Abstract
RGK proteins belong to the Ras superfamily of monomeric G-proteins, and currently include four members– Rad, Rem, Rem2, and Gem/Kir. RGK proteins are broadly expressed, and are the most potent known intracellular inhibitors of high-voltage-activated Ca2+ (CaV1 and CaV2) channels. Here, we review and discuss the evidence in the literature regarding the functional mechanisms, structural determinants, physiological role, and potential practical applications of RGK-mediated inhibition of CaV1/CaV2 channels.
1. INTRODUCTION
RGK (Rad, Rem, Rem2, Gem/Kir) proteins are a four-member subfamily of the Ras superfamily of monomeric G-proteins. Rad was first discovered as a protein over-expressed in skeletal muscle of type II diabetic humans [1]; Gem as a mitogen-induced gene in human T cells [2]; Rem was originally cloned using a degenerate PCR strategy based on similarity to Rad and Gem [3]; and Rem2 was cloned from a rat brain cDNA library [4]. Functionally, individual RGKs have been linked to diverse functions in different cell types and tissues including (but not limited to): promotion of cell shape remodeling via regulation of cytoskeletal dynamics (Gem) [5-9]; induction of apoptosis in cardiac myocytes (Rad) [10]; regulation of synapse development and dendritic morphology (Rem2) [11, 12]; control of neuronal proliferation and apoptosis during embryogenesis, and survival of human embryonic stem cells (Rem2) [13, 14].
All RGK proteins powerfully inhibit high-voltage-activated Ca2+ (CaV1/CaV2) channels [15-17]. In this review, we discuss the experimental evidence that underlies current understanding of the mechanisms and structural determinants underlying RGK regulation of CaV1/CaV2 channels, its potential physiological role, and the state of efforts to exploit this channel regulation for practical applications.
2. BASIC STRUCTURE-FUNCTION OF RGK PROTEINS
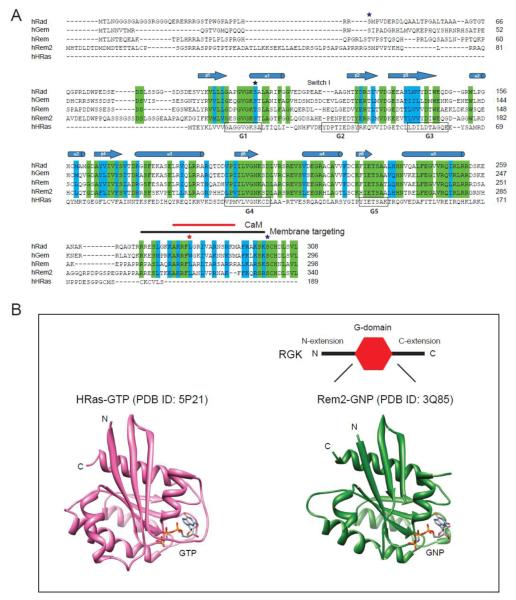
Similar to other Ras superfamily proteins, RGKs contain a guanine nucleotide binding domain (G-domain) [18-23]. In comparison to Ras, RGK proteins have relatively large N– and C–termini extensions, and non-conservative substitutions in the G-domain of residues critical for GTP binding and hydrolysis [1-4] (Fig. 1). Within the RGK family, the N-termini extensions have variable lengths and display low sequence conservation. The functional significance of the N-terminus extensions in RGKs is unknown. The C-terminus extensions consist of a distal conserved region (~40 residues) separated from the G-domain by a relatively short (12 – 22 residues) variable linker sequence. The C-termini of RGK proteins lack the CAAX (C= cysteine, A= aliphatic, X= any amino acid) prenylation motif that is common to many Ras superfamily proteins, and directs their anchoring to membranes [18]. Nevertheless, RGKs target to the plasma membrane using basic and hydrophobic residues in their C-terminus extensions [24, 25]. The membrane-targeting region in the C-termini of RGK proteins overlaps with a calmodulin (CaM) binding domain (CBD) that mediates RGK interactions with Ca2+-CaM [26-29] (Fig. 1A). 14-3-3 proteins bind as dimers to all four RGKs, and this requires phosphorylation of two distinct serines in the N- and C-termini extensions, respectively [6, 26-28, 30] (Fig.1A). The functional significance of CaM and 14-3-3 binding to RGKs is unknown, though it has been suggested that these interactions may regulate the subcellular localization or stability of RGK proteins [6, 27, 28].
Figure 1. Structural features of RGK proteins.
(A) Sequence alignment of human RGK proteins and H-Ras. Identical residues in RGKs are shaded green, and similar residues shaded in cyan. ★ residues homologous to RasS17; ★ hydrophobic residues important for CaM binding and membrane targeting; ★serine residues important for 14-3-3 binding. (B) Crystal structures of GTP-bound H-Ras (PDB ID: 5P21) and GNP-bound Rem2 G-domain (PDB ID: 3Q85).
The G-domains of all RGKs have been functionally demonstrated to be bona fide guanine nucleotide binding proteins, although there may be quantitative differences within the family [1-4, 22, 23]. For example, Rad displays higher affinity (Kd ≈ 100 nM) for guanine nucleotides compared to Gem (Kd ≈ 1 – 20 μM) [23]. Moreover, Gem displays a 5- to 10-fold higher affinity for GDP vs GTP, whereas Rad displays no such preference [23]. Crystal structures of RGK proteins confirm that their G-domains adopt a fold comprised of a six-stranded β-sheet surrounded by five α-helices [20-22], similar to that found in other Ras superfamily members [18, 19] (Fig. 1B). In Ras superfamily proteins, five loops within the G-domain (G1 – G5) are highly conserved and form the guanine nucleotide-binding site (Fig. 1). The G1-box (or P-loop) with consensus sequence GXXXXGKS/T is conserved in RGKs, and similar to Ras, engages in interactions that co-ordinate the α/β phosphates and Mg2+ [20-22]. Similarly, G4 (NKXD) and G5 (ETSA) motifs are conserved in RGKs and participate in recognition of the guanine base. In Ras, G2 (XTX) and G3 (DXAG) motifs reside within regions referred to as Switch I and Switch II, respectively. In Ras-GDP, Switch I and Switch II are disordered, suggesting a high flexibility of these regions. The Thr35 and Gly60 residues in Ras G2 and G3, respectively, act as sensors for the γ phosphate of GTP. Hence, in Ras, GTP binding leads to a conformational change in which switches I and II are stabilized [31]. Since RGKs do not have the equivalent of Thr35 in switch I and show non-conservative substitutions in G3 (DXWE instead of DXAG), it has long been suggested that they may not display the canonical GTP-regulated switch mechanism evident in most Ras superfamily G-proteins [31]. Indeed, crystal structures of Rad and Rem2 bound to a non-hydrolyzable GTP analog showed little structural changes from the GDP-bound forms, with no evidence of a GTP-mediated stabilization of switch I and II [23]. A caveat that must be mentioned is that all RGK protein crystal structures to date are of truncated proteins in which the N- and C-termini extensions have been removed (Fig. 1B). In some other G-proteins such as Ran and Arf, the canonical switch mechanism is modified by additional conformation changes in C- and N-termini extensions, respectively [31]. To this point, a crystal structure of GDP-bound Gem that includes the initial part of the C-terminus extension has been reported [22]. In this structure, the proximal C-terminus extension forms a helix that contacts with an interswitch region between the β2 and β3 strands of the G-domain, as well as the α5 helix. Moreover, in functional assays, the presence of the N- and C-termini extensions in Gem increases its GTPase activity by 20-fold [22].
The rate of intrinsic GTP hydrolysis has been measured for Rad [23, 32] and Gem [22, 23]. Purified Rad has a low rate of intrinsic GTPase activity which can be dramatically increased by cytosolic fractions derived from different tissues [32]. Sub-fractionation of liver cytosol led to the identification of nucleoside diphosphate kinase (nm23 or NDPK) as a GTPase activating protein (GAP) for Rad and Gem (but not Rem) [33]. In an unusual twist, nm23 also acted as a functional guanine nucleotide exchange factor (GEF) for Gem due to its diphosphate kinase catalyzing the transfer of phosphate from ATP to a bound GDP in Gem [33]. To date, nm23 is the only known GAP (and GEF) for any RGK protein.
3. BASIC STRUCTURE-FUNCTION OF CaV CHANNELS
Ca2+ influx through voltage-dependent Ca2+ (CaV) channels plays a critical role in various biological functions including muscle contraction, synaptic transmission, hormone secretion, and gene expression [34, 35]. CaV channels are divided into two main families based on their threshold for activation: low-voltage-activated (LVA) Ca2+ channels and high-voltage-activated (HVA) Ca2+ channels. There are three types of LVA Ca2+ channels (T-type: CaV3.1-3.3) [36], and seven types of HVA Ca2+ channels (L-type: CaV1.1–1.4; P/Q-type: CaV2.1; N-type: CaV2.2; R-type: CaV2.3) [34, 37].
HVA CaV channels are hetero-multimeric proteins comprised of pore-forming α1 subunits and auxiliary β, α2δ, and sometimes γ subunits. So far, seven genes encoding HVA α1 subunits (CaV1.1 [α1S]; CaV1.2 [α1C]; CaV1.3 [α1D]; CaV1.4 [α1F]; CaV2.1 [α1A]; CaV2.2 [α1B]; CaV2.3 [α1E]) [37], four genes encoding CaVβ (CaVβ1-4), and four genes encoding α2δ (α2δ1-4) have been identified [34]. Pore-forming α1 subunits are all comprised of four homologous domains (I–IV) each with six transmembrane segments (S1–S6). CaVβs are required for efficient targeting of α1 subunits to the plasma membrane [38-42], enhancing channel open probability (Po) [41], normalizing the voltage-dependence of channel activation [43-46], and modulating inactivation [43, 47-50]. CaVβs bind with high affinity to a conserved 18-residue sequence (the α interaction domain, or AID) located in the loop connecting domains I and II (I-II loop) of α1 subunits [51-53].
4. RGK INHIBITION OF VOLTAGE-DEPENDENT CALCIUM CHANNELS
4.1. Discovery and basic properties
The separate fields of RGK proteins and CaV channels intersected when a yeast two hybrid screen of insulin-secreting MIN6 cells identified Gem/Kir as a CaVβ3-binding protein [15]. Co-expression of recombinant CaV1.3 or CaV1.2 channels with Gem in Xenopus oocytes resulted in a complete and constitutive inhibition of both channel types [15]. Subsequently, it was found that the ability to inhibit CaV1.2 channels applied to all RGK proteins [16, 54]. Since these seminal studies, RGK proteins have been shown to indiscriminately and potently inhibit all high-voltage-activated channels tested including, CaV1.1 [55], CaV1.2 [17, 26, 28, 54, 56-60], CaV1.3 [15], CaV2.1 [15, 61, 62], and CaV2.2 [15, 56, 63]. By contrast, low-voltage-activated T-type (CaV3.1 – CaV3.3) channels are unaffected by RGK proteins [16, 63]. RGK inhibition of CaV1 and CaV2 channels takes place in all cell types studied to date, including heterologous expression systems (e.g. HEK cells, Xenopus oocytes), cell lines containing endogenous CaV channels (e.g. PC12 cells, MIN6 cells), and primary cells (heart, skeletal muscle and neurons).
4.2. RGKs use multiple mechanisms to inhibit CaV channels
The question of how RGKs inhibit CaV1 and CaV2 channels has been intensely studied by several groups. The whole-cell calcium current (ICa) is related to microscopic channel properties by the relation: ICa = N × FA × i × Po, where N is the total number of channels in the surface membrane, FA is the fraction of activatable channels, i is the unitary current amplitude, and Po is the single-channel open probability. In principle, RGKs could inhibit ICa by reducing any one of the four parameters or a combination of them. To address whether RGKs reduce N, Beguin and colleagues used a CaV1.2 pore-forming α1C subunit harboring a hemagluttinin (HA) epitope tag in an extracellular loop. Combining immunofluorescence and confocal microscopy, they found that all four RGKs prevent surface expression of HA-tagged CaV1.2 channels reconstituted in either PC12 or HEK 293 cells [15, 26, 27]. By contrast, using a surface biotinylation/Western blot detection approach, Finlin et al, found that Rem2 inhibited ICa in mouse insulinoma MIN6 cells without reducing the number of endogenous CaV1.2 channels at the membrane [54]. Similarly, Ikeda and colleagues found that Rem2 inhibits CaV2.2 channels stably expressed in tsA201 cells without decreasing channel surface density as determined by radio-labeled ω-conotoxin GVIA binding assays [63]. These two latter reports suggested that Rem2 inhibited CaV1.2 and CaV2.2 channels directly at the cell surface, although the exact mechanisms were not investigated.
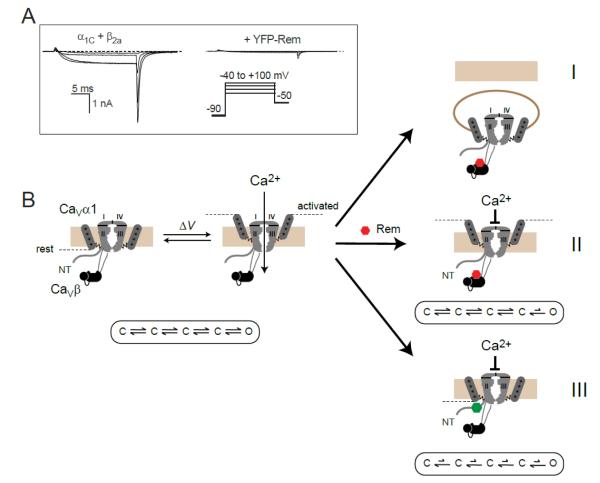
By combining optical detection of surface epitope-tagged α1C subunits with quantum dot and high throughput flow cytometry measurements, our group discovered that Rem partially decreases (by 60%) the surface density of recombinant CaV1.2 channels reconstituted in HEK 293 cells [57]. This decrease was completely prevented by co-expressing dominant negative dynamin, suggesting that Rem increased the rate of dynamin-dependent CaV1.2 endocytosis, rather than interfered with forward trafficking of the channel [57] (Fig. 2, mechanism I). Interestingly, even when Rem-induced decrease in channel surface density was completely reversed with dominant negative dynamin, the inhibition of ICa was not rescued, suggesting that Rem could also block the activity of surface channels. We distinguished two separate mechanisms Rem used to block CaV1.2 channels at the cell surface. First, Rem could decrease ICa by diminishing channel Po without accompanying reductions in voltage sensor movement (Fig. 2, mechanism II). Targeting the Rem G-domain to the membrane either by the Rem C-terminus or a generic membrane targeting module is sufficient to reconstitute mechanism II [57]. Second, we discovered that Rem reduced maximal gating charge (Qmax) of CaV1.2 channels in a manner that was not accounted for by a decrease in N. This result suggested that Rem partially immobilizes CaV1.2 channel voltage sensors (Fig. 2, mechanism III). On the assumption that all four voltage sensors are required to move for the channel to open, the decreased Qmax suggests that Rem reduces the fraction of activatable (FA) CaV1.2 channels on the cell surface. Overall, this study established that within the same experimental system Rem utilized at least three separable mechanisms to inhibit recombinant CaV1.2 channels [57, 64].
Figure 2. Rem inhibits CaV1.2 channels using multiple mechanisms.
(A) Left, exemplar whole-cell currents from HEK 293 cells expressing CaV1.2 α1C + β2a. Right, representative α1C + β2a channel currents in the presence of Rem. (B) Rem inhibits ICa using three independent mechanisms: by reducing channel surface density via enhanced dynamin-dependent endocytosis (mechanism I); by reducing channel Po independently of channel voltage sensor movement (mechanism II); by partially immobilizing channel voltage sensors as reported by a decrease in maximal gating charge (Qmax; mechanism III). Mechanisms I and II persist with RemT94N, suggesting they do not require GTP to be bound to the Rem nucleotide binding domain (represented as a red hexagon). By contrast, mechanism III is selectively lost with RemT94N suggesting a requirement for GTP binding to Rem (represented as a green hexagon). Figure modified from [57] and [17].
Several groups have reported that over-expressing distinct RGKs in cardiac myocytes or skeletal myotubes dramatically decreases endogenous ICa,L [55, 58-60]. In three of these studies the impact of RGKs on Qmax was also measured. Murata et al [60] found that over-expressing Gem in adult guinea pig ventricular myocytes profoundly inhibited CaV1.2 channel Qmax (70% reduction). By contrast, over-expressing Rem in guinea pig ventricular myocytes [59] or skeletal myotubes [55] yielded smaller reductions in CaV1.2 (33% reduction) and CaV1.1 (44% reduction) Qmax, respectively. RGK mediated decrease in CaV1.1/CaV1.2 channel Qmax could result from a decrease in the surface density of channels or an ability of RGKs to partially immobilize channel voltage sensors. The impact of RGKs on ICa,L in cardiac myocytes has often been interpreted to reflect a decrease in the surface density of CaV1.2 channels [58, 60, 65]. However, we discovered that acute treatment of control and Rem-over-expressing myocytes with the CaV1.2 channel agonist, BAY K 8644, eliminated the inhibitory effect of Rem and resulted in ICa,L of comparable amplitude between the two conditions [59]. This result not only demonstrated that Rem-inhibited ICa,L can be rescued pharmacologically, but also indicated that the majority of CaV1.2 channels must be present at the cell surface in cardiac myocytes over-expressing Rem. This contrasts with the finding that Rem significantly reduces the surface density of recombinant CaV1.2 channels reconstituted in PC12 or HEK 293 cells [17, 57], suggesting that although RGKs can inhibit CaV1.2 channels using multiple distinct mechanisms, only particular subsets of these may be available and used in different cellular contexts.
5. STRUCTURAL DETERMINANTS ON RGKs IMPORTANT FOR ICa INHIBITION
5.1. Role of the RGK C-terminus
Much work has focused on defining the important structural elements on RGKs that mediate their potent inhibition of CaV1/CaV2 channels. Deleting the N-terminus extension from different RGKs does not abolish their ability to inhibit ICa [61-63], suggesting that this non-conserved feature is not critical for this effect. By contrast, several investigators have demonstrated that deleting the distal C-terminus of individual RGKs generates truncated versions (e.g. Rem265, Rad276, Gem264) that do not block ICa [16, 56, 57, 59, 62, 63, 66]. Hence, the distal C-terminus of RGKs is clearly critical for the mechanism of CaV channel block. Nevertheless, the precise manner in which the C-termini of RGKs participate in ICa inhibition is not completely clear, though the available evidence suggests multiple mechanisms may be in play. Ambiguities arise in part due to the overlapping roles of RGK C-termini– as membrane-targeting modules, as CaM binding sites, and as bearers of nuclear localization signals (NLS) (Fig. 1A).
5.2. Is RGK C-terminus sufficient to inhibit ICa?
Several investigators have sought to determine whether the C-terminus of different RGK proteins is sufficient to inhibit ICa with mixed results. Ikeda and colleagues found that over-expressing the C-terminus of Rem2 in SCG neurons did not block endogenous CaV2.2 channels, whereas full-length Rem2 strongly inhibited ICa [63]. Similarly, expression of the Rem distal C-terminus (final 32 residues) did not inhibit recombinant CaV1.2 channels in tsA201 cells [66]. By contrast, Leyris et al found that the final 75 residues of Gem, which includes the entire C-terminus extension, was sufficient to fully inhibit recombinant CaV2.1 channels reconstituted in Xenopus oocytes [67]. Recently, a 12-amino acid peptide containing residues K265–K276 in the Gem C-terminus was shown to be sufficient to acutely inhibit CaV2.1 channels expressed in Xenopus oocytes, albeit at high concentrations [62]. In this study, the Gem K265–K276 peptide was directly applied to CaV2.1 in the inside-out patch configuration, explicitly demonstrating that the inhibitory effect was achieved at the level of surface channels. Remarkably, a scrambled Gem K265–K276 peptide, which contained the same amino acid residues as the original but in different positions, also effectively inhibited CaV2.1 in inside-out patches [62], suggesting that the amino acid content of this region rather than its sequence was important for channel inhibition. The region corresponding to Gem residues K265-K276 is quite well conserved amongst RGK proteins (Fig. 1A). Therefore, the inability of Rem2 and Rem C-termini to inhibit CaV2.2 channels in SCG neurons [63] and CaV1.2 in tsA201 cells [66], respectively, may indicate that the sufficiency of this region to block ICa, as observed with Gem K265–K276 inhibition of CaV2.1 channels [62, 67] may not be a general property. Alternatively, one possible explanation for the discrepancies could be that the effective concentration of the expressed C-terminal fragment was different in these studies-higher in oocyte studies and lower in cell lines. Further work is needed to clarify this issue, as well as the mechanism K265-K276 peptide uses to decrease CaV2.1.
5.3 Role of RGK membrane targeting
In many cell types, RGK proteins autonomously target to the inner leaflet of the plasma membrane via their C-termini interacting with membrane phosphatidylinositol lipids [24, 25, 56, 63, 66]. Deleting the distal C-terminus of RGKs eliminates both their ability to inhibit ICa and their membrane targeting [56, 63, 66]. The potential importance of RGK membrane targeting in the mechanism of ICa inhibition has been investigated by several groups. Replacing the C-termini of either Rem2 [63] or Rem [66] with the tail of K-Ras4B which consists of a polybasic region and a CAAX prenylation motif, restored both membrane targeting and inhibition of CaV2.2 and CaV1.2 channels, respectively. For Rem2, the polarity of the membrane targeting domain appeared to be important, as attaching the first 10 residues from Gαi1, which contains myristoylation and palmitoylation motifs, to the N-terminus of truncated Rem2 (Rem2ΔC) restored membrane targeting but not inhibition of CaV2.2 channels [63]. By contrast, attaching a palmitoylated peptide to the N-terminus of truncated Rem (Rem265) restored both membrane targeting and inhibition of CaV2.2 channels stably expressed in tsA201 cells [56]. Overall, the available data suggest that one way the RGK C-terminus participates in ICa inhibition depends on its ability to target the RGK G-domain to the plasma membrane. We exploited this feature to create a small-molecule-inducible CaV1/CaV2 channel inhibitor by fusing the C1 domain from protein kinase Cγ to either the N- or C-terminus of YFP-Rem265 [17, 56, 57]. The resulting constructs, C1PKCγ-YFP-Rem265 or YFP-Rem265-C1PKCγ, are cytosolic when expressed in HEK 293 cells, but are rapidly recruited to the membrane upon exposure to phorbol-12,13-dibutyrate (PdBu) [56, 57]. Inhibition of CaV1.2 and CaV2.2 channels occurs concomitantly with the dynamic translocation of C1PKCγ-YFP-Rem265 or YFP-Rem265-C1PKCγ to the plasma membrane [17, 56, 57]. These molecules were termed genetically encoded molecules for inducibly inactivating CaV channels (GEMIICCs) [56]. GEMIICCs acutely inhibited ICa without affecting Qmax, suggesting they lowered current solely by decreasing channel Po [56, 57] (Fig. 2, mechanism II). Beyond providing insights into the mechanism by which membrane targeting of RGKs results in ICa inhibition, GEMIICCs provide a proof-of-concept that new functionalities (in this case inducible inhibition) can be engineered into RGKs.
5.4. Role of CaM binding and nuclear localization of RGKs
In addition to mediating targeting to the membrane, the C-terminus of RGKs binds CaM and possesses nuclear localization signals (Fig. 1A). A useful mutation widely used in the field converts a hydrophobic residue in the RGK C-terminus (corresponding to GemW269 RemL271 RadL281 Rem2L317) to glycine (Fig. 1A). The effects of this mutation are complex since it diminishes CaM binding [26-28], prevents membrane targeting [57], and dramatically increases nuclear localization of RGKs [26-28, 57]. Hence, the functional consequences of this mutation in RGKs need to be interpreted carefully. GemW269G consistently shows a diminished ability to inhibit ICa compared to wild-type Gem. This effect has been demonstrated for GemW269G inhibition of CaV1.2 channels in PC12 cells [15, 28] and cardiac myocytes [60], and CaV2.2 channels in SCG neurons [6]. Similarly, RadL281G displayed a diminished ability to inhibit CaV1.2 channels in PC12 cells, whereas RemL271G retained full inhibitory activity in the same system [26]. The precise reason for the functional differences between GemW269G/RadL281G and RemL271G is not clear. Because these mutations markedly increase the nuclear localization of the respective RGK protein, it was suggested that nuclear sequestration of CaVβ by RadL281G and RemL271G could represent a mechanism to regulate surface CaV1.2 channels [26]. An ambiguity with these experiments is that though the mutant RGKs are enriched in the nucleus, a significant portion remains in the cytosol. Hence, in the case of RemL271G it was unclear whether inhibition of CaV1.2 channels was achieved via the nuclear or cytosolic pools of the mutant protein. We examined this question by examining the effect of RemL271G on CaV1.2 channels reconstituted in HEK 293 cells [57]. YFP-RemL271G in HEK cells was present in both the nucleus and cytosol and caused a partial inhibition of ICa,L when compared to wild type Rem. Attaching a nuclear localization signal (NLS) to YFP-RemL271G localized it exclusively to the nucleus. Interestingly, NLS-YFP-RemL271G was completely inert with respect to ICa,L inhibition. Conversely, attaching a nuclear export signal (NES) to YFP-RemL271G targeted it exclusively to the cytosol, and NES-YFP-RemL271G completely blocked ICa,L, explicitly demonstrating that the cytosolic pool is the active component for channel inhibition. These results support three consequential conclusions. First, the ineffectiveness of GemW269G and RadL281G to inhibit ICa may be because their nuclear localization reduces their active concentration in the cytosol. This interpretation is consistent with the finding that RGK inhibition of ICa is dose-dependent [68]. Second, that CaM binding is not necessary for RGK inhibition of ICa. Third, that membrane targeting of RGKs is not an absolute requirement for ICa inhibition, since the non-membrane-targeted NES-YFP-RemL271G is an effective blocker of CaV1.2 channels.
5.5 Role of nucleotide binding and hydrolysis in RGK inhibition of ICa
A canonical feature of Ras superfamily G-proteins is that they function as guanine nucleotide-regulated molecular switches, cycling between inactive GDP-bound and active GTP-bound conformations [18]. There is tremendous ambiguity as to whether and how this basic defining property plays a role in RGK regulation of CaV1/CaV2 channels. All RGKs have been demonstrated to be bona fide guanine nucleotide binding proteins though there may be quantitative differences among them with respect to relative affinities for GDP and GTP, and the intrinsic rate of GTP hydrolysis. Comparing crystal structures of GTP- and GDP-bound Rad and Rem2 suggest RGKs do not undergo the classical switch mechanism observed in Ras, which involves a GTP-mediated stabilization of otherwise disordered Switch I and Switch II regions [23, 31]. An important approach used to investigate the potential role of GTP binding and hydrolysis of RGKs is to introduce point mutations that decrease their affinities for guanine nucleotides. In Ras, a S17N mutation locks the protein in a GDP-bound state [69]. RasS17N has a dominant negative effect on Ras signaling in cells because it has a higher affinity for, and sequesters, Ras-GEFs [69]. The residue corresponding to Ras S17 is conserved in all RGKs (RadS105, GemS89, RemT94, Rem2S129) (Fig. 1A). Similar to the S17N mutation in Ras, GTP binding is abolished in RadS105N and GemS89N, although the affinity to GDP is also reduced [23, 32].
Over a series of papers, Beguin and colleagues demonstrated using pull-down assays that RadS105N, GemS89N, RemT94N, and Rem2S129N displayed decreased binding to CaVβ subunits compared to their wild-type counterparts [26-28]. This was interpreted as indicating that GTP binding was necessary for RGKs to bind CaVβs. However, the impact of these mutations on the ability of the distinct RGKs to inhibit ICa was not evaluated in these studies. A different conclusion regarding the role of nucleotide binding in RGKs was reached in a study where the Rem2/CaVβ2a interaction was unaffected when Rem2 was loaded with either GDP or GTP [24].
A number of studies have focused on evaluating the functional impact of the nucleotide binding state of RGKs on their ability to inhibit ICa, with mixed results Chen et al found that Rem2S129N down-regulated ICa to the same extent as wild type Rem2 in sympathetic neurons [63]. However, the same group also found that GemS89N lost the ability to inhibit ICa in sympathetic neurons [6]. This discrepancy hints at a fundamental difference between these two RGKs with respect to the role of nucleotide binding in ICa inhibition in neurons. RadS105N has been reported to have no effect on recombinant CaV1.2 channels reconstituted in HEK 293 cells, but to exert a dominant negative effect in cardiac myocytes, increasing endogenous ICa,L [70]. By contrast, we found that RemT94N potently inhibited CaV1.2 channels in HEK 293 cells, but with an interesting difference from wild-type Rem. Whereas wild-type Rem significantly decreased gating currents, RemT94N blocked ICa without impacting Qmax [57]. This result suggested that the ability of Rem to inhibit voltage sensor movement is selectively dependent on GTP binding (Fig. 2, mechanism III). Surprisingly, over-expressing RemT94N in heart cells had no impact on endogenous ICa,L, in contrast to wild-type Rem which markedly decreased ICa,L [59]. Given that RemT94N does inhibit CaV1.2 channels in HEK 293 cells, this result suggests the existence of a cardiac specific mechanism that inactivates the ability of GDP-bound Rem to inhibit ICa,L in heart. A caveat of all the functional studies described here is they rely on mutations predicted to lock RGK proteins in a GDP-bound state. It is possible that these mutations may also have some unanticipated effects that could confound interpretation of results. To circumvent this problem, Chen et al attempted to reverse Rem2 inhibition of ICa in sympathetic neurons by dialyzing in GDP-β-S via the patch pipette [63]. They observed no reversal of ICa inhibition over 20 minutes of GDP-β-S dialysis, suggesting that GTP binding may not be necessary for Rem2 inhibition of ICa in sympathetic neurons.
6. STRUCTURAL DETERMINANTS ON CaV1/CaV2 CHANNELS IMPORTANT FOR RGK INHIBITION
6.1. Role of auxiliary β subunits in RGK regulation of CaV channels
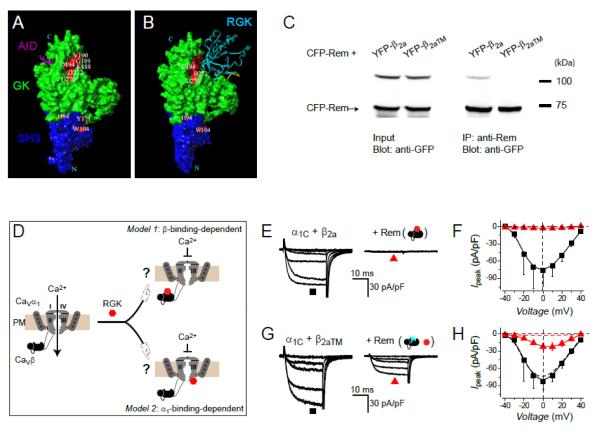
All RGK proteins bind CaVβ subunits [15-17, 26, 56, 71-73]. The affinity of RGK/CaVβ association is about an order of magnitude lower than the interaction of CaVβ with the AID present in the I-II loop of CaV1/CaV2 pore-forming α1 subunits [56]. The relatively low affinity of the RGK/CaVβ interaction may explain the lack of a high resolution crystal structure for this complex. Beguin et al. conducted an extensive mutagenesis screen of CaVβs and RGKs to identify mutations that interrupted their mutual interaction without disrupting their global tertiary structures [72]. Mutations on CaVβ that eliminated interaction with RGKs clustered at a hotspot region that was distinct from the α-binding pocket that binds the AID [74-76] (Fig. 3A). Based on results from this mutagenesis screening and the known structures of RGKs and CaVβs, the authors generated a homology model in which Gem was docked to the identified hotspot on CaVβ (Fig. 3B).
Figure 3. Role of CaVβ-binding in Rem inhibition of CaV1.2 channels.
(A) Mutations on CaVβ that disrupt binding to RGK proteins aggregate at a hotspot that is separate from the α1-binding pocket that binds AID. Modified from [72]. (B) Computational model showing docking of Gem to the CaVβ RGK-binding hotspot. Modified from [72]. (C) Co-immunoprecipitation assay demonstrating that a mutated β2a, β2aTM, no longer interacts with Rem. (D) Cartoon showing two possible modes of RGK inhibition of CaV1.2 channels– β-binding-dependent and α1-binding-dependent. (E) Exemplar currents from HEK 293 cells expressing α1C + β2a in the absence (left) and presence (right) of Rem. (F) Population current-voltage (I-V) relationships for α1C + β2a in the absence (■) and presence (▲) of Rem. (G, H) Data for α1C + β2aTM ±Rem. Same format as E and F. Figure reproduced from [17].
It was initially suggested that RGKs bind to CaVβs and prevent their interaction with α1 subunits, thereby compromising their chaperone function and severely limiting channel trafficking to the membrane [15, 26, 77]. However, subsequent work has shown that RGKs do not disrupt the α1-β interaction, and there is consensus that RGK inhibition of ICa involves a ternary α1/β/RGK complex [24, 54, 56, 57, 63]. Within this ternary complex framework, there are two possible ways in which CaVβ could play a role in RGK inhibition of ICa. In the first scenario, a direct RGK/CaVβ interaction is not necessary for ICa inhibition. However, CaVβ could play a role by promoting a permissive conformation of the channel complex that is necessary for functional interaction with RGKs. Alternatively, a direct RGK/CaVβ interaction could be obligatory for the mechanism of RGK inhibition of ICa. Evidence has been provided for both scenarios, and there are indications that there may be specificity for different RGK/CaV channel combinations as discussed below. An important tool that made these advances possible was the discovery of specific mutations in CaVβs that selectively eliminated binding to RGKs without compromising functional regulation of CaV α1 subunit trafficking and gating [17, 61, 72] (Fig. 3C).
Jian Yang’s group used a mutated CaVβ that no longer binds RGKs to demonstrate that direct interaction with CaVβ was not necessary for Gem to acutely inhibit CaV2.1 channels in Xenopus oocytes [61]. Nevertheless, they further showed that β binding to α1A was necessary for Gem to downregulate CaV2.1. This latter effect was demonstrated using a β with weakened affinity for α1A, which could be readily washed off in inside-out patches. With the β washed off, Gem no longer inhibited the channel, though it could still bind α1A. On the basis of these results, they proposed a model where the presence of β exposes an inhibitory site on the channel complex that is then engaged by Gem to block CaV2.1 [53, 61]. The location of this putative inhibitory site on the CaV2.1 channel complex that binds Gem is currently unknown.
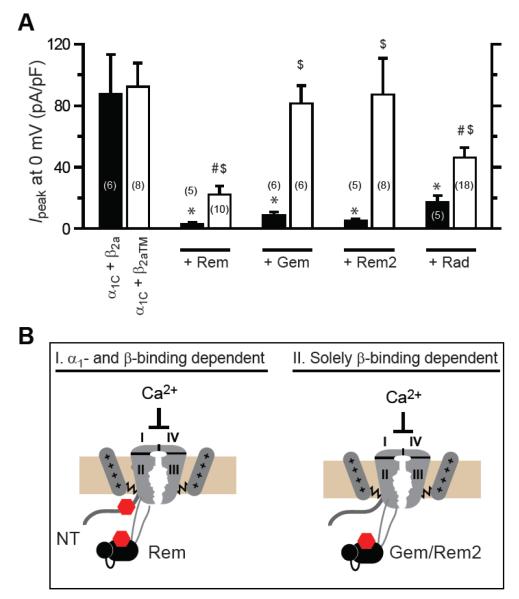
We investigated whether the RGK/β interaction has any role in the mechanism of ICa inhibition, or merely represents an unrelated epiphenomenon, by examining Rem inhibition of CaV1.2 channels in HEK 293 cells [17]. We found that CaV1.2 channels containing a β2a mutant that selectively loses binding to RGK proteins (Fig. 3C), are less potently inhibited (74% inhibition) by Rem than channels containing wild-type β2a (96% inhibition), suggesting the prevalence of both β-binding dependent and independent modes of inhibition [17] (Fig. 3, E-H). We further found that two mechanistic signatures of Rem inhibition of CaV1.2 channels (decreased N and Po), but not a third (reduced Qmax), depend on Rem binding to CaVβ. Surprisingly, we discovered a functional dichotomy amongst the RGKs– while Rem and Rad used both β-binding-dependent and independent mechanisms to inhibit CaV1.2, Gem and Rem2 solely utilized a β-binding-dependent method to do so [17] (Fig. 4). These findings may explain why CaV1.2 channels expressed in the absence of β subunits are partially blocked by Rem in HEK 293 cells [78], but minimally affected by Gem or Rem2 in Xenopus oocytes [15, 68, 79]. Finally, we found that Rem inhibition of CaV2.2 channels was completely dependent on the Rem/CaVβ interaction, in contrast with CaV1.2 [17]. Therefore, the mechanisms of RGK inhibition of CaV1/CaV2 channels are customized at the levels of both the RGK and channel type. Overall, these data suggest a dualistic view for RGK inhibition of CaV channels. First, all RGKs can inhibit all CaV1/CaV2 channels via mechanisms that depend on direct RGK/CaVβ interactions. Because CaVβs are necessary for the formation of all functionally mature CaV channels, this could explain the promiscuity of RGKs in blocking all CaV1/CaV2 channel types. Second, distinct RGKs may initiate β-binding-independent channel inhibition through selective interaction with specific CaV1/CaV2 channel pore-forming α1 subunits [17].
Figure 4. Distinct RGKs differentially use β-binding-dependent and β-binding-independent mechanisms to inhibit CaV1.2 channels.
(A) Impact of distinct RGKs on wild-type (α1C+β2a) and mutant (α1C+β2aTM) CaV1.2 channels. *, #, $ P < 0.05 when compared to α1C+β2a, α1C+ β2aTM, or α1C+β2a+RGK, respectively, using two-tailed unpaired Student’s t test. (B) Cartoon showing dichotomy in determinants used by distinct RGKs to inhibit CaV1.2 channels. Rem can inhibit CaV1.2 by both β-binding-dependent and α1-binding-dependent mechanisms. Gem and Rem2 inhibit CaV1.2 solely using a β-binding-dependent mechanism. The stoichiometry of Rem binding to the CaV1.2 complex has not been investigated. One possibility is that two Rem proteins can simultaneously associate with a single CaV1.2 channel. Alternatively, it is also possible that within a single CaV1.2 channel complex, the interaction of Rem with α1C N-terminus or β subunit is mutually exclusive, thus ensuring a 1:1 binding stoichiometry. Figure reproduced from [17].
6.2. Direct interactions of RGKs with CaV α1 subunits
A logical explanation for the observation that specific RGKs can inhibit CaV2.1 and CaV1.2 channels without binding to β subunits is that particular RGKs directly interact with individual CaV1/CaV2 α1 subunits. Consistent with this idea, Gem co-immunoprecipitates with CaV2.1 α1A in the absence of CaVβ [61]. Nevertheless, the exact Gem binding sites on α1A that underlie channel inhibition are unknown. By exchanging corresponding fragments between P/Q- (α1A) and T-type channels (CaV3.1 α1G), Fan et al. showed that the region comprised of the S1-S3 transmembrane segments of domain II conferred Gem sensitivity [61]. However, since Gem is an intracellular protein, it is most likely that IIS1-IIS3 may be involved in the transduction of the effect, rather than being a binding site for Gem [61, 79].
There have also been efforts to identify direct RGK binding sites on CaV1.2 α1C subunit. Pang et al. [80] reported that Rem (as well as Rad and Rem2) directly binds to proximal and distal regions C-terminus of α1C. The proximal α1C C-terminus contains structural elements, including a CaM binding domain and an EF hand motif, that are essential for Ca2+-dependent regulation (inactivation and facilitation) of CaV1.2 [81-86]. Ca2+-CaM was found to block the Rem/α1C C-terminus interaction in vitro. This interplay was not due to Ca2+-CaM interaction with the Rem C-terminus, suggesting that Rem directly competes with Ca2+-CaM for α1C C-terminus [80]. Furthermore, co-overexpression of CaM was found to partially relieve Rem inhibition of CaV1.2 channels in tsA201 cells. The authors concluded that direct binding to the proximal α1C C-terminus is important for Rem-mediated regulation of CDI and ICa,L blockade in CaV1.2 [80]. Results from our group support a different conclusion. Based on the idea that the putative Rem binding site was most likely localized within an intracellular region of the channel, we conducted an unbiased screen for potential Rem interaction sites on α1C (N- and C-termini, I-II, II-III, and III-IV loops) using three independent approaches (fluorescence resonance energy transfer, co-localization analyses, and co-immunoprecipitation assays) [17]. We found that Rem interacts solely with the α1C N-terminus in the region immediately upstream of transmembrane segment I in domain I (IS1). Moreover, over-expressing α1C N-terminus completely rescued Rem-mediated β-binding-independent inhibition of CaV1.2 channels, suggesting that Rem/α1C N-terminus interaction underlies this mode of regulation. Neither Rem2 nor Gem bound α1C N-terminus, providing an explanation for why they exhibit only a β-binding-dependent mechanism of CaV1.2 inhibition. Although the distal N-terminus shows homology among distinct CaV1/CaV2 α1-subunits (60% identical residues or conservative substitutions), Rem does not bind CaV2.2 α1B N-terminus [17]. This explains why Rem inhibits CaV2.2 channels solely through a β-binding-dependent mechanism.
7. CROSSTALK OF RGK AND PROTEIN KINASE SIGNALING ON CaV1.2 CHANNELS
In heart, up-regulation of CaV1.2 channels by protein kinase A (PKA) is an important physiological modulation that contributes to sympathetic regulation of the heartbeat, a critical component of the flight-or-fight mechanism [87, 88]. Two reports have documented an interesting cross talk between RGKs and PKA signaling at the level of CaV1.2 channels in heart. Specifically, in primary cardiac myocytes over-expressing either Rad [58] or Rem [59], the remaining ICa,L was completely insensitive to PKA modulation. By contrast, the CaV1.2 channel agonist BAY K 8644 robustly and acutely rescued ICa,L in myocytes over-expressing Rem [59]. The mechanism by which RGKs prevent PKA-mediated modulation of CaV1.2 channels in heart is unknown.
A recent study found that α1-adrenergic receptor stimulation could attenuate Rem inhibition of CaV1.2 channels in both HEK 293 cells and cardiac myocytes [65]. The mechanism was found to involve activation of protein kinase D1 (PKD1) and subsequent phosphorylation of Rem at residue Ser18. The authors propose that this leads to sequestration of Rem by 14-3-3 proteins and permits CaV1.2 channels trapped intracellularly to traffick to the cell surface [65].
8. PRACTICAL APPLICATIONS OF RGK INHIBITION OF CaV CHANNELS
Inhibition of CaV1/CaV2 channels is an important or potential therapy for many cardiovascular and neurological diseases including: hypertension, cardiac arrhythmias, neuropathic pain, Alzheimer’s disease, and Parkinson’s disease [89-92]. Under certain circumstances, intracellular genetically encoded CaV channel inhibitors may have advantages over traditional small-molecule blockers [93]. For example, localized expression of RGKs may permit a more restricted inhibition of CaV channels, permitting a targeted therapeutic result while minimizing unwanted off-target effects [93]. In a proof of concept demonstration of this principle, focal gene delivery of Gem to the atrioventricular (AV) node slowed AV nodal conduction and reduced the heart rate in a porcine atrial fibrillation model [60].
The potential practical applications of RGKs may be widely expanded if new functionalities such as inducibility and selectivity could be engineered into them. To this end, we have developed engineered derivatives of Rem which are inert but can be acutely activated by small molecules to inhibit ICa [56]. Recently, a caveolae-targeted Rem has been developed that selectively inhibits caveolae-localized Cav1.2 channels in heart cells [94]. This molecule selectively inhibits hypertrophic Ca2+ signaling pathways in cardiac myocytes without impairing contractility.
9. PHYSIOLOGICAL SIGNIFICANCE OF RGK INHIBITION OF ICa
In contrast to the steady progress in evaluating the mechanisms by which RGKs inhibit CaV1/CaV2 channels, the physiological role of this channel regulation remains somewhat mysterious. Many different excitable cell types co-express RGKs and specific CaV1/CaV2 channel isoforms. For example, Rad and Rem are expressed in cardiac and skeletal muscle [3, 16, 95], Rem2 is expressed in neurons and pancreatic β-cells [4, 11, 54, 96], and Gem is present in mitogen-activated T cells and pancreatic β cells [2, 15, 97]. This leads to the expectation that RGK inhibition of CaV channels plays a physiological role in these cells. However, this is difficult to prove for two main reasons.
First, RGKs have been shown to interact with many other signaling molecules besides CaV channel subunits. Therefore, it is difficult to specifically assign any (patho)physiological consequences that arise from knockout of individual RGKs to their effect on CaV channels. Knockout mice for Rad [95], Gem [97], and Rem [98] have been generated. Rad knockout mice display an increased susceptibility to transverse aortic constriction (TAC) induced hypertrophy, and down-regulation of Rad is correlated with development of heart failure in humans [95]. ICa,L has not been measured in these mice so it is unknown whether this contributes to the observed phenotype. However, deciphering the potential role of ICa,L is further complicated by the fact that Rad functionally interacts with CaMKII [29, 95] and Rho kinase [99, 100], two signaling molecules well known to be involved in the development of pathological cardiac hypertrophy [101, 102]. Gem knockout mice displayed glucose intolerance, impaired insulin secretion in response to high glucose, and abnormal Ca2+ handling in pancreatic β-cells [97]. ICa in β-cells was not measured, precluding any inferences about a possible role of CaV channel dysregulation in these abnormalities. Rem knockout mice exhibit a moderately increased ICa in cardiac myocytes, without showing any overt cardiac phenotype [98]. Interpretation of the results may be confounded by compensatory mechanisms as well as the fact that other RGKs present in heart may provide a redundant pathway for ICa inhibition. To date, the most direct evidence that an RGK may mediate constitutive inhibition of ICa in an excitable cell comes from a study which demonstrated that knockdown of Rad with shRNA resulted in an increase in ICa,L in cultured cardiac myocytes [58]. Similarly RNAi knockdown of Rem2 in neurons demonstrated a role in synaptic development and dendritic morphology [11, 12], and reduced the frequency of miniature excitatory postsynaptic currents [96]. However, the Rem2 knockdown in hippocampal neurons had no impact on ICa, suggesting that other effectors underlie the observed functional effects.
A second reason for the difficulty in evaluating the physiological role of RGK inhibition of CaV channels in different systems has to do with the likely constitutive, background nature of this regulation. Development of approaches that can selectively and acutely relieve RGK inhibition of CaV channels would appear to be necessary for progress in determining the physiological role of this form of CaV1/CaV2 channel regulation.
10. CONCLUSION
All RGKs powerfully and promiscuously inhibit all CaV1 and CaV2 channels. Surprisingly, this seemingly homogenous and simple phenomenon is underlain by a rich variety of mechanisms and structural determinants [57, 68]. The mechanisms and structural determinants of RGK inhibition of CaV channels appear to be customized based on the RGK type, CaV1/CaV2 channel isoform, and cellular context. This may have contributed to apparently contradictory reports in the field. Further work is needed to define the precise mechanisms that different RGKs use to inhibit distinct CaV1/CaV2 channel isoforms in specific cell types. Nailing down the physiological role and importance of RGK inhibition of CaV channels has proven difficult due to the constitutive nature of this inhibition, the fact that RGKs functionally interact with other important signaling molecules, and the existence of compensatory mechanisms and redundant pathways in knockout mice. New tools that permit acute relief of RGK inhibition of CaV channels are needed to probe the physiological significance of this phenomenon. Finally, RGKs and engineered derivatives have potential utility as therapeutics and useful molecular tools. Exploring these dimensions of the RGK/CaV channel functional interaction is an exciting area for future research.
Highlights.
RGK proteins inhibit voltage-dependent CaV1 and CaV2 channels.
RGKs use multiple mechanisms and determinants to inhibit CaV1/CaV2 channels.
The mechanisms of channel inhibition are customized for different RGK/CaV channel types.
New functionalities can be engineered into RGKs for practical applications.
ACKNOWLEDGEMENTS
This work was supported by NIH grant RO1 HL069911 (to H.M.C.). HMC is an Established Investigator of the American Heart Association. The authors thank Donald Chang for comments on the manuscript and Dr. Oliver Clarke for help in displaying the protein structures in Fig. 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- [2].Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- [3].Finlin BS, Andres DA. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]
- [4].Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem J. 2000;347(Pt 1):223–231. [PMC free article] [PubMed] [Google Scholar]
- [5].Leone A, Mitsiades N, Ward Y, Spinelli B, Poulaki V, Tsokos M, Kelly K. The Gem GTP-binding protein promotes morphological differentiation in neuroblastoma. Oncogene. 2001;20:3217–3225. doi: 10.1038/sj.onc.1204420. [DOI] [PubMed] [Google Scholar]
- [6].Ward Y, Spinelli B, Quon MJ, Chen H, Ikeda SR, Kelly K. Phosphorylation of critical serine residues in Gem separates cytoskeletal reorganization from down-regulation of calcium channel activity. Mol Cell Biol. 2004;24:651–661. doi: 10.1128/MCB.24.2.651-661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pan JY, Fieles WE, White AM, Egerton MM, Silberstein DS. Ges, A human GTPase of the Rad/Gem/Kir family, promotes endothelial cell sprouting and cytoskeleton reorganization. J Cell Biol. 2000;149:1107–1116. doi: 10.1083/jcb.149.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hatzoglou A, Ader I, Splingard A, Flanders J, Saade E, Leroy I, Traver S, Aresta S, de Gunzburg J. Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to down-regulate the Rho pathway. Mol Biol Cell. 2007;18:1242–1252. doi: 10.1091/mbc.E06-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sun Z, Zhang J, Chen C, Du Q, Chang L, Cao C, Zheng M, Garcia-Barrio MT, Chen YE, Xiao RP, Mao J, Zhu X. Rad GTPase induces cardiomyocyte apoptosis through the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun. 2011;409:52–57. doi: 10.1016/j.bbrc.2011.04.104. [DOI] [PubMed] [Google Scholar]
- [11].Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ghiretti AE, Paradis S. The GTPase Rem2 regulates synapse development and dendritic morphology. Dev Neurobiol. 2011;71:374–389. doi: 10.1002/dneu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Edel MJ, Menchon C, Menendez S, Consiglio A, Raya A, Belmonte J.C. Izpisua. Rem2 GTPase maintains survival of human embryonic stem cells as well as enhancing reprogramming by regulating p53 and cyclin D1. Genes Dev. 2010;24:561–573. doi: 10.1101/gad.1876710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Edel MJ, Boue S, Menchon C, Sanchez-Danes A, Izpisua Belmonte JC. Rem2 GTPase controls proliferation and apoptosis of neurons during embryo development. Cell Cycle. 2010;9:3414–3422. doi: 10.4161/cc.9.17.12719. [DOI] [PubMed] [Google Scholar]
- [15].Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- [16].Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci U S A. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang T, Puckerin A, Colecraft HM. Distinct RGK GTPases Differentially Use alpha(1)- and Auxiliary beta-Binding-Dependent Mechanisms to Inhibit Ca(V)1.2/Ca(V)2.2 Channels. PLoS One. 2012;7:e37079. doi: 10.1371/journal.pone.0037079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- [20].Opatowsky Y, Sasson Y, Shaked I, Ward Y, Chomsky-Hecht O, Litvak Y, Selinger Z, Kelly K, Hirsch JA. Structure-function studies of the G-domain from human gem, a novel small G-protein. FEBS Lett. 2006;580:5959–5964. doi: 10.1016/j.febslet.2006.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yanuar A, Sakurai S, Kitano K, Hakoshima T. Crystal structure of human Rad GTPase of the RGK-family. Genes Cells. 2006;11:961–968. doi: 10.1111/j.1365-2443.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- [22].Splingard A, Menetrey J, Perderiset M, Cicolari J, Regazzoni K, Hamoudi F, Cabanie L, El Marjou A, Wells A, Houdusse A, de Gunzburg J. Biochemical and structural characterization of the gem GTPase. J Biol Chem. 2007;282:1905–1915. doi: 10.1074/jbc.M604363200. [DOI] [PubMed] [Google Scholar]
- [23].Sasson Y, Navon-Perry L, Huppert D, Hirsch JA. RGK family G-domain:GTP analog complex structures and nucleotide-binding properties. J Mol Biol. 2011;413:372–389. doi: 10.1016/j.jmb.2011.08.017. [DOI] [PubMed] [Google Scholar]
- [24].Correll RN, Botzet GJ, Satin J, Andres DA, Finlin BS. Analysis of the Rem2 - voltage dependant calcium channel beta subunit interaction and Rem2 interaction with phosphorylated phosphatidylinositide lipids. Cell Signal. 2008;20:400–408. doi: 10.1016/j.cellsig.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, Seino Y, Hunziker W. Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [27].Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Kuwamura N, Yamada Y, Seino Y, Hunziker W. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Takahashi A, Yamada Y, Seino Y, Hunziker W. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J Cell Sci. 2005;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- [29].Moyers JS, Bilan PJ, Zhu J, Kahn CR. Rad and Rad-related GTPases interact with calmodulin and calmodulin-dependent protein kinase II. J Biol Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- [30].Finlin BS, Andres DA. Phosphorylation-dependent association of the Ras-related GTP-binding protein Rem with 14-3-3 proteins. Arch Biochem Biophys. 1999;368:401–412. doi: 10.1006/abbi.1999.1316. [DOI] [PubMed] [Google Scholar]
- [31].Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- [32].Zhu J, Reynet C, Caldwell JS, Kahn CR. Characterization of Rad, a new member of Ras/GTPase superfamily, and its regulation by a unique GTPase-activating protein (GAP)-like activity. J Biol Chem. 1995;270:4805–4812. doi: 10.1074/jbc.270.9.4805. [DOI] [PubMed] [Google Scholar]
- [33].Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, Kahn CR. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci U S A. 1999;96:14911–14918. doi: 10.1073/pnas.96.26.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- [35].Tsien RW, Tsien RY. Calcium channels, stores and oscillations. Annual Review of Cell Biology. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- [36].Perez-Reyes E, Lory P. Molecular biology of T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:605–609. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- [37].Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- [38].Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- [39].Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- [40].Yamaguchi H, Okuda M, Mikala G, Fukasawa K, Varadi G. Cloning of the beta(2a) subunit of the voltage-dependent calcium channel from human heart: cooperative effect of alpha(2)/delta and beta(2a) on the membrane expression of the alpha(1C) subunit. Biochem Biophys Res Commun. 2000;267:156–163. doi: 10.1006/bbrc.1999.1926. [DOI] [PubMed] [Google Scholar]
- [41].Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of beta-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci U S A. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fang K, Colecraft HM. Mechanism of auxiliary beta-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J Physiol. 2011;589:4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- [44].Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- [45].Waard M. De, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved beta subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- [46].Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal alpha1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].De Waard M, Campbell KP. Subunit regulation of the neuronal alpha 1A Ca2+ channel expressed in Xenopus oocytes. J Physiol. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta 2 subunit splice variants on L-type calcium channel gating. Biophysical Journal. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- [51].Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- [52].Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- [53].Buraei Z, Yang J. The {beta} Subunit of Voltage-Gated Ca2+ Channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- [55].Bannister RA, Colecraft HM, Beam KG. Rem inhibits skeletal muscle EC coupling by reducing the number of functional L-type Ca2+ channels. Biophys J. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- [57].Yang T, Xu X, Kernan T, Wu V, Colecraft HM. Rem, a member of the RGK GTPases, inhibits recombinant CaV1.2 channels using multiple mechanisms that require distinct conformations of the GTPase. J Physiol. 2010;588:1665–1681. doi: 10.1113/jphysiol.2010.187203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang G, Zhu X, Xie W, Han P, Li K, Sun Z, Wang Y, Chen C, Song R, Cao C, Zhang J, Wu C, Liu J, Cheng H. Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart. Circ Res. 2010;106:317–327. doi: 10.1161/CIRCRESAHA.109.208272. [DOI] [PubMed] [Google Scholar]
- [59].Xu X, Marx SO, Colecraft HM. Molecular mechanisms, and selective pharmacological rescue, of Rem-inhibited CaV1.2 channels in heart. Circ Res. 2010;107:620–630. doi: 10.1161/CIRCRESAHA.110.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- [61].Fan M, Buraei Z, Luo HR, Levenson-Palmer R, Yang J. Direct inhibition of P/Q-type voltage-gated Ca2+ channels by Gem does not require a direct Gem/Cavbeta interaction. Proc Natl Acad Sci U S A. 2010;107:14887–14892. doi: 10.1073/pnas.1007543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fan M, Zhang WK, Buraei Z, Yang J. Molecular Determinants of Gem Protein Inhibition of P/Q-type Ca2+ Channels. J Biol Chem. 2012;287:22749–22758. doi: 10.1074/jbc.M111.291872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Flucher BE. Rem-induced inhibition of Ca2+ channels--a three-pronged assault. J Physiol. 2010;588:1801–1802. doi: 10.1113/jphysiol.2010.191247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jhun BS, J OU, Wang W, Ha CH, Zhao J, Kim JY, Wong C, Dirksen RT, Lopes CM, Jin ZG. Adrenergic signaling controls RGK-dependent trafficking of cardiac voltage-gated L-type Ca2+ channels through PKD1. Circ Res. 2012;110:59–70. doi: 10.1161/CIRCRESAHA.111.254672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Correll RN, Pang C, Finlin BS, Dailey AM, Satin J, Andres DA. Plasma membrane targeting is essential for Rem-mediated Ca2+ channel inhibition. J Biol Chem. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Leyris JP, Gondeau C, Charnet A, Delattre C, Rousset M, Cens T, Charnet P. RGK GTPase-dependent CaV2.1 Ca2+ channel inhibition is independent of CaVbeta-subunit-induced current potentiation. FASEB J. 2009;23:2627–2638. doi: 10.1096/fj.08-122135. [DOI] [PubMed] [Google Scholar]
- [68].Seu L, Pitt GS. Dose-dependent and isoform-specific modulation of Ca2+ channels by RGK GTPases. J Gen Physiol. 2006;128:605–613. doi: 10.1085/jgp.200609631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- [70].Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- [71].Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. J Biol Chem. 2006;281:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- [72].Beguin P, Ng YJ, Krause C, Mahalakshmi RN, Ng MY, Hunziker W. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel beta-subunits via an uncommon effector binding domain. J Biol Chem. 2007;282:11509–11520. doi: 10.1074/jbc.M606423200. [DOI] [PubMed] [Google Scholar]
- [73].Flynn R, Chen L, Hameed S, Spafford JD, Zamponi GW. Molecular determinants of Rem2 regulation of N-type calcium channels. Biochem Biophys Res Commun. 2008;368:827–831. doi: 10.1016/j.bbrc.2008.02.020. [DOI] [PubMed] [Google Scholar]
- [74].Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- [76].Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- [77].Sasaki T, Shibasaki T, Beguin P, Nagashima K, Miyazaki M, Seino S. Direct inhibition of the interaction between alpha-interaction domain and beta-interaction domain of voltage-dependent Ca2+ channels by Gem. J Biol Chem. 2005;280:9308–9312. doi: 10.1074/jbc.M413773200. [DOI] [PubMed] [Google Scholar]
- [78].Crump SM, Correll RN, Schroder EA, Lester WC, Finlin BS, Andres DA, Satin J. L-type calcium channel alpha-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol. 2006;291:H1959–1971. doi: 10.1152/ajpheart.00956.2005. [DOI] [PubMed] [Google Scholar]
- [79].Flynn R, Zamponi GW. Regulation of calcium channels by RGK proteins. Channels (Austin) 2010;4:434–439. doi: 10.4161/chan.4.6.12865. [DOI] [PubMed] [Google Scholar]
- [80].Pang C, Crump SM, Jin L, Correll RN, Finlin BS, Satin J, Andres DA. Rem GTPase interacts with the proximal Ca(V)1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) 2010;4 doi: 10.4161/chan.4.3.11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- [82].Peterson BZ, Lee JS, Mulle JG, Wang Y, de Leon M, Yue DT. Critical determinants of Ca(2+)-dependent inactivation within an EF-hand motif of L-type Ca(2+) channels. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Van Petegem F, Chatelain FC, Minor DL., Jr. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- [85].Zuhlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the(alpha)1C subunit. J Biol Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]
- [86].Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–754. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- [87].McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- [88].Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Triggle DJ. Calcium channel antagonists: clinical uses--past, present and future. Biochem Pharmacol. 2007;74:1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- [90].Simuni T, Borushko E, Avram MJ, Miskevics S, Martel A, Zadikoff C, Videnovic A, Weaver FM, Williams K, Surmeier DJ. Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study. Mov Disord. 2010;25:2863–2866. doi: 10.1002/mds.23308. [DOI] [PubMed] [Google Scholar]
- [91].Kochegarov AA. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium. 2003;33:145–162. doi: 10.1016/s0143-4160(02)00239-7. [DOI] [PubMed] [Google Scholar]
- [92].Anekonda TS, Quinn JF. Calcium channel blocking as a therapeutic strategy for Alzheimer’s disease: the case for isradipine. Biochim Biophys Acta. 2011;1812:1584–1590. doi: 10.1016/j.bbadis.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xu X, Colecraft HM. Engineering proteins for custom inhibition of Ca(V) channels. Physiology (Bethesda) 2009;24:210–218. doi: 10.1152/physiol.00010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, Sun Z, Zhu X, Cui T, Youker KA, Yang Q, Day SM, Kahn CR, Chen YE. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation. 2007;116:2976–2983. doi: 10.1161/CIRCULATIONAHA.107.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang HG, Wang C, Pitt GS. Rem2-targeted shRNAs reduce frequency of miniature excitatory postsynaptic currents without altering voltage-gated Ca(2)(+) currents. PLoS One. 2011;6:e25741. doi: 10.1371/journal.pone.0025741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gunton JE, Sisavanh M, Stokes RA, Satin J, Satin LS, Zhang M, Liu SM, Cai W, Cheng K, Cooney GJ, Laybutt DR, So T, Molero JC, Grey ST, Andres DA, Rolph MS, Mackay CR. Mice Deficient in GEM GTPase Show Abnormal Glucose Homeostasis Due to Defects in Beta-Cell Calcium Handling. PLoS One. 2012;7:e39462. doi: 10.1371/journal.pone.0039462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Magyar J, Kiper CE, Sievert G, Cai W, Shi GX, Crump SM, Li L, Niederer S, Smith N, Andres DA, Satin J. Rem-GTPase regulates cardiac myocyte L-type calcium current. Channels (Austin) 2012;6:166–173. doi: 10.4161/chan.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Fu M, Zhang J, Tseng YH, Cui T, Zhu X, Xiao Y, Mou Y, Leon H. De, Chang MM, Hamamori Y, Kahn CR, Chen YE. Rad GTPase attenuates vascular lesion formation by inhibition of vascular smooth muscle cell migration. Circulation. 2005;111:1071–1077. doi: 10.1161/01.CIR.0000156439.55349.AD. [DOI] [PubMed] [Google Scholar]
- [100].Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:H287–296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]