Abstract
Rationale
Preclinical studies suggest that stress potentiates cue-induced cocaine seeking, and that this effect is more pronounced in females. These findings have not been characterized in clinical populations.
Objectives
The objectives of this study were to examine the impact a pharmacological stressor, alpha-2 adrenergic receptor antagonist yohimbine, on the subjective, endocrine and physiologic responses to drug-paired cues cocaine-dependent men and women.
Methods
In a double-blind placebo controlled cross-over study, cocaine-dependent men (n=32), cocaine-dependent women (n=30), control men (n=32) and control women (n=25) received either yohimbine or placebo prior to two cocaine cue exposure sessions.
Results
Yohimbine increased ratings of anxiety both before (p<0.001) and after (p=0.035) cues, and the post-cue increase in anxiety was more pronounced in women (p=0.001). Yohimbine also significantly increased craving, compared to placebo (p<0.05), following the cue presentation, and this effect was greater in women than men (gender by treatment interaction; p=0.006). Yohimbine also increased salivary cortisol (p<0.001) and DHEA (p=0.003) levels, regardless of diagnostic group. Women had a significantly greater heart rate response following yohimbine as compared to men (p<0.001).
Conclusions
Stress may increase the salience of cocaine-cues for cocaine-dependent women as compared to men. This suggests gender differences in vulnerability to craving and relapse under stressful conditions.
Keywords: cocaine-dependence, stress, cues, noradrenaline, yohimbine, sex differences
Introduction
Both stress and drug-related environmental cues contribute to the high rates of relapse among cocaine-dependent individuals (Alleweireldt et al. 2001; Erb et al. 1996; Preston and Epstein 2011; Sinha et al. 1999; Sinha et al. 2000). Although both have been studied separately, few studies have investigated their interaction, especially in cocaine-dependent individuals. Moreover, although both stress and cue reactivity differ in cocaine-dependent men and women (Back et al. 2005; Fox et al. 2006; McKay et al. 1996; Waldrop et al. 2009), few studies have examined the relationships between drug-related cues and stress in men versus women.
Preclinical studies demonstrate that stress and drug-paired cues interactively influence drug-seeking behavior in rodents trained to self-administer drugs. For example, pharmacological provocation of the noradrenergic stress system with the alpha-2 adrenergic receptor antagonist yohimbine potentiates cue-induced reinstatement of both cocaine and heroin seeking behavior (Banna et al. 2010; Feltenstein and See 2006), and footshock stress potentiates cue-induced reinstatement of both cocaine and ethanol-seeking behavior (Buffalari and See 2009; Liu and Weiss 2002). These preclinical data suggest that the combined effects of stress and drug cues may increase the vulnerability of substance-dependent individuals to relapse.
There is some evidence that there are sex differences in the role in the relapse (McKay et al. 1996). Compared with cocaine-dependent men, cocaine-dependent women report greater stress and nervousness in response to laboratory stress tasks (Back et al. 2005; Waldrop et al. 2009). In rodents, female animals exhibit greater drug seeking behavior in response to pharmacological stressors than male rodents (Anker and Carroll 2010; Buffalari et al. 2012). Of note, yohimbine potentiates cue-induced reinstatement of cocaine seeking behavior to a greater extent in female, compared to male rodents (Feltenstein et al. 2011). Sex differences in the interaction between stress and drug-paired cues have not previously been investigated in clinical populations of cocaine-dependent men and women.
The primary focus of the present study was to examine the effects of the pharmacological stressor, yohimbine, on subjective, endocrine and physiologic responses to drug-related cues in a clinical population of cocaine-dependent men and women. We hypothesized that yohimbine would increase reactivity to cues (compared to placebo), and that this effect would be greater in women than men.
Methods
Study Participants
Study participants were recruited primarily via media advertisements over a 48-month period. Written informed consent was obtained before study assessments were administered. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received Institutional Review Board (IRB) approval. Inclusion criterion for the cocaine-dependent groups included (1) Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for cocaine-dependence in the 90-days prior to the study. Exclusion criterion for the cocaine-dependent groups included (1) DSM-IV criteria for substance dependence except caffeine, nicotine, alcohol or marijuana within the past 60 days. Exclusion criteria for the control groups included (1) DSM-IV criteria for current or lifetime dependence on alcohol or any drugs of abuse except nicotine and caffeine and (2) DSM-IV criteria for current abuse of alcohol or any illicit drugs. General exclusion criteria included (1) pregnancy, nursing, or ineffective means of birth control; (2) premenstrual dysphoric disorder; (3) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (4) history of or current psychotic, panic, eating, or bipolar affective disorders; (5) current major depressive and PTSD; (6) history of or current medical conditions that might affect HPA axis activity; (7) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (8) psychotropic medications with the exception of selective serotonin reuptake inhibitors, opiates or opiate antagonists, benzodiazepines, antipsychotics, b-blockers and other medications that might interfere with HPA axis activity or physiologic measurements; (9) acute illness or fever; (10) body mass index > 35 and (11) unwillingness or inability to maintain abstinence from alcohol and other drugs of abuse (except nicotine) for three days prior to the cue-reactivity sessions.
Assessment
Subjects meeting preliminary screen criteria were evaluated for study eligibility with the Mini-International Neuropsychiatric Interview (MINI) which permits accurate diagnosis of current psychiatric disorders using DSM-IV criteria (Sheehan et al. 1998). The substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV), was used for current and lifetime substance use disorder diagnosis (First et al. 1994). Substance use in the ninety days prior to study enrollment and throughout the study period was assessed using the Time-Line Follow-Back (Sobell and Sobell 1992). If a participant met criteria for alcohol dependence, the Severity of Alcohol Dependence Questionnaire was administered (Stockwell et al. 1983). Participants scoring ten or higher were evaluated by a clinician prior to enrollment. Menstrual history data was collected from female participants. A medical history and physical examination, including electrocardiogram were completed to assess for medical exclusions. Following baseline assessments, participants meeting inclusion criteria and no exclusion criteria were scheduled to complete the laboratory procedures.
Laboratory Procedures
Subjects participated in two cue exposure sessions on consecutive days. On the first day of testing, participants arrived at the Clinical and Translational Research Center (CTRC) of the Medical University of South Carolina at 10:00 a.m. Upon arrival, urine pregnancy tests were administered to female participants. Smokers were provided with a nicotine patch. Subjects were required to abstain from alcohol or other substance use (except nicotine and caffeine) for a minimum of three days prior to testing. Abstinence was assessed using self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri) which were administered prior to each cue reactivity session. If the pregnancy and drug tests were negative (with the exception of THC), study procedures continued.
The study was conducted using a double-blind placebo-controlled design. At 11:00 a.m., baseline salivary cortisol and dehydroepiandrosterone (DHEA) samples were collected. At 12:00 p.m., subjects received either yohimbine hydrochloride (Spectrum Laboratories, Inc., New Brunswick, New Jersey) 21.6 mg or a matching placebo capsule. Treatment order was counterbalanced across the two cue reactivity sessions. Participants were randomized to treatment by sex and by diagnostic group, such that half of the participants in each group received yohimbine prior to the first cue reactivity session and half of the participants received placebo. Identical yohimbine and placebo capsules were compounded by the MUSC Investigational Drug Service who managed the study randomization procedures and recorded treatment assignment. The dose and timing of administration were selected based on previous studies (O’Carroll et al. 1999; Swann et al. 2005). At 12:15 p.m. the participants were provided with a standard lunch and were seated in the CTRC testing room where they were allowed to read until the testing procedures began. At 1:30 p.m., a blood pressure cuff was placed on the subject’s arm. Subjective, endocrine (DHEA and cortisol) and heart rate data were collected, one at 1:40 p.m. and the second at 1:55 p.m. A modified version of the Within Session Rating Scale was used to assess subjective responses (Childress et al. 1986). This 100 mm visual analogue scale is anchored with the adjectival modifiers (“not at all, mildly, moderately and extremely”). There are four items assessing domains of craving (want/need/craving/ability to resist). Other items include anxiety, distress, and mood. At 2:00, the subject participated in a scripted imagery exercise regarding cocaine use. The scripted imagery session began with a brief relaxation exercise during which the subject was instructed to release any tension in the shoulders, back and neck and to breathe deeply. The subject was then instructed to recall a time when they were using cocaine, and to visualize the experience with as much sensory detail as possible including their physical response (increased heart rate and breathing), and the rush of excitement at the first hit of cocaine. Participants were then asked to view and handle cocaine-related paraphernalia. The cocaine cues consisted of a small bag of simulated crack cocaine, the subject’s preferred style of crack pipe, a lighter, and money ($20 bill) for those who use crack cocaine. For powder or intravenous users, simulated cocaine and money were used. The cocaine cues were placed on the table directly in front of the subject. The subject was asked to inspect and handle the cues for a two-minute period. Following the completion of the “in vivo” exposure, the subjects viewed a five-minute film depicting cocaine use in a variety of settings. This combination of “in-vivo” cues and cocaine use depiction has produced significant craving and physiologic activity in previous studies (Coffey et al. 2002; Saladin et al. 2003). The cue-paradigm lasted for 10 minutes. Heart rate, subjective and endocrine data were collected immediately after the cues, and again 5-, 30-, and 60-minutes post-cue exposure. Afterwards, participants were escorted to a private room in the Medical University Hospital (MUH) where they spent the night. The following morning, participants were escorted back to the CTRC and repeated the study procedures described above with the alternate medication. Participants spent a second night in the MUH. At 11:00 a.m. the following morning, the subjects were debriefed, compensated for their participation and discharged from the CTRC.
Saliva samples were assayed by enzyme-linked immunosorbent assay using commercially available kits (Salimetrics, LLC, State College, PA) in the CTRC laboratory. The DHEA assay has a lower sensitivity limit of 5 pg/ml and a DHEA serum-saliva correlation of (r=0.857). The cortisol kit has a lower sensitivity level of <0.003 μg/dL. The correlation between salivary and serum cortisol has been shown to be high (r=0.91).
Statistical Analysis
This study was powered to determine if cocaine-dependent subjects would demonstrate greater cocaine craving after receiving yohimbine followed by exposure to cocaine-related cues as compared to cocaine-related cue exposure following administration of placebo. Sample size estimates were based on our own preliminary data from cocaine-dependent women. It was determined that 30 women and 30 men per group would be sufficient to achieve 80% power with a type I error rate of 5% to detect a 2 point (40%) increase in cocaine craving.
A Wilcoxon Rank sum test was used to evaluate continuous baseline demographic and clinical measures across groups while the Pearson Chi-Square test was used to assess the association among categorical and ordinal variables. To assess the association of treatment with yohimbine and response to cocaine cue, linear mixed effects models were developed that included all serial measurements made at each time point following administration of yohimbine or placebo (including the pre-cue/post treatment time points) (Patterson 1971). Models were fitted using SAS PROC MIXED using Restricted Maximum Likelihood approach to account for unbalanced data and model-based estimates were used to test the planned hypotheses. Pair-wise comparisons between treatment and groups were assessed at time points immediately prior to and following the presentation of the cue. Interactions were tested using all post cue time points while controlling for pre cue response levels. Since the controls were not expected to crave cocaine, the analysis of the craving data was restricted to cocaine dependent men and women. Cortisol and DHEA levels collected prior to the administration of yohimbine or placebo were collected and used as covariates in the analysis models. To achieve normality, the endocrine outcomes were log10 transformed. All comparisons and statistical analysis were adjusted for baseline levels when available. Effects of drug administration order and cue-reactivity session (day 1 vs. day 2) were tested in all models. No effects of the order of drug administration were determined to significantly affect the model estimates. Cue-reactivity session had trend-level significant effects on subjective responses, thus we controlled for cue-reactivity session the analyses.
All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, N.C.). Significance for all planned pair-wise comparisons was set at a 2-sided alpha level of 0.05 and no correction for multiple testing was applied to reported values.
Results
Study Participants
Data were collected from groups of cocaine-dependent men and women and non-dependent healthy control groups of men and women. Baseline demographic and clinical characteristics by cocaine use status and gender are presented in Table 1. There were no significant differences in race, education, marital status or smoking status between the cocaine-dependent and control groups. There were significant differences in age and employment status, cocaine-dependent participants were older and reported greater unemployment levels than controls. Within the cocaine-dependent cohort, there were no differences in treatment status, years of use or time since last use between men and women. There were no differences between cocaine-dependent and control women in day of the menstrual cycle at the study visit.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | Cocaine Subjects
|
Control Subjects
|
||||
|---|---|---|---|---|---|---|
| All Subjects (n=62) | Male (n=32) | Female (n=30) | All Subjects (n=57) | Male (n=32) | Female (n=25) | |
| Age | 41.1 ± 10.0 | 39.7 ± 9.9 | 42.7 ± 9.9 | 33.1± 12.6* | 32.2 ± 12.4 | 34.3 ± 13.1 |
| Male (n) | 51.6 (32) | 100.0 (32) | 0.0 (0) | 56.1 (32) | 100.0 (32) | 0.0 (0) |
| Caucasian (n) | 37.1 (23) | 31.3 (10) | 43.3 (13) | 45.6 (26) | 46.9 (15) | 44.0 (11) |
| Married (n) | 12.9 (8) | 12.5 (4) | 13.3 (4) | 17.5 (10) | 12.5 (4) | 24.0 (6) |
| HS Graduate (n) | 43.6 (27) | 43.8 (14) | 43.3 (13) | 59.6 (34) | 40.6 (13) | 84.0 (21) |
| Unemployed (non-student) (n) | 71.0 (44) | 62.5 (20) | 80.0 (24) | 45.6 (26)* | 46.9 (15) | 44.0 (11) |
| Smoker | 88.7 (55) | 84.4 (27) | 93.3 (28) | 73.7 (42) | 84.4 (27) | 60.0 (15) |
| Menstrual Cycle Day | 17.0 ± 3.9 | 15.5 ± 2.3 | ||||
| Alcohol Dependent | 12.9 (8) | 12.5 (4) | 13.3 (4) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Currently in Treatment | 3.2 (2) | 6.3 (2) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Treatment in the Past1 | 45.9 (28) | 50.0 (16) | 40.0 (12) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Total Years of Use | 14.7 ± 8.6 | 15.5 ± 8.8 | 13.9 ± 7.2 | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Days Since Last Use | 17.4 ± 16.9 | 20.5 ± 20.7 | 14.1 ± 10.9 | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Pre Medication Levels2 | ||||||
| Cortisol (ng/ml) | 2.22 ± 1.3 | 2.40 ± 1.5 | 2.01 ± 0.9 | 2.90 ± 2.3 | 3.32 ± 2.4 | 2.42 ± 2.1 |
| DHEA (ng/ml) | 0.21 ± 0.1 | 0.24 ± 0.2 | 0.18 ± 0.1 | 0.25 ± 0.2 | 0.29 ± 0.2 | 0.20 ± 0.1 |
| Cortisol/DHEA Molar Ratio | 10.8 ± 8.2 | 10.8 ± 9.7 | 10.9 ± 56.2 | 12.1 ± 9.1 | 11.3 ± 7.7 | 12.9 ± 10.5 |
| Post Placebo Levels | ||||||
| Craving | 1.80 ± 2.2 | 1.52 ± 2.1 | 2.11 ± 2.2 | 0* | 0 | 0 |
| Anxiety | 1.37 ± 1.9 | 1.26 ± 1.9 | 1.50 ± 1.8 | 0.55 ± 1.4* | 0.65 ± 1.3 | 0.44 ± 1.6 |
| Cortisol (ng/ml) | 1.76 ± 1.0 | 1.80 ± 0.8 | 1.71 ± 1.11 | 2.09 ± 1.6 | 2.44 ± 2.0 | 1.69 ± 1.0 |
| DHEA (ng/ml) | 0.20 ± 0.1 | 0.23 ± 0.2 | 0.17 ± 0.1 | 0.21 ± 0.1 | 0.24 ± 0.1 | 0.18 ± 0.1 |
| Cortisol/DHEA Molar Ratio | 9.0 ± 6.1 | 8.9 ± 6.9 | 9.0 ± 5.3 | 9.5 ± 7.0 | 9.1 ± 6.6 | 10.0 ± 7.5 |
| Post Yohimbine Levels | ||||||
| Craving | 2.48 ± 2.4 | 1.70 ± 2.1 | 3.32 ± 2.4¥ | 0* | 0 | 0 |
| Anxiety | 2.62 ± 2.6^ | 2.43 ± 2.8^ | 2.82± 2.5^ | 1.11 ± 2.1* | 1.19 ± 2.1 | 1.00 ± 2.1 |
| Cortisol (ng/ml) | 3.59 ± 3.2 | 3.00 ± 2.3 | 4.28 ± 4.0 | 3.01 ± 2.3 | 3.32 ± 2.4 | 2.67 ± 2.0 |
| DHEA (ng/ml) | 0.21 ± 0.1 | 0.23 ± 0.1 | 0.20 ± 0.1 | 0.23 ± 0.1 | 0.27 ± 0.1 | 0.20 ± 0.1 |
| Cortisol/DHEA Molar Ratio | 17.1 ± 16.7 | 16.1 ± 17.6 | 18.2 ± 15.9 | 12.7 ± 11.4 | 12.3 ±10.7 | 13.0 ± 12.0 |
p<0.01 as compared to post-placebo anxiety
p <0.05 as compared to all cocaine subjects
p =0.056 as compared post-placebo craving
treatment for cocaine abuse or dependence
pre-medication levels taken prior to administration of study drug on the 1st day of cue exposure
categorical values represented as % (n); continuous variables represented by mean (sd)
Subjective Responses
Yohimbine produced a significant increase in pre-cue anxiety in cocaine-dependent subjects but not in healthy controls (t212=4.13, p<0.001) (Table 1). This finding was consistent for both cocaine-dependent men (t212=3.34, p=0.001) and women (t212=2.50, p=0.01). In cocaine-dependent subjects, yohimbine increased anxiety, immediately (t212=4.81, p<0.001), five (t212=4.66, p<0.001) and 30-minutes (t212=2.37, p<0.05) after cue exposure (overall f1,53=4.7, p=0.035; Fig. 1a). There was no effect of yohimbine on post-cue anxiety in control subjects (Fig. 1a). In cocaine-dependent subjects, a significant gender by treatment interaction was found (f1,53=11.9, p=0.001). Compared with cocaine-dependent men, cocaine-dependent women reported greater anxiety in response to yohimbine immediately (t212=2.41, p=0.017) and five (t212=2.68, p=0.008) minutes following cue presentation (Fig. 1b).
Fig. 1.
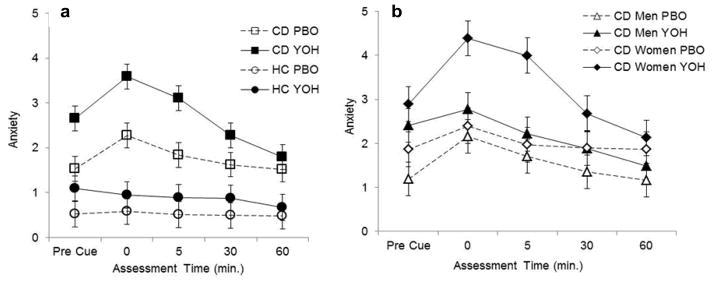
Comparison of mean (se) anxiety following pre-treatment with placebo (PBO) or yohimbine (YOH) between (a) cocaine-dependent subjects (CD) and healthy controls (HC) and (b) CD men and CD women; pre-, immediately post- (0), 5, 30, and 60 minutes after exposure to the cocaine cue
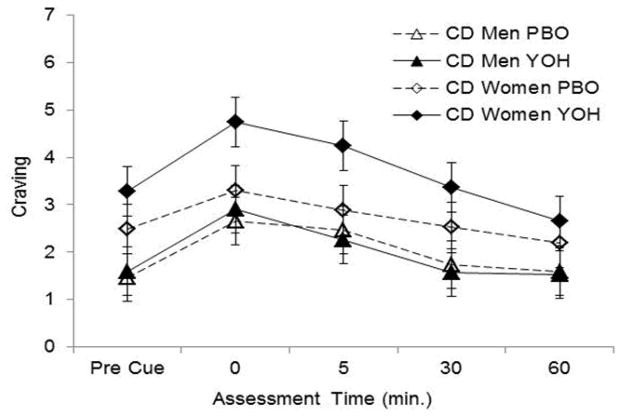
Yohimbine produced a trend level significant increase in pre-cue craving in cocaine-dependent women (t212=1.92, p=0.056) but not in cocaine-dependent men (Table 1). Exposure to the cue produced a significant increase in craving (t238=6.00, p<0.001) (Fig. 2). Yohimbine produced a significantly greater craving response as compared to placebo, immediately (t212=2.95, p<0.01) and five minutes (t212=2.05, p<0.05) following cue presentation. A significant gender by treatment interaction was found (f1, 53=8.2, p=0.006). Compared with cocaine-dependent men, cocaine-dependent women reported greater craving in response to yohimbine immediately (t212=2.10, p=0.037) and five (t212=2.74, p=0.007) minutes following cue presentation (Fig 2).
Fig. 2.
Comparison of mean (se) craving following pre-treatment with PBO (PBO) or yohimbine (YOH) between cocaine-dependent (CD) men and CD women; pre-, immediately post- (0), 5, 30, and 60 minutes after exposure to the cocaine cue
Endocrine Responses
Yohimbine produced a significant increase in salivary cortisol levels (t95=14.3, p<0.001) in both cocaine-dependent (t373=7.30, p<0.001) and control (t373=4.10, p=0.002) groups (Fig. 3a). There was no significant effect of cue presentation on salivary cortisol levels in either the cocaine-dependent or control groups, regardless of treatment or gender. Yohimbine produced a significant increase in salivary DHEA levels (t92=3.05, p=0.003) in both the cocaine-dependent (t751=2.20, p=0.028) and control (t751=2.11, p=0.036) groups (Fig. 3b). There was no effect of cue presentation on salivary DHEA levels in either cocaine-dependent or control groups, regardless of treatment or gender.
Fig. 3.
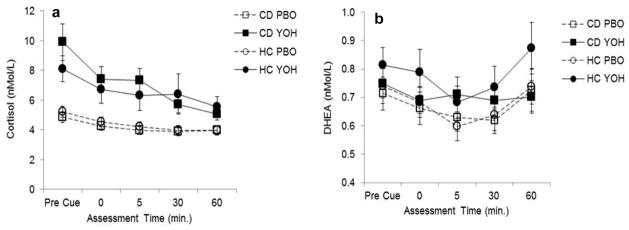
Comparison of mean (se) (a) cortisol and (b) DHEA following pretreatment with placebo (PBO) or yohimbine (YOH) between cocaine-dependent (CD) and healthy controls (HC); pre-immediately post (0), 5, 30, and 60 minutes after exposure to the cocaine cue
Heart Rate Responses
There was no effect of yohimbine on pre-cue heart rate (t875=0.83, p=0.407), however women had higher heart rates than men regardless of treatment (t875=4.90, p<0.001) (Figs. 4a and 4b). Yohimbine produced a significant increase in post-cue heart rate in all subjects (f1, 103=11.7, p<0.001). Yohimbine produced a significantly greater post-cue heart rate response in women as compared to men (f1,649=9.4, p=0.002).
Fig. 4.
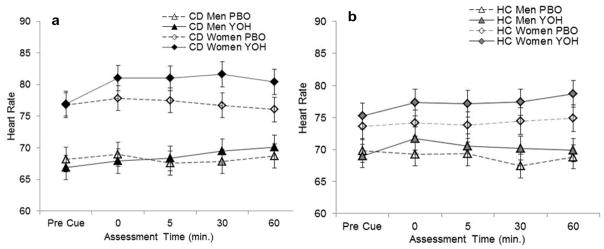
Comparison of mean (se) heart rate following pre-treatment with placebo (PBO) or yohimbine (YOH) between (a) cocaine-dependent (CD) men and CD women (b) healthy control (HC) men and women; pre- immediately post (0), 5, 30, and 60 minutes after exposure to the cocaine cue
Discussion
In the present study we investigated the impact of the pharmacological stressor yohimbine on subjective, physiologic and endocrine responses to a drug-paired cue in cocaine-dependent men and women and sex-matched control groups. Yohimbine increased salivary cortisol and DHEA levels and there were no differences between cocaine-dependent subjects and sex-matched control groups in the magnitude of these responses. Yohimbine produced a significant increase in heart rate; however, women had a greater response as compared to men. Yohimbine increased anxiety in cocaine-dependent subjects but not in healthy controls. Consistent with our hypothesis and the preclinical literature, yohimbine appeared to potentiate subjective responses to a cocaine-related cue (Banna et al. 2010; Feltenstein and See 2006). In addition, cocaine-dependent women reported greater anxiety and craving responses to yohimbine and the cue as compared to cocaine-dependent men (Feltenstein et al. 2011). To our knowledge, this is the first study to investigate endocrine, physiologic and subjective responses a pharmacologic stressor combined with a drug-related cue in cocaine-dependent men and women and to systematically investigate sex differences in these responses.
Relative to placebo, cocaine-dependent participants reported significantly higher anxiety following yohimbine administration even before exposure to the cocaine cue. These data are consistent with the findings of McDougle and colleagues (1994) that yohimbine administration to cocaine-dependent individuals during early discontinuation of cocaine use increased nervousness and panic-like symptoms (McDougle et al. 1994). The anxiogenic effects of yohimbine were not observed in healthy controls, which is consistent with a clinical study that found no effect of yohimbine on subjective responses in healthy controls (Swann et al. 2005). To our knowledge, this is the first clinical study to demonstrate significant differences in subjective responses to provocation of the NA system between cocaine-dependent individuals and healthy controls. Moreover, these findings support the preclinical studies suggesting that chronic cocaine use increases central NA tone and subsequently drives basal levels of anxiety during drug withdrawal (Harris and Aston-Jones 1993; Rudoy and Van Bockstaele 2007). We also found that yohimbine increased cue-induced anxiety and craving. Clinical studies have found that drug cues elicit anxiety in cocaine-dependent individuals (Fox et al. 2012; Sinha et al. 2003). The present findings suggest that yohimbine exacerbated the anxiogenic effects of the cue.
Stress potentiation of cocaine cue-induced anxiety and craving was greater in cocaine-dependent women as compared to cocaine-dependent men. These data are consistent with the preclinical findings that yohimbine, acting as a pharmacologic stressor, produces greater cue-induced reinstatement of cocaine-seeking behavior in female rodents compared with male rodents, as increased craving in the drug cue exposure paradigm is considered a proxy for an increased propensity to relapse in clinical situations (Feltenstein et al. 2011). In addition, there was a trend towards increased pre-cue craving following yohimbine in cocaine-dependent women, but not in men. Female rodents exhibit greater cocaine-seeking behavior in response to a yohimbine challenge than male rodents (Anker and Carroll 2010). Compared with male rodents, female rodents exhibit greater cross-sensitization to stimulants and stress, thus the present findings of elevated anxiety and craving in cocaine-dependent women but not in men may reflect sex differences in overlap between central stress and reward systems (Camp and Robinson 1988; Holly et al. 2012). This is particularly important since stress and drug-cues have traditionally been treated as independent factors in the clinical relapse process (Sinha et al. 2000). Although clinical studies of cocaine-dependent individuals suggest that stress is a significant risk factor for relapse in women (Back et al. 2005; McKay et al. 1996; Waldrop et al. 2009), the findings of the present study suggest that the stress-relapse connection is more complex and stress may enhance the salience of cocaine cues for cocaine-dependent women. As such, this study supports the idea that stress followed by drug-related cues could enhance the likelihood of relapse and provides a reasonable human laboratory model to explore sex differences in the stress-cue-relapse connection. Symptoms of cocaine withdrawal including, stress and drug-cue induced craving can be attenuated by medications that inhibit NA transmission (Fox et al. 2012; Jobes et al. 2011). Of note, a recent clinical study found that the alpha-2 agonist guanfacine attenuated craving, anxiety and negative affect responses to both stress and drug-cues in cocaine-dependent women but not in cocaine-dependent men (Fox et al. 2014). Thus, taken together with the present findings these data suggest that inhibition of NA transmission may be an effective treatment option for attenuating craving and risk for relapse in cocaine-dependent women.
Following drug cue exposure, yohimbine-treated subjects (both cocaine-dependent and healthy controls) exhibited significantly higher heart rate, cortisol and DHEA responses as compared with placebo administration. Noradrenergic transmission to the hypothalamus and hippocampus stimulates the hypothalamic pituitary adrenal (HPA) axis (Maccari et al. 1992a; Maccari et al. 1992b). Thus, the increase in adrenal steroid levels found in this study was not surprising. Since drug cues also increase NA and HPA axis activity in cocaine-dependent individuals, we expected that cocaine-dependent subjects would exhibit exacerbated heart rate and adrenal steroid responses to yohimbine and the drug cue (Sinha et al. 2003; Waldrop et al. 2009). However, there were no group differences in the magnitude of the physiologic or endocrine responses to the stressor and the drug cue. It is possible that the yohimbine challenge produced a ceiling effect precluding us from observing any additional increase in HPA axis or NA activity. Of note, cocaine-dependent and healthy controls exhibit similar cortisol levels in response to a corticotropin releasing hormone (CRH) challenge (Brady et al. 2009). Thus, taken with the present findings, it appears that adrenal regulation of steroid levels in response to pharmacological stress remains intact in cocaine-dependent men and women.
Of note, there were no group differences in heart rate responses to yohimbine. In a previous study, heart rate responses to a corticotropin releasing hormone challenge were significantly higher in cocaine-dependent women than control women (Brady et al. 2009). Thus, we expected that cocaine-dependent women would exhibit a greater heart rate response to yohimbine than control women. Perhaps lower doses of yohimbine would have elucidated differences in the sensitivity of peripheral NA tone between cocaine-dependent and healthy control women. The finding of heightened anxiety and craving response to yohimbine without any effects on either heart rate or HPA hormones suggests dysregulation in central NA tone. The disparity between the biologic and subjective responses to yohimbine is consistent with studies of substance-dependent individuals exposed to laboratory stress tasks (Back et al. 2005; Brady et al. 2009). Sinha and colleagues proposed that the cardiovascular, endocrine and subjective responses are semi-independent and represent different components of the stress response (Sinha et al. 2003). Specifically, subjective responses may represent emotional and behavioral adaptation; while endocrine and heart rate responses represent the biologic adaptation required to maintain homoeostatic balance. Neuroimaging studies may provide additional insight into the role of the NA system in mediating stress and cue reactivity in cocaine dependence.
These findings should be considered in light of some limitations. Subjective ratings and heart rate data were not collected prior to placebo and yohimbine administration, thus the results are based on pre-cue rather than pre-yohimbine assessments and therefore difficult to discern yohimbine effects independent of the cue. We did not control for ovarian hormone status. Elevated progesterone levels have been associated with subjective responses to laboratory stressors and cocaine cues in cocaine-dependent women and attenuates stress-induced potentiation of cue-induced cocaine-seeking behavior in female rodents (Feltenstein et al. 2011; Sinha et al. 2007). However, there was no difference between the groups of women in menstrual cycle day and both groups of women completed the study visit during the early luteal phase of the cycle, just prior to the rise in progesterone. Moreover, three of the healthy controls and none of the cocaine-dependent women reported taking oral contraceptives. Thus, it is unlikely that the present findings were confounded by ovarian hormone levels or oral contraceptives. Duration of abstinence from cocaine may have a significant impact on DHEA levels (Buydens-Branchey et al. 2002). Thus, variability in the duration of abstinence may have prevented us from detect group differences DHEA levels. Finally, yohimbine has moderate affinity for both serotonin and dopamine receptors and non-specific effects as a monoamine oxidase inhibitor (MAOI), it is possible that the present findings were not specific to the NA system (Bhattacharya et al. 1991; Millan et al. 2000). However, the anxiogenic effects of yohimbine were opposite of those that would be expected if yohimbine were acting as an MAOI.
Despite these limitations, this is the first study of stress potentiation of cue-reactivity in a clinical population of cocaine-dependent men and women. The present findings are in agreement with the preclinical literature suggesting that stress may increase the craving response to drug cues and this interaction may be particularly important in cocaine-dependent women. Importantly, these findings expand a growing literature suggesting important sex/gender differences in risk factors for relapse in cocaine dependence and suggest that medications that attenuate NA activity may be particularly effective treatment strategies for cocaine-dependent women.
Acknowledgments
Funding: This work was supported by National Institutes of Health; National Institute on Drug Abuse and the Office of Research on Women’s Health P50DA016511, National Institute of Child Health and Human Development K12 HD055885, National Center for Advancing Translational Sciences UL1TR000062.
Footnotes
Conflict of Interest: Authors Moran-Santa Maria, Baker and Ramakrishnan declare no conflict of interest. Kathleen Brady lists: Consultant AstraZeneca Pharmaceuticals Aimee McRae lists: Forest Pharmaceuticals medication provided for separate NIH grant.
References
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–60. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–7. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behavioural brain research. 2010;208:144–8. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Clow A, Przyborowska A, Halket J, Glover V, Sandler M. Effect of aromatic amino acids, pentylenetetrazole and yohimbine on isatin and tribulin activity in rat brain. Neuroscience letters. 1991;132:44–6. doi: 10.1016/0304-3940(91)90429-w. [DOI] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Archives of general psychiatry. 2009;66:422–30. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiology & behavior. 2012;105:209–14. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiology & behavior. 2009;98:614–7. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Dorota Majewska M. Perturbations of plasma cortisol and DHEA-S following discontinuation of cocaine use in cocaine addicts. Psychoneuroendocrinology. 2002;27:83–97. doi: 10.1016/s0306-4530(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behavioural brain research. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Conditioned responses in a methadone population. A comparison of laboratory, clinic, and natural settings. J Subst Abuse Treat. 1986;3:173–9. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–27. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- First MB, Frances AJ, Pincus HA, Vettorello N, Davis WW. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 2006;185:348–57. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R. Sex Differences in Guanfacine Effects on Drug Craving and Stress Arousal in Cocaine-Dependent Individuals. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. Journal of psychopharmacology. 2012;26:958–72. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–6. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology. 2012;224:179–88. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–8. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:7856–61. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Mormede P, Piazza PV, Simon H, Angelucci L, Le Moal M. Hippocampal type I and type II corticosteroid receptors are modulated by central noradrenergic systems. Psychoneuroendocrinology. 1992a;17:103–12. doi: 10.1016/0306-4530(92)90049-d. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Rouge-Pont F, Angelucci L, Simon H, le Moal M. Noradrenergic regulation of type-I and type-II corticosteroid receptors in amygdala and hypothalamus. Brain research. 1992b;587:313–8. doi: 10.1016/0006-8993(92)91013-5. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994;51:713–9. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. The Journal of nervous and mental disease. 1996;184:616–22. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Drysdale E, Cahill L, Shajahan P, Ebmeier KP. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999;29:1083–8. doi: 10.1017/s0033291799008703. [DOI] [PubMed] [Google Scholar]
- Patterson HD, Thompson R. Recovery of inter-block information when block sizes are unequal. Biometrika. 1971;58:545–564. [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:1119–29. doi: 10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Drobes DJ, Coffey SF, Dansky BS, Brady KT, Kilpatrick DG. PTSD symptom severity as a predictor of cue-elicited drug craving in victims of violent crime. Addict Behav. 2003;28:1611–29. doi: 10.1016/j.addbeh.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–51. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–52. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and biological methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stockwell T, Murphy D, Hodgson R. The severity of alcohol dependence questionnaire: its use, reliability and validity. Br J Addict. 1983;78:145–55. doi: 10.1111/j.1360-0443.1983.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005;57:1209–11. doi: 10.1016/j.biopsych.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, Spratt EG, Kreek MJ, Brady KT. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]