Abstract
Aims/hypothesis
Children participating in longitudinal type 1 diabetes prediction studies were reported to have less severe disease at diabetes diagnosis. Our aim was to investigate children who from birth participated in the Diabetes Prediction in Skåne (DiPiS) study for metabolic status at diagnosis and then continued to be followed for two years of regular clinical care.
Methods
Children, followed in DiPiS before diagnosis, were compared to children in the same birth cohort who did not participate in follow-up. Metabolic status, symptoms at diagnosis as well as HbA1c and doses of insulin at 3, 6, 12 and 24 months after diagnosis were compared.
Results
Children, followed in DiPiS and diagnosed at 2–12 years of age, had 0.8% (9 mmol/mol) lower HbA1c at diagnosis than those who were not followed (p=0.006). At diagnosis, fewer DiPiS children had symptoms (p=0.014) and ketoacidosis at diagnosis were reduced (2% compared to 18%, p=0.005). During regular clinical care, HbA1c levels for the DiPiS children remained lower both at 12 (0.4% (4 mmol/mol); p=0.009) and 24 months (0.8% (9 mmol/mol) p <0.001) after diagnosis, despite no difference in total daily insulin between the two groups.
Conclusions
Participation in prospective follow-up before diagnosis of type 1 diabetes leads to earlier diagnosis with fewer symptoms, decreased incidence of ketoacidosis as well as better metabolic control up to two years after diagnosis. Our data indicate that metabolic control at the time of diabetes diagnosis is important for early metabolic control possibly affecting the risk of long-term complications.
Keywords: Type 1 Diabetes, HbA1c, Diabetic Ketoacidosis, Follow-up studies
Introduction
The triggering event for islet autoimmunity eventually resulting in the clinical onset of type 1 diabetes, is still unknown. This is true for both the initial insult of the autoimmune process but also for factors governing the time it takes for autoimmunity to lead to clinical disease. Prediction of who will develop the disease and when is critical for attempts to stall and perhaps to stop the disease process. The human leukocyte antigen (HLA) DQ locus is presumed to account for 50% of the genetic risk of type 1 diabetes (1). More than 40 additional non-HLA risk genes have been identified in recent genome wide association studies (2). Islet autoantibodies are used to estimate the risk for diabetes and it is well established that the risk is increasing with an increasing number of autoantibodies (3).
Children born with increased genetic risk for type 1 diabetes have been followed in several prospective longitudinal studies. The focus has been the development and maintenance of the beta-cell autoimmune process. Only a few studies have been published on the status of the participants both at the time of diagnosis of diabetes and during follow-up after diagnosis. Participants in longitudinal studies have been shown to have fewer metabolic abnormalities at diagnosis and a lower frequency of diabetic ketoacidosis (4–6) as well as having a milder clinical course in the first year after diagnosis (7). This may be due to intense follow-up with measures of plasma glucose, HbA1c or glucose tolerance tests. However, the parent´s knowledge of the risk of disease may also result in an earlier diagnosis through increased vigilance regarding symptoms.
DiPiS is a prospective study on type 1 diabetes prediction in Sweden. The aim of this study was to investigate the disease at diagnosis and the metabolic control during two years of regular clinical care after diagnosis, after participation in the DiPiS study (8). We compared the DiPiS children with children born during the same years who developed diabetes outside of the DiPiS study.
Methods
Participants
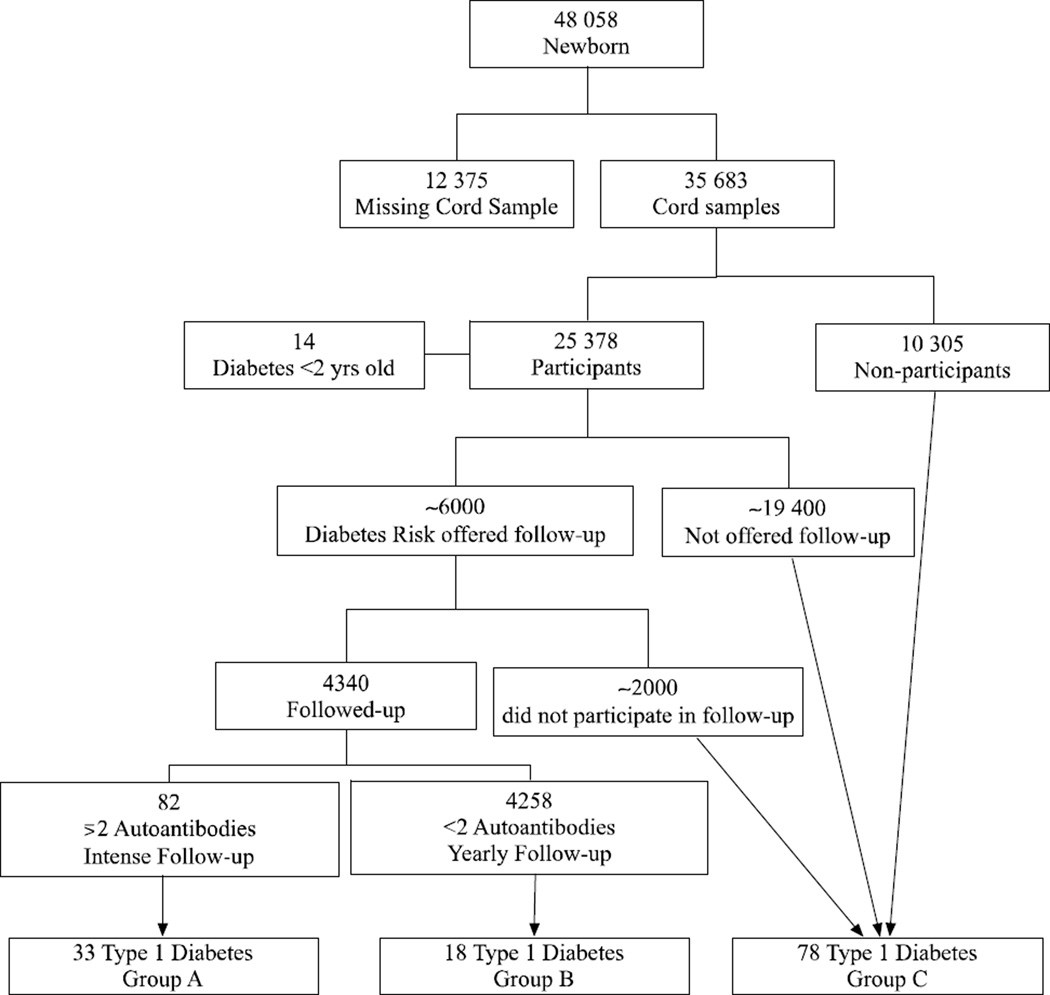
In the Diabetes Prediction in Skåne (DiPiS) study, 2500 children with genetic risk for type 1 diabetes in southern Sweden are prospectively studied in a 15-year longitudinal investigation (9). Between September 2000 and August 2004 approximately 48 000 children were born in the five participating hospitals and after oral consent from the mother, 35 683 of those children had umbilical cord blood samples collected, for HLA genotyping (Figure 1). When the child was two months old, the parents were invited to participate in DiPiS by giving written consent and answering questionnaires regarding, among other things, pregnancy, socioeconomic factors, birth weight and length, hereditary factors and stressors during pregnancy. A total of 25 378 questionnaires (71%) were returned by the parents of the study participants of which 92% wanted to participate.
Figure 1.
Study design and selection of participants.
A risk score was developed based on HLA risk genotype for type 1 diabetes, presence of maternal infections during pregnancy, maternal diabetes, cord blood autoantibodies and high or low relative birth weight. Based upon this risk score approximately 6000 children who had answered the two months questionnaire were selected for annual follow-up. Only parents of the 3680 children who returned the 2-year questionnaire received HLA-based risk information. Participants are screened yearly, from the age of two years, with a questionnaire and blood sampling for islet autoantibody analysis. Those children who develop two or more autoantibodies are offered follow-up every three months by a pediatrician, with autoantibody sampling, random plasma glucose, HbA1c, growth parameters, questionnaire and OGTT annually.
Up to July 2013 a total of 143 of the children with cord blood samples taken at birth had developed type 1 diabetes. Of those, 14 children developed diabetes before two years of age and were therefore not given risk information or the possibility to participate in the follow-up. After exclusion of children who had developed other types of diabetes and those diagnosed before the age of two, 129 children remained in the following three subgroups (Figure 1): Group A: DiPiS children participating in intense follow-up. This group is comprised of children who had developed multiple autoantibodies and have participated in follow-up every 3 months (n=33).
Group B: DiPiS children who participated in annual autoantibody sampling and questionnaires. This group has answered the two-year questionnaire, have been invited to the DiPiS follow-up and have received information about the risk for type 1 diabetes (n=18).
Group C: Children with umbilical cord samples but who declined participation in follow up and risk information or where not invited to the study due to low HLA risk for type 1 diabetes. None of these children received information about type 1 diabetes risk (n=78).
The analysis in this study was made as a comparison between children in groups A and B together (Follow-up group (FU)) with group C (No Follow-Up group (NFU)) who were not involved in any kind of follow-up.
Compliance to follow-up in group A was good as only 15% (n=12) of the autoantibody positive subjects have dropped out. The dropout rate is variable, as some of the dropout children have opted to return to a yearly follow-up. Group B has 2160 subjects in active follow-up out of the original 4258, representing a dropout rate of 49%. Group A and B children who stopped follow-up have not been included in the NFU group and most importantly none of the children in the NFU group have received any information on HLA-DQ related risk for type 1 diabetes. Group A children who switched to annual follow-up has been included in group B.
A total of 32 participants have not reached 24 months of clinical follow-up at the present time: 8 in group A, 6 in group B and 18 in group C. The exact number of samples available for analysis are indicated in figure 2.
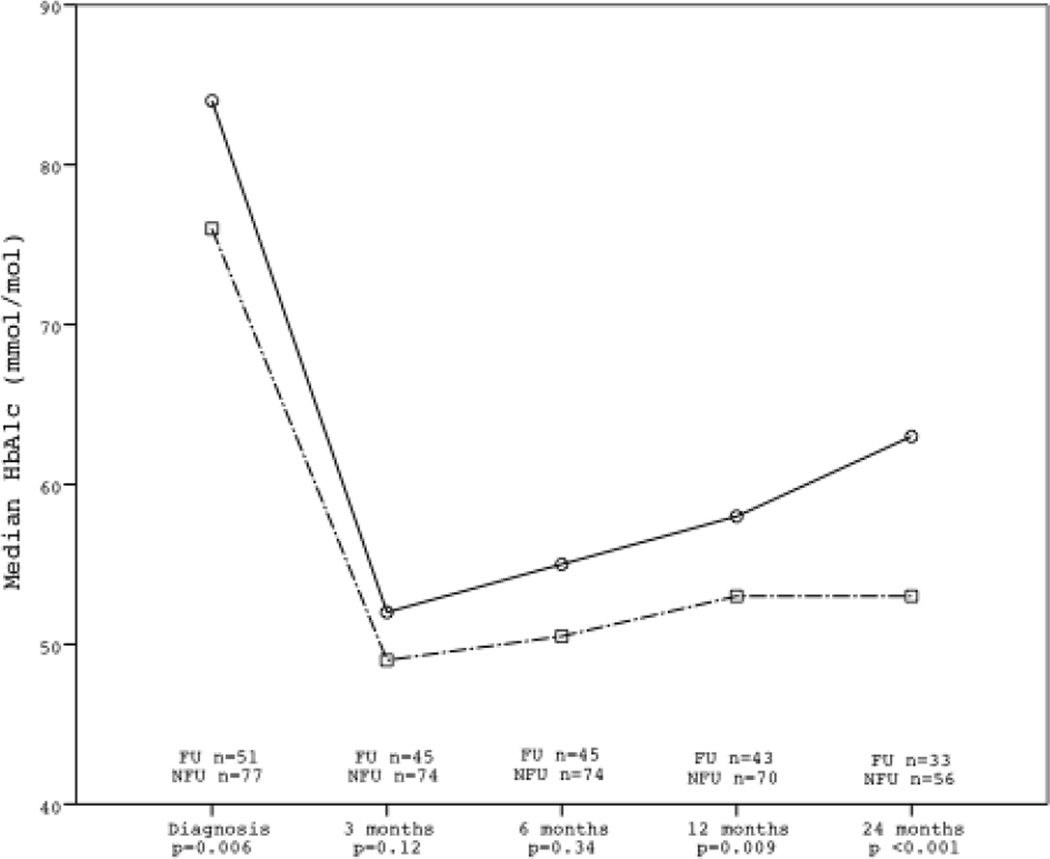
Figure 2.
HbA1c levels at diagnosis and the first two years after diagnosis in the FU and NFU Groups.
Definition of DKA
Diabetic ketoacidosis was defined as a blood pH <7.3 and severe diabetic ketoacidosis as blood pH <7.1 at diagnosis. Samples were drawn at diagnosis in an emergency room setting.
HLA DQ typing
HLA DQ genotyping was performed on cord blood with allele specific HLA DQ A1 and B1 probes as described elsewhere (9). HLA was classified as HLA-DQA1*0501-DQB1*0201 (DQ2) or HLA-DQA*0301-DQB1*0302 (DQ8) and stratified into 4 risk groups: DQ 2/8, DQ8/8 or 8/X, DQ2/2 or 2/X or DQ X/X (X is neither DQ2 nor DQ8).
Autoantibody analysis
Follow-up samples and samples from diagnosis of diabetes were analyzed for autoantibodies to GAD65 and IA-2 with radio immune assays described elsewhere (10) and with a commercially available ELISA kit, according to the manufacturers instructions (RSR Limited, Cardiff, UK). Samples from follow up visits and diabetes diagnosis were additionally analyzed for autoantibodies to insulin (IAA) and radio immune assay of the three amino acid variants of zinc transporter 8 (ZnT8RA, arginine 325 zinc transporter 8 autoantibody; ZnT8WA, tryptophan 325 zinc transporter 8 autoantibody; ZnT8QA, glutamine 325 zinc transporter 8 autoantibody) autoantibodies.. For IAA performed before 2011 and all ZnT8 autoantibodies the method is described elsewhere (11). For IAA samples analyzed after 2010 the following alterations to the assay has been made: To the buffer used for incubation (TRIS-buffer (pH 8.0) with 1 % (v/v) Tween 20) 1% w/v bovine serum albumin were added to prevent non-specific binding. In the competitive assay, instead of arbitrary units, U/mL has been calculated using a standard curve. The standard curve represented seven different concentrations (3–358 U/mL) and the concentration was plotted against cpm values on a Log2 scale.
Data collection
Pediatricians at the pediatric clinics in the Region Skåne continuously reported children from the DiPiS cohort when they developed diabetes. Additional information was gained from on-going studies, including all children diagnosed in Skåne (the Skåne study) and Sweden (the Better Diabetes Diagnosis study)(12). Data regarding metabolic parameters, symptoms at diagnosis and clinical post diagnosis follow-up data on HbA1c and insulin doses were retrieved from individual electronic patient records (Melior, Siemens AG, Berlin, Germany). Insulin doses were analyzed as total units of insulin per kg and day. Follow-up data was recorded after diagnosis at 3, 6, 12 and 24 ± one month. The clinical care of the diagnosed patients is ongoing, and some children with type 1 diabetes have therefore yet to reach 24 months after diagnosis.
Statistical Methods
Data were analyzed using SPSS version 21 (Spss inc, Chicago, Il). Differences in categorical variables were tested using Pearson´s Chi-squared test or Fisher’s exact test when appropriate. Mann – Whitney U test was used for continuous variables. Correlation analysis between HbA1c and insulin doses was performed using Spearman’s rho. All analyses were performed using IFCC (mmol/mol) and results were then recalculated to NGSP (%). Missing data was excluded on a variable-by-variable basis to maximize use of the data. Bonferroni correction for multiple comparisons was performed where applicable. P-Values <0.05 were considered statistically significant.
The Regional Ethics Review Board in Lund, Sweden, approved the study.
Results
Baseline characteristics
The baseline characteristics of the 129 DiPiS children diagnosed with type 1 diabetes are summarized in Table 1. The FU/NFU groups did not differ in gender (p=0.38) or mean age at diagnosis of diabetes (p=0.45). Two children were negative for islet autoantibodies at diagnosis of diabetes (Table 1). One was antibody positive in control samples one year after diagnosis and the other had a clinically typical type 1 diabetes. The HLA risk genotypes DQ 2/8, DQ 8/8, DQ 8/X, DQ2/2 and DQ2/X were found in 97% in the children with intense follow-up (group A), 100% in the group with some follow-up (group B) and 86% in the group (group C) without follow-up (Table 1).
Table 1.
Baseline characteristics of the DiPiS study groups at diagnosis
| Studygroup in DiPiS | ||||
|---|---|---|---|---|
| A | B | C | ||
| n | 33 | 18 | 78 | |
| Age at diagnosis (yrs) | Mean ±SD | 6.5±2.6 | 7.3±3.2 | 6.5±2.6 |
| Gender (n) | Female | 20 (61%) | 9 (50%) | 38 (49%) |
| Male | 13 (39%) | 9 (50%) | 40 (51%) | |
| HLA-DQ (n) | 2/8 | 15 (46%) | 6 (33%) | 34 (44%) |
| 8/8, 8/X (X is not 2) | 14 (42%) | 9 (50%) | 18 (23%) | |
| 2/2, 2/X (X is not 8) | 3 (9%) | 3 (17%) | 15 (19%) | |
| X/X (X is not 2 or 8) | 1 (3%) | 0 | 11 (14%) | |
| Autoantibody pos. (n) | GAD65A | 24 (73%) | 13 (72%) | 52 (67%) |
| IAA-A | 11 (33%) | 8 (44%) | 33 (42%) | |
| IA2-A | 28 (85%) | 13 (72%) | 51 (65%) | |
| ZnT8RA | 17 (52%) | 8 (44%) | 36 (46%) | |
| ZnT8WA | 18 (55%) | 8 (44%) | 29 (37%) | |
| ZnT8QA | 12 (36%) | 5 (28%) | 18 (23%) | |
| Antibodycount (n) | Negative | 0 | 0 | 2 (3%) |
| Single | 4 (12%) | 4 (22%) | 12 (15%) | |
| Multiple | 27 (82%) | 14 (78%) | 60 (77%) | |
| Missing | 2 (6%) | 0 | 4 (5%) | |
Clinical characteristics at diagnosis
The presence of the following symptoms was recorded at diagnosis: polyuria, polydipsia, weight loss and ketoacidosis. The presence of at least 1 symptom was less in the FU (84%) compared to the NFU group (97%; OR 0.14 (95% CI 0.03–0.70 ; p=0.014)), Bonferroni corrected p-value 0.028) (Table 2).
Table 2.
Incidence of diabetes related symptoms at diagnosis in the FU and NFU group
| Follow-Up | No Follow-Up | ||||||
|---|---|---|---|---|---|---|---|
| n | Incidence (%) | Incidence (%) | pa | pb | OR | 95% CI | |
| Symptoms | 129 | 84 | 97 | 0.014 | 0.028 | 0.141 | 0.029–0.696 |
| -polydipsia | 129 | 80 | 96 | ||||
| -polyuria | 129 | 76 | 96 | ||||
| -weightloss | 122 | 46 | 65 | ||||
| DKA | 129 | 2 | 18 | 0.005 | 0.01 | 0.091 | 0.012–0.719 |
| -severe DKA | 129 | 2 | 5 | ||||
pa p-value of Mann-Whitney U-test
pb Bonferroni corrected p-value
DKA, diabetic ketoacidosis (pH <7.3)
Severe DKA, diabetic ketoacidosis (pH <7.1)
Diabetic Ketoacidosis
The proportion of children presenting with diabetic ketoacidosis (pH <7.3) was lower in the FU (2%) compared to the NFU group (18%; OR 0.091 (95% CI 0.01–0.80; p=0.005) Bonferroni corrected p-value 0.010). Regarding severe ketoacidosis (pH <7.1) no significant differences could be seen with an incidence of 2% in the FU and 5% in the NFU group. None of the patients enrolled in intense follow-up presented with diabetic ketoacidosis.
HbA1c
At the time of diabetes diagnosis the FU group had a lower median HbA1c, 9.2% (77 mmol/mol) compared to 10.0% (87 mmol/mol) in the NFU group (p=0.006) (Table 2). At three and six months after diagnosis no significant differences were observed. At 12 months after diagnosis the children in the FU group had lower HbA1c, 7.0% (53 mmol/mol) compared to 7.4% (57mmol/mol) in the NFU group (p=0.009) (Table 2, Figure 2). At 24 months after diagnosis this difference increased with the FU group having median HbA1c 7.0% (53 mmol/mol) compared to 7.8% (62 mmol/mol) in the NFU group (p <0.001). All differences remained statistically significant after correction for multiple comparisons (Table 3, Figure 2). In the total cohort at diagnosis, patients, with ketoacidosis (11.5% (102mmol/mol) had a higher median HbA1c than patients without (9.6% (81mmol/mol); p <0.001). However, HbA1c did not differ between these two groups during the first two years after diagnosis (3 months p=0.386 ; 6 months p=0.080 ; 12 months p=0.126 ; 24 months p=0.793).
Table 3.
HbA1c at onset and HbA1c and total daily dose of insulin for the 2 years following diabetes diagnosis in the FU and NFU groups.
| Follow-up | No Follow-Up | |||||
|---|---|---|---|---|---|---|
| n | Median (IQR) | Median (IQR) | pa | pb | ||
| HbA1c at diagnosis | % (mmol/mol) | 128 | 9.2 (8.0–10.5) | 10.0 (9.0–11.2) | 0.006 | 0.03 |
| (77 (64–91)) | (86 (75–99)) | |||||
| HbA1c, 3 months | % (mmol/mol) | 119 | 6.5 (6.1–7.0) | 6.7 (6.2–7.5) | 0.120 | 0.60 |
| (48 (43–53)) | (50 (44–58)) | |||||
| HbA1c, 6 months | % (mmol/mol) | 119 | 6.6 (6.0–7.5) | 7.1 (6.2–7.6) | 0.344 | >1 |
| (49 (43–59)) | (54 (44–60)) | |||||
| HbA1c, 12 months | % (mmol/mol) | 113 | 7.0 (6.5–7.4) | 7.4 (6.5–7.4) | 0.009 | 0.045 |
| (53 (48–57)) | (57 (51–65)) | |||||
| HbA1c, 24 months | % (mmol/mol) | 89 | 7.0 (6.5–7.7) | 7.8 (7.1–8.4) | <0.001 | <0.001 |
| (53 (48–61)) | (62 (54–69)) | |||||
| TDD, 3 months | U kg−1 day−1 | 125 | 0.47 (0.30–0.58) | 0.52 (0.39–0.65) | 0.026 | 0.13 |
| TDD, 6 months | U kg−1 day−1 | 120 | 0.62 (0.41–0.76) | 0.62 (0.41–0.76) | 0.443 | >1 |
| TDD, 12 months | U kg−1 day−1 | 113 | 0.75 (0.52–0.89) | 0.75 (0.59–0.90) | 0.511 | >1 |
| TDD, 24 months | U kg−1 day−1 | 89 | 0.76 (0.63–0.93) | 0.89 (0.63–0.93) | 0.098 | 0.49 |
pa p-value of Mann-Whitney U-test
pb Bonferroni corrected p-value
IQR Interquartile range
TDD Total daily dose of insulin
Insulin Dose and Remission
Three months after diagnosis, children in the FU group had a lower total daily dose of insulin (0.47 U kg−1 day−1) compared to the NFU group (0.52 U kg−1 day−1 ; p=0.026), while no differences were observed at 6, 12 and 24 months after diagnosis (Table 3). No differences in total daily insulin dose (TDD) were observed after Bonferroni correction for multiple analyses. A weak but significant correlation between insulin dose and HbA1c was detected in the total study group 24 months post diagnosis (ρ=0.376, p <0.001). No differences in remission, defined as TDD <0.5 U kg−1 day−1, were observed between the groups at any time.
Discussion
The main findings in this study is that children participating in a prospective long-term follow-up before the diagnosis of type 1 diabetes are healthier at diabetes diagnosis and have better metabolic control in standard clinical care up to two years after diagnosis, compared to children who have not been followed in a study. Specifically, we found that children participating in follow-up more often presented without any reported diabetes related symptoms. Children in follow-up (FU) therefore had decreased rates of polydipsia polyuria and weight loss, between 15 and 20 % less, than the No follow-up (NFU) group. These findings are in line with earlier diabetes prediction studies such as DPT-1, DAISY and the BABYDIAB (5, 7, 13). The TEDDY study described 11.3% ketoacidosis which was lower than similar longitudinal studies or registries (6). It was therefore of interest that the NFU group in our study had similar rates of ketoacidosis at diagnosis compared to data from the Swedish pediatric diabetes registry from 2012 (14) whereas our FU group had only 2% ketoacidosis at diagnosis. This very low frequency of ketoacidosis is in part explained by the fact that our DiPiS children were not followed until they were two years of age.
Reducing the incidence of ketoacidosis is important as it carries a risk of mortality as well as reduced beta cell function (15–17). Since DiPiS was designed to follow children and give risk information from 2 years of age, children diagnosed with diabetes before this age (n=14) were missed. It is known that children in the youngest age group have a higher frequency of diabetic ketoacidosis at diagnosis (16, 18). It cannot be excluded that our frequency of ketoacidosis in the NFU group might have been higher if the DiPiS study design would have included children from an earlier age. It is possible that the younger age group may have had the greatest benefit of having parents who were informed about the risk of diabetes in their child.
Our results implicate that children participating in follow-up before diagnosis of type 1 diabetes have better metabolic control up to two years after diagnosis, measured as a lower HbA1c. Since HbA1c is strongly associated to the risk of long-term diabetes complications (19, 20), this is an important finding not previously described for a timeframe as long as 2 years after participating in a longitudinal follow-up study. Furthermore, our finding that HbA1c was improved in the FU group was unexpected. We can only speculate why the study subjects showed an improved metabolic control for up to two years. One possibility is that the families were better prepared for type 1 diabetes treatment and may have informed themselves in anticipation of a diagnosis. Another possibility is that the early diagnosis resulted in fewer metabolic abnormalities. This may have led to a prolonged period of partial remission due to better beta cell function at diagnosis. Previous studies have also addressed psychological problems related to early screening of infants and children, however, without finding serious adverse effects (21, 22). In this study we show a beneficial effect to the participants with less severe status at diagnosis and an improved metabolic control up to two years after diagnosis. These results may be important to the recruitment of study subjects for future follow-up studies.
A potential weakness of the present study was that the recording of data and the follow up was not part of the original DiPiS protocol. For example, fasting or stimulated C-peptide at the time of diabetes diagnosis could not be done. During follow-up after diagnosis, c-peptide sampling was not a part of the regular clinical follow-up. However, no differences in remission, defined as total daily insulin dose <0,5 U kg−1 day−1, could be seen between the FU and NFU groups. Also, the number of study participants with type 1 diabetes in this study may be regarded as rather small. This has to be taken into account when interpreting the data.
It could be argued that families willing to participate in the DiPiS study were more motivated, have a higher educational level or are more anxious about their child’s health. Socioeconomic data is only available for the 25 0000 families who filled out the 2-month questionnaire but not for the approximately 10 000 families who did not, Previous analyses in DiPiS have found that families were less likely to participate if the child was born in a hospital in a large city, the mother was either less than 25 or older than 40 years of age, was premature or twin. Mothers with diabetes were less likely to participate. (4–6, 21). It is possible that other socioeconomic factors influenced the outcome after diagnosis, which was outside of the scope of this study.
The present study represents a large part of the population since cord blood samples were obtained from 80% of the children born during four years in the southernmost part of Sweden. The incidence of diabetes in the study cohort is 40/100 000 at the present time. The children diagnosed with diabetes are all cared for by public healthcare in six pediatric clinics and all physicians are using the same electronic patient records. It was therefore possible easily to access the patient charts. Differences in care are presumed to be small since all centers adhere to national guidelines on the management of pediatric type 1 diabetes and regular meetings are held in the region to ensure equal care.
In conclusion, our study shows that the DiPiS children who were enrolled at two years of age in a longitudinal study on the prediction of diabetes were diagnosed at an early stage of the disease as HbA1c was lower and there were fewer symptoms including a lower frequency of ketoacidosis. Additionally, the DiPiS children had better metabolic control after diagnosis when subjected to standard clinical care, demonstrating significantly lower HbA1c levels 12 and 24 months after diagnosis. Screening at birth for type 1 diabetes genetic risk and informing the parents may be sufficient to increase the awareness of diabetes symptoms to permit a diagnosis with less symptoms.
Acknowledgements
Other co-investigators in the DiPiS study group are: Cecilia Andersson, Maria Ask, Jenny Bremer, Charlotte Brundin, Corrado Cilio, Carina Hansson, Gertie Hansson, Sten Ivarsson, Berglind Jonsdottir, Bengt Lindberg, Barbro Lernmark, Zeliha Mestan, Anita Ramelius, Ingrid Wigheden and Ulla-Marie Carlsson. We thank all the participating parents and children in the DiPiS study.
Funding:
Our research is supported in part by the Swedish Research Council (grant no. 14064), Juvenile Diabetes Research Foundation, Wallenberg Foundation, Swedish Childhood Diabetes Foundation, Swedish Diabetes Association, Nordisk Insulin Fund, National Institutes of Health (DK26190), SUS funds, as Terry & Louise Gregg Diabetes in Pregnancy Award from the American Diabetes Association, Lion Club International, district 101-S, SUS foundations and the Skåne County Council Foundation for Research and Development.
Footnotes
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript
Contribution statement
ML analyzed the data and wrote the manuscript. ÅS and CS were involved in early interpretation, collection and analysis of data. IJ was involved in updating, collecting and early interpretation of data. AC, EC, BJ, KL, ÅL, JN and TV were involved in study design, data collection and critically revising the manuscript for important intellectual content. HEL designed the study, was involved in data collection, interpreted data and contributed to and edited the manuscript. All authors gave final approval of the version to be published.
References
- 1.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res. 2001;56:69–89. doi: 10.1210/rp.56.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notkins AL. Immunologic and genetic factors in type 1 diabetes. J Biol Chem. 2002;277:43545–43548. doi: 10.1074/jbc.R200012200. [DOI] [PubMed] [Google Scholar]
- 4.Larsson HE, Larsson HE, Vehik KK, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatric Diabetes. 2013 doi: 10.1111/pedi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler C, Schober E, Ziegler A-G, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatric Diabetes. 2012;13:308–313. doi: 10.1111/j.1399-5448.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsson HE, Larsson HE, Vehik KK, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34:2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27:1399–1404. doi: 10.2337/diacare.27.6.1399. [DOI] [PubMed] [Google Scholar]
- 8.Larsson K, Elding Larsson H, Cederwall E, et al. Genetic and perinatal factors as risk for childhood type 1 diabetes. Diabetes Metab Res Rev. 2004;20:429–437. doi: 10.1002/dmrr.506. [DOI] [PubMed] [Google Scholar]
- 9.Larsson HE, Lynch K, Lernmark B, et al. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50:1161–1169. doi: 10.1007/s00125-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 10.Lynch KF, Lernmark B, Merlo J, et al. Cord blood islet autoantibodies and seasonal association with the type 1 diabetes high-risk genotype. Journal of Perinatology. 2008;28:211–217. doi: 10.1038/sj.jp.7211912. [DOI] [PubMed] [Google Scholar]
- 11.Andersson C, Andersson C, Vaziri-Sani F, et al. Triple specificity of ZnT8 autoantibodies in relation to HLA and other islet autoantibodies in childhood and adolescent type 1 diabetes. Pediatric Diabetes. 2013;14:97–105. doi: 10.1111/j.1399-5448.2012.00916.x. [DOI] [PubMed] [Google Scholar]
- 12.Delli AJ, Vaziri-Sani F, Lindblad BB, et al. Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the better diabetes diagnosis study. Diabetes. 2012;61:2556–2564. doi: 10.2337/db11-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triolo TM, Chase HP, Barker JM DPT-1 Study Group. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32:769–773. doi: 10.2337/dc08-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swedish Childhood Diabetes Registry SWEDIABKIDS, Swedish Childhood Diabetes Registry SWEDIABKIDS. Swediabkids Annual report 2012. Nationellt register för barn och ungdomsdiabetes. 2013:1–54. [Google Scholar]
- 15.Hara N, Alkanani AK, Ir D, et al. The role of the intestinal microbiota in type 1 diabetes. Clin Immunol. 2013;146:112–119. doi: 10.1016/j.clim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Archives of Disease in Childhood. 2001;85:16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA: The Journal of the American Medical Association. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 18.Rewers A, Klingensmith GJ, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. PEDIATRICS. 2008;121:e1258–e1266. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 19.Shalitin S, Phillip M. Which factors predict glycemic control in children diagnosed with type 1 diabetes before 6.5 years of age? Acta Diabetol. 2012;49:355–362. doi: 10.1007/s00592-011-0321-x. [DOI] [PubMed] [Google Scholar]
- 20.Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care. 2004;27:955–962. doi: 10.2337/diacare.27.4.955. [DOI] [PubMed] [Google Scholar]
- 21.Lernmark B, Elding Larsson H, Hansson G, et al. Parent responses to participation in genetic screening for diabetes risk. Pediatric Diabetes. 2004;5:174–181. doi: 10.1111/j.1399-543X.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson J, Ludvigsson M, Sepa A. Screening for prediabetes in the general child population: maternal attitude to participation. Pediatric Diabetes. 2001;2:170–174. doi: 10.1034/j.1399-5448.2001.20405.x. [DOI] [PubMed] [Google Scholar]