer itself is useful for normalization of the conjugation- and DNA replication-proficiency of this donor/recipient pair (190).
2.1.2. Generalized transduction
Bacteriophages infect bacterial cell and utilize its material to replicate and package their genomes, then lyse the emptied bacterium to infect new cells (32). Bacteriophages are specifically designed to either shred the host DNA to utilize its nucleotides or, if the host chromosome is not degraded, at least to avoid packaging pieces of it, but many of them do it at low frequency anyway (218). When such a “phage” carrying only a piece of bacterial chromosome infects a cell of the same or closely-related bacterial species, the injected sub-chromosomal piece can incorporate via homologous recombination into the resident chromosome, bringing in new genes or different alleles of resident genes. Such a transfer of genetic information from one bacterial cell to another with the help of a bacteriophage is called “generalized transduction” (218). Generalized transduction can be observed with any virulent or lytically-developing temperate bacteriophage (although with most of them its detection requires either special phage mutants or conditions) and transfers any chromosomal piece with a low and more-or-less equal probability.
Certain phages can be mutated to corrupt the specificity of their packaging machinery — this makes them better “transducing phages” (312). The widely-used transducing phage in E. coli is a derivative of P1 (capsid takes about 100 kbp worth of DNA), for Salmonella it is a derivative of P22 (capsid takes about 44 kbp of DNA) (218). While in typical transducing phages the fraction of transducing particles is less than 1% and never more than 5%, mutant phages are known in which up to 50% of the virions can be filled with the random pieces of host DNA (289, 332).
Although the injected piece in the case of P1 transduction is full 100 kbp, the median size of the integrated pieces is only 20% of the total size, ~20 kbp (218). Still, markers separated by 60 kbp co-integrate with 0.1x frequency of the integration of a single marker. For a much smaller P22 of Salmonella, the numbers relating integration frequencies with the length of integrated pieces are surprisingly similar (218). Integration is again in “one chunk” (78, 236). Generalized transduction is by far the most common and easiest way to transfer mutations between strains in E. coli and Salmonella. Moreover, it can be also used to select for (or to generate?) duplications in the recipient chromosome; counterintuitively, the duplicated chromosomal segment in this case can be much longer than the transduced piece itself (6, 118). In experienced hands, transduction becomes a kind of genomic Lego, as complex chromosomal rearrangements can be assembled in several transducing steps from suitable donors (268).
2.1.3. Phage-by-chromosome events and specialized transduction
“Specialized transduction” is a phenomenon observed with a sub-class of temperate phages that, as lysogens, reside in the host chromosome. As a result of improper excision events, these phages occasionally spawn hybrid variants carrying stretches of contiguous chromosomal regions (337). Lysogenization with such a hybrid phage via the regular site-specific recombination at the attB site generates a bacterium with a duplication of the captured region, and the majority of the specialized transduction events are of this type, which has nothing to do with homologous recombination. However, lysogenic induction of phage Lambda, for example, also generates defective phage particles, called Lambda doc, that result from attempted packaging of the Lambda copy that is still in the chromosome, and therefore approximately half of DNA in these particles is the neighboring bacterial DNA, either to the right or to the left of the prophage (337). Recombination of such a hybrid phage with the chromosome is by chromosomal homology (338) and resembles generalized transduction, only it is specific to the regions contiguous with the prophage — hence the term “specialized transduction”.
The read-out in transduction is always formation of a recombinant bacterium, so the fate of the phage DNA, originally attached to the transduced chromosomal piece, is unknown. However, the situation can be turned around, when the chromosomal homology is constructed into the phage genome, and the recombination is followed by detecting recombinant phages that “picked up” markers from the chromosome, in either heteroimmune or homoimmune crosses (25, 177). Probably one of the more dramatic examples of such recombination is the formation of the Lambda-reverse phage. A mutant lambda, deficient in its own phage recombination functions, cannot grow well on the wild type E. coli. This growth defect in wild type E. coli is suppressed when the mutant lambda picks up the recET genes of the defective Rac prophage from the host chromosome (94, 97, 136). Although Rac is mostly heterologous with lambda, short regions of shared homology apparently provide enough information to make a successful recombinant, as proposed for other phage-by-phage crosses (38).
2.1.4. Transformation
Although a few bacteria are naturally transformable with exogenous DNA from the environment (195, 313), most bacteria do not take in exogenous DNA unless artificially forced to become “competent”, and E. coli is not an exception. E. coli cells can be made competent (= transformable, capable of taking in DNA) by treatment with divalent cations (especially Ca2+) (105, 106, 214). Calcium phosphate has a low solubility, and DNA, being a polyphosphate in nature, “precipitates” on the cell surface LPS in the presence of Ca2+ (252), which facilitates subsequent DNA internalization, via still unknown mechanisms, triggered by a transient heat-shock. However, the best way to introduce DNA into E. coli remains electroporation in the extremely high and extremely brief electric fields (106, 237), when DNA molecules become projectiles and forcefully penetrate into bacterial cells (138).
Once the problem of DNA uptake is overcome in E. coli, a liner DNA that wants to incorporate into E. coli chromosome by homology faces another problem: degradation by the RecBCD exonuclease (19, 330). This makes recombination after exogenous DNA introduction by either chemical or physical means not a very efficient process in wild type E. coli (although still the last chance, if P1 transduction cannot be done) (80), but the situation can be significantly improved in recBC sbcBC or recD mutants of E. coli, in which alternative recombination pathways operate (52, 119, 275, 331), or if the host recombination enzymes are replaced altogether with those of lambdoid bacteriophages (244, 355, 358). In fact, the chromosomal incorporation of homologous DNA by phage recombination functions is so efficient, that PCR products (68) and even oligonucleotides (53, 359) are now routinely used to design new chromosomal DNA sequences in vivo (287, 321) (see Eco-Sal III Module 7.2.9. λ Recombination and Recombineering).
2.2. Chromosome-based stable merozygotes
Merozygotes are stable when two (or more) homologous sequences can stably propagate together in the same cell. This situation is realized when there are plasmids in the cell carrying pieces of the chromosomal DNA, or there are repeats on the same chromosome. The format of this recombination is circular-by-circular, although mechanistically it is likely to be a mixture of circular-by-circular and circular-by-linear events (ends of the double-strand breaks). Some of the resulting viable recombination products can be formed by single crossovers.
2.2.1. Plasmid-by-chromosome events
When plasmids, — autonomously-replicating and much smaller than the host chromosome DNA molecules, — carry segments of the chromosomal DNA, recombination between the plasmid and the chromosome can insert the former into the latter (Fig. 5A) or can exchange plasmid markers into the chromosome and vise versa, if they carry distinctive markers. The frequency of these events is 10-4, so their detection requires extensive screening (173), unless damage to plasmid DNA is used to stimulate recombination (242). Conveniently, two types of powerful selection are available to reveal the plasmid integration events.
One way to select for plasmid integration is to use dnaA(Ts) mutants at the non-permissive temperature. DnaA is the origin-recognition and replication-initiation protein in E. coli and Salmonella. In the absence of initiation from oriC, origins of the integrated plasmids provide an alternative route for initiation of the chromosomal replication. Both high copy number plasmids (215, 351) and low copy number plasmids can integratively suppress this dnaA(Ts) initiation defect; however, fully-functional origins of multicopy plasmids are tolerated in the chromosome only in the presence of the free plasmid copies of the same origin (351). Apparently, in the absence of multiple copies of free plasmid to titrate the diffusible plasmid initiator, multiple initiations from the plasmid origin within the chromosome kill the cell. In contrast, chromosomal insertions of plasmids with the copy number 1-2 per chromosome, like F or R, are well-tolerated (67) and fully suppress the chromosomal replication defect of the host without preconditions (217, 247).
The other way to select for plasmid integration into the chromosome by homology is to move the cells to conditions non-permissive for plasmid replication, while keeping selection for plasmid genes (93, 104). For example, since ColE1-type plasmid replicons depend on DNA polymerase I for initiation of their replication, shifting polA(Ts) mutants to the non-permissive temperature while maintaining selection for a plasmid gene (usually antibiotic-resistance) forces the plasmid to seek integration into the chromosome, which can be facilitated by prior cloning of a chromosomal sequence onto the plasmid (103). Returning the cells to conditions permissive for plasmid replication forces the chromosome to shed the plasmid, because even an integrated replicon with 6-10 copies per chromosome significantly slows cells down (104). This way, polA mutants can be used to pick up the chromosomal markers onto plasmids (276). A more efficient alternative is provided by P1 transduction of a plasmid that carries homology to the host chromosome with differences in alleles: the transduced plasmid is enriched for the chromosomal allele, apparently via transient plasmid integration into the chromosome (176).
As already mentioned above (2.1.1.), the F-plasmid can integrate into the chromosome (via transposition, or more frequently, via homologous recombination with pre-existing IS elements (70)), forming an Hfr strain, but then can sometimes leave the chromosome, carrying the piece of the latter, to form “primary” F’. Strains with “primary” F’ frequently cannot lose the enlarged episome, because the still haploid chromosomal region, now on the plasmid, carries essential genes. Once transferred to a wild-type F– strain, F’ becomes “secondary”, meaning that it confers diploidy on the chromosomal region it carries (201). A secondary F’ can either exchange markers with the chromosome (if there are distinct markers to begin with) (115, 133, 345) or it can integrate into the chromosome. Integration of secondary F’s by homology into the chromosome is the reason F’-strains mobilize the chromosome during conjugation with high frequency (254, 288, 341).
2.2.2. Repeat-mediated recombination
When the chromosome carries repeats, — stretches of DNA that have homologies in either direct or inverted orientation someplace else on the same chromosome, single crossover between these repeats in non-replicating chromosomes can lead to two classes of chromosomal rearrangements: 1) deletion between direct repeats (Fig. 5B); 2) inversion between inverted repeats (Fig. 6A). In the replicating chromosomes, two more rearrangements are possible: 3) direct repeats can cause unequal sister chromatid exchange (Fig. 6B), while inverted repeats can cause dimerization with inversion (Fig. 6C and 11), the latter most likely lethal in the chromosome, because it has never been detected (10) (1.3., but see 2.3.2. below for viable products of this recombination). In contrast, double crossovers between repeats will only exchange markers within the repeats themselves, without rearranging the chromosome (Fig. 7). Finally, homozigotization of repeats (when genetic differences between the two repeats are erased in favor of one of the repeats) can also happen and is referred to as “gene conversion” (280) (Fig. 7).
Although bacterial genomes are generally considered non-repetitous (29), they do carry around 1% of their DNA in repeats, which, in the genomes of E. coli and its relatives, are of four kinds: 1) ribosomal RNA (rRNA) operons — both E. coli and Salmonella have seven of these 6 kbp-long repeats (26, 224); 2) the mysterious Rhs elements (up to eight in some strains, belonging to up to five different lineages) that share a 3.7 kb-long core (333); 3) insertion sequences, 0.8-2.0 kb-long, whose actual copy number vary from strain to strain of E. coli or Salmonella, but for any given element is usually below 10 (70, 327); 4) small extragenic repeats (BIMEs/REPs, IRUs, box C sequences, RSA sequences) (11), that are too short and imperfect to be efficient recombination mediators, but whose influence is still felt because of their shear numbers that for some of them run into hundreds.
Recombination between rRNA operons creating duplications of chromosomal segments is argued to be quite frequent, on the order of 10-4 to 10-3 in the population, but too deleterious to change the chromosomal structure in E. coli or Salmonella in the absence of a strong selection for the resulting duplication (6, 7, 116). Deletions between rRNA operons would be too deleterious for survival, but inversions, especially those that do not significantly change the position of the replication origin, are well-tolerated (117). It is surprising, therefore, that the natural E. coli and Salmonella isolates keep the same gene order in the origin-proximal chromosomal half, delineated by the rRNA operons (116), especially so since the high degree of homology between these ancient duplications (“concerted evolution”) suggests regular homologous recombination events in the form of gene conversion (175). Interestingly, in contrast to this “set-in stone” chromosomal frame of most E. coli strains and Salmonella serovars, Salmonella typhi chromosomes are notoriously rearranged by inversions and transpositions between rRNA operons (183, 278).
Recombination between the related Rhs elements can be as frequent as the one between rRNA operons (179). Recombination between insertion sequences can invert significant chromosomal segments (108) and whole chromosomal arms (197), integrate F-plasmid to generate Hfr strains and produce F’ plasmids, carrying chromosomal pieces (327) (discussed in (70); see also 2.2.1.). Recombination between REP elements is also documented (143, 299).
Deletions between closely-spaced direct repeats that do not remove essential genes are detected on the chromosome (35, 199), but recombination between widely-spaced direct repeats can go only through double-crossovers or gene conversion, to avoid deletion of essential genes. The same is true for the majority of inversions that do not include either the replication origin or the terminus: single crossovers are only allowed if the inverted repeats are not too far away from each other (291), while both formation and tolerance of longer inversions are governed by several restrictions (33, 291). One classic example of exclusively double crossover / gene conversion events mediated by widely-spaced inverted repeats is the Konrad system for detection of hyper-rec mutants (147, 360, 361), in which neither origin, nor terminus is included between repeats. On the other hand, recombination between the two 99%-homologous genes (tufA and tufB), coding for EF-Tu translation elongation factor and present in the chromosomes of both E. coli and Salmonella, can go by either single crossover or by double crossover / gene conversion, because the two repeats are positioned almost symmetrically around the replication origin in the inverted orientation. In Salmonella, recombination between genetically-marked tufA and tufB genes goes by both single crossover or double crossover / gene conversion (1, 126), and the non-reciprocal events predominate in the latter case, suggesting gene conversion, rather than actual double crossovers (9).
2.3. Extra-chromosomal systems
These offer not only an added flexibility, since the smaller size of the extrachromosomal substrates facilitates their physical handling and reconstruction, but also one more substrate configuration: linear-by-linear, in phage crosses. Moreover, the substrate input can be tightly controlled in this case, while the product output tends to be orders of magnitude higher than in other systems, reaching absolute numbers comparable with those of initial substrates. Because of this unusual efficiency, the extra-chromosomal systems are favored by those operating genetically at the lower limits of homologous recombination mechanisms (“extreme recombination”), as well as by those studying the in vivo molecular mechanisms with physical techniques (“in vivo biochemistry”).
2.3.1. Phage-by-phage recombination
Recombination is both robust (due to several recombining phage genomes per each cell) and synchronous (due to synchronous and rapid injection) among phages, making this the most powerful recombination format known, equally suitable for investigations with genetic, biochemical or physical techniques (163, 240, 319). Phage recombination is generally robust because phage DNA replication generates a lot of waste, so phages like lambda use recombination in part to recycle this waste, maximizing the yield of their packageable DNA (163). This ensures close to one exchange per progeny lambda chromosomes, which is perfect for genetic analysis. Other phages, like T4, depend on homologous recombination to prime their late DNA replication and will stop their development if homologous recombination is blocked by mutation (241). These phages enjoy several exchanges per every packaged phage genome, which makes their recombination almost “too hot” for genetic analysis at the genome scale, yet perfect for physical detection of in vivo recombination products (156) or for fine genetic analysis at the scale of a single gene (18, 301). And, of course, the frugal yet sophisticated (rather than the primitive phage-like) biochemistry of T4 homologous recombination yields a bonanza of insights about the enzymology of homologous strand exchange, not only in bacteria, but also in eukaryotes (14, 153).
Small bacteriophages tend to replicate prudently and therefore recombine much less than their bigger cousins (274), but their recombination can be induced in the presence of recombination systems of other phages (243) or by DNA damage (17). In fact, the format of the phage cross (or a simple infection), in which phages are pretreated with DNA damaging agents before infection, is a perfect way to circumvent the uncertainty principle of the DNA repair research. The uncertainty derives from two contradictory requirements (as in quantum mechanics, where the first uncertainty principle was ever formulated). To understand the DNA repair mechanisms, ideally one would like to know how the cell repairs a few “pure” DNA lesions. However, in order to reliably detect DNA lesions and their subsequent repair in vivo with physical techniques, one needs to introduce massive DNA damage. Massive DNA damaging treatments perturb the normal cell metabolism because of the unavoidable damage to other cellular components, like RNA, proteins and membranes, and because of the cellular responses to massive DNA damage that can change the enzymology of repair itself (like SOS response in E. coli (see Eco-Sal III Module 5.4.3. “The SOS Regulatory Network”, and Module 7.2.8. “The SOS Response”)). Using phages, it becomes possible, without treating cell with DNA-damaging agents, to task the cell with a considerable (= easily detectable) DNA repair / recombination effort on phage DNA that was pre-treated before infection.
Use of temperature-sensitive phage mutants, or suppressor-sensitive phage mutants in conjunction with suppressing and non-suppressing host bacteria, makes crosses between two or more phages easy to set up (84). Alternatively, a single phage parent carrying repeats can be used, scoring deletions or triplications with direct repeats (16, 112) or inversions with inverted repeats (82, 140). Phage systems can be also used to study how inhibitors of various aspects of the host cell metabolism influence recombination (151, 325). Finally, recombination between related phages can be studied (15). Another unique advantage of the phage-by-phage crosses is the fact that recombination acts are separated from subsequent analysis of recombinants via “rescuing” the products of recombination through packaging. The packaged products are later analyzed in host cells that could have either the same or different genotypes, to facilitate scoring of various phage markers. Such a separation of the recombination act from subsequent analysis allows one, for example, to completely block DNA replication during the cross and still retrieve a few recombinant particles, revealing the important role of replication in homologous recombination (309). Moreover, since the final recombination product is packaged linear, relieving the critical requirement for regeneration of the circular chromosome, not only linear-by-circular, but also linear-by-linear crosses are productive.
One of the early favorites in the study of the general principles of homologous recombination (catalyzed by phage-encoded enzymes), the phage cross can also be used to study E. coli's homologous recombination, by employing recombination-deficient phages (261). Phages like T4 depend on their own hyper-efficient homologous recombination for phage DNA replication (241) — therefore, they cannot be used to study host cells’ recombination. On the other hand, phage lambda can in principle replicate without recombination (83), while recombination-deficient lambdas recombine happily using the host homologous recombination pathways (307). Thus, the repressed phage lambda chromosome is a perfect substrate to study the relations between such complex phenomena as double-strand break repair by host recombination system and DNA replication (167), or even phage own recombination, when the recombination functions are expressed from plasmids (257, 309). In fact, were it not for phage crosses catalyzed by the host enzymes, such an important aspect of the regulation of the bacterial homologous recombination as turning off the RecBCD-catalyzed DNA degradation at Chi-sites (reviewed in (245)), would have been discovered only 20 years later (66).
2.3.2. Plasmid-by-plasmid recombination
Although evidence of recombination was periodically surfacing in natural-replicon-based plasmids, like a deletion between IS2 direct repeats within F’ (71) or recombination intermediates in ColE1 (259, 260), this strictly circular-by-circular format of homologous recombination between or within small extrachromosomal circles became really popular with the advent of genetic engineering, allowing one to design experimental systems targeting detection of specific events of homologous recombination at the very limits of detection (31, 45). In fact, in plasmids based on engineered replicons devoid of the multimer-resolution systems, the most graphic and easily-obtained physical evidence of homologous recombination is plasmid multimerization (13, 87, 356) and demultimerization (145).
Two general classes of substrates for genetic detection of plasmid-by-plasmid recombination are employed: one class consists of two compatible (stably maintained together in the same strain) plasmids carrying regions of mutual homology (135, 169, 170, 200) (this corresponds to multimer formation in the physical assays), while the other class is a single plasmid with repeats of various lengths, either in direct (21, 72, 199, 222) or inverted (20, 212, 352) orientation (corresponding to demultimerization in physical assays). While the first class has to recombine intermolecularly, the second class can recombine either inter- or intramolecularly, with both types of events detectable (199, 352). Sometimes the repeated sequences carry heterologies, the setup that allows one to detect gene conversion-type events (74, 86). Finally, since plasmids are relatively small, their introduction into the host cells by transformation is rather efficient compared with the chromosomal DNA, which allows one to transform bacterial cells with linearized plasmids or even their pieces (both would be unable to replicate) to explore the mechanisms of double-strand break-stimulated recombination by isolating replication-competent circular products of the forced reassembly (69, 142).
Although plasmid-by-plasmid recombination sounds like a little (=inadequate) brother to the chromosomal recombination, miniaturization of the substrates gives plasmid recombination at least two advantages over its big brother. First, physical analysis of the overall configuration of the recombination products becomes possible, providing interesting insights about the structure of the plasmid dimers (21, 72, 199, 222). For example, in addition to the expected reciprocal 1+3 dimers, direct repeats also mediate formation of the mysterious non-reciprocal 1+2 dimers (72, 222), which are also observed in mitochondrial DNA rearrangements in higher eukaryotes (162). Another interesting example is formation of the outlandish-looking head-to-head dimers, mediated by inverted repeats (Fig. 12) (20, 206, 212). Such analysis is impossible with the chromosome, in which tandem dimers, even if they form, are quickly resolved into monomers by the XerCD/dif system in preparation for segregation and cell division (296), while inverted dimers (Fig. 12) are simply inviable (10).
Fig. 12.
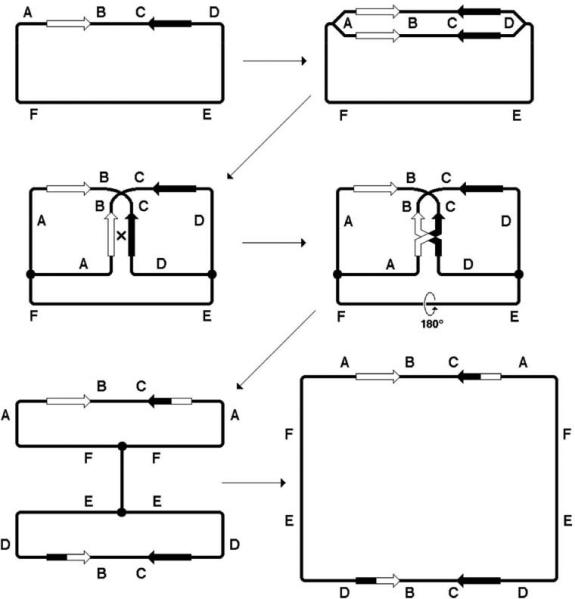
Formation of the head-to-head plasmid dimer, mediated by inverted repeats. This is, basically, a full-chromosome version of Fig. 6C.
And second, analysis of the products of plasmid recombination is not restricted to the cell in which they originally formed: plasmid DNA can be isolated and introduced for analysis into a different strain (usually, a recombination-deficient one to suppress further recombination) (13, 74, 135, 260). This trick is especially useful with multicopy plasmids, in which, due to their enormous copy number, selection for the rare (recombinant) variants is inefficient (223). This property of crosses in the high-copy number plasmids should be kept in mind when interpreting obligatory dimerization of the recombinant plasmids (162), since dimerization accelerates replication of plasmids, regulated by a common diffusible inhibitor (315) and, therefore, enhances the selection.
2.3.3. Plasmid-by-phage events
Plasmids can form as by-products of aborted phage infection (like lambda-dv (219)) and can also recombine with the infecting phages if they carry segments of mutual homology. Genetically, if the phages are the final read-out, two outcomes are possible: either plasmid integration by a single cross-over (the plasmid is small, while the phage chromosome has space) (139, 221, 295, 320), or the phage picking up a marker from the plasmid as a result of a double crossover (301, 306, 320) (especially if the plasmid is too big to splice into the phage). Double crossovers are the only outcome detectable if the restoration of the plasmid is the read-out, as in the phage-mediated transduction of plasmids, carrying segments of the phage genome (154, 248). Physical analysis of the products of plasmid-by-phage recombination is possible if the size of the products is expected to be different from the size of the substrates: the small size of both recombining molecules facilitates detection of differences using standard DNA separation techniques like gel electrophoresis (256). Alternatively, stimulation of plasmid replication by an infecting phage, if they share homology, can be observed (155, 220).
Due to the robust and efficient nature of crosses involving bacteriophages (see 2.3.1.), even physical detection of otherwise elusive recombination intermediates becomes possible. Importantly, some of these intermediates are dead-end products, undetectable genetically, and their physical detection offers recombination studies the depth of resolution which is only contemplated in other DNA metabolism studies, like mutagenesis (see Eco-Sal III Module 7.2.1. Mutagenesis). For example, recombination with the phage stimulates rolling-circle replication in a plasmid, which is detectable physically, but cannot yield stable final products, detectable genetically. The plasmid-byphage setup was the first to demonstrate recombination-dependent replication, an indication that homologous recombination generates functional replication forks (155, 220). Physical demonstration of this event in the chromosome would be close to impossible due to much lower frequency of these events there. In fact, plasmid-by-phage recombination is so robust and efficient that it was successfully used to study the minimal sequence length requirements of homologous recombination in E. coli, which turned out to be around 30 nucleotides (139, 295, 301, 336).
3. Genetic analysis of HR reveals distinct pathways
Homologous recombination is a complex phenomenon with mechanisms that are still not completely understood, especially in the areas of generation of recombination substrates and also where recombination connects with DNA replication. Genetic characterization of the distinct pathways of homologous recombination in E. coli was facilitated by the availability of diverse experimental systems (see 2.) and by the strong physiological phenotypes of recombination-deficient mutants. Recombination-deficient mutants are sensitive to DNA damage, which not only offers a convenient read-out, but also indicates that the main physiological function of homologous recombination is repair of chromosomal damage ((163); also see below and Eco-Sal III Module 7.2.7. Recombinational Repair — Enzymes and Pathways). However, DNA damage sensitivity of a mutant does not automatically qualify it as a recombinational repair one, — it is more likely to be a bona fide DNA repair mutant (see Eco-Sal III Module 7.2.4. DNA Damage Reversal and Excision Repair), so homologous recombination tests (see Section 2. above) should be used to verify that the mutant indeed affects homologous recombination. And even in the case of a DNA damage-sensitive and homologous recombination-defective mutant, care should be taken to separate true recombination functions from those of DNA replication and chromosome condensation and segregation. Indeed, recombination operates on DNA organized in nucleoids and creates substrates for subsequent chromosomal replication, — therefore detection of recombinant chromosomes usually assumes their successful replication, condensation and segregation. Likewise, survival of DNA damage critically depends on restoration of DNA replication, likely in the context of the nucleoid. Thus, partial defects in DNA synthesis elongation, like dnaE (27), or restart, like priA insertion mutants (144, 279), or in the nucleoid-associated proteins, like HU (75, 137, 174), may behave like homologous recombination mutants without being those as such; these chromosomal replication and organization functions are discussed elsewhere in Eco-Sal III (128, 157).
3.1. Isolation of mutants in homologous recombination
There are very few circumstances under which homologous recombination becomes poisonous to the cell, and these already involve some other defects in homologous recombination, making selection or even enrichment for recombination-deficient mutants in otherwise wild type E. coli generally unavailable, and leaving only the possibility of brute-force screens. Some mutants in homologous recombination (mnemonic “rec”), notably recA (40) and recJ (198), were isolated by screening for defective recombinantion after conjugation (2.1.1.) on replica plates (reviewed in (36, 39)). There were also other mutants defective in conjugational recombination, but since they also had defects in other DNA transactions, they were not specific for homologous recombination. However, most of the early mutants were isolated differently, by screening for the X-ray or gamma-ray-sensitivity and then also checking their recombination-proficiency; mutants in recA, recB, recC, ruv(ABC) and recG were isolated this way (81, 125, 187, 250, 328). Since the major chromosomal challenge after X-ray or gamma-ray radiation is repair of double-strand DNA breaks, while conjugational recombination proficiency also depends on double-strand break repair, it is not surprising that all the early mutants were defective or deficient in this repair.
Once a rec mutant was isolated in otherwise wild type cells, the next step was to attempt isolation of its extragenic suppressors that would restore recombination-proficiency. In this way, it was found that the recA mutants could not be suppressed indirectly, but recB, recC and ruv mutants were easily suppressed, usually by activation of analogous functions residing on defective prophages. Specifically, the sbcA suppressors returned recombination-proficiency after conjugation to both recB and recC mutants by activating the RecE exonuclease and the RecT strand annealing protein (12, 95) of the Rac prophage (346), whereas the rus suppressors did the same to the ruv mutants (294) by activating an alternative resolvase RusA of the DLP12 prophage (211). Curiously, the recG defect in recombination and DNA damage sensitivity was suppressed by inactivation of the non-essential helicase activity of the otherwise nearly-essential primosome-loading protein PriA (3), suggesting that RecG is not a true recombination function, but instead controls some potentially poisoning pathways associated with DNA repair.
Once the restoration of recombination proficiency was achieved, a new search for recombination-deficient mutants, now in a double mutant background, became possible. These mutants would now reveal the functions important for the alternative pathway that was suppressed in the wild type cells. Mutations inactivating homologous recombination in the recB sbcA or recC sbcA background include the expected recA (95), recE (42, 95) and recT (42), but also recF (95), recJ (198), recO (146), recR (209) and ruvC (190). The only mutation that was found to inactivate homologous recombination in the ruv rus background (besides the expected recA and recBC defects) was recG (211, 213). Symmetrically, the ruv defect returned DNA damage-sensitivity to the recG priA(helicase-minus) double mutants (134).
Quite a different logic of extragenic suppression is exemplified by the recBC sbcBC recombination-proficient mutant combination. In this case, suppression is conferred by two unlinked mutations, sbcB (317) and sbcC (188) (or sbcD (92)), and the mechanism of it can be considered opposite to the recBC sbcA suppression: instead of activating an analogous function to compensate for the recBC defect, the sbcBC suppressors dramatically modify the metabolism of linear DNA in E. coli cells. The modification includes inactivation of the nuclease domain of ExoI, the ssDNA-specific exonuclease and the product of sbcB/xonA gene, turning it into a 3’-ssDNA-end-protecting protein (322), as well as complete inactivation of the SbcCD nuclease that attacks DNA hairpins and can also trim ssDNA overhangs of a duplex via its ss-endo activity (49, 50). However, the recombination-deficient mutants, isolated in the recombination-proficient recBC sbcBC genetic background, are the same as for the recBC sbcA background, sans recE and recT, the two Rac prophage recombination functions that are not activated in the sbcBC mutants in the first place. Specifically, recA (123), recF (123), recJ (123, 198), recO (146), recR (209) and all three ruv genes (186, 213) are needed for high conjugational recombination in the recBC sbcBC background. In addition, the recG (187), recN (190), recQ (231, 246) and uvrD, the latter one either alone (123) or together with helD (231), genes also participate, emphasizing the differences between the two pathways. The list of rec- mutants in the recBC sbcBC background includes only those whose specific defect was in homologous recombination and in DNA damage repair. Conjugational-recombination-deficiency indices for all the recombination mutants in wild type, recBC sbcA and recBC sbcBC backgrounds are shown in Table 2.
Table 2.
Conjugation recombination deficiency indices for various E. coli mutants in homologous recombination in wild type, as well as in recBC sbcA and recBC sbcBC backgrounds. Instead of showing precise numbers, that can be found in the papers cited in section 3.1., the table shows magnitude of effects to expect from specific mutations in three recombinational repair proficient backgrounds (WT, recBC sbcA and recBC sbcBC).
| Background | |||
|---|---|---|---|
| Mutant | WT | recBC sbcA | recBC sbcBC |
| Rec+ | — | — | —@ |
| recA | 000 | 000 | 000 |
| recB # | 00 | na | na |
| recC # | 00 | na | na |
| recD # | — | na | na |
| recE | — | 00 | na |
| recF | — | 00 | 00 |
| recG | 0 | 0 | 0 |
| recJ | — | 0 | 0 |
| recN | — | 0 | 00 |
| recO | — | 00 | 00 |
| recQ | — | 0 | 0 |
| recR | — | 00 | 00 |
| recT | — | 00 | * |
| priA* | 0 | (0) | (0) |
| ruv | 0 | 0 | 0 |
— - no effect
@ - although the final recombination frequencies are 50% of the wild type in the recBC sbcBC mutants, the kinetics of recombinant formation is extremely slow compared with wild type (194).
na - not applicable (mutant)
0 - approximately one order of magnitude down
00 - approximately two orders of magnitude down
000 - approximately three orders of magnitude down
# - the RecBCD proteins work as a single complex
* - PriA is a replication restart function, connecting recombinational repair with DNA replication.
Isolation of the secondary recombination-deficient mutations in the suppressed rec mutants naturally lead to the idea of several recombinational pathways in E. coli, the major one (RecBC) and two minor ones (RecE and RecF), the latter two normally suppressed in the wild type cells (36, 37, 41). It is worth noting in retrospect that, although the original “recombination system” with three pathways turned out to be too “conjugation-centric” to be universal, the general idea of separate recombinational pathways was fully validated (see 3.2. and 3.3.). Specifically, the RecBC pathway recombines two homologous chromosomes if at least one of them is linear (= has end(s)), the RecF pathway recombines two circular chromosomes, while the RecE pathway catalyzes phage recombination. Subsequent biochemical analysis (see 5.) revealed the two-fold meaning of the recBC sbcA and recBC sbcBC alternative pathways: 1) alternative ways of producing 3′-overhangs at double-strand ends; 2) alternative ways of loading RecA protein on these overhangs. In other words, in the wild type cells, only one way of double-strand end processing and RecA loading at this end is available (controlled by RecBC), while the RecF pathway loads RecA at single-strand gaps.
Recently, due to the central role of homologous recombination in repair of double-strand DNA breaks (also see below (4.3. and 5.2)), an entirely different approach — a color screen — was used to isolate recombination-affected mutants. Starting with rdgB or dut mutants, that have elevated levels of spontaneous chromosomal fragmentation due to, correspondingly, hypoxanthine-DNA or uracil-DNA incorporation and excision, secondary mutations were isolated that made the original single mutants inviable (synthetically lethal with the rdgB or dut defects) (204, 324). Among such RdgB- or Dut-dependent mutants, there were multiple hits inactivating all the known six genes of recombinational repair of double-strand breaks in E. coli: recA, recB, recC, ruvA, ruvB and ruvC (204, 324). The prediction is that, if performed in the recBC sbcA or recBC sbcBC backgrounds, such a screen should yield all other known rec mutants, as well as (hopefully) some still unknown ones. As an approach, identification of synthetic lethal mutant combinations shows its power in unraveling complicated pathways of the DNA metabolism around recombinational repair (270, 357), although care should be exercised with interpretation of quadruple and quintuple mutants, as the comfort zone of typical human thinking is limited to three dimensions.
3.2. Substrate analysis
Another level of complexity of homologous recombination in E. coli was revealed when the defect (or its absence) of the isolated mutants in conjugational recombination was compared with their defect in plasmid recombination (both in the otherwise wild type background). The idea behind such a comparison is to change the substrate format from linear-by-circular for conjugational recombination to circular-by-circular for plasmid recombination. This substrate analysis revealed that some of the mutants, like recA, recG and ruv, were equally defective in both types of recombination, while some other mutants, like recD, recN and recQ, were wild type in both (reviewed in (39, 207, 303, 304)). The interesting mutants showed defect with one pair of substrates, while no defect with the other pair (Table 3). To illustrate the main results of this analysis, let us first make Table 3 clearer by 1) eliminating the mutants with no effect in the otherwise wild type strain in either format (recD, recE, recN, recQ and recT); 2) setting aside the recA mutants, which show the strongest effect in either format; 3) grouping together mutants with a strong effect in one format, but no effect in the other (recB and recC versus recF, recJ, recO and recR); 4) grouping together mutants with weak effects in either format (recG and ruvABC).
Table 3.
Quantitative illustration of substrate analysis: conjugational (linear × circular) versus plasmid (circular × circular) recombination deficiency indices for various E. coli single mutants in homologous recombination. Again, instead of showing precise numbers, that can be found in the papers cited in section 3.2., the table shows magnitude of effects to expect from specific mutations in the two substrate pair setups.
| linear × circular | circular × circular | |
|---|---|---|
| Rec+ | — | — |
| recA | 000 | 00 |
| recB | 00 | — |
| recC | 00 | — |
| recF | — | 00 |
| recJ | — | 00 |
| recO | — | 00 |
| recR | — | 00 |
| recG | 0 | 0 |
| ruv | 0 | 0 |
Clarified in this way, the results of substrate analysis suggest that: 1) RecA catalyzes both conjugational and plasmid recombination — likely by being responsible for the critical step in both processes (homology-guided strand exchange?); 2) RecB and RecC catalyze conjugational, but not plasmid recombination; 3) RecF, RecJ, RecO and RecR catalyze plasmid, but not conjugational recombination; 4) RecG and RuvABC are important, but not critical, for both conjugational and plasmid recombination. Since we have an idea about the nature of the substrates, we can conclude that the RecBC pathway catalyzes (RecA-mediated) exchanges between two DNAs if at least one of them has free ends (like the linear subchromosomal piece after conjugation), while the RecFJOR pathway catalyzes (RecA-mediated) exchanges between chromosomes without ends, for example, between two circular plasmids. We can also say that both RecG and Ruv functions help recombination, but the specificity of their action is unclear. However, since these mutants are insensitive to the substrate configuration, we can tentatively conclude that both RecG and Ruv functions do not recognize the initial recombination substrates (this recognition is, apparently, the function of RecBC and RecFJOR), but work at a later phase of the reaction.
The results of this idealized substrate analysis look convincing and satisfying, but the real life situations are usually more complicated and harder to interpret, stressing the importance of basing one's interpretations in this analysis on the precise information, instead of assumptions, about the format of the substrates. For example, in the Konrad system that detects events between two distant inverted repeats in the chromosome (147), recombination depends on RecBC, rather than on RecF (360), suggesting that it is due to linear-by-circular type of events, rather than the assumed circular-by-circular ones. In a different chromosomal recombination system, again based on distant repeats, recombination depends on both RecBC and RecF, suggesting that the format of this reaction can be either linear-by-circular or circular-by-circular, depending on the situation (90). Finally, deletion formation at tandem repeats in the chromosome happens at unexpectedly high frequencies in the absence of RecA (285), forcing to classify this recombination as “homology-driven”, rather than “homologous”. The generic mechanism of such homology-driven recombination is single-strand annealing (reviewed in (160, 163)).
3.3. Epistatic analysis
Epistatic analysis involves combining in a single organism two mutations affecting the same process and monitoring the resulting phenotype; its application to sorting out DNA repair mutants in Saccharomyces cerevisiae is considered classic (28, 55, 91, 110). “Epistasis” means “covering over”, and originally epistatic analysis was designed to find genes acting together in the same pathway by looking for double mutants with a phenotype of one of the single mutants. Remarkably, epistatic analysis turned out to have more distinguishing power than at first thought, because it can also reveal distinct pathways within one broad mechanism. Suppose there are two mutants, each showing a 30% decrease of some measurable phenotype. The three kinds of epistatic interactions are: 1) the double mutant possesses the phenotype of the single mutants (still shows only 30% drop), — this classic epistasis suggests that the two genes work in the same pathway; 2) the double mutant shows an “additive” (actually, multiplicative) effect (50% decrease), — the two genes must be working in separate pathways, and there are more functional pathways left; 3) the double mutant shows a synergistic effect (99% down) — there are only two pathways, and the two mutations inactivate both (55, 110).
To see how epistatic analysis works for E. coli mutants in homologous recombination, let us first change the readout from recombination deficiency index in the two substrate configurations to something more general. As already mentioned, mutants in homologous recombination are sensitive to DNA damage, for example, to ultraviolet (UV) irradiation. If we take determinations of UV sensitivity of double mutants in comparison with UV sensitivity of the corresponding single mutants, for example, from papers of Lloyd and colleagues (99, 184, 187, 189, 192, 210), we can generate a matrix. To keep it simple, let us take a particular low dose of UV (say, 10 J/m2, which will be sublethal for wild type cells) and to approximate sensitivity of any given mutant to this dose to the nearest order of magnitude (Table 4).
Table 4.
Quantitative illustration of epistatic analysis using resistance to UV-irradiation as a read-out. In the following matrix, the values show how many orders of magnitude mutants are more UV-sensitive than WT cells at a particular dose of UV (10 J/m2), sublethal for the wild type cells. Again, instead of showing precise numbers, that can be found in these papers (99, 184, 187, 189, 192, 210), the table shows magnitude of effects to expect from specific mutations or their pairwise combinations.
| WT | recA | recB | recC | recD | recF | recG | recJ | recO | recR | ruv | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 1 | ||||||||||
| recA | 1000 | 1000 | |||||||||
| recB | 10 | 1000 | 10 | ||||||||
| recC | 10 | 1000 | 10 | 10 | |||||||
| recD | 1 | 1000 | 10 | 10 | 1 | ||||||
| recF | 10 | 1000 | 1000 | 1000 | 10 | 10 | |||||
| recG | 10 | 1000 | 100 | 100 | 10 | 10 | 10 | ||||
| recJ | 10 | 1000 | 100 | 100 | 100 | 10 | 10 | 10 | |||
| recO | 10 | 1000 | 1000 | 1000 | 10 | 10 | 10 | 10 | 10 | ||
| recR | 10 | 1000 | 1000 | 1000 | 10 | 10 | 10 | 10 | 10 | 10 | |
| ruv | 10 | 1000 | 10 | 10 | 10 | 100 | 1000 | 10 | 100 | 100 | 10 |
Such epistatic analysis shows that in E. coli:
by themselves, most rec mutations decrease the resistance ~10-fold (the first column). Only recA mutations make cells very sensitive to UV. This suggests several pathways of recombinational repair, with RecA protein playing the key role.
To confirm the key role of RecA, we combine recA mutation with other mutants pairwise (the second column): indeed, the recA mutant sensitivity is obtained for all double mutants => therefore, recA must control the limiting step common to all pathways of recombinational repair.
Combining all other mutations pairwise produces the following picture:
-
3
recB mutation does not change UV sensitivity when combined with recC or ruv mutations, => RecB protein works together with the corresponding proteins.
-
4
recB mutant becomes quite UV-sensitive if combined with recG and recJ mutations and extremely UV-sensitive if combined with recF, recJ, recO and recR mutations, => the RecFJOR group defines a pathway alternative to the RecBC-Ruv pathway, while RecGJ group may compensate for the recB defect in some way.
-
5
recC mutant combinations confirm the recB analysis.
-
6
recF mutation does not change UV sensitivity when combined with recG, recJ, recO and recR defects, but it makes cells more UV-sensitive if combined with the ruv defect and extremely UV-sensitive when combined with recB or recC defects, => RecFGJOR group works together and is separate from the RecBC group, while the Ruv function may play a compensatory role.
Now we again see the pattern of RecBC versus RecFJOR pathways, but the situation with RecG and Ruv is less clear, because although they both group with a particular pathway, their combinations with the other pathway have only moderate effects. However, when the recG and ruv mutants are combined together, an extremely sensitive double mutant is produced again! In summary, the results with recG and ruv mutants show that RecG and Ruv proteins 1) work at the stage of recombinational repair that is different from the stage at which RecBC and RecFOR work; 2) define the two alternative pathways at that other stage; 3) the early RecBC pathway preferentially feeds into the late Ruv pathway, while the early RecFOR pathway preferentially feeds into the late RecG pathway (although there is also a significant cross-feeding between the early and the late pathways).
If we combine the results of epistatic analysis with the results of the substrate analysis above, we arrive at the tentative formal scheme of homologous recombination in E. coli (Fig. 13):
recA is the control gene of HR, catalyzing the central reaction of the process, which is the formation of recombinational intermediate.
RecBC and RecFOR complexes define the two alternative early pathways, working before the RecA-promoted stage, most probably to assist RecA.
RuvABC and RecG enzymes represent the two alternative late pathways, working after the RecA-promoted stage, most probably by resolving recombination intermediates (for example, like in Fig. 3 and 4).
Fig. 13.
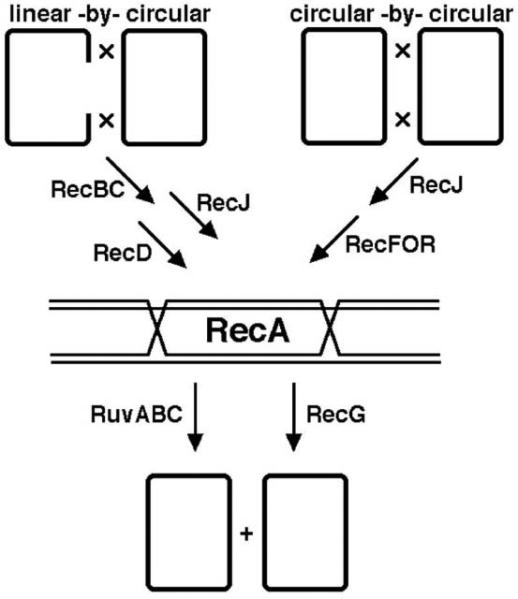
The formal genetic scheme of homologous recombination in E. coli. The scheme is based on the result of the substrate analysis and of the epistatic analysis. The chromosomal duplex DNA is shown as a single line, with the exception of the double Holliday junction, which is shown double-stranded. Explanations in the text.
4. Evolution versus Survival
Homologous recombination may be important for evolution, but 1) it is stimulated by DNA damage; 2) although DNA damage does induce cellular (SOS) response, homologous recombination is stimulated directly by DNA lesions, rather than by overproduction of recombination functions as a part of the SOS response; 3) both homologous recombination and SOS response are induced by one-strand DNA damage after it has been aggravated by DNA replication, apparently spreading to the opposite DNA strand and becoming two-strand DNA damage; 4) recombination mutants are sensitive to DNA-damaging treatments and have reduced viability. Therefore, the physiological function of homologous recombination is to repair two-strand DNA lesions, which are, in effect, “chromosomal lesions”.
4.1. Stimulation of homologous recombination by DNA damage
The mechanism of homologous recombination proposed by Holliday (121, 260) (1.2.) provides a framework for thinking about the configuration of recombination intermediates and their resolution. However, the mechanism of Holliday does not provide the reason why two DNA duplexes should exchange strands in the first place and how the position for such an exchange is selected. In fact, with coincident nicks initiating the exchange, the original Holliday scheme is a perfect description of what we know about site-specific recombination (100), rather than homologous recombination. Then how and where is homologous recombination initiated?
To answer this question, one can examine what stimulates homologous recombination, assuming that recombination-stimulating conditions mostly increase the frequency of spontaneous recombination-inducing events, rather than generate novel classes of such events. Soon after homologous recombination was discovered in E. coli, it was found to be stimulated by DNA-damaging treatments (43, 132). Stimulation of homologous recombination in a variety of experimental systems by a variety of DNA damaging treatments is readily observed (65, 114, 203) and is documented beyond doubt (reviewed in (120)). The genetic requirements of the DNA damage-induced recombination are the same as those of spontaneous recombination ((111, 242), reviewed in (120)), implying that spontaneous recombination (i) has the same mechanisms as recombination induced by DNA damage; (ii) is itself induced by spontaneous DNA damage. Most DNA lesions affect only one DNA strand at any point; such “one-strand” DNA lesions are efficiently removed by one of the several excision-repair systems (reviewed in (89); also see Eco-Sal III Module 7.2.4. DNA Damage Reversal and Excision Repair). Since such repair would obviate any need for remedial interaction between homologs, the observed stimulation of homologous recombination by one-strand DNA damage might reasonably be attributed to induction of a cell-wide hyper-recombinogenic state. In other words, recombination may be an indirect consequence of cell's reaction to DNA damage, rather than direct consequence of DNA damage repair.
It is well known that treatment of cells with one DNA damaging agent, followed by incubation in growth conditions, makes them better prepared to repair DNA lesions from subsequent exposures to different DNA damaging treatments (216, 255). This inducible response to DNA damage, called the SOS response by Miroslav Radman (262), is best demonstrated by the effect of split-dose DNA damaging treatments, during which repair-proficient cells show better survival after a particular dose of DNA damaging treatment if the treatment is delivered in several sub-doses separated by short time intervals (77, 277). The SOS response (182, 262) includes increased expression of about 30 genes in the E. coli chromosome and decreased expression of some 20 more genes and operons (54, 85). The products of induced genes participate in DNA damage repair (removal of DNA lesions) and tolerance (when cells live with DNA lesions without removing them); the products of suppressed genes participate in metabolism of various carbon sources, as well as in cell division. The current SOS reviews include Eco-Sal III Modules 5.4.3. “The SOS Regulatory Network” and 7.2.8. “The SOS Response”.
The idea that DNA damage induces homologous recombination mostly via induction of a cell-wide hyper-recombinogenic state predicts that hyper-recombination mutants (“hyper-recs”) in E. coli, if isolated, should be affected in the regulation of recombinational enzymes. For example, overproduction of the RecA recombinase may reasonably be expected to increase the frequency of spontaneous strand exchange between otherwise intact homologous DNA duplexes, increasing HR, but it does not (146). In fact, no recA gene upregulation mutants were ever isolated as hyper-recs in E. coli. Instead, all of the several hyper-recombination mutants found to date, with a single exception, increase the level of endogenous DNA damage (147, 361) or chromosomal damage (in the shape of disintegrated replication forks) (285, 286), suggesting that, independently of whether recombination enzymes are overproduced or not, DNA lesions are required directly to stimulate recombination. The single exception are uvrD mutants (reviewed in (163)), which may potentially accumulate extra endogenous DNA damage because of the UvrD helicase involvement in nucleotide excision repair and mismatch repair pathways (reviewed in (89)), but which are also demonstrably hyper-rec for a different reason. UvrD helicase diassembles RecA filaments (see 5.3.) both in vitro (238, 329) and in vivo (34), so RecA filaments are stabilized in uvrD mutants, increasing the overall recombination. However, this exception does not challenge the rule, because even in the absence of UvrD recombination still initiates at the sites of DNA damage. Thus, no hyper-recombinogenic state can be induced in wild type cells without DNA damage. Also, if damaged DNA is introduced into cells, the increased recombination is detected only between chromosomes that share homology with the damaged chromosome (96, 267), further arguing against the idea of indirect induction.
4.2. The idea of replication-dependent DNA lesions and of their subsequent recombinational repair
The exquisite sensitivity to DNA damage of the first isolated recombination-deficient mutants (40, 109) suggested that E. coli uses homologous recombination as the only way to repair certain DNA lesions. Still, it could be argued that homologous recombination happens independently of the DNA lesions, with the survivors being the lucky few that inherited, after recombination, only the damage-free segments of the genome, as was originally proposed to explain the multiplicity reactivation phenomenon in bacteriophage (205). This idea predicts that the induction of homologous recombination by DNA damage should always be accompanied by a decrease in viability, with recombinants being more frequent only among the surviving chromosomes. However, moderate doses of DNA damaging agents that would be lethal for recombination-deficient mutants, yet sublethal for recombination-proficient cells, do induce recombination in the latter (318). Also, in experimental systems where survival of the damaged DNA does not depend on the rec status of the host strain (for example, transformation with a chemically-modified plasmid), the extent of DNA damage-induced recombination (transfer of the plasmid allele to the chromosome) does so greatly (22, 203). Thus, it seems certain that repair by homologous recombination results from an active mechanism rather than from a random redistribution of lesions.
Although the mechanisms of formation and the final structure of lesions requiring recombination for repair are still debated (58, 152, 163, 234), the specificity of the inducible response provides a clue. When the original DNA damage affects only one strand of the DNA duplex (like UV), the inducible response to this damage requires DNA replication (283, 354). Similarly, induction of homologous recombination by treatments that affect only one strand of the DNA duplex requires replication of the damaged DNA (51, 178), suggesting that one-strand lesions spread to the opposite strands by replication, and the resulting two-strand lesions induce both the SOS response and homologous recombination. Accordingly, “recombinational repair” is thought to be recruited to repair two-strand lesions, because the individual DNA strands cannot be mended by one-strand (excision) repair in this case (Fig. 14A) (124, 163). To make repair possible, the strands of an affected duplex are aligned by homology with the strands of an intact homologous duplex and then exchanged to form the classic recombination intermediate (compare Fig. 14B with Fig. 2, right, or Fig. 4A). As a result, both lesions become single-stranded (Fig. 14C) and can now be removed from the hybrid duplexes by one-strand (excision) repair (Fig. 14D).
Fig. 14.
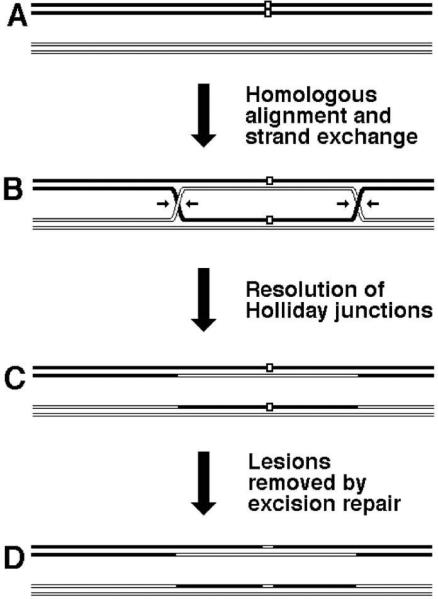
The idea of two-strand DNA repair. A. A DNA molecule with a two-strand lesion (small open rectangles in the filled duplex) is shown homologously aligned with an intact homolog (open duplex). B. The two sequences have exchanged strands, converting the two-strand lesion into a pair of one-strand lesions. C. Holliday junction resolution (the nicking of strands is shown in “B”) separates the chromosomes from each other. D. Excision repair removes the one-strand lesions, completing the overall repair reaction. Note that if the black and white “parental” DNAs are homologs (carry genetically-scorable differences), rather than identical sisters, the resulting chromosomes may be detectably recombinant.
4.3. Sensitivity to DNA damage and decreased viability of rec mutants
The various rec mutants show varying degree of sensitivity to DNA damage, their relative sensitivities being dependent on the DNA damaging agent used (Table 5). UVC-light (the short-wavelength UV) generates mostly pyrimidine dimers within the same DNA strand and is considered a typical one-strand DNA lesion-inducing agent (89). Nalidixic acid interferes with the function of DNA gyrase, which apparently leads to induction of double-strand DNA breaks (225, 230), so DNA gyrase can be viewed as an agent that induces predominantly two-strand DNA lesions. Ionizing radiation generates mostly one-strand DNA damage (oxidized bases, nicks), but also produces a measurable amount of direct double-strand breaks (89, 334), so ionizing radiation can be viewed as an agent inducing both one-strand and two-strand DNA lesions. Generally, the stronger the defect in homologous recombination, the higher the sensitivity to DNA damage.
Table 5.
Viability of homologous recombination mutants and their sensitivity to different kinds of DNA damaging treatments. The doses of the DNA damaging treatments are in all cases sublethal for the WT cells (survival 80% or higher). (Disclaimer: The references were selected for mutant-to-mutant consistency and, in some cases, are neither original demonstrations, nor the wild type background. Moreover, since this is a compilation from different studies done in different laboratories at different times, the conditions and backgrounds are variable, limiting usefulness of this comparison to providing only the most general guidance.)
| Mutation | DNA damaging treatment | ||||
|---|---|---|---|---|---|
| UVa | NAb | IRc | Viad | Ref. | |
| None (wild type) | 0.8 | 0.8 | 0.8 | 1.0 | N/A |
| recA | <0.0001 | 0.005 | 0.002 | 0.5 | (98, 253, 344) |
| recBC | 0.003 | 0.005 | 0.01 | 0.3 | (98, 209) |
| recD | 1.0 | 1.0 | (189) | ||
| recFOR | 0.05 | 1.0 | 0.8-1.0 | (190, 192, 209) | |
| recG | 0.2 | 0.1 | 0.8 | (187) | |
| recJ | 0.3 | 0.9 | (209) | ||
| recL (uvrD) | 0.001 | 0.005 | 0.6 | (8, 185) | |
| recN | 0.5 | 0.5 | 1.0 | (253) | |
| recQ | 1.0 | 1.0 | (189) | ||
| ruv | 0.002 | 0.1 | 0.6 | (184, 250) | |
| priA | 0.02 | 0.02 | 0.1e | (144) | |
| xerCD or dif | 1.0 | 0.6-0.9 | (235) | ||
| recBC recF | 0.00001 | 0.003 | 0.25 | (191, 192, 209) | |
| ruv recG | 0.00001 | 0.25 | (184) | ||
Ultraviolet light sensitivity is shown as the ratio of survival of the mutant to the survival of the WT cells at the dose of 20 J/m2, cells seeded on LB plates.
Nalidixic acid sensitivity (225) is shown as the ratio of survival of the mutant to the survival of the WT cells after incubation in LB with 100 μg/ml of the drug for one hour at 37°C.
Ionizing radiation sensitivity is shown as the ratio of survival of the mutant to the survival of the WT cells at the dose of 8 Krads.
Viability is measured as colony-forming units per volume of a rapidly-growing culture at a particular OD600, divided by the same value for the wild type cells.
minimal media only.
The increased sensitivity of Rec- mutants to these highly artificial exogenous DNA damaging treatments does not by itself prove an important adaptive role for recombination in DNA repair, especially if the natural niche of an organism keeps it protected against exogenous DNA damage. However, the natural life cycle of E. coli alternates between periods of stable growth with little DNA damage (in the gut) and periods of little growth with severe DNA damage (in natural waters) (163, 284), making the repair role of homologous recombination highly relevant. Furthermore, all mutants deficient in homologous recombination (recA, recBC recF, ruv recG) have decreased viability even when grown in benign laboratory conditions (Table 5), suggesting that normal cellular metabolism generates endogenous DNA damage that requires recombination for repair and making it likely that homologous recombination ability is continuously selected for its role in repairing this otherwise lethal DNA damage. What kind of DNA damage can be so lethal?
5. Chromosomal lesions and their repair via homologous strand exchange
Homologous recombination is the only known way to faithfully repair chromosomal lesions — the most severe, incapacitating DNA lesions possible. Most chromosomal lesions are formed when the cell replicates the damaged template DNA. Since an unrepaired chromosomal lesion is a “kiss-of-death” for the cell, homologous recombination represents a critical DNA repair mechanism the cell relies upon when replicating damaged DNA templates. Chromosomal lesions come in two basic flavors, explaining why E. coli has two distinct pathways of homologous recombination (163). By their biochemical properties, the recombinational repair proteins can be subdivided into three teams: 1) the strand-exchange (catalysis) team, responsible for the central step in recombinational repair; 2) the specificity teams, responsible for chromosomal lesion recognition and for the licensing of RecA polymerization at these lesions; 3) the cleaning-up teams, responsible for removal of spent RecA filaments and DNA junctions from recombination intermediates.
5.1. Physical detection and classification of chromosomal lesions
Although one-strand DNA lesions do compromise the accuracy of genetic information and can interfere with the progress of individual replisomes, they neither block the overall chromosomal replication or segregation, nor do they threaten chromosome integrity. Besides, all one-strand DNA lesions are reparable by either direct DNA damage reversal or by excision repair (see Eco-Sal III Module 7.2.4. DNA Damage Reversal and Excision Repair). In contrast, two-strand DNA lesions cannot be repaired by excision, because there is no intact template within the affected duplex segment. At the same time, two-strand DNA lesions, like double-strand DNA breaks (DSB), cannot be left unrepaired either, because of their lethal consequences for the cell (Fig. 15): 1) DSBs cause loss of genetic material due to DNA degradation from the exposed ends; 2) DSBs block replication of the chromosomal DNA, 3) DSBs interfere with segregation of the daughter chromosomes. Other types of two-strand DNA lesions share all or some of these lethal consequences with double-strand DNA breaks, — therefore, they all can be viewed as “chromosomal lesions”.
Fig. 15.
The lethal consequences of chromosomal lesions, exemplified by chromosomal fragmentation due to double-strand DNA breaks. The chromosome is shown by a double-line square. Yellow highlights in red frames: either double-strand breaks or their consequences. Brown circles with black arrows nearby, centromeres and the direction they are going. Blue arrows, normal cellular processes (chromosomal replication and segregation). Magenta arrows, chromosomal fragmentation. Red arrows, consequences of chromosomal fragmentation. A. Chromosome. B. Initiation of chromosomal replication. C. Initiation of sister chromosome segregation. D. Fragmentation of non-replicating chromosome. E. Fragmentation of replicating chromosome F. Fragmentation of segregating chromosome. G. Gene loss due to DNA degradation form unprotected ends. H. Inability to replicate the chromosomal segment, separated from oirC by double-strand breaks. I. Inability to segregate chromosomal segment, separated from centromere by double-strand breaks.
As is already clear from the example of the two-ended double-strand breaks (Fig. 15), not all two-strand lesions require DNA replication for their formation. Treatments with ionizing radiation produce double-strand breaks, which promptly reveal themselves by chromosome fragmentation (23, 98) and render chromosomes susceptible to degradation. Treatments with bifunctional alkylating agents result in DNA that can be rapidly renatured after experimental denaturation, suggesting interstrand crosslinks (264). Such crosslinks completely block DNA replication in their locality and prevent subsequent chromosomal segregation. Because double-strand breaks and interstrand crosslinks affect both DNA strands opposite each other, but arise independently of DNA replication, they can be classified as direct chromosomal lesions.
Different kinds of chromosomal lesions form at replication forks that are trying to traverse single-strand DNA lesions (56, 233). In excision-repair-deficient mutants, single-strand gaps are found in DNA strands synthesized after UV-irradiation (272). The average size of the gaps is half the length of Okazaki fragments (131), while the number of gaps (at low UV doses) is similar to the number of original UV lesions (272). The gaps are thought to form when replication forks traverse pyrimidine dimers in template DNA (Fig. 4B) (124). Such gaps should form naturally in the lagging strand, due to the multiple priming events there; however, since all nascent DNA in vivo is first synthesized as Okazaki fragments (5, 249), the same gaps should also form in the leading strand. Interestingly, in vitro reinitiation downstream of the lesion in the leading strand is robust, even though in the absence of template strand lesions the leading strand is synthesized continuously in this system (113). Also, the demonstration that DNA polymerase III holoenzyme carries three catalytic assemblies makes it possible that replication forks shed individual replisomes (catalytic assemblies) that are stalled at lesions (229). In summary, the available evidence suggests that, when a replication forks encounters a non-coding lesion in one of the template strands, it reinitiates downstream, leaving behind single-strand gaps opposite the lesions (Fig. 16, left) (further reviewed in (163, 353)). These so-called daughter-strand gaps, if left unrepaired, preclude subsequent DNA replication, because both DNA strands now have lesions that would block the replisome progress. Daughter-strand gaps represent “blocked single-strand gap”, which is one general type of two-strand (chromosomal) lesions.
Fig. 16.
The two basic types of replication-dependent two-strand DNA lesions. A replication fork is moving from left to right along the template DNA with unrepaired one-strand lesions. The template on the left contains a non-coding lesion (T=T, thymine dimer), while the template on the right has a single-strand interruption. Replication fork encounter with a non-coding lesion generates a daughter-strand gap (left), while replication fork encounter with a nick generates a one-ended double-strand break (= double-strand end, right)
Events after UV-irradiation in uvr+ cells, which efficiently excise UV-induced pyrimidine dimers, reveal the other class of replication-dependent chromosomal lesions. In such cells, chromosomal DNA is fragmented; this fragmentation depends both on the ongoing excision repair and on DNA replication (reviewed in (163)). It is proposed that replication forks in these cells collapse when they run into transient excision gaps (Fig. 16, right) (107, 165). When both replication forks of a bubble collapse, this generates a linear chromosome with redundancy at the ends or a circular chromosome plus a subchromosomal fragment. The lesions in this case come in the format of a one-ended double-strand break.
Daughter-strand gaps and collapsed replication forks are replication-dependent chromosomal lesions and as such look quite different from direct chromosomal lesions — two-ended (direct) double-strand breaks and interstrand crosslinks. However, the four lesions can be grouped into two major classes. When a cell tries to repair a crosslink, it first excises one-strand portion of it, but the repair is then stalled, because the second strand cannot be used as a template to resynthesize the excised DNA strand (48). Therefore, excision repair converts interstrand crosslink into a persistent, blocked single-strand gap and, in this quality, the first intermediate in interstrand crosslink repair resembles daughter-strand gaps (Fig. 17).
Fig. 17.
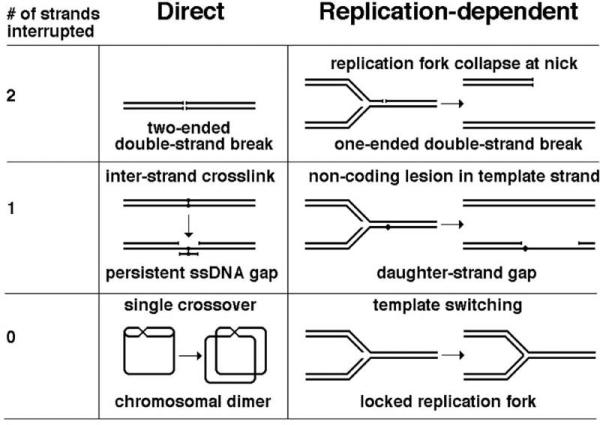
One possible classification of chromosomal lesions. This classification is based, on the one hand, on whether formation of these lesions depends or not on DNA replication (“direct” versus “replication-dependent”) and, on the other hand, on the number of DNA strands interrupted in the final format of the lesion. In E. coli, the repair of chromosomal lesions with either one or two strands interrupted completely depends on recombinational repair. Chromosomal dimers are resolved by FtsK-XerCD/dif resolution system (site-specific recombination). Locked replication fork is still a hypothetical lesion, consistent with certain gross-chromosomal rearrangements, and whose (hypothetical) repair is likely to be dependent on homologous recombination.
In contrast to a direct, two-ended double-strand break, replication fork collapse generates one-ended double-strand end. However, a double-strand break can be viewed as a special case of a more general replication fork collapse involving two replication forks converging on a nick, an event that generates two double-strand ends. Thus, we can group the four chromosomal lesions as follows: interstrand crosslinks and DSGs into the group of blocked single-strand gaps (one strand interrupted), while DSBs and RFCs into the group of double-strand ends (two strands interrupted) (Fig. 17). Finally, to complete the classification, there are also chromosomal lesions that do not involve any DNA damage and yet they still interfere with chromosomal replication and/or segregation. The direct lesions of this kind are chromosomal dimers (296), while the replication-dependent lesions without DNA damage are (hypothetical) locked replication forks (102, 168) (Fig. 17).
5.2. The inferred pathways of recombinational repair
In E. coli, chromosomal lesions (two-strand DNA lesions) that involve DNA damage are repaired by homology-guided strand exchange between sister chromatids. The evidence in support of this notion comes in three flavors (reviewed in (163)). First, physical connections between parental and daughter strands, associated with lesion repair, can be detected. Formation of such hybrid strands is the most direct physical evidence of strand exchange; such evidence is available for daughter-strand gap repair (273) and for the repair of crosslinks (47). Second, repair of chromosomal lesions is not observed in recA mutants. Such evidence is available for double-strand breaks (150), daughter-strand gaps (305) and crosslinks (46), as well as for the yet-to-be-physically-confirmed lesions such as collapsed replication forks (reviewed in (159, 161)). Third, DNA damage stimulates homologous recombination (4.1.), although the structure of chromosomal lesions in this case is not specified. Also, resolution of recombinational repair intermediates between sister duplexes in the E. coli chromosome is expected to generate sister-chromatid exchanges, detected as chromosome dimerization; formation of dimeric chromosomes is stimulated in E. coli mutants with increased DNA damage and is eliminated in single recA or in double recB recF mutants (310, 311).
As argued above (5.1.) the final configuration of chromosomal lesions with associated DNA damage belongs to one of the two basic types (Fig. 17): either a blocked single-strand gap (a daughter-strand gap or the product of an attempted excision repair of a crosslink) or a double-strand end (a pair of ends in the case of a direct double-strand break, a solitary end in the case of replication fork collapse). Double-strand end repair is not observed in recA or in recBC mutants (150, 281), suggesting that it is catalyzed by the RecBC pathway of homologous recombination (3.). Similarly, repair of blocked (persistent) single-strand gaps is not observed in recA or recFOR mutants (269, 305, 326), suggesting that it is catalyzed by the RecFOR pathway (3.). The central role of RecA in this repair suggests a common critical step. Both types of lesions disappear with normal kinetics in recG or ruv mutants, indicating that the products of these genes are not involved in repair of broken strands per se. However, both recG and ruv mutants have a specific problem after DNA damage: their nucleoids cannot untangle, as if kept together by unresolved recombinational repair intermediates (129, 130), consistent with the postulated late role of the RecG and Ruv enzymes in recombinational repair reactions, specifically in resolution of DNA junctions.
A word of caution here: although the proposed individual pathways are supposed to cover all possible chromosomal lesions, they most likely do not. Some complex DNA lesions may require combination of several pathways to complete repair. For example, repair of interstand crosslinks requires cooperation of at least nucleotide-excision repair (see Eco-Sal III Module 7.2.4. DNA Damage Reversal and Excision Repair) and both RecBC and RecFOR pathways of recombinational repair (41, 47, 48, 300). In general, repair of complex DNA lesions or clustered DNA lesions (tight groups of ones-strand lesions) is a barely explored area.
5.3. The mechanisms of recombinational repair
Plausible schemes for the two recombinational repair reactions are shown in Fig. 18. In both cases, the affected DNA region, with the help of RecA protein, invades the homologous region on the intact (sister) chromosome. After the resolution of DNA junctions and repair of one-strand lesions, the replication is restarted, either to fill the gaps or to resume a replication fork. It should be stressed, though, that, while genetic analysis is powerful enough to figure out the entire pathway of a particular reaction in terms of genes and hypothetical reaction stages (Fig. 13), it must rely on biochemical characterization of reactions, catalyzed by the corresponding proteins in vitro, to translate a formal pathway scheme into a detailed mechanism. The biochemical characterization of the Rec and Ruv proteins showed the following (see (163, 168) for more in-depth discussion):
Fig. 18.
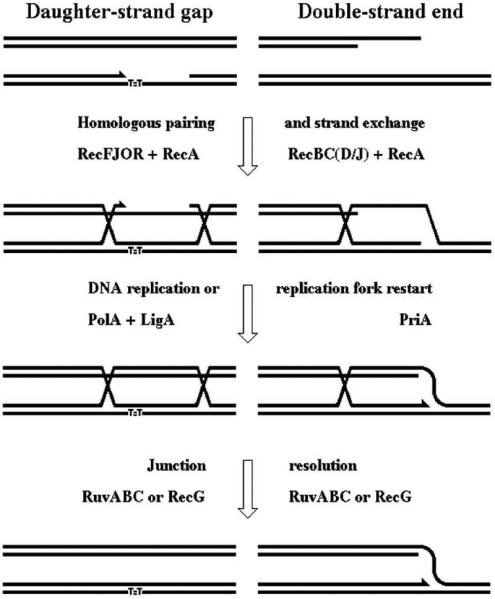
The two pathways of recombinational repair in E. coli. On the left, the daughter-strand gap repair pathway is shown, while the double-strand end repair pathway is on the right. Stages are marked with enzymes that catalyze them.
RecA protein polymerizes into spiral filaments on ssDNA in the 5’—>3’ direction, making RecA filaments more stable on the 3’-ends of linear ssDNA than on the 5’-ends (180, 263, 292). Such a ssDNA-RecA filament is the homologous pairing and strand exchange entity (reviewed in (148, 265)) that catalyzes single-strand invasion into a circular duplex (63, 226, 297). Two-strand DNA lesions (in which DNA strands are interrupted) are always associated with ssDNA or are processed to become associated with ssDNA (168), so this ssDNA invasion catalyzed by RecA is precisely what is needed to form a recombinational repair intermediate. Thus, RecA should be the central activity of recombinational repair, catalyzing the all-important strand exchange step, which is fully consistent with the conclusion of genetic analysis. RecA filaments also serve as a scaffold for binding and activation of a peculiar cryptic autoprotease called LexA (122, 181), which is a repressor for the SOS regulon, critical for the overall resistance of E. coli to DNA damage (see Eco-Sal III Module 5.4.3. The SOS Regulatory Network, and Module 7.2.8. The SOS Response). For in-depth discussion of the in vitro properties and structure of RecA, please consult section 2 of the Eco-Sal III Module 7.2.7. “Homologous Recombination — Enzymes and Pathways”.
Since in vitro RecA polymerizes on ssDNA even in the presence of SSB protein (under certain conditions) (149, 172, 323), it may be unclear what keeps RecA off ssDNA regions, which are always present at replication forks, in the absence of DNA damage in vivo? There is an argument, based on the intracellular nucleotide and Mg2+ concentrations, that normal physiological conditions are suboptimal for RecA polymerization in the presence of SSB (158). There is also a kinetic argument that in vivo RecA cannot polymerize on ssDNA that does not need recombinational repair (283) because the kinetics of RecA polymerization on SSB-complexed ssDNA in vitro has a lag, while the polymerization itself, once started, is fast (61, 323). This bi-phasic property of RecA polymerization, or its stimulation by increasing Mg2+ concentration, suggest the existence of a limiting step, — the nucleation event. In other words, in the cell, RecA apparently needs to be licensed to polymerize on ssDNA. What is the nature of this license and does it also provide any specificity for RecA polymerization? To answer these questions, we should keep in mind that the enzymes catalyzing the early phase of recombinational repair should not only promote RecA polymerization, but should also be able to recognize specific chromosomal lesions (that is, possess substrate-specificity).
Of all the proteins in E. coli cells, RecBCD complex has the highest affinity toward double-strand DNA ends (on the order of 1 nM, or 0.1 ends per cell) (266, 316), making it the first enzyme to bind to the ends of a double-strand break. Besides securing the ends, the RecBCD enzyme has several other relevant activities in vitro: 1) DNA end-specific helicase; 2) ATP-dependent dsDNA and ssDNA exonuclease; 3) stimulator of RecA polymerization on a particular DNA strand (reviewed in (73)). Circular duplex DNAs or SSB-complexed circular single-stranded DNAs are completely resistant to the action of RecBCD. After binding to a double-strand DNA end, RecBCD uses its helicase/exonuclease activities to generate a 3’-overhang of ssDNA, which is instantly complexed by SSB. RecBCD eventually stops and promotes RecA polymerization on the generated ss-overhang by providing a nucleation push for RecA and thus aiding it in the displacement of SSB (reviewed in (73)). For in-depth discussion of the in vitro properties and structure of the RecBCD enzyme, please consult section 3 of the Eco-Sal III Module 7.2.7. “Homologous Recombination — Enzymes and Pathways”.
RecF, RecO and RecR proteins in vitro can bind ssDNA, as well as duplex DNA (reviewed in (59)). In addition, RecO and RecR proteins stimulate RecA polymerization on SSB-complexed ssDNA. Lastly, both RecF and RecO bind RecR protein, but not each other (reviewed in (59)). As a group, if not a single complex, RecF, RecO and RecR proteins help RecA to polymerize on DNA with ss-gaps (239). It has been speculated that RecF has affinity to DNA with ss-gaps in vivo, and, using its affinity to RecR, it targets RecOR proteins to these gaps (163). The ss-gaps in vivo are, of course, complexed by SSB, but RecOR proteins provide a nucleation signal for RecA (reviewed in (59)), so RecA displaces SSB on ssDNA gaps, if licensed by RecFOR. For in-depth discussion of the in vitro properties and structures of RecF, RecO and RecR proteins, please consult section 4 of the Eco-Sal III Module 7.2.7. “Homologous Recombination — Enzymes and Pathways”.
The late recombinational repair functions, encoded by recG and ruv genes, can be better understood as the opposites of the early functions, RecBCD and RecFOR. Whereas the two main roles of the early functions are (i) to recognize specific chromosomal lesions; (ii) to license RecA polymerization on these lesions, the two main roles of the late functions are (i) to recognize and remove generic recombination intermediates; (ii) to disassemble RecA filaments associated with these recombination intermediates (discussed in (163, 168)).
Since the most basic recombinational repair intermediate should contain Holliday junctions, the RecG and Ruv proteins were expected to have affinity to Holliday junctions and to catalyze reactions with them. The ruv locus turned out to be a complex one, comprising three genes: ruvA, ruvB and ruvC (293, 298). RuvA protein in vitro indeed binds completely double-stranded four-way DNA junctions (Holliday junctions) and changes their conformation from the folded one (difficult to branch-migrate) to the square planar one (easy to branch-migrate) (reviewed in (339)). RuvB in vitro forms hexameric doughnuts around duplex DNA, interacts with RuvA and, while holding on to RuvA, can “pump” duplex DNA through the central cavity of the hexameric ring (reviewed in (339)). RuvB is a distant relative of hexameric helicases, but its “pumping” action actually anneals DNA strands, separated at the Holliday junction, rather than unwinds DNA duplexes. RuvAB complex also depolymerizes the RecA filaments, associated with Holliday junctions (2). RuvC is another protein that binds Holliday junctions, again changing their conformation to the planar one, and then symmetrically cleaves a pair of opposite strands at special sites to “resolve” the junctions (reviewed in (339)). RuvABC, working as a single complex, “resolvasome”, binds, isomerizes, protects, branch-migrates and resolves Holliday junctions (reviewed in (164, 166, 339)). For in-depth discussion of the in vitro properties and structures of RuvA, RuvB and RuvC proteins, please consult section 5 of the Eco-Sal III Module 7.2.7. “Homologous Recombination — Enzymes and Pathways”.
RecG helicase binds on one side of three-way or four-way DNA junctions and branch-migrates them by effectively “squeezing out” the DNA on the opposite side of the junction (reviewed in (163)). RecG can also depolymerize RecA filaments associated with DNA junctions (340). Genetic data suggest that RecG, like RuvABC, participates in the DNA junction removal (184), but the mechanisms of its action are poorly understood (228, 271). One idea is that RecG works on the double Holliday junctions to migrate them toward each other until they come together and are, thus, eliminated (“dissolved” into each other) (163). Such “dissolution” will also require the action of DNA gyrase between the two junctions (or Topo IV outside), to remove the DNA coils. To learn more about the in vitro properties and structure of RecG helicase, please consult section 6 of the Eco-Sal III Module 7.2.7. “Homologous Recombination — Enzymes and Pathways”.
To summarize, the recombinational repair proteins can be subdivided into three groups: 1) The strand-exchange proteins — the catalysis team, responsible for the central step in recombinational repair. RecA in bacteria, assisted by SSB. 2) The enzymes recognizing chromosomal lesions, targeting them for RecA polymerization and licensing RecA polymerization on SSB-complexed ssDNA — the specificity teams. These include RecBC and RecFOR activities. 3) The enzymes removing spent RecA filaments and DNA junctions from hybrid duplexes — the cleaning-up teams. These include the RuvABC resolvasome and the RecG helicase.
Conclusion
Homologous recombination in E. coli is a complex phenomenon with clear evolutionary implications and an important everyday metabolic purpose. For the students of homologous recombination, both E. coli and Salmonella offer solid understanding of the core events, several formats of elaborate experimental systems to address its various aspects, well-developed genetics with several pathways, and unparalleled biochemistry. This chapter strived to cover all important aspects of homologous recombination in E. coli outside the biochemical and structural aspects of it, which receive separate in-depth coverage in the recombinational repair chapter by Michel and Leach (Eco-Sal III Module 7.2.7. Recombinational Repair — Enzymes and Pathways).
Since the study of homologous recombination laid the groundwork for the subsequent successful study of recombinational repair, it may be unclear why biochemistry of homologous recombination in E. coli should be discussed in a separate chapter and under a different title, apart from its phenomenology and genetics. The answer comes naturally from considering the phenomena of “homologous recombination” vis-à-vis “recombinational repair”. “Recombinational repair” in bacteria is a particular metabolic transaction, with specific substrates (non-functional chromosomes) and products (functional chromosomes), defined enzymatic steps and important outcome for the cell. An ideal recombinational repair transaction is genetically silent, just like an ideal DNA replication round, a part of which the former actually is. In contrast, “homologous recombination” is always a result, rather than a specific process, a peculiar and unintended consequence of sojourning of two homologous DNA sequences in the same cell, a rearrangement of the genetic material that might advance the lineage in the evolutionary race, but more likely will be without effect or will have deleterious consequences due to changes in the chromosomal structure. Studies of homologous recombination illuminate the future of recombinational repair, but do not yield the physiologically-relevant mechanisms of DNA metabolism the latter is famous for. The two indeed have different purposes, practice different philosophies and provide different insights, — therefore they deserve separate dedicated chapters.
Preamble.
The introduction (part 1) to this module is written with a beginner graduate student in mind: it starts with a thorough definition of homologous recombination in its relation to other types of recombination, spends quality time on recombination intermediates, goes through the basic types of rearrangements due to homologous recombination, explains the peculiarity of mapping by recombination in prokaryotes and finally briefly goes over recombination detection. Homologous recombination aficionados, who will likely find such an introduction unnecessarily trivial, should start with part 2.
Acknowledgements
I wish to thank Bob Lloyd, Sue Lovett, Benedicte Michel, Steve Sandler and Frank Stahl for critical reading and helpful suggestions on this Chapter. While I was writing it, research in my laboratory was supported by grant # RSG-05-135-01-GMC from the American Cancer Society and by grant # GM 073115 from the National Institutes of Health.
Literature Cited
- 1.Abdulkarim F, Hughes D. Homologous recombination between the tuf genes of Salmonella typhimurium. J. Mol. BIol. 1996;260:506–522. doi: 10.1006/jmbi.1996.0418. [DOI] [PubMed] [Google Scholar]
- 2.Adams DE, Tsaneva IR, West SC. Dissociation of RecA filament from duplex DNA by the RuvA and RuvB DNA repair proteins. Proc. Natl. Acad. Sci. USA. 1994;91:9901–9905. doi: 10.1073/pnas.91.21.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Deib AA, Mahdi AA, Lloyd RG. Modulation of recombination and DNA repair by the RecG and PriA helicases of Escherichia coli K-12. J. Bacteriol. 1996;178:6782–6789. doi: 10.1128/jb.178.23.6782-6789.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertini AM, Hofer M, Calos MP, Miller JH. On the formation of spontaneous deletions: The importance of short sequence homologies in the generation of large deletions. Cell. 1982;29:319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- 5.Amado L, Kuzminov A. The replication intermediates in Escherichia coli are not the product of DNA processing or uracil excision. J. Biol. Chem. 2006;281:22635–22646. doi: 10.1074/jbc.M602320200. [DOI] [PubMed] [Google Scholar]
- 6.Anderson P, Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc. Natl. Acad. Sci. USA. 1981;78:3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson RP, Roth JR. Tandem genetic duplications in phage and bacteria. Annu. Rev. Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- 8.Arthur HM, Lloyd RG. Hyper-recombination in uvrD mutants of Escherichia coli K-12. Mol. Gen. Genet. 1980;180:185–191. doi: 10.1007/BF00267368. [DOI] [PubMed] [Google Scholar]
- 9.Arwidsson O, Hughes D. Evidence against reciprocal recombination as the basis for tuf gene conversion in Salmonella enterica serovar Typhimurium. J. Mol. Biol. 2004;338:463–467. doi: 10.1016/j.jmb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Bachellier S, Gilson E, Hofnung M, Hill CW. Repeated Sequences. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2012–2040. [Google Scholar]
- 11.Bachellier S, Saurin W, Perrin D, Hofnung M, Gilson E. Structural and functional diversity among bacterial interspersed mosaic elements (BIMEs). Mol. Microbiol. 1994;12:61–70. doi: 10.1111/j.1365-2958.1994.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 12.Barbour SD, Nagaishi H, Templin A, Clark AJ. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of Rec− mutations. Proc. Natl. Acad. Sci. USA. 1970;67:128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedbrook JR, Ausubel FM. Recombination between bacterial plasmids leading to the formation of plasmid multimers. Cell. 1976;9:707–716. doi: 10.1016/0092-8674(76)90134-3. [DOI] [PubMed] [Google Scholar]
- 14.Beernink HTH, Morrical SW. RMPs: recombination/replication mediator proteins. Trends Biochem. Sci. 1999;24:385–389. doi: 10.1016/s0968-0004(99)01451-6. [DOI] [PubMed] [Google Scholar]
- 15.Beier H, Hausmann R. T3 × T7 phage crosses leading to recombinant RNA polymerases. Nature. 1974;251:538–540. doi: 10.1038/251538a0. [DOI] [PubMed] [Google Scholar]
- 16.Bellett AJD, Busse HG, Baldwin RL. Tandem genetic duplications in a derivative of phage lambda. In: Hershey AD, editor. The Bacteriophage Lambda. Cold Spring Harbor Laboratory Press; 1971. pp. 501–513. [Google Scholar]
- 17.Benbow RM, Zuccarelli AJ, Sinsheimer RL. A role for single- strand breaks in bacteriophage ϕX174 genetic recombination. J. Mol. Biol. 1974;88:629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- 18.Benzer S. On the topography of the genetic fine structure. Proc. Nat. Acad. Sci. U.S.A. 1961;47:403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benzinger R, Enquist LW, Skalka A. Transfection of Escherichia coli spheroplasts. V. Activity of RecBC nuclease in rec+ and rec minus spheroplasts measured with different forms of bacteriophage DNA. J. Virol. 1975;15:861–871. doi: 10.1128/jvi.15.4.861-871.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi X, Liu LF. DNA rearrangements mediated by inverted repeats. Proc. Natl. Acad. Sci. USA. 1996;93:819–823. doi: 10.1073/pnas.93.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi X, Liu LF. recA-independent and recA-dependent intramolecular plasmid recombination. Differential homology requirements and distance effect. J. Mol. Biol. 1994;235:414–423. doi: 10.1006/jmbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 22.Bichara M, Pinet I, Origas M, Fuchs RP. Inactivation of recG stimulates the RecF pathway during lesion-induced recombination in E. coli. DNA Repair. 2006;5:129–137. doi: 10.1016/j.dnarep.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Billen D, Hewitt R. Concerning the dynamics of chromosome replication and degradation in a bacterial population exposed to X-rays. Biochim. Biophys. Acta. 1967;138:587–595. doi: 10.1016/0005-2787(67)90554-0. [DOI] [PubMed] [Google Scholar]
- 24.Birge EA, Low KB. Detection of transcribable recombination products following conjugation in Rec+, RecB−, and RecC− strains of Escherichia coli K12. J. Mol. Biol. 1974;83:447–457. doi: 10.1016/0022-2836(74)90506-3. [DOI] [PubMed] [Google Scholar]
- 25.Blanco M, Armengod ME. Role of the bacterial and phage recombination systems and of DNA replication in genetic recombination of UV-irradiated phage Lambda. Mol. Gen. Genet. 1976;146:51–54. doi: 10.1007/BF00267982. [DOI] [PubMed] [Google Scholar]
- 26.Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 27.Blinkowa A. The role of polymerase III in conjugation between E. coli K12 donor and recepient strains carrying dnaEts mutation. Acta Microbiol. Pol. 1976;25:95–108. [PubMed] [Google Scholar]
- 28.Brendel M, Haynes RH. Interactions among genes controlling sensitivity to radiation and alkylation in yeast. Mol. Gen. Genet. 1973;125:197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- 29.Britten RJ, Kohne DE. Repeated sequences in DNA. Science. 1968;161:529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- 30.Broker TR, Lehman IR. Branched DNA molecules: intermediates in T4 recombination. J. Mol. Biol. 1971;60:131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- 31.Bzymek M, Lovett ST. Instability of repetitive DNA sequences: the role of replication in multiple mechanisms. Proc. Natl. Acad. Sci. USA. 2001;98:8319–8325. doi: 10.1073/pnas.111008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calendar R, Abedon ST, editors. The Bacteriophages. second ed. Oxford University Press; New York: 2006. [Google Scholar]
- 33.Capiaux H, Cornet F, Corre J, Guijo MI, Pérals K, Rebollo JE, Louarn JM. Polarization of the Escherichia coli chromosome. A view from the terminus. Biochimie. 2001;83:161–170. doi: 10.1016/s0300-9084(00)01202-5. [DOI] [PubMed] [Google Scholar]
- 34.Centore RC, Sandler SJ. UvrD limits the number and intensities of RecA-green fluorescent protein structures in Escherichia coli K-12. J. Bacteriol. 2007;189:2915–2920. doi: 10.1128/JB.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chumley FG, Roth JR. Rearrangement of the bacterial chromosome using Tn10 as a region of homolgy. Genetics. 1980;94:1–14. [Google Scholar]
- 36.Clark AJ. Recombination deficient mutants of E. coli and other bacteria. Annu. Rev. Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- 37.Clark AJ. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu. Rev. Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- 38.Clark AJ, Inwood W, Cloutier T, Dhillon TS. Nucleotide sequence of coliphage HK620 and the evolution of lambdoid phages. J. Mol. Biol. 2001;311:657–679. doi: 10.1006/jmbi.2001.4868. [DOI] [PubMed] [Google Scholar]
- 39.Clark AJ, Low KB. Pathways and systems of homologous recombination in Escherichia coli. In: Low KB, editor. The Recombination of Genetic Material. Academic Press; San Diego: 1988. pp. 155–215. [Google Scholar]
- 40.Clark AJ, Margulies AD. Isolation and characterization of recombination-deficient mutants of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA. 1965;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark AJ, Sandler SJ. Homologous recombination: the pieces begin to fall into place. Crit. Rev. Microbiol. 1994;20:125–142. doi: 10.3109/10408419409113552. [DOI] [PubMed] [Google Scholar]
- 42.Clark AJ, Sharma V, Brenowitz S, Chu CC, Sandler S, Satin L, Templin A, Berger I, Cohen A. Genetic and molecular analyses of the C-terminal region of the recE gene from the Rac prophage of Escherichia coli K-12 reveal the recT gene. J. Bacteriol. 1993;175:7673–7682. doi: 10.1128/jb.175.23.7673-7682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark JB, Haas F, Stone WS, Wyss O. The stimulation of gene recombination in Escherichia coli. J. Bacteriol. 1950;59:375–379. doi: 10.1128/jb.59.3.375-379.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clowes RC, Moody EE. Chromosomal transfer from “recombination-deficient” strains of Escherichia coli K-12. Genetics. 1966;53:717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen A, Silberstein Z, Broido S, Laban A. General genetic recombination of bacterial plasmids. Basic Life Sci. 1985;30:505–519. doi: 10.1007/978-1-4613-2447-8_36. [DOI] [PubMed] [Google Scholar]
- 46.Cole RS. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J. Bacteriol. 1971;107:846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole RS. Repair of DNA containing interstand crosslinks in Escherichia coli: sequential excision and recombination. Proc. Natl. Acad. Sci. USA. 1973;70:1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole RS, Levitan D, Sinden RR. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J. Mol. Biol. 1976;103:39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- 49.Connelly JC, de Leau ES, Leach DR. DNA cleavage and degradation by the SbcCD protein complex from Escherichia coli. Nucleic Acids Res. 1999;27:1039–1046. doi: 10.1093/nar/27.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connelly JC, Leach DR. The sbcC and sbcD genes of Escherichia coli encode a nuclease involved in palindrome inviability and genetic recombination. Genes Cells. 1996;1:285–291. doi: 10.1046/j.1365-2443.1996.23024.x. [DOI] [PubMed] [Google Scholar]
- 51.Cordone L, Sperandeo-Mineo RM, Mannino S. UV-induced enhancement of recombination among lambda bacteriophages: relation with replication of irradiated DNA. Nucleic Acid Res. 1975;2:1129–1142. doi: 10.1093/nar/2.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cosloy SD, Oishi M. Genetic transformation in Escherichia coli K12. Proc. Natl. Acad. Sci. USA. 1973;70:84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costantino N, Court DL. Enhanced levels of lambda Red- mediated recombinants in mismatch repair mutants. Proc. Natl. Acad. Sci. USA. 2003;100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox B, Game J. Repair systems in Saccharomyces. Mutat. Res. 1974;26:257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- 56.Cox MM. A broadening view of recombinational DNA repair in bacteria. Genes Cells. 1998;3:65–78. doi: 10.1046/j.1365-2443.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- 57.Cox MM. Motoring along with the bacterial RecA protein. Nat. Rev. Mol. Cell Biol. 2007;8:127–138. doi: 10.1038/nrm2099. [DOI] [PubMed] [Google Scholar]
- 58.Cox MM. Recombinational DNA repair of damaged replication forks in Escherichia coli: Questions. Annu. Rev. Genet. 2001;35:53–82. doi: 10.1146/annurev.genet.35.102401.090016. [DOI] [PubMed] [Google Scholar]
- 59.Cox MM. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 2007;42:41–63. doi: 10.1080/10409230701260258. [DOI] [PubMed] [Google Scholar]
- 60.Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 61.Cox MM, Lehman IR. RecA protein-promoted DNA strand exchange. Stable complexes of recA protein and single-stranded DNA formed in the presence of ATP and single-stranded DNA binding protein. J. Biol. Chem. 1982;257:8523–8532. [PubMed] [Google Scholar]
- 62.Cullum J, Broda P. Chromosome transfer and Hfr formation by F in rec+ and recA strains of Escherichia coli K12. Plasmid. 1979;2:358–365. doi: 10.1016/0147-619x(79)90019-2. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham RP, DasGupta C, Shibata T, Radding CM. Homologous pairing in genetic recombination: RecA protein makes joint molecules of gapped circular DNA and closed circular DNA. Cell. 1980;20:223–235. doi: 10.1016/0092-8674(80)90250-0. [DOI] [PubMed] [Google Scholar]
- 64.Curtiss R. r. Bacterial conjugation. Annu. Rev. Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- 65.Curtiss R. r. Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968;58:9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dabert P, Ehrlich SD, Gruss A. χ sequence protects against RecBCD degradation of DNA in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danilevich VN, Stepanshin YG, Volozhanstev NV, Golub EI. Transposon-mediated insertion of R factor into bacterial chromosome. Mol. Gen. Genet. 1978;161:337–339. doi: 10.1007/BF00331010. [DOI] [PubMed] [Google Scholar]
- 68.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Degryse E. In vivo intermolecular recombination in Escherichia coli: application to plasmid constructions. Gene. 1996;170:45–50. doi: 10.1016/0378-1119(95)00858-6. [DOI] [PubMed] [Google Scholar]
- 70.Deonier RC. Native insertion sequence elements: locations, distributions and sequence relatioships. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2000–2011. [Google Scholar]
- 71.Deonier RC, Mirels L. Excision of F plasmid sequences by recombination at directly repeated insertion sequence 2 elements: involvement of recA. Proc. Natl. Acad. Sci. USA. 1977;74:3965–3969. doi: 10.1073/pnas.74.9.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dianov GL, Kuzminov AV, Mazin AV, Salganik RI. Molecular mechanisms of deletion formation in Escherichia coli plasmids. I. Deletion formation mediated by long direct repeats. Mol. Gen. Genet. 1991;228:153–159. doi: 10.1007/BF00282460. [DOI] [PubMed] [Google Scholar]
- 73.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 2008;72:642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doherty MJ, Morrison PT, Kolodner R. Genetic recombination of bacterial plasmid DNA. Physical and genetic analysis of the products of plasmid recombination in Escherichia coli. J. Mol. Biol. 1983;167:539–560. doi: 10.1016/s0022-2836(83)80097-7. [DOI] [PubMed] [Google Scholar]
- 75.Dri A-M, Moreau PL, Rouviere-Yaniv J. Role of the histone-like proteins OsmZ and HU in homologous recombination. Gene. 1992;120:11–16. doi: 10.1016/0378-1119(92)90003-8. [DOI] [PubMed] [Google Scholar]
- 76.Dutra BE, Sutera VAJ, Lovett ST. RecA-independent recombination is efficient but limited by exonucleases. Proc. Nat. Acad. Sci. U.S.A. 2007;104:216–221. doi: 10.1073/pnas.0608293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dzidic S, Salaj-Smic E, Trgovcevic Z. The relationship between survival and mutagenesis in Escherichia coli after fractionated ultraviolet irradiation. Mutat. Res. 1986;173:89–91. doi: 10.1016/0165-7992(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 78.Ebel-Tsipis J, Fox MS, Botstein D. Generalized transduction by bacteriophage P22 in Salmonella typhimurium. II. Mechanism of integration of transducing DNA. J. Mol. Biol. 1972;71:449–469. doi: 10.1016/0022-2836(72)90362-2. [DOI] [PubMed] [Google Scholar]
- 79.Egel R. RecA-DNA filament topology: the overlooked alternative of an unconventional syn-syn duplex intermediate. DNA Repair. 2007;6:669–675. doi: 10.1016/j.dnarep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 80.El Karoui M, Amundsen SK, Dabert P, Gruss A. Gene replacement with linear DNA in electroporated wild-type Escherichia coli. Nucleic Acids Res. 1999;27:1296–1299. doi: 10.1093/nar/27.5.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emmerson PT. Recombination deficient mutants of Escherichia coli K12 that map between thyA and argA. Genetics. 1968;60:19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ennis DG, Amundsen SK, Smith GR. Genetic functions promoting homologous recombination in Escherichia coli: a study of inversion in phage λ. Genetics. 1987;115:11–24. doi: 10.1093/genetics/115.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enquist LW, Skalka A. Replication of bacteriophage λ DNA dependent on the function of host and viral genes. I. Interaction of red, gam, and rec. J. Mol. Biol. 1973;75:185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- 84.Epstein RH, Bolle A, Steinberg CM, Kellenberger E, Boy de la Tour E, Chevalley R, Edgar RS, Susman M, Denhardt GH, Lielausis A. Physiological studies of conditional lethal mutants of bacteriophage T4D. Cold. Spring harbor Symp. Quant. Biol. 1964;28:375–394. [Google Scholar]
- 85.Fernández de Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, Woodgate R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 2000;35:1560–1572. doi: 10.1046/j.1365-2958.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 86.Feschenko VV, Lovett S. Slipped misalignment mechanisms of deletion formation: analysis of deletion endpoints. J. Mol. Biol. 1998;276:559–569. doi: 10.1006/jmbi.1997.1566. [DOI] [PubMed] [Google Scholar]
- 87.Fishel RA, James AA, Kolodner R. RecA-independent general recombination of plasmids. Nature. 1981;294:184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- 88.Fox MS. On the mechanism of integration of transforming deoxyribonucleate. J. Gen. Physiol. 1966;49:183–196. doi: 10.1085/jgp.49.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Washington, D.C.: 2006. [Google Scholar]
- 90.Galitski T, Roth JR. Pathways for homologous recombination between chromosomal direct repeats in Salmonella typhimurium. Genetics. 1997;146:751–767. doi: 10.1093/genetics/146.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Game JC. The Saccharomyces repair genes at the end of the century. Mutat. Res. 2000;451:277–293. doi: 10.1016/s0027-5107(00)00055-5. [DOI] [PubMed] [Google Scholar]
- 92.Gibson FP, Leach DRF, Lloyd RG. Identification of sbcD mutations as cosupressors of recBC that allow propagation of DNA palindromes in Escherichia coli K-12. J. Bacteriol. 1992;174:1222–1228. doi: 10.1128/jb.174.4.1222-1228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gil D, Bouché J-P. ColE1-type vectors with fully repressible replication. Gene. 1991;105:17–22. doi: 10.1016/0378-1119(91)90508-9. [DOI] [PubMed] [Google Scholar]
- 94.Gillen JR, Karu AE, Nagaishi H, Clark AJ. Characterization of the deoxyribonuclease determined by lambda reverse as exonuclease VIII of Escherichia coli. J. Mol. BIol. 1977;113:27–41. doi: 10.1016/0022-2836(77)90039-0. [DOI] [PubMed] [Google Scholar]
- 95.Gillen JR, Willis DK, Clark AJ. Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J. Bacteriol. 1981;145:521–532. doi: 10.1128/jb.145.1.521-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golub EI, Low KB. Indirect stimulation of genetic recombination. Proc. Natl. Acad. Sci. USA. 1983;80:1401–1405. doi: 10.1073/pnas.80.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gottesman MM, Gottesman ME, Gottesman S, Gellert M. Characterization of bacteriophage λ reverse as an Escherichia coli phage carrying a unique set of host-derived recombination functions. J. Mol. Biol. 1974;88:471–487. doi: 10.1016/0022-2836(74)90496-3. [DOI] [PubMed] [Google Scholar]
- 98.Gray WJH, Green MHL, Bridges BA. DNA synthesis in gamma-irradiated recombination deficient strains of Escherichia coli. J. Gen. Microbiol. 1972;71:359–366. doi: 10.1099/00221287-71-2-359. [DOI] [PubMed] [Google Scholar]
- 99.Gregg AV, McGlynn P, Jaktaji RP, Lloyd RG. Direct rescue of stalled DNA replication forks via the combined action of PriA and RecG helicase activities. Mol. Cell. 2002;9:241–251. doi: 10.1016/s1097-2765(02)00455-0. [DOI] [PubMed] [Google Scholar]
- 100.Grindley ND, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006;75:567–605. doi: 10.1146/annurev.biochem.73.011303.073908. [DOI] [PubMed] [Google Scholar]
- 101.Grohmann E, Muth G, Espinosa M. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67:277–301. doi: 10.1128/MMBR.67.2.277-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guild WR. The “fork-and-knife” model of the replication point mechanism. Cold Spring Harbor Symp. Quant. Biol. 1969;33:143. [Google Scholar]
- 103.Gutterson NI, Koshland DE. Replacement and amplification of bacterial genes with sequences altered in vitro. Proc. Natl. Acad. Sci. USA. 1983;80:4894–4898. doi: 10.1073/pnas.80.16.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamilton CM, Aldea M, Washburn BK, Babitzke P, Kushner SR. New method for generating deletions and gene replacements in Escherichia coli. J. Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 106.Hanahan D, Bloom FR. Mechanisms of DNA Transformation. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2449–2459. [Google Scholar]
- 107.Hanawalt PC. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem. Photobiol. 1966;5:1–12. [PubMed] [Google Scholar]
- 108.Handa N, Nakayama Y, Sadykov M, Kobayashi I. Experimental genome evolution: large-scale genome rearrangements associated with resistance to replacement of a chromosomal restriction-modification gene complex. Mol. Microbiol. 2001;40:932–940. doi: 10.1046/j.1365-2958.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 109.Harm W. On the control of UV-sensitivity of phage T4 by the gene x. Mutat. Res. 1964;1:344–354. [Google Scholar]
- 110.Haynes RH, Kunz BA. DNA repair and mutagenesis in yeast. In: Strathern JN, Jones EW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces. Vol. 1. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1981. pp. 371–414. [Google Scholar]
- 111.Hays JB, Duncan BK, Boehmer S. Recombination of uracil-containing lambda bacteriophages. J. Bacteriol. 1981;145:306–320. doi: 10.1128/jb.145.1.306-320.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hays JB, Zagursky RJ. Generalized recombination in tandem duplications of bacteriophage lambda. Mol. Gen. Genet. 1978;160:325–330. [Google Scholar]
- 113.Heller RC, Marians KJ. Replication fork reactivation downstream of a blocked nascent leading strand. Nature. 2006;439:557–562. doi: 10.1038/nature04329. [DOI] [PubMed] [Google Scholar]
- 114.Helling RB. Ultraviolet light-induced recombination. Biochem. Biophys. Res. Commun. 1973;55:752–757. doi: 10.1016/0006-291x(73)91208-4. [DOI] [PubMed] [Google Scholar]
- 115.Herman RK. Identification of recombinant chromosomes and F-merogenotes in merodiploids of Escherichia coli. J. Bacteriol. 1968;96:173–179. doi: 10.1128/jb.96.1.173-179.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hill CW. Large genomic sequence repetitions in bacteria: lessons from rRNA operons and Rhs elements. Res. Microbiol. 1999;150:665–674. doi: 10.1016/s0923-2508(99)00125-4. [DOI] [PubMed] [Google Scholar]
- 117.Hill CW, Harnish BW. Inversions between ribosomal RNA genes of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1981;78:7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hill CW, Schiffer D, Berg P. Transduction of merodiploidy: induced duplication of recipient genes. J. Bacteriol. 1969;99:274–278. doi: 10.1128/jb.99.1.274-278.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoekstra WP, Bergmans JE, Zuidweg EM. Role of recBC nuclease in Escherichia coli transformation. J. Bacteriol. 1980;143:1031–1032. doi: 10.1128/jb.143.2.1031-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoffmann GR. Bacterial assays for recombinagens. Mutat. Res. 1992;284:125–146. doi: 10.1016/0027-5107(92)90028-z. [DOI] [PubMed] [Google Scholar]
- 121.Holliday R. A mechanism for gene conversion in fungi. Genet. Res. 1964;5:282–304. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- 122.Horii T, Ogawa T, Nakatani T, Hase T, Matsubara H, Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleavage by the RecA protein. Cell. 1981;27:515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- 123.Horii Z-I, Clark AJ. Genetic analysis of the RecF pathway of genetic recombination in Escherichia coli K-12: isolation and characterization of mutants. J. Mol. Biol. 1973;80:327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- 124.Howard-Flanders P. DNA repair and recombination. Brit. Med. Bull. 1973;29:226–235. doi: 10.1093/oxfordjournals.bmb.a071012. [DOI] [PubMed] [Google Scholar]
- 125.Howard-Flanders P, Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966;53:1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hughes D. Co-evolution of the tuf genes links gene conversion with the generation of chromosomal inversions. J. Mol. Biol. 2000;297:355–364. doi: 10.1006/jmbi.2000.3587. [DOI] [PubMed] [Google Scholar]
- 127.Ippen-Ihler KA, Minkley EG., Jr. The conjugation system of F, the fertility factor of Escherichia coli. Annu. Rev. Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- 128.Ishihama A. Module 2.6. The nucleoid: an overview. In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D, editors. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; Washington, DC: 2009. http://www.ecosal.org. [Google Scholar]
- 129.Ishioka K, Fukuoh A, Iwasaki H, Nakata A, Shinagawa H. Abortive recombination in Escherichia coli ruv mutants blocks chromosome partitioning. Genes Cells. 1998;3:209–220. doi: 10.1046/j.1365-2443.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- 130.Ishioka K, Iwasaki H, Shinagawa H. Roles of the recG gene product of Escherichia coli in recombinational repair: Effects of the ΔrecG mutation on cell division and chromosome partition. Genes Genet. Syst. 1997;72:91–99. doi: 10.1266/ggs.72.91. [DOI] [PubMed] [Google Scholar]
- 131.Iyer VN, Rupp WD. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim. Biophys. Acta. 1971;228:117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- 132.Jacob F, Wollman EL. Étude génétique d'un bactériophage tempéré d'Escherichia coli. III. Effet du rayonnement ultraviolet sur la recombinaison génétique. Annales de L'Institut Pasteur. 1955;88:724–749. [PubMed] [Google Scholar]
- 133.Jacob F, Wollman EL. Sexuality and the Genetics of Bacteria. Academic Press; New York: 1961. [Google Scholar]
- 134.Jaktaji RP, Lloyd RG. PriA supports two distinct pathways for replication restart in UV-irradiated Escherichia coli cells. Mol. Microbiol. 2003;47:1091–1100. doi: 10.1046/j.1365-2958.2003.03357.x. [DOI] [PubMed] [Google Scholar]
- 135.James AA, Morrison PT, Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J. Mol. Biol. 1982;160:411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- 136.Kaiser K, Murray NE. Physical characterization of the “Rac prophage” in E. coli K-12. Mol. Gen. Genet. 1979;175:159–174. doi: 10.1007/BF00425532. [DOI] [PubMed] [Google Scholar]
- 137.Kano Y, Imamoto F. Requirement of integration host factor (IHF) for growth of Escherichia coli deficient in HU protein. Gene. 1990;89:133–137. doi: 10.1016/0378-1119(90)90216-e. [DOI] [PubMed] [Google Scholar]
- 138.Kimoto H, Taketo A. Initial stage of DNA-electrotransfer into E. coli cells. J. Biochem. 1997;122:237–242. doi: 10.1093/oxfordjournals.jbchem.a021734. [DOI] [PubMed] [Google Scholar]
- 139.King SR, Richardson JP. Role of homology and pathway specificity for recombination between plasmids and bacteriophage λ. Mol. Gen. Genet. 1986;204:141–147. doi: 10.1007/BF00330201. [DOI] [PubMed] [Google Scholar]
- 140.Kleckner N, Ross DG. recA-dependent genetic switch generated by transposon Tn10. J. Mol. Biol. 1980;144:215–221. doi: 10.1016/0022-2836(80)90033-9. [DOI] [PubMed] [Google Scholar]
- 141.Kline BC. A review of mini-F plasmid maintenance. Plasmid. 1985;14:1–16. doi: 10.1016/0147-619x(85)90027-7. [DOI] [PubMed] [Google Scholar]
- 142.Kobayashi I, Takahashi N. Double-stranded gap repair of DNA by gene conversion in Escherichia coli. Genetics. 1988;119:751–757. doi: 10.1093/genetics/119.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kofoid E, Bergthorsson U, Slechta ES, Roth JR. Formation of an F' plasmid by recombination between imperfectly repeated chromosomal Rep sequences: a closer look at an old friend (F'128 pro lac). J. Bacteriol. 2003;185:660–663. doi: 10.1128/JB.185.2.660-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kogoma T, Cadwell GW, Barnard KG, Asai T. The DNA replication priming protein, PriA, is required for homologous recombination and double-strand break repair. J. Bacteriol. 1996;178:1258–1264. doi: 10.1128/jb.178.5.1258-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kolodner R. Genetic recombination of bacterial plasmid DNA: electron microscopic analysis of in vitro intramolecular recombination. Proc. Natl. Acad. Sci. USA. 1980;77:4847–4851. doi: 10.1073/pnas.77.8.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kolodner R, Fishel RA, Howard M. Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J. Bacteriol. 1985;163:1060–1066. doi: 10.1128/jb.163.3.1060-1066.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Konrad EB. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J. Bacteriol. 1977;130:167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kowalczykowski SC, Dixon DA, Eggleston AK, Lauder SD, Rehrauer WM. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kowalczykowski SC, Krupp RA. Effects of Escherichia coli SSB protein on the single-stranded DNA dependent ATPase activity of Escherichia coli RecA protein. Evidence that SSB protein facilitates the binding of RecA protein to regions of secondary structure within single-stranded DNA. J. Mol. Biol. 1987;193:97–113. doi: 10.1016/0022-2836(87)90630-9. [DOI] [PubMed] [Google Scholar]
- 150.Krasin F, Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J. Mol. Biol. 1977;116:81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- 151.Kreuzer KN. A bacteriophage model system for studying topoisomerase inhibitors. Adv. Pharmacol. 1994;29B:171–186. doi: 10.1016/s1054-3589(08)61137-0. [DOI] [PubMed] [Google Scholar]
- 152.Kreuzer KN. Interplay between DNA replication and recombination in prokaryotes. Annu. Rev. Microbiol. 2005;59:43–67. doi: 10.1146/annurev.micro.59.030804.121255. [DOI] [PubMed] [Google Scholar]
- 153.Kreuzer KN. Recombination-dependent DNA replication in phage T4. Trends Biochem. Sci. 2000;25:165–173. doi: 10.1016/s0968-0004(00)01559-0. [DOI] [PubMed] [Google Scholar]
- 154.Kreuzer KN, Alberts BM. Characterization of a defective phage system for the analysis of bacteriophage T4 DNA replication origins. J. Mol. Biol. 1986;188:185–198. doi: 10.1016/0022-2836(86)90303-7. [DOI] [PubMed] [Google Scholar]
- 155.Kreuzer KN, Saunders M, Weislo LJ, Kreuzer HWE. Recombination-dependent DNA replication stimulated by double-strand breaks in bacteriophage T4. J. Bacteriol. 1995;177:6844–6853. doi: 10.1128/jb.177.23.6844-6853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kreuzer KN, Yap WY, Menkens AE, Engman HW. Recombination-dependent replication of plasmids during bacteriophage T4 infection. J. Biol. Chem. 1988;263:11366–11373. [PubMed] [Google Scholar]
- 157.Kurth I, O'Donnell M. Module 4.4.2. Replisome Dynamics during Chromosome Duplication. In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D, editors. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; Washington, DC: 2009. http://www.ecosal.org. [Google Scholar]
- 158.Kuzminov A. A mechanism for induction of the SOS response in E. coli: insights into the regulation of reversible protein polymerization in vivo. J. Theor. Biol. 1995;177:29–43. doi: 10.1006/jtbi.1995.0222. [DOI] [PubMed] [Google Scholar]
- 159.Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol. Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 160.Kuzminov A. DNA replication meets genetic exchange: chromosomal damage and its repair by homologous recombination. Proc. Natl. Acad. Sci. USA. 2001;98:8461–8468. doi: 10.1073/pnas.151260698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kuzminov A. Instability of inhibited replication forks in E. coli. BioEssays. 1995;17:733–741. doi: 10.1002/bies.950170810. [DOI] [PubMed] [Google Scholar]
- 162.Kuzminov A. Mutant fixation via plasmid dimerization and its relation to human diseases. TIG. 1996;12:246–249. doi: 10.1016/0168-9525(96)30058-9. [DOI] [PubMed] [Google Scholar]
- 163.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuzminov A. RuvA, RuvB and RuvC proteins: cleaning-up after recombinational repairs in E. coli. BioEssays. 1993;15:355–358. doi: 10.1002/bies.950150511. [DOI] [PubMed] [Google Scholar]
- 165.Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc. Natl. Acad. Sci. USA. 2001;98:8241–8246. doi: 10.1073/pnas.131009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuzminov A. Unraveling the late stages of recombinational repair: metabolism of DNA junctions in Escherichia coli. BioEssays. 1996;18:757–765. doi: 10.1002/bies.950180911. [DOI] [PubMed] [Google Scholar]
- 167.Kuzminov A, Stahl FW. Double-strand end repair via the RecBC pathway in Escherichia coli primes DNA replication. Genes Dev. 1999;13:345–356. doi: 10.1101/gad.13.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kuzminov A, Stahl FW. Overview of homologous recombination and repair machines. In: Higgins NP, editor. The Bacterial Chromosome. ASM Press; Washington, D.C.: 2005. pp. 349–367. [Google Scholar]
- 169.Laban A, Cohen A. Interplasmidic and intraplasmidic recombination in Escherichia coli K-12. Mol. Gen. Genet. 1981;184:200–207. doi: 10.1007/BF00272905. [DOI] [PubMed] [Google Scholar]
- 170.Laban A, Silberstein Z, Cohen A. The effect of nonhomologous DNA sequences on interplasmidic recombination. Genetics. 1984;108:39–52. doi: 10.1093/genetics/108.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lane HE. Replication and incompatibility of F and plasmids in the IncFI group. Plasmid. 1981;5:100–126. doi: 10.1016/0147-619x(81)90079-2. [DOI] [PubMed] [Google Scholar]
- 172.Lavery PE, Kowalczykowski SC. Properties of RecA441 protein-catalyzed DNA strand exchange can be attributed to an enhanced ability to compete with SSB protein. J. Biol. Chem. 1990;265:4004–4010. [PubMed] [Google Scholar]
- 173.Lee CA, Saier MH. Use of cloned mtl genes of Escherichia coli to introduce mtl deletion mutations into the chromosome. J. Bacteriol. 1983;153:685–692. doi: 10.1128/jb.153.2.685-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li S, Waters R. Escherichia coli strains lacking protein HU are UV sensitive due to a role of HU in homologous recombination. J. Bacteriol. 1998;180:3750–3756. doi: 10.1128/jb.180.15.3750-3756.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liao D. Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J. Mol. Evol. 2000;51:305–317. doi: 10.1007/s002390010093. [DOI] [PubMed] [Google Scholar]
- 176.Liljeström P, Pirhonen M, Palva ET. In vivo transfer of chromosomal mutations onto multicopy plasmids by transduction with bacteriophage P1. Gene. 1985;40:241–246. doi: 10.1016/0378-1119(85)90046-0. [DOI] [PubMed] [Google Scholar]
- 177.Lin P-F, Bardwell E, Howard-Flanders P. Initiation of genetic exchanges in λ phage-prophage crosses. Proc. Natl. Acad. Sci. USA. 1977;74:291–295. doi: 10.1073/pnas.74.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin P-F, Howard-Flanders P. Genetic exchanges caused by ultraviolet photoproducts in phage λ DNA molecules: the role of DNA replication. Mol. Gen. Genet. 1976;146:107–115. doi: 10.1007/BF00268079. [DOI] [PubMed] [Google Scholar]
- 179.Lin RJ, Capage M, Hill CW. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J. Mol. Biol. 1984;177:1–18. doi: 10.1016/0022-2836(84)90054-8. [DOI] [PubMed] [Google Scholar]
- 180.Lindsley JE, Cox MM. Dissociation pathway for RecA nucleoprotein filaments formed on linear duplex DNA. J. Mol. Biol. 1989;205:695–711. doi: 10.1016/0022-2836(89)90315-x. [DOI] [PubMed] [Google Scholar]
- 181.Little JW, Edmiston SH, Pacelli LZ, Mount DW. Cleavage of the Escherichia coli LexA protein by the RecA protease. Proc. Natl. Acad. Sci. USA. 1980;77:3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Little JW, Mount DW. The SOS regulatory system of Escherichia coli. Cell. 1982;29:11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- 183.Liu SL, Sanderson KE. Highly plastic chromosomal organization in Salmonella typhi. Proc. Natl. Acad. Sci. USA. 1996;93:10303–10308. doi: 10.1073/pnas.93.19.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lloyd RG. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J. Bacteriol. 1991;173:5414–5418. doi: 10.1128/jb.173.17.5414-5418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lloyd RG. lexA dependent recombination in uvrD strains of Escherichia coli. Mol. Gen. Genet. 1983;189:157–161. doi: 10.1007/BF00326069. [DOI] [PubMed] [Google Scholar]
- 186.Lloyd RG, Benson FE, Shurvinton CE. Effect of ruv mutations on recombination and DNA repair in Escherichia coli K12. Mol. Gen. Genet. 1984;194:303–309. doi: 10.1007/BF00383532. [DOI] [PubMed] [Google Scholar]
- 187.Lloyd RG, Buckman C. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J. Bacteriol. 1991;173:1004–1011. doi: 10.1128/jb.173.3.1004-1011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lloyd RG, Buckman C. Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J. Bacteriol. 1985;164:836–844. doi: 10.1128/jb.164.2.836-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lloyd RG, Buckman C. Overlapping functions of recD, recJ and recN provide evidence of three epistatic groups of genes in Escherichia coli recombination and DNA repair. Biochimie. 1991;73:313–320. doi: 10.1016/0300-9084(91)90218-p. [DOI] [PubMed] [Google Scholar]
- 190.Lloyd RG, Buckman C, Benson FE. Genetic analysis of conjugational recombination in Escherichia coli K12 strains deficient in RecBCD enzyme. J. Gen. Microbiol. 1987;133:2531–2538. doi: 10.1099/00221287-133-9-2531. [DOI] [PubMed] [Google Scholar]
- 191.Lloyd RG, Evans NP, Buckman C. Formation of recombinant lacZ+ DNA in conjugational crosses with a recB mutant of Escherichia coli K12 depends on recF, recJ and recO. Mol. Gen. Genet. 1987;209:135–141. doi: 10.1007/BF00329848. [DOI] [PubMed] [Google Scholar]
- 192.Lloyd RG, Porton MC, Buckman C. Effect of recF, recJ, recN, recO and ruv mutations on ultraviolet survival and genetic recombination in a recD strain of Escherichia coli K12. Mol. Gen. Genet. 1988;212:317–324. doi: 10.1007/BF00334702. [DOI] [PubMed] [Google Scholar]
- 193.Lloyd RG, Thomas A. A molecular model for conjugational recombination in Escherichia coli K12. Mol. Gen. Genet. 1984;197:328–336. doi: 10.1007/BF00330981. [DOI] [PubMed] [Google Scholar]
- 194.Lloyd RG, Thomas A. On the nature of the RecBC and RecF pathways of conjugal recombination in Escherichia coli. Mol. Gen. Genet. 1983;190:156–161. doi: 10.1007/BF00330339. [DOI] [PubMed] [Google Scholar]
- 195.Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lotan D, Yagil E, Bracha M. Bacterial conjugation: an analysis of mixed recombinant clones. Genetics. 1972;72:381–391. doi: 10.1093/genetics/72.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Louarn JM, Bouché JP, Legendre F, Louarn J, Patte J. Characterization and properties of very large inversions of the E. coli chromosome along the origin-to-terminus axis. Mol. Gen. Genet. 1985;201:467–476. doi: 10.1007/BF00331341. [DOI] [PubMed] [Google Scholar]
- 198.Lovett ST, Clark AJ. Genetic analysis of the recJ gene of Escherichia coli K-12. J. Bacteriol. 1984;157:190–196. doi: 10.1128/jb.157.1.190-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lovett ST, Drapkin PT, Sutera VA, Gluckman-Peskind TJ. A sister-strand exchange mechanism for recA-independent deletion of repeated DNA sequences in Escherichia coli. Genetics. 1993;135:631–642. doi: 10.1093/genetics/135.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lovett ST, Hurley RL, Sutera VAJ, Aubuchon RH, Lebedeva MA. Crossing over between regions of limited homology in Escherichia coli. RecA-dependent and RecA-independent pathways. Genetics. 2002;160:851–859. doi: 10.1093/genetics/160.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Low KB. Escherichia coli K-12 F-prime factors, old and new. Bacteriol. Rev. 1972;36:587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Low KB, Porter DD. Modes of gene transfer and recombination in bacteria. Annu. Rev. Genet. 1978;12:249–287. doi: 10.1146/annurev.ge.12.120178.001341. [DOI] [PubMed] [Google Scholar]
- 203.Luisi-DeLuca C, Porter RD, Taylor WD. Stimulation of recombination between homologous sequences on plasmid DNA and chromosomal DNA in Escherichia coli by N-acetoxy-2-acetylaminofluorene. Proc. Natl. Acad. Sci. USA. 1984;81:2831–2835. doi: 10.1073/pnas.81.9.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lukas L, Kuzminov A. Chromosomal fragmentation is the major consequence of the rdgB defect in Escherichia coli. Genetics. 2006;172:1359–1362. doi: 10.1534/genetics.105.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Luria SE. Reactivation of irradiated bacteriophage by transfer of self-reproducing units. Proc. Natl. Acad. Sci. USA. 1947;33:253–264. doi: 10.1073/pnas.33.9.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lyu YL, Lin CT, Liu LF. Inversion/dimerization of plasmids mediated by inverted repeats. J. Mol. Biol. 1999;285:1485–1501. doi: 10.1006/jmbi.1998.2419. [DOI] [PubMed] [Google Scholar]
- 207.Mahajan SK. Pathways of homologous recombination in Escherichia coli. In: Kucherlapati R, Smith GR, editors. Genetic Recombination. American Society for Microbiology; Washington, D.C.: 1988. pp. 87–140. [Google Scholar]
- 208.Mahan MJ, Roth JR. Role of recBC function in formation of chromosomal rearrangements: a two-step model for recombination. Genetics. 1989;121:433–443. doi: 10.1093/genetics/121.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mahdi AA, Lloyd RG. Identification of the recR locus of Escherichia coli K-12 and analysis of its role in recombination and DNA repair. Mol. Gen. Genet. 1989;216:503–510. doi: 10.1007/BF00334397. [DOI] [PubMed] [Google Scholar]
- 210.Mahdi AA, Lloyd RG. The recR locus of Escherichia coli K-12: molecular cloning, DNA sequencing and identification of the gene product. Nucleic Acids Res. 1989;17:6781–6794. doi: 10.1093/nar/17.17.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mahdi AA, Sharples GJ, Mandal TN, Lloyd RG. Holliday junction resolvases encoded by homologous rusA genes in Escherichia coli K-12 and phage 82. J. Mol. Biol. 1996;257:561–573. doi: 10.1006/jmbi.1996.0185. [DOI] [PubMed] [Google Scholar]
- 212.Malagón F, Aguilera A. Genetic stability and DNA rearrangements associated with a 2 × 1.1-Kb perfect palindrome in Escherichia coli. Mol. Gen. Genet. 1998;259:639–644. doi: 10.1007/s004380050858. [DOI] [PubMed] [Google Scholar]
- 213.Mandal TN, Mahdi AA, Sharples GJ, Lloyd RG. Resolution of Holliday intermediates in recombination and DNA repair: indirect suppression of ruvA, ruvB, and ruvC mutations. J. Bacteriol. 1993;175:4325–4334. doi: 10.1128/jb.175.14.4325-4334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mandel M, Higa A. Calcium-dependent bacteriophage DNA infection. J. Mol. Biol. 1970;53:159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- 215.Mao Y-M, Shi Q, Li Q-G, Shen Z-J. recA gene dependence of replication of the Escherichia coli chromosome initiated by plasmid pUC13 integrated at predetermined sites. Mol. Gen. Genet. 1991;225:234–240. doi: 10.1007/BF00269854. [DOI] [PubMed] [Google Scholar]
- 216.Martignoni KD. Inhibition of x-ray-induced protection of Escherichia coli K-12 cells against the lethal effects of ultra-violet light by nitrofurantoin. Int. J. Radiat. Biol. 1978;33:577–585. doi: 10.1080/09553007814550491. [DOI] [PubMed] [Google Scholar]
- 217.Martin RR, Thorlton CL, Unger L. Formation of Escherichia coli Hfr strains by integrative suppression with the P group plasmid RP1. J. Bacteriol. 1981;145:713–721. doi: 10.1128/jb.145.2.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Masters M. Generalized transduction. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2421–2441. [Google Scholar]
- 219.Matsubara K. Replication control system in lambda dv. Plasmid. 1981;5:32–52. doi: 10.1016/0147-619x(81)90076-7. [DOI] [PubMed] [Google Scholar]
- 220.Mattson T, Van Houwe G, Bolle A, Epstein R. Fate of cloned bacteriophage T4 DNA after phage T4 infection of clone-bearing cells. J. Mol. Biol. 1983;170:343–355. doi: 10.1016/s0022-2836(83)80152-1. [DOI] [PubMed] [Google Scholar]
- 221.Mattson T, Van Houwe G, Epstein R. Recombination between bacteriophage T4 and plasmid pBR322 molecules containing cloned T4 DNA. J. Mol. Biol. 1983;170:357–379. doi: 10.1016/s0022-2836(83)80153-3. [DOI] [PubMed] [Google Scholar]
- 222.Mazin AV, Kuzminov AV, Dianov GL, Salganik RI. Mechanisms of deletion formation in Escherichia coli plasmids. II. Deletions mediated by short direct repeats. Mol. Gen. Genet. 1991;228:209–214. doi: 10.1007/BF00282467. [DOI] [PubMed] [Google Scholar]
- 223.Mazin AV, Timchenko TV, Saparbaev MK, Mazina OM. Dimerization of plasmid DNA accelerates selection for antibiotic resistance. Mol. Microbiol. 1996;20:101–108. doi: 10.1111/j.1365-2958.1996.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 224.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 225.McDaniel LS, Rogers LH, Hill WE. Survival of recombination-deficient mutants of Escherichia coli during incubation with nalidixic acid. J. Bacteriol. 1978;134:1195–1198. doi: 10.1128/jb.134.3.1195-1198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McEntee K, Weinstock GM, Lehman IR. Initiation of general recombination catalyzed in vitro by the RecA protein of Escherichia coli. Proc. Nat. Acad. Sci. U.S.A. 1979;76:2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.McGavin S. Models of specifically paired like (homologous) nucleic acid structures. J. Mol. Biol. 1971;55:293–298. doi: 10.1016/0022-2836(71)90201-4. [DOI] [PubMed] [Google Scholar]
- 228.McGlynn P, Lloyd RG. Genome stability and the processing of damaged replication forks by RecG. Trends Genet. 2002;18:413–419. doi: 10.1016/s0168-9525(02)02720-8. [DOI] [PubMed] [Google Scholar]
- 229.McInerney P, Johnson A, Katz F, O'Donnell M. Characterization of a triple DNA polymerase replisome. Mol. Cell. 2007;27:527–538. doi: 10.1016/j.molcel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 230.McPartland A, Green L, Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980;20:731–737. doi: 10.1016/0092-8674(80)90319-0. [DOI] [PubMed] [Google Scholar]
- 231.Mendonca VM, Klepin HD, Matson SW. DNA helicases in recombination and repair: construction of a ΔuvrD ΔhelD ΔrecQ mutant deficient in recombination and repair. J. Bacteriol. 1995;177:1326–1335. doi: 10.1128/jb.177.5.1326-1335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Meselson M, Weigle JJ. Chromosome breakage accompanying genetic recombination in bacteriophage. Proc. Natl. Acad. Sci. USA. 1961;47:857–868. doi: 10.1073/pnas.47.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Michel B. Replication fork arrest and DNA recombination. Trends Biochem. Sci. 2000;25:173–178. doi: 10.1016/s0968-0004(00)01560-7. [DOI] [PubMed] [Google Scholar]
- 234.Michel B, Boubakri H, Baharoglu Z, LeMasson M, Lestini R. Recombination proteins and rescue of arrested replication forks. DNA Repair. 2007;6:967–980. doi: 10.1016/j.dnarep.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 235.Michel B, Recchia GD, Penel-Colin M, Ehrlich SD, Sherratt DJ. Resolution of Holliday junctions by RuvABC prevents dimer formation in rep mutants and UV-irradiated cells. Mol. Microbiol. 2000;37:180–191. doi: 10.1046/j.1365-2958.2000.01989.x. [DOI] [PubMed] [Google Scholar]
- 236.Miesel L, Roth JR. Salmonella recD mutations increase recombination in a short sequence transduction assay. J. Bacteriol. 1994;176:4092–4103. doi: 10.1128/jb.176.13.4092-4103.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miller JF. Bacterial transformation by electroporation. Methods Enzymol. 1994;235:375–385. doi: 10.1016/0076-6879(94)35156-2. [DOI] [PubMed] [Google Scholar]
- 238.Morel P, Hejna JA, Ehrlich SD, Cassuto E. Antipairing and strand transferase activities of E. coli helicase II (UvrD). Nucleic Acids Res. 1993;21:3205–3209. doi: 10.1093/nar/21.14.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Morimatsu K, Kowalczykowski SC. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell. 2003;11:1337–1347. doi: 10.1016/s1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 240.Mosig G. Homologous recombination. In: Karam JD, editor. Molecular Biology of Bacteriophage T4. American Society for Microbiology; Washington, D.C.: 1994. pp. 54–82. [Google Scholar]
- 241.Mosig G. The essential role of recombination in phage T4 growth. Annu. Rev. Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- 242.Mudgett JS, Buckholt M, Taylor WD. Ultraviolet light-induced plasmid-chromosome recombination in Escherichia coli: the role of recB and recF. Gene. 1991;97:131–136. doi: 10.1016/0378-1119(91)90020-c. [DOI] [PubMed] [Google Scholar]
- 243.Munekiyo R, Sekiguchi M. Recombination of bacteriophage phi X174 by the red function of bacteriophage lambda. J. Virol. 1979;29:438–442. doi: 10.1128/jvi.29.2.438-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Murphy KC, Campellone KG, Poteete AR. PCR-mediated gene replacement in Escherichia coli. Gene. 2000;246:321–330. doi: 10.1016/s0378-1119(00)00071-8. [DOI] [PubMed] [Google Scholar]
- 245.Myers RS, Stahl FW. χ and the RecBCD enzyme of Escherichia coli. Annu. Rev. Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- 246.Nakayama H, Nakayama K, Nakayama R, Irino N, Nakayama Y, Hanawalt PC. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol. Gen. Genet. 1984;195:474–480. doi: 10.1007/BF00341449. [DOI] [PubMed] [Google Scholar]
- 247.Nishimura Y, Caro L, Berg CM, Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J. Mol. Biol. 1971;55:441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- 248.Noguchi T, Takahashi H. Transactivation of a plasmid-borne bacteriophage T4 late gene. Mol. Gen. Genet. 1993;239:393–399. doi: 10.1007/BF00276937. [DOI] [PubMed] [Google Scholar]
- 249.Okazaki R, Okazaki T, Sakabe K, Sugimoto K, Kainuma R, Sugino A, Iwatsuki N. In vivo mechanism of DNA chain growth. Cold Spring Harbor Symp. Quant. Biol. 1968;33:129–143. [Google Scholar]
- 250.Otsuji N, Iyehara H, Hideshima Y. Isolation and characterization of Escherichia coli ruv mutant which forms nonseptate filaments after low doses of ultraviolet light irradiation. J. Bacteriol. 1974;117:337–344. doi: 10.1128/jb.117.2.337-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ou JT. Recombinant clone heterogeneity in Escherichia coli conjugation: effect of pH and partially replicated recipient deoxyribonucleic acid. Genetics. 1975;80:401–419. doi: 10.1093/genetics/80.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Panja S, Aich P, Jana B, Basu T. Plasmid DNA binds to the core oligosaccharide domain of LPS molecules of E. coli cell surface in the CaCl2-mediated transformation process. Biomacromolecules. 2008;9:2501–2509. doi: 10.1021/bm8005215. [DOI] [PubMed] [Google Scholar]
- 253.Picksley SM, Attfield PV, Lloyd RG. Repair of DNA double-strand breaks in Escherichia coli K12 requires a functional recN product. Mol. Gen. Genet. 1984;195:267–274. doi: 10.1007/BF00332758. [DOI] [PubMed] [Google Scholar]
- 254.Pittard J, Ramakrishnan T. Gene transfer by F' strains of Escherichia coli. IV. Effect of a chromosomal deletion on chromosome transfer. J. Bacteriol. 1964;88:367–373. doi: 10.1128/jb.88.2.367-373.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pollard EC, Fugate JKJ. Relative rates of repair of single-strand breaks and postirradiation DNA degradation in normal and induced cells of Escherichia coli. Biophys. J. 1978;24:429–437. doi: 10.1016/S0006-3495(78)85393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Poteete AR. Involvement of DNA replication in phage lambda Red-mediated homologous recombination. Mol. Microbiol. 2008;68:66–74. doi: 10.1111/j.1365-2958.2008.06133.x. [DOI] [PubMed] [Google Scholar]
- 257.Poteete AR, Fenton AC. Efficient double-strand break-stimulated recombination promoted by the general recombination systems of phages ¬ and P22. Genetics. 1993;134:1013–1021. doi: 10.1093/genetics/134.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Potter H, Dressler D. DNA recombination: in vivo and in vitro studies. Cold Spring Harbor Symp. Quant. Biol. 1979;43:969–985. doi: 10.1101/sqb.1979.043.01.106. [DOI] [PubMed] [Google Scholar]
- 259.Potter H, Dressler D. On the mechanism of genetic recombination: electron microscopic observation of recombination intermediates. Proc. Natl. Acad. Sci. USA. 1976;73:3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Potter H, Dressler D. On the mechanism of genetic recombination: the maturation of recombination intermediates. Proc. Natl. Acad. Sci. USA. 1977;74:4168–4172. doi: 10.1073/pnas.74.10.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pugh JC, Ritchie DA. Formation of phage T1 concatemers by the RecE recombination pathway of Escherichia coli. Virology. 1984;135:200–206. doi: 10.1016/0042-6822(84)90130-2. [DOI] [PubMed] [Google Scholar]
- 262.Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. In: Hanawalt PC, Setlow RB, editors. Molecular Mechanisms for Repair of DNA. A. Plenum Press; New York: 1975. pp. 355–367. [DOI] [PubMed] [Google Scholar]
- 263.Register JC, III, Griffith J. The direction of RecA protein assembly onto single-strand DNA is the same as the direction of strand assimilation during strand exchange. J. Biol. Chem. 1985;260:12308–12312. [PubMed] [Google Scholar]
- 264.Roberts JJ. The repair of DNA modified by cytotoxic, mutagenic, and carcinogenic chemicals. Adv. Radiat. Biol. 1978;7:211–436. [Google Scholar]
- 265.Roca AI, Cox MM. RecA protein: structure, function, and role in recombinational DNA repair. Prog. Nucleic Acid Res. Mol. Biol. 1997;56:129–223. doi: 10.1016/s0079-6603(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 266.Roman L, Kowalczykowski SC. Characterization of the helicase activity of the Escherichia coli RecBCD enzyme using a novel helicase assay. Biochemistry. 1989;28:2863–2873. doi: 10.1021/bi00433a018. [DOI] [PubMed] [Google Scholar]
- 267.Ross P, Howard-Flanders P. Initiation of recA+-dependent recombination in Escherichia coli (λ). II. Specificity in the induction of recombination and strand cutting in undamaged covalent circular bacteriophage 186 and lambda DNA molecules in phage-infected cells. J. Mol. Biol. 1977;117:159–174. doi: 10.1016/0022-2836(77)90029-8. [DOI] [PubMed] [Google Scholar]
- 268.Roth JR, Benson N, Galitski T, Haack K, Lawrence JG, Miesel L. Rearrangements of the bacterial chromosome: formation and applications. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2256–2276. [Google Scholar]
- 269.Rothman RH, Kato T, Clark AJ. The beginning of an investigation of the role of recF in the pathways of metabolism of ultraviolet-irradiated DNA in Escherichia coli. In: Hanawalt PC, Setlow RB, editors. Molecular Mechanisms for Repair of DNA. A. Plenum Press; New York: 1975. pp. 283–291. [DOI] [PubMed] [Google Scholar]
- 270.Rudolph CJ, Mahdi AA, Upton AL, Lloyd RG. RecG protein and single-strand DNA exonucleases avoid cell lethality associated with PriA helicase activity in Escherichia coli. Genetics. 2010;186:473–492. doi: 10.1534/genetics.110.120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rudolph CJ, Upton AL, Briggs GS, Lloyd RG. Is RecG a general guardian of the bacterial genome? DNA Repair. 2010;9:210–223. doi: 10.1016/j.dnarep.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 272.Rupp WD, Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 1968;31:291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- 273.Rupp WD, Wilde CE, III, Reno DL, Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J. Mol. Biol. 1971;61:25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- 274.Rush MG, Warner RC. Multiple length rings of ϕX174 and S13 replicative forms. III. A possible intermediate in recombination. J. Biol. Chem. 1968;243:4821–4826. [PubMed] [Google Scholar]
- 275.Russell CB, Thaler DS, Dahlquist FW. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J. Bacteriol. 1989;171:2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Saarilahti HT, Palva ET. In vivo transfer of chromosomal mutations onto multicopy plasmids utilizing polA strains: cloning of an ompR2 mutation in Escherichia coli K-12. FEMS Microbiology Letters. 1985;26:27–33. [Google Scholar]
- 277.Salaj-Smic E, Dzidic S, Trgovcevic Z. The effect of a split UV dose on survival, division delay and mutagenesis in Escherichia coli. Mutat. Res. 1985;144:127–130. doi: 10.1016/0165-7992(85)90127-7. [DOI] [PubMed] [Google Scholar]
- 278.Sanderson KE, Liu SL. Chromosomal rearrangements in enteric bacteria. Electrophoresis. 1998;19:569–572. doi: 10.1002/elps.1150190417. [DOI] [PubMed] [Google Scholar]
- 279.Sandler SJ, Samra HS, Clark AJ. Differential suppression of priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA, and dnaC. Genetics. 1996;143:5–13. doi: 10.1093/genetics/143.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Santoyo G, Romero D. Gene conversion and concerted evolution in bacterial genomes. FEMS Microbiol. Rev. 2005;29:169–183. doi: 10.1016/j.femsre.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 281.Sargentini NJ, Smith KC. Quantitation of the involvement of the recA, recB, recC, recF, recJ, recN, lexA, radA, radB, uvrD, and umuC genes in the repair of X-ray-induced DNA double-strand breaks in Escherichia coli. Radiat. Res. 1986;107:58–72. [PubMed] [Google Scholar]
- 282.Sarkari JF, Mahajan SK. Detection of free cytoplasmic circles of transposon Tn9 multimers in Escherichia coli. Mol. Biol. Rep. 1990;14:223–229. doi: 10.1007/BF00429889. [DOI] [PubMed] [Google Scholar]
- 283.Sassanfar M, Roberts JW. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 1990;212:79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 284.Savageau MA. Escherichia coli habitats, cell types, and molecular mechanisms of gene control. Amer. Nat. 1983;122:732–744. [Google Scholar]
- 285.Saveson CJ, Lovett ST. Enhanced deletion formation by aberrant DNA replication in Escherichia coli. Genetics. 1997;146:457–470. doi: 10.1093/genetics/146.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Saveson CJ, Lovett ST. Tandem repeat recombination induced by replication fork defects in Escherichia coli requires a novel factor, RadC. Genetics. 1999;152:5–13. doi: 10.1093/genetics/152.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sawitzke JA, Thomason LC, Costantino N, Bubunenko M, Datta S, Court DL. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 2007;421:171–199. doi: 10.1016/S0076-6879(06)21015-2. [DOI] [PubMed] [Google Scholar]
- 288.Scaife J, Gross JD. The mechanism of chromosome mobilization by an F-prime factor in Escherichia coli K12. Genet. Res. 1963;4:328–331. [Google Scholar]
- 289.Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 290.Schofield MA, Agbunag R, Miller JH. DNA inversions between short inverted repeats in Escherichia coli. Genetics. 1992;132:295–302. doi: 10.1093/genetics/132.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Segall AM, Mahan J, Roth JR. Rearrangements of the bacterial chromosome: forbidden inversions. Science. 1988;241:1314–1318. doi: 10.1126/science.3045970. [DOI] [PubMed] [Google Scholar]
- 292.Shaner SL, Flory J, Radding CM. The distribution of Escherichia coli RecA protein bound to duplex DNA with single-stranded ends. J. Biol. Chem. 1987;262:9220–9230. [PubMed] [Google Scholar]
- 293.Sharples GJ, Benson FE, Illing GT, Lloyd RG. Molecular and functional analysis of the ruv region of Escherichia coli K-12 reveals three genes involved in DNA repair and recombination. Mol. Gen. Genet. 1990;221:219–226. doi: 10.1007/BF00261724. [DOI] [PubMed] [Google Scholar]
- 294.Sharples GJ, Chan SN, Mahdi AA, Whitby MC, Lloyd RG. Processing of intermediates in recombination and DNA repair: identification of a new endonuclease that specifically cleaves Holliday junctions. EMBO J. 1994;13:6133–6142. doi: 10.1002/j.1460-2075.1994.tb06960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shen P, Huang HV. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986;112:441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sherratt DJ, Arciszewska LK, Blakely G, Colloms S, Grant K, Leslie N, McCulloch R. Site-specific recombination and circular chromosome segregation. Phil. Trans. R. Soc. Lond. 1995;347:37–42. doi: 10.1098/rstb.1995.0006. [DOI] [PubMed] [Google Scholar]
- 297.Shibata T, DasGupta C, Cunningham RP, Radding CM. Purified Escherichia coli RecA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc. Nat. Acad. Sci. U.S.A. 1979;76:1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shinagawa H, Makino K, Amemura M, Kimura S, Iwasaki H, Nakata A. Structure and regulation of the Escherichia coli ruv operon involved in DNA repair and recombination. J. Bacteriol. 1988;170:4322–4329. doi: 10.1128/jb.170.9.4322-4329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shyamala V, Schneider E, Ames GF. Tandem chromosomal duplications: role of REP sequences in the recombination event at the join-point. EMBO J. 1990;9:939–946. doi: 10.1002/j.1460-2075.1990.tb08192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sinden RR, Cole RS. Repair of cross-linked DNA and survival of Escherichia coli treated with psoralen and light: effects of mutations influencing genetic recombination and DNA metabolism. J. Bacteriol. 1978;136:538–547. doi: 10.1128/jb.136.2.538-547.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Singer BS, Gold L, Gauss P, Doherty DH. Determination of the amount of homology required for recombination in bacteriophage T4. Cell. 1982;31:25–33. doi: 10.1016/0092-8674(82)90401-9. [DOI] [PubMed] [Google Scholar]
- 302.Smith GR. Conjugational recombination in E. coli: myths and mechanisms. Cell. 1991;64:19–27. doi: 10.1016/0092-8674(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 303.Smith GR. Homologous recombination in prokaryotes. Microbiol. Rev. 1988;52:1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Smith GR. Mechanism and control of homologous recombination in Escherichia coli. Annu. Rev. Genet. 1987;21:179–201. doi: 10.1146/annurev.ge.21.120187.001143. [DOI] [PubMed] [Google Scholar]
- 305.Smith KC, Meun DHC. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J. Mol. Biol. 1970;51:459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- 306.Smith RD, Miller RC. Replication and plasmid-bacteriophage recombination. I. Marker rescue analysis. Virology. 1981;115:223–236. doi: 10.1016/0042-6822(81)90106-9. [DOI] [PubMed] [Google Scholar]
- 307.Stahl FW, McMilin KD, Stahl MM, Crasemann JM, Lam S. The distribution of crossovers along unreplicated lambda bacteriophage chromosomes. Genetics. 1974;77:395–408. doi: 10.1093/genetics/77.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Stahl FW, McMilin KD, Stahl MM, Nozu Y. An enhancing role for DNA synthesis in formation of bacteriophage lambda recombinants. Proc. Natl. Acad. Sci. USA. 1972;69:3598–3601. doi: 10.1073/pnas.69.12.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Stahl MM, Thomason L, Poteete AR, Tarkowski T, Kuzminov A, Stahl FW. Annealing vs. invasion in phage λ recombination. Genetics. 1997;147:961–977. doi: 10.1093/genetics/147.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Steiner WW, Kuempel PL. Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli. Mol. Microbiol. 1998;27:257–268. doi: 10.1046/j.1365-2958.1998.00651.x. [DOI] [PubMed] [Google Scholar]
- 311.Steiner WW, Kuempel PL. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J. Bacteriol. 1998;180:6269–6275. doi: 10.1128/jb.180.23.6269-6275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sternberg NL, Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol. 1991;204:18–43. doi: 10.1016/0076-6879(91)04004-8. [DOI] [PubMed] [Google Scholar]
- 313.Stewart GJ, Carlson CA. The biology of natural transformation. Annu. Rev. Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- 314.Sugawara N, Haber JE. Repair of DNA double strand breaks: in vivo biochemistry. Methods Enzymol. 2006;408:416–429. doi: 10.1016/S0076-6879(06)08026-8. [DOI] [PubMed] [Google Scholar]
- 315.Summers DK, Beton CWH, Withers HL. Multicopy plasmid instability: the dimer catastrophe hypothesis. Mol. Microbiol. 1993;8:1031–1038. doi: 10.1111/j.1365-2958.1993.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 316.Taylor AF, Smith GR. Substrate specificity of the DNA unwinding activity of the RecBC enzyme of Escherichia coli. J. Mol. Biol. 1985;185:431–443. doi: 10.1016/0022-2836(85)90414-0. [DOI] [PubMed] [Google Scholar]
- 317.Templin A, Kushner SR, Clark AJ. Genetic analysis of mutations indirectly suppressing recB and recC mutations. Genetics. 1972;72:205–215. [PMC free article] [PubMed] [Google Scholar]
- 318.Tenenbaum L, Quinto I, Faelen M. The E. coli multitest: a set of strains to characterize diverse genotoxic effects. Mutat. Res. 1988;203:415–426. doi: 10.1016/0165-1161(88)90014-3. [DOI] [PubMed] [Google Scholar]
- 319.Thaler DS, Stahl FW. DNA double-chain breaks in recombination of phage l and of yeast. Annu. Rev. Genet. 1988;22:169–197. doi: 10.1146/annurev.ge.22.120188.001125. [DOI] [PubMed] [Google Scholar]
- 320.Thaler DS, Stahl MM, Stahl FW. Tests of the double-strand-break repair model for red-mediated recombination of phage lambda and plasmid lambda dv. Genetics. 1987;116:501–511. doi: 10.1093/genetics/116.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. 2007 doi: 10.1002/0471142727.mb0116s78. Chapter 1:Unit 1.16. [DOI] [PubMed] [Google Scholar]
- 322.Thoms B, Borchers I, Wackernagel W. Effects of single-strand DNases ExoI, RecJ, ExoVII, and SbcCD on homologous recombination of recBCD+ strains of Escherichia coli and roles of SbcB15 and XonA2 ExoI mutant enzymes. J. Bacteriol. 2008;190:179–192. doi: 10.1128/JB.01052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Thresher RJ, Christiansen G, Griffith JD. Assembly of presynaptic filaments. Factors affecting the assembly of RecA protein onto single-stranded DNA. J. Mol. Biol. 1988;201:101–113. doi: 10.1016/0022-2836(88)90442-1. [DOI] [PubMed] [Google Scholar]
- 324.Ting H, Kouzminova EA, Kuzminov A. Synthetic lethality with the dut defect in Escherichia coli reveals layers of DNA damage of increasing complexity due to uracil incorporation. J. Bacteriol. 2008;190:5841–5854. doi: 10.1128/JB.00711-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Tomizawa JI. Molecular mechanisms of genetic recombination in bacteriophage. I. Effect of KCN on genetic recombination of phage T4. J. Mol. Biol. 1964;8:508–515. doi: 10.1016/s0022-2836(64)80008-5. [DOI] [PubMed] [Google Scholar]
- 326.Tseng Y-C, Hung J-L, Wang T-CV. Involvement of the RecF pathway recombination genes in postreplication repair in UV-irradiated Escherichia coli cells. Mutat. Res. 1994;315:1–9. doi: 10.1016/0921-8777(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 327.Umeda M, Ohtsubo E. Mapping of insertion elements IS1, IS2 and IS3 on the Escherichia coli K-12 chromosome. Role of the insertion elements in formation of Hfrs and F' factors and in rearrangement of bacterial chromosomes. J. Mol. Biol. 1989;208:601–614. doi: 10.1016/0022-2836(89)90151-4. [DOI] [PubMed] [Google Scholar]
- 328.van de Putte P, Zwenk H, Rörsch A. Properties of four mutants of Escherichia coli defective in genetic recombination. Mutat. Res. 1966;3:381–392. doi: 10.1016/0027-5107(66)90048-0. [DOI] [PubMed] [Google Scholar]
- 329.Veaute X, Delmas S, Selva M, Jeusset J, Le Cam E, Matic I, Fabre F, Petit MA. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2005;24:180–189. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wackernagel W. An improved spheroplast assay for lambda-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972;48:94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- 331.Wackernagel W. Genetic transformation in E. coli: the inhibitory role of the RecBC DNase. Biochem. Biophys. Res. Commun. 1973;51:306–311. doi: 10.1016/0006-291x(73)91257-6. [DOI] [PubMed] [Google Scholar]
- 332.Wall JD, Harriman PD. Phage P1 mutants with altered transducing abilities for Escherichia coli. Virology. 1974;59:532–544. doi: 10.1016/0042-6822(74)90463-2. [DOI] [PubMed] [Google Scholar]
- 333.Wang YD, Zhao S, Hill CW. Rhs elements comprise three subfamilies which diverged prior to acquisition by Escherichia coli. J. Bacteriol. 1998;180 doi: 10.1128/jb.180.16.4102-4110.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 335.Waters VL. Conjugative transfer in the dissemination of beta-lactam and aminoglycoside resistance. Front. Biosci. 1999;4:D433–D456. doi: 10.2741/waters. [DOI] [PubMed] [Google Scholar]
- 336.Watt VM, Ingles CJ, Urdea MS, Rutter WJ. Homology requirements for recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1985;82:4768–4772. doi: 10.1073/pnas.82.14.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Weisberg RA. Specialized Transduction. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. ASM Press; Washington, D.C.: 1996. pp. 2442–2448. [Google Scholar]
- 338.Weisberg RA, Sternberg N. Transduction of recB− host is promoted by λ red+ function. In: Grell RF, editor. Mechanisms in Recombination. Plenum Press; New York: 1974. pp. 107–109. [Google Scholar]
- 339.West SC. Processing of recombination intermediates by the RuvABC proteins. Annu. Rev. Genet. 1997;31:213–244. doi: 10.1146/annurev.genet.31.1.213. [DOI] [PubMed] [Google Scholar]
- 340.Whitby MC, Ryder L, Lloyd RG. Reverse branch migration of Holliday junctions by RecG protein: a new mechanism for resolution of intermediates in recombination and DNA repair. Cell. 1993;75:341–350. doi: 10.1016/0092-8674(93)80075-p. [DOI] [PubMed] [Google Scholar]
- 341.Wilkins BM. Chromosome transfer from F-lac+ strains of Escherichia coli K-12 mutant at recA, recB, or recC. J. Bacteriol. 1969;98:599–604. doi: 10.1128/jb.98.2.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wilkins BM, Howard-Flanders P. The genetic properties of DNA transferred from ultraviolet-irradiated Hfr cells of Escherichia coli K-12 during mating. Genetics. 1968;60:243–255. doi: 10.1093/genetics/60.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Willetts N, Skurray R. The conjugation system of F-like plasmids. Annu. Rev. Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- 344.Willetts NS, Clark AJ. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J. Bacteriol. 1969;100:231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Willetts NS, Mount DW. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J. Bacteriol. 1969;100:923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Willis DK, Satin LH, Clark AJ. Mutation-dependent suppression of recB21 recC22 by a region cloned from the Rac prophage of Escherichia coli K-12. J. Bacteriol. 1985;162:1166–1172. doi: 10.1128/jb.162.3.1166-1172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wilson JH. Nick-free formation of reciprocal heteroduplexes: a simple solution to the topological problem. Proc. Natl. Acad. Sci. USA. 1979;76:3641–3645. doi: 10.1073/pnas.76.8.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wood TH. Effects of temperature, agitation, and donor strain on chromosome transfer in Escherichia coli K-12. J. Bacteriol. 1968;96:2077–2084. doi: 10.1128/jb.96.6.2077-2084.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wood TH. Genetic recombination in Escherichia coli: clone heterogeneity and the kinetics of segregation. Science. 1967;157:319–321. doi: 10.1126/science.157.3786.319. [DOI] [PubMed] [Google Scholar]
- 350.Wood TH, Walmsley RH. Conjugation in Escherichia coli K-12 and its modification by irradiation. Biophys. J. 1969;9:391–420. doi: 10.1016/S0006-3495(69)86393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yamaguchi K, Tomizawa J. Establishment of Escherichia coli cells with an integrated high copy number plasmid. Mol. Gen. Genet. 1980;178:525–533. doi: 10.1007/BF00337857. [DOI] [PubMed] [Google Scholar]
- 352.Yamamoto K, Yoshikura H, Takahashi N, Kobayashi I. Apparent gene conversion in an Escherichia coli rec+ strain is explained by multiple rounds of reciprocal crossing-over. Mol. Gen. Genet. 1988;212:393–404. doi: 10.1007/BF00330842. [DOI] [PubMed] [Google Scholar]
- 353.Yao NY, O'Donnell M. Replisome dynamics and use of DNA trombone loops to bypass replication blocks. Mol. Biosyst. 2008;4:1075–1084. doi: 10.1039/b811097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yarmolinsky MB, Stevens E. Replication-control functions block the induction of an SOS response by a damaged P1 bacteriophage. Mol. Gen. Genet. 1983;192:140–148. doi: 10.1007/BF00327659. [DOI] [PubMed] [Google Scholar]
- 355.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zaman MM, Boles TC. Chi-dependent formation of linear plasmid DNA in exonuclease-deficient recBCD+ strains of Escherichia coli. J. Bacteriol. 1994;176:5093–5100. doi: 10.1128/jb.176.16.5093-5100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhang J, Mahdi AA, Briggs GS, Lloyd RG. Promoting and avoiding recombination: contrasting activities of the Escherichia coli RuvABC Holliday junction resolvase and RecG DNA translocase. Genetics. 2010;185:23–37. doi: 10.1534/genetics.110.114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 359.Zhang Y, Muyrers JP, Rientjes J, Stewart AF. Phage annealing proteins promote oligonucleotide-directed mutagenesis in Escherichia coli and mouse ES cells. BMC Mol. Biol. 2003;4 doi: 10.1186/1471-2199-4-1. online doi: 10.1186/1471-2199-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zieg J, Kushner SR. Analysis of genetic recombination between two partially deleted lactose operons of Escherichia coli K12. J. Bacteriol. 1977;131:123–132. doi: 10.1128/jb.131.1.123-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zieg J, Maples VF, Kushner SR. Recombination levels of Escherichia coli K-12 mutants deficient in various replication, recombination, or repair genes. J. Bacteriol. 1978;134:958–966. doi: 10.1128/jb.134.3.958-966.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


