Abstract
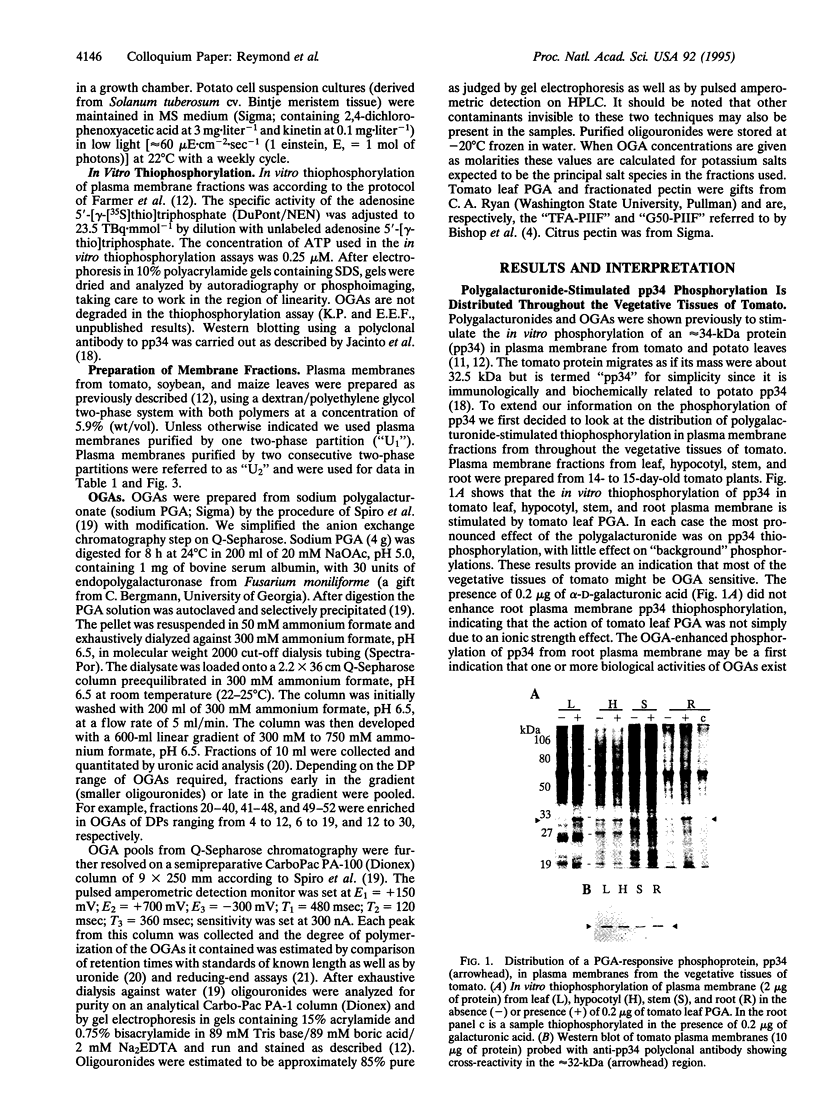
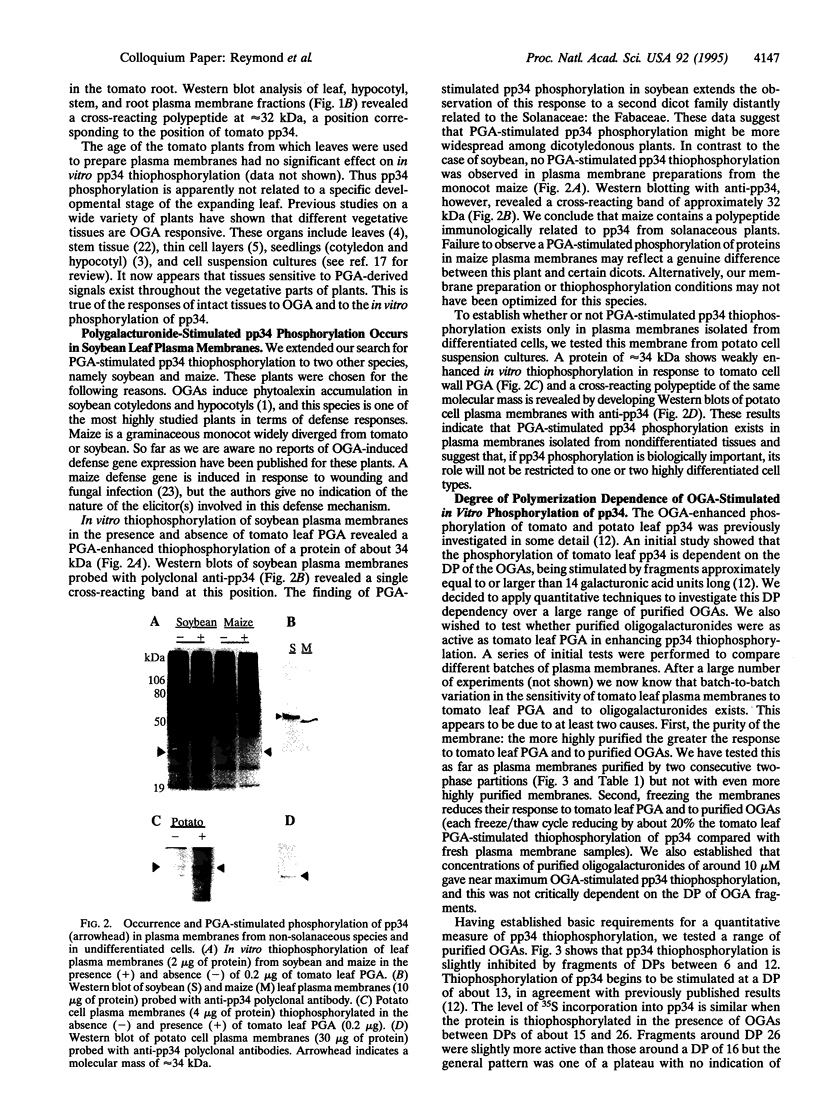
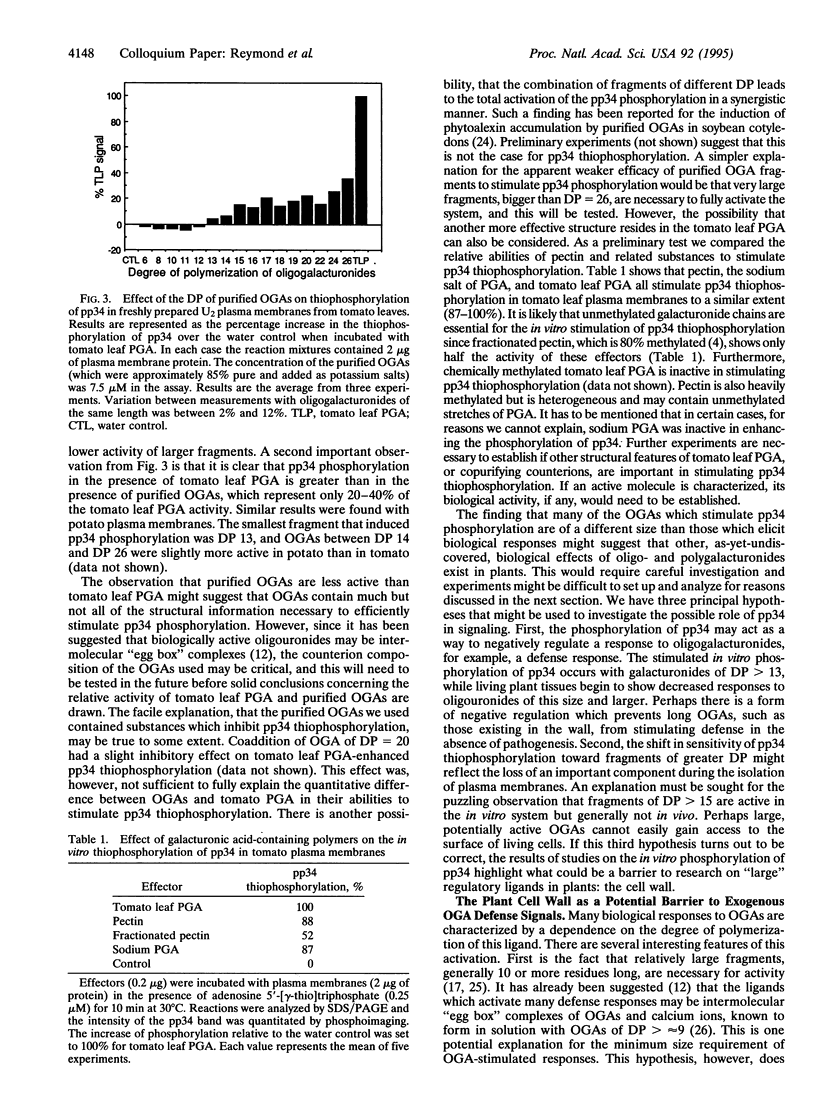
Oligogalacturonides are plant cell wall-derived regulatory molecules which stimulate defense gene expression during pathogenesis. In vitro, these compounds enhance the phosphorylation of an approximately 34-kDa protein (pp34) in purified plasma membranes from potato and tomato leaves. We now show that polygalacturonate-enhanced phosphorylation of pp34 occurs in plasma membranes purified from tomato roots, hypocotyls, and stems and from undifferentiated potato cells. Furthermore, a similar phosphorylation is detected in leaf plasma membranes from soybean, a plant distantly related to tomato. Purified oligogalacturonides 13 to at least 26 residues long stimulate pp34 thiophosphorylation in vitro. This stimulation pattern differs from the induction of many known defense responses in vivo, where a narrower range of smaller fragments, between approximately 10 and 15 residues long, are active. On the basis of these differences we suggest that observed effects of applied exogenous oligogalacturonides on defense responses may not necessarily reflect the situation during pathogenesis. The cell wall could act as a barrier to many exogenous oligo- and polygalacturonides as well as other large regulatory ligands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Cordero M. J., Raventós D., San Segundo B. Expression of a maize proteinase inhibitor gene is induced in response to wounding and fungal infection: systemic wound-response of a monocot gene. Plant J. 1994 Aug;6(2):141–150. doi: 10.1046/j.1365-313x.1994.6020141.x. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992 Oct;4(5):772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Darvill A., Augur C., Bergmann C., Carlson R. W., Cheong J. J., Eberhard S., Hahn M. G., Ló V. M., Marfà V., Meyer B. Oligosaccharins--oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology. 1992 Jun;2(3):181–198. doi: 10.1093/glycob/2.3.181. [DOI] [PubMed] [Google Scholar]
- Davis K. R., Darvill A. G., Albersheim P., Dell A. Host-Pathogen Interactions : XXIX. Oligogalacturonides Released from Sodium Polypectate by Endopolygalacturonic Acid Lyase Are Elicitors of Phytoalexins in Soybean. Plant Physiol. 1986 Feb;80(2):568–577. doi: 10.1104/pp.80.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Farmer E. E., Moloshok T. D., Saxton M. J., Ryan C. A. Oligosaccharide signaling in plants. Specificity of oligouronide-enhanced plasma membrane protein phosphorylation. J Biol Chem. 1991 Feb 15;266(5):3140–3145. [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Regenass M., Spanu P., Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Yoshida K. Cell expansion and single-cell separation induced by colchicine in suspension-cultured soybean cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2618–2622. doi: 10.1073/pnas.85.8.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto T., Farmer E. E., Ryan C. A. Purification of Potato Leaf Plasma Membrane Protein pp34, a Protein Phosphorylated in Response to Oligogalacturonide Signals for Defense and Development. Plant Physiol. 1993 Dec;103(4):1393–1397. doi: 10.1104/pp.103.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L., Rueter S., Heinstein P. F., Low P. S. Characterization of the Oligogalacturonide-Induced Oxidative Burst in Cultured Soybean (Glycine max) Cells. Plant Physiol. 1993 May;102(1):233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972 May;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Mohnen D., Eberhard S., Marfà V., Doubrava N., Toubart P., Gollin D. J., Gruber T. A., Nuri W., Albersheim P., Darvill A. The control of root, vegetative shoot and flower morphogenesis in tobacco thin cell-layer explants (TCLs). Development. 1990 Jan;108(1):191–201. doi: 10.1242/dev.108.1.191. [DOI] [PubMed] [Google Scholar]
- Mohnen D., Eberhard S., Marfà V., Doubrava N., Toubart P., Gollin D. J., Gruber T. A., Nuri W., Albersheim P., Darvill A. The control of root, vegetative shoot and flower morphogenesis in tobacco thin cell-layer explants (TCLs). Development. 1990 Jan;108(1):191–201. doi: 10.1242/dev.108.1.191. [DOI] [PubMed] [Google Scholar]