Abstract
Background
Alternatively Spliced Tissue Factor (asTF) is a novel isoform of full-length Tissue Factor (fl-TF) that exhibits angiogenic activity. Although asTF has been detected in human plaques, it is unknown whether its expression in atherosclerosis causes increased neovascularization and an advanced plaque phenotype.
Methods and Results
Carotid (n=10) and coronary specimens (n=8), from patients with stable or unstable angina, were classified as complicated or uncomplicated based on plaque morphology. Analysis of asTF expression and cell type –specific expression revealed a strong expression and co-localization of asTF with macrophages and neovessels within complicated, but not un-complicated, human plaques. Our results showed that the angiogenic activity of asTF is mediated via HIF-1α up-regulation through integrins and activation of phosphatidylinositol-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK) pathways. HIF-1α up-regulation by asTF also was associated with increased VEGF expression in primary human endothelial cells, and VEGF-Trap significantly reduced the angiogenic effect of asTF in vivo. Furthermore, asTF gene transfer significantly increased neointima formation and neovascularization following carotid wire injury in ApoE−/− mice.
Conclusions
The results of this study provide strong evidence that asTF promotes neointima formation and angiogenesis in an experimental model of accelerated atherosclerosis. Herein, we demonstrate that the angiogenic effect of asTF is mediated via the activation of the HIF-1/VEGF signaling. This mechanism may be relevant to neovascularization, progression and associated complications of human atherosclerosis as suggested by the increased expression of asTF in complicated vs. uncomplicated human carotid and coronary plaques.
Keywords: angiogenesis, atherosclerosis, hypoxia-inducible factor 1, vascular endothelial growth factor, tissue factor, alternative splicing
Introduction
Atherosclerosis is a leading cause of death and disability worldwide with 17.3 million deaths per year1–3, and is projected to increase significantly over the next two decades4. Approximately 75% of acute coronary events and 60% of symptomatic carotid artery disease are caused by rupture of vulnerable plaques5–8. Over the last decade, angiogenesis has been the target of intense investigation as it is the critical source of plaque hemorrhage9 and rupture8, 10, 11, ultimately responsible for the sudden onset of myocardial infarction and stroke9, 12.
Therefore, identifying new angiogenic factors and signaling pathways that can facilitate the development of tailored therapies for plaque stabilization is of great necessity.
Tissue Factor (TF), also known as Full Length-Tissue Factor (fl-TF), is an integral-membrane glycoprotein (47kDa) that is implicated in tumor growth, angiogenesis and metastasis13–15 and also abundantly present in the lipid-rich core of plaques where it is considered a major determinant of plaque thrombogenicity upon rupture16.
More recently, a naturally occurring isoform of human fl-TF named Alternatively Spliced Tissue Factor (asTF) was described. Through alternative splicing of the primary RNA transcript the transmembrane and cytoplasmic domains of fl-TF are replaced with a unique COOH-terminal domain in asTF17. asTF is a non-membrane anchored molecule of 27kDa that, while retaining most of the contact sites for coagulation factor VIIa, lacks large portions of an exosite for macromolecular substrates18. As a result, asTF has no appreciable procoagulant activity and it is believed to play a more prominent role in promoting angiogenesis19, 20. Unlike fl-TF, asTF exhibits angiogenic activity via integrin ligation and activation of Focal Adhesion Kinase (FAK) in a factor VIIa and Protease-Activated Receptor-2 (PAR-2) independent manner19, 21–23. In addition to its expression in pancreatic, lung, breast and cervical cancer19, 21, 24, asTF has been detected in human plaques25, 26; however, limited evidence exists beyond analysis of lipid-rich aortic lesions26. Whereas asTF is expressed by monocytes/macrophages and vascular smooth muscle cells17, 27, the main cellular components of plaques, it is unknown whether asTF expression in atherosclerotic plaques is related to a more advanced phenotype and if so, what its functional relevance is. To test the hypothesis that asTF plays a role in atherosclerosis, we analyzed its expression and cellular distribution in complicated and uncomplicated human atherosclerotic plaques. Secondly, we investigated the integrin signal transduction pathways to identify new angiogenic signaling and effector(s) of asTF. Finally, we evaluated the impact of asTF overexpression on neointima formation and neovascularization in a murine model of accelerated atherosclerosis.
METHODS
Please refer to the Methods section in the Data Supplements for a detailed description and Supplemental Table 1 for primer sequences used for conventional PCR.
Human Samples
Human carotid specimens (n=10) were obtained from patients undergoing carotid endarterectomy and the Institutional Biorepository/Biospecimen Bank. Their use was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai and subjects gave informed, written consent. Immediately after surgery, carotid plaques were processed by transverse dissection at the site of maximum plaque diameter28, 29. One cross-sectional half of the entire plaque was fixed in formalin and embedded in paraffin for histopathology. The other half was flash frozen in liquid nitrogen and stored at −80°C for further analysis. Paraffin blocks of coronary plaques (n=8) were from patients presenting with stable angina (n=4) or acute coronary events (ACS) (n=4) undergoing percutaneous directional atherectomy. Informed written consent for the analysis of tissue samples was obtained from all patients prior to revascularization and their use was approved by the Institutional Review Board of the University of Bonn. All specimens were classified as complicated and uncomplicated according to validated pathological criteria as previously reported30–33.
Animals
Eight-weeks-old, male C57Bl6 mice (The Jackson Laboratory, Bar Harbor, Maine, USA) were used for the in vivo Matrigel Plug model. ApoE −/− mice (8-week old, male, C57BL/6 background), obtained from The Jackson Laboratory, were used for the in vivo carotid artery wire injury and lentiviral transduction experiments. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and carried out in compliance with Institutional Standards for Humane Care and Use of Laboratory Animal experiments.
Effect of asTF on HIF-1α expression
Briefly, endothelial cells were treated with asTF (10 nM) or vehicle for 6–24 hours and HIF-1α protein and mRNA levels were measured by Western blot and PCR respectively. In additional experiments endothelial cells were treated with asTF (10 nM), fl-TF (10 nM) or vehicle for 24–72 hours and HIF-1α protein expression was measured by immunofluorescence and Western blot analysis.
Role of integrin signaling on HIF-1α induction by asTF
Endothelial cells were pre-incubated with blocking antibodies against αv, α6, β3 or β1 integrins (10 μg/mL) for 30 minutes prior to treatment with asTF (10 nM) for 6 hours and HIF-1α expression was measured by western blot of cell lysates. Phosphorylation of FAK and total FAK expression following asTF stimulation of endothelial cells were detected by western blot analysis. Following pre-incubation with the FAK inhibitor PP2 (10 μM) for 30 minutes, endothelial cells were then treated with asTF (10 nM) for 6 hours and HIF-1α up-regulation was measured by western blot analysis. Endothelial cells were also transduced with Ad.dnAkt or Ad.βgal (MOI of 100) and after 24 hours treated for 8 hours with asTF (10 nM) and HIF-1α up-regulation was measured by western blot analysis.
In vivo Matrigel Plug Assay
Eight weeks old C57Bl6 mice were anesthetized and 0.5 mL ice-cold Matrigel (growth factors reduced) was injected s.c.. Matrigel was either supplemented with 10 nM of asTF, fl-TF or PBS (vehicle). VEGF (50 ng/mL) was used as positive control. After 10 days the animals were euthanized, Matrigel plugs harvested and processed for immunohistochemical analysis and vessel density (N/mm2) quantification34.
In separate experiments Matrigel was supplemented with asTF (10 nM) or PBS (Vehicle) either in the presence or absence of anti-αv, anti-α6, anti-β1, anti-β3 integrin subunit blocking antibodies or specific kinase inhibitors. Ten days after injection, matrigel was harvested for neovessel quantification and HIF-1α immunostaining.
Another series of experiments designed to test the hypothesis that VEGF is an angiogenic effector of asTF. Twenty-four hours before Matrigel injection Ad.VEGF-Trap (1011 vp) or Ad. Lacz (1011 vp) were systemically injected as we previusly reported 34. Animals from each groups (Ad.VEGF-Trap or Ad.LacZ) were then randomized to receive Matrigel supplemented with asTF (10 nM), VEGF (50 ng/mL) or vehicle.
Mouse Model of Carotid Artery Wire Injury and Lentiviral Transduction
ApoE −/− mice (8-week old, C57BL/6 background) were fed with a Western-type diet (Harlan Laboratories) from 2 weeks before surgery continuing throughout the experiment. Transluminal wire injury of the left common carotid artery (LCCA) was performed as previously described35. Immediately after injury, LCCA was cannulated and the biclamped segment incubated with 20 μl of lentivirus encoding asTF-GFP (2 × 106 and 8 × 106 IU/mouse) or GFP (2 × 106 IU/mouse) for 30 minutes.
Statistical analysis
All experiments were performed in triplicate (unless otherwise specified) from at least three independent experiments, and data are shown as mean ± SD or median (minimum-maximum), as appropriate. Intra and inter assay coefficients of variation (CV) were calculated to measure variations of results within one experiment and between replicates, respectively. Intra-assay CV were <10% and inter-assay CV were <15% for experiments performed. Normality was assessed using Kolmogorov-Smirnov and Shapiro-Wilk tests. When only two groups were compared, statistical differences were assessed with unpaired two-tailed Student’s t-test or Mann-Whitney test for the comparison of normally or not normally distributed variables, respectively. Otherwise, statistical significance was determined using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test or Kruskal-Wallis test, as appropriate. Relationships between variables were determined by the Pearson’s or Spearman’s rho correlation coefficients. P<0.05 was considered statistically significant. Computations were performed with IBM SPSS 19.0 statistical software (SPSS Inc., Chicago, Ill).
Results
asTF is highly expressed within complicated carotid plaques and coronary lesions from patients with acute coronary events
Carotid plaques were classified as type IV–V (atheroma or fibroatheroma, respectively; n=5) and type VI (type IV or V plaques complicated by disruption, hemorrhage or thrombosis; n=5). Coronary specimens were classified as type IV (n=4) and type VI (n=4) primary lesions. As expected33, type IV lesions were from patients with stable angina while type VI specimens from patients with acute coronary events.
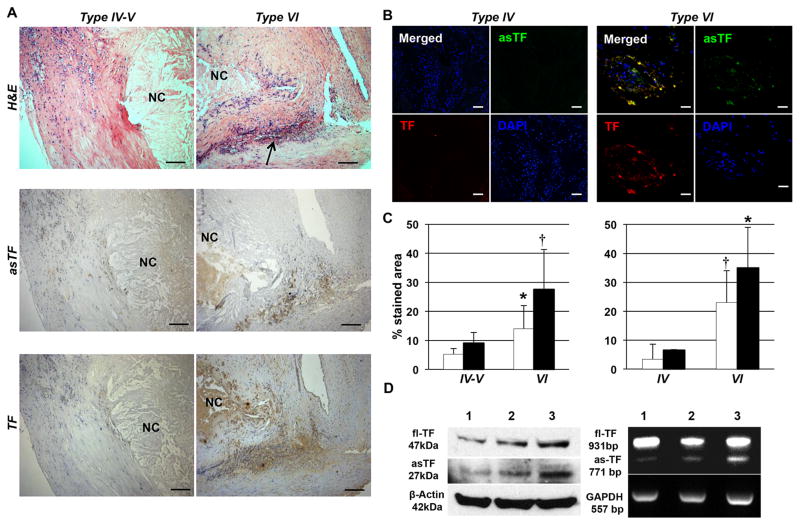
Compared to Type IV–V, Type VI (complicated) carotid lesions showed a significantly (p=0.03) increased macrophage density (13.4±8.6 vs. 3.0±3.1% of stained area). Significantly (p=0.02) greater neovascularization was observed in complicated vs. uncomplicated carotid plaques (178.7±28.3 vs. 82.1±1.3 neovessels/plaque; 28.0±4.8 vs. 9.9±0.8 neovessels/mm2). In addition, complicated lesions showed a significantly (p=0.03) lower vascular smooth muscle cell density (15.8±6.6 vs. 29.2±11.0% of stained area) and collagen content (28.0±8.7 v. 42.5±6.2 % of stained area) compared to uncomplicated ones (Supplemental Figure 1). Of note, we observed a greater expression of both asTF and TF in complicated vs. uncomplicated carotid plaques (Figure 1A and C). Similarly, complicated (type VI) and clinically unstable coronary lesions exhibited increased expression of asTF and TF vs. uncomplicated (type IV) and clinically stable specimens (Figure 1B and C). Quantitatively, asTF mRNA levels (RT-PCR) increased 2.9-fold and protein levels (Western blot) increased 3.7-fold in complicated vs. uncomplicated carotid lesions (Figure 1D).
Figure 1.
asTF expression is greater in type VI (complicated) lesions than type IV–V carotid and type IV coronary (uncomplicated) plaques. A. H&E stained sections (upper panel) of type IV–V (left; n=5) and type VI (right; n=5) carotid plaques. Necrotic Core (NC) and hemorrhage (arrow) of type VI lesions (right) are clearly visible. asTF (middle panel) and TF (bottom panel) immunostaining pattern of type VI (left) and IV–V (right) carotid plaques. Magnification: 100x. Scale bar=100 μm. B. Double immunofluorescence staining for asTF and TF of type IV (left; n=4) and VI (right; n=4) human coronary lesions. A greater expression of both asTF and TF and their co-localization was clear in Type VI vs. Type IV lesions. Magnification 400x. Scale Bar: 20 μm. C. Bars show asTF (white bars) and TF (black bars) quantification in type IV–V (n=5) and VI carotid (n=5; left panel) and in type IV (n=4) and VI coronary (n=4; right panel) plaques. *P=0.04 and †P=0.02 vs. type IV–V carotid or type IV coronary plaques. D. Western blot and PCR analysis of type IV–V (lines 1 and 2) and Type VI (line 3) carotid plaques shows asTF and fl-TF protein and mRNA expression in human plaques.
asTF is expressed by macrophages and neovessels within complicated carotid and coronary plaques
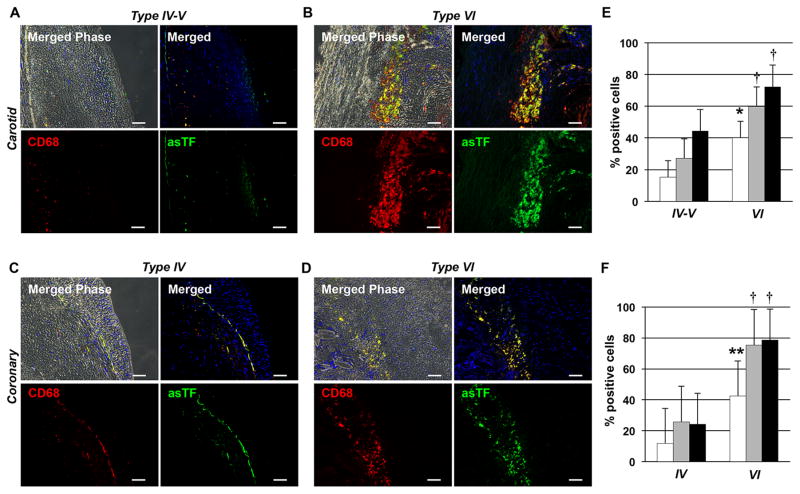
To analyze asTF expression in macrophages and vascular endothelial cells, we performed double immunofluorescence labeling for asTF and CD68 (macrophages) as well as asTF and CD31 (vascular endothelial cells) in carotid and coronary human specimens. In agreement with published results, we observed a greater CD68+ cell density in both complicated carotid and coronary plaques vs. uncomplicated plaques (Figure 2A–D). Importantly, asTF+ cell density was higher in complicated vs. uncomplicated lesions. Furthermore, image analysis revealed a strong colocalization of asTF expression with CD68+ cells in complicated plaques. Notably, a specific fraction of asTF+/CD68+subset of all CD68+ cells was significantly higher in complicated vs. uncomplicated lesions (Figure 2E and F).
Figure 2.
asTF is highly expressed by macrophages within complicated human atherosclerotic plaques. Figures show pattern of double immunofluorescence of uncomplicated (type IV–V) and complicated (type VI) carotid (A and B) and coronary (C and D) plaques. A greater expression of asTF and macrophages (CD68+ cells) was observed in complicated carotid (B; n=5) and coronary (D; n=4) lesions vs. uncomplicated carotid (A; n=5) and coronary (C; n=4) plaques, respectively. asTF showed a strong co-localization with macrophages (CD68+ cells) in type VI lesions (B and D). Magnification 200x. Scale Bar: 40 μm. Quantification of asTF/CD68+ double positive cells (white bars), total asTF+ (grey bars) and total CD68+ (black bars) cells in carotid (E) and coronary (F) plaques. *P=0.006; **P=0.007, †P=0.04 vs. type IV–V or type IV lesions.
We also observed a significantly higher density of CD31+ cells in both complicated carotid and coronary plaques compared to uncomplicated specimens with a marked colocalization of asTF with CD31+ cells in complicated carotid and coronary lesions (Supplemental Figure 2A–D). Specifically, the percentage of asTF+/CD31+ of all CD31+ cells was significantly higher in complicated vs. uncomplicated lesions (Supplemental Figure 2E and F).
asTF promotes endothelial cell proliferation, migration and tube formation
Proliferation
Proliferation was measured by BrdU incorporation assay. Results showed significantly increased endothelial cell proliferation when exposed to asTF and fl-TF (0.1–10 nM) vs. vehicle (Supplemental Figure 3A). Increased expression of the proliferation marker Ki67 in cells treated with asTF and fl-TF was also observed (Supplemental Figure 3B). Cyclin D1 up-regulation at 2 and 6 hours was followed by a down-regulation at 12 hours in cells treated with asTF (Supplemental Figure 3C). A strong co-localization of asTF and fl-TF with the proliferation marker Ki67 was seen in complicated plaques (Supplemental Figure 3D).
Migration
To measure migration we used the Boyden Chamber Assay. A similar 2-fold increase in cell migration to that of fl-TF at 24 hours was observed when asTF (10nM) was present in the lower compartment (Supplemental Figure 4A). No migration was observed at 6 hours (data not shown). Separate experiments were performed to investigate the effect of coating transwells with asTF- on cell migration. These experiments showed that the increased migration induced by the presence of asTF in the lower compartment was further increased when transwells were also coated with asTF (Supplemental Figure 4B).
Cytotoxicity and cell viability
Significant cytotoxicity was observed in cells exposed to asTF and fl-TF at concentrations of 100 and 200nM (Supplemental Figure 4C). MTT assay showed an increased viability of cells exposed to asTF and fl-TF at up to 10nM vs. controls at 24, 48 and 72 hours (Supplemental Figure 4D). Based on these findings 10nM concentration was employed for all further studies.
In vitro Matrigel assay
A 2.5-fold of increase in capillary-like formation was induced by asTF and fl-TF vs. vehicle. This effect was similar to that of VEGF, which was used as positive control (Supplemental Figure 5, A and B). PAR-2 inhibition did not significantly affect asTF-induced angiogenesis (Supplemental Figure 5C). In contrast, no capillary formation was observed when asTF and fl-TF were denatured by boiling at 95°C for 5 minutes, demonstrating that the observed angiogenic activity was protein specific and not dependent on endotoxin contamination (Supplemental Figure 4E).
In Vivo Matrigel assay
The angiogenic effects of asTF, fl-TF were also investigated using the in vivo Matrigel assay. The results of these experiments confirmed significantly higher neovessel formation induced by asTF, fl-TF and VEGF vs. vehicle. However, asTF exhibits greater angiogenic activity vs. fl-TF and VEGF (Supplemental Figure 5D).
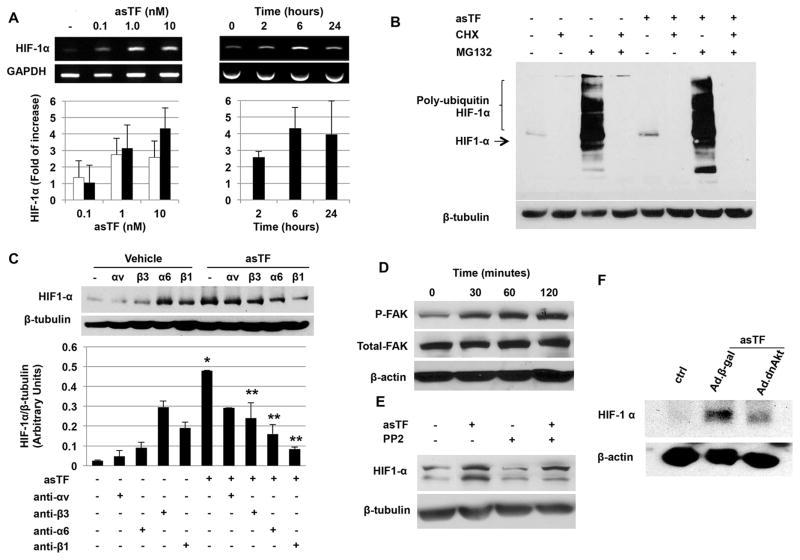
asTF induces HIF-1α up-regulation
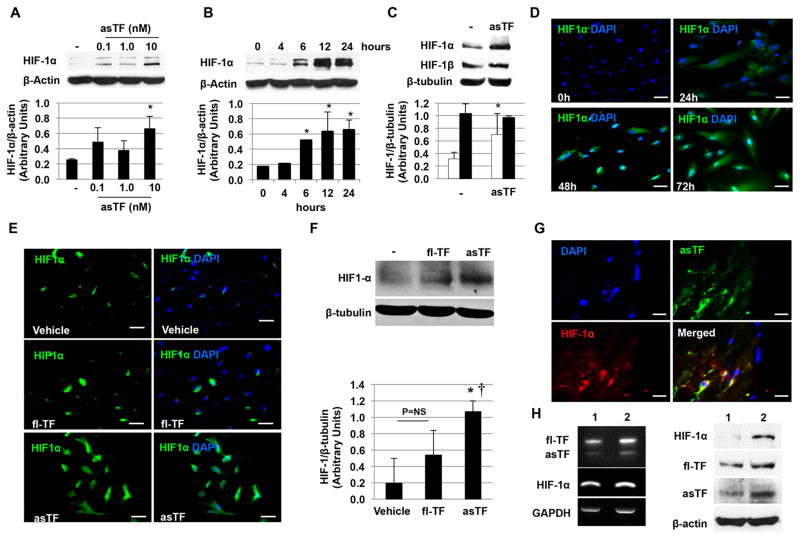
Since the angiogenic activity of asTF is mediated via binding to integrins19 and integrin signal transduction pathways have been implicated in Hypoxia-Inducible Factor-1α (HIF-1α) expression in tumors36, 37, we investigated the possibility that asTF promotes HIF-1α expression. A significant HIF-1α protein up-regulation was detected in endothelial cells treated with asTF but not with fl-TF. This increase was dose-dependent (Figure 3A) and was apparent as early as 6 hours with a maximum expression at 12–24 hours (Figure 3B). In contrast, no effect was observed on HIF-1β (Figure 3C). For the first 24 hours the increase of HIF-1α was restricted to the cytoplasm followed by a progressive translocation to the nucleus of the cells (48 and 72 hours; Figure 3D). No significant induction was observed when cells were treated with fl-TF (Figure 3E and F). Significant up-regulation of HIF-1α induced by asTF was confirmed in vivo as shown by the greater percentage of HIF-1α+ cells detected in plugs containing asTF vs. vehicle (Figure 4). A positive linear correlation between HIF-1α and neovessel density (r=0.29; p=0.036) was also found. Increased HIF-1α expression was observed in complicated human lesions that expressed high levels of asTF. Of note, a strong co-localization between HIF-1α and asTF was observed in complicated plaques (Figure 3G and H).
Figure 3.
asTF induces HIF-1α up-regulation. A. Western blot analysis of endothelial cell lysates shows an asTF-dose-dependent increase of HIF-1α vs. control (vehicle). B. Time course studies showed an increase in HIF-1α as early as 6 hours with a peak at 12–24h vs. control (vehicle). C. Unlike HIF-1α (white bars), no significant effect of asTF on HIF-1β (black bars) was observed. D. Immunofluorescence staining showing the time course of HIF-1α expression (Alexa Fluor 488) in endothelial cells treated with asTF (10 nM) vs. control (vehicle). DAPI was used as counterstaining. E. Up-regulation of HIF-1α was also observed in HAEC treated with asTF (10nM) for 24 hours (left). No significant effect was observed when HAEC were treated with fl-TF (right). Magnification 200x. Scale bar=50 μm. F. Similar results were confirmed by western blot analysis of cell lysates treated with either fl-TF (10nM) or asTF (10nM) for 24 hours. *P=0.02 vs. vehicle, † P=0.04 vs. fl-TF. G. Immunofluorescence staining showing co-localization of HIF-1α (Alexa Fluor 594) and asTF (Alexa Fluor 488) in complicated (type VI) carotid plaques. DAPI was used as counterstaining. Magnification 1000x. Scale bar= 10μm. H. asTF mRNA was identified by PCR in carotid plaques. Sample 1 is from type IV–V plaques (n=5), sample 2 is from type VI lesions (n=5). An increase in HIF-1α expression was observed in sample 2 also characterized by a higher asTF gene expression. Western blot analysis of the same samples confirmed the findings at the protein level. Data from in vitro experiments are the average of triplicates of single experiments that were repeated 3 times.
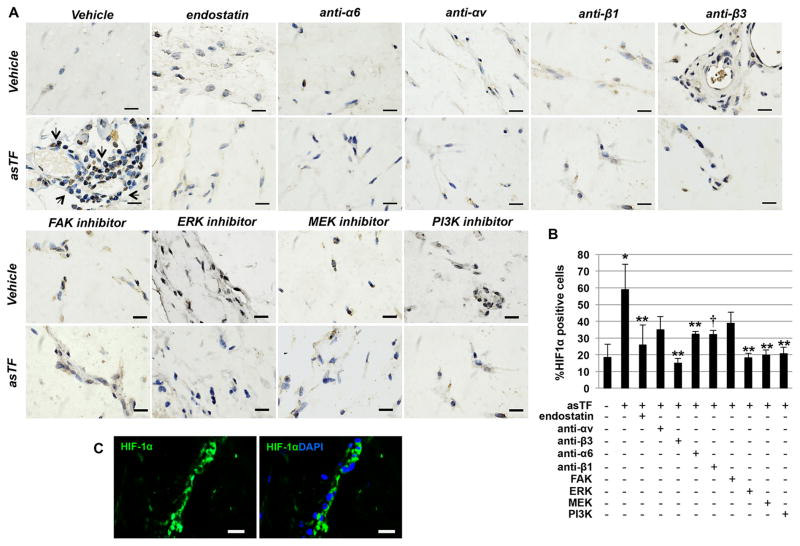
Figure 4.
asTF induces HIF-1α expression in vivo, and this increase is inhibited by specific integrin blocking antibodies and intracellular kinases inhibitors. A. HIF-1α staining of sections of Matrigel plugs shows an increase in HIF-1α positive cells in plugs containing asTF (10nM). HIF-1α expression induced by asTF in vivo was significantly inhibited when specific neutralizing antibodies and kinase inhibitors were co-administered with asTF in the preparation. Magnification 1000x. Scale bar=10 μm. B. Bars show the percentage of HIF-1α positive cells in the plugs. C. Immunofluorescence staining showing HIF-1α (Alexa Fluor 488) positive cells forming a tube-like structure in a Matrigel plug admixed with asTF (10nM). DAPI was used as counterstaining for nuclei. *P<0.0001 vs. ctrl; **P<0.01, † P=0.058 vs. asTF. Magnification 1000x. Scale bar=10 μm. (n=2 plugs/mouse; n=3 mice/group).
To elucidate whether the increased levels of HIF-1α induced by asTF were the result of increased transcription, we first measured HIF-1α mRNA levels by RT-PCR. We observed a dose-dependent up-regulation (4-fold of increase) of HIF-1α mRNA which appeared to plateau at 6–24 hours (Figure 5A).
Figure 5.
Mechanisms involved in asTF-induced HIF-1α up-regulation. A. Dose-dependent up-regulation of HIF-1α mRNA by asTF was detected (left) at 2 hours (white bars) and 6 hours (black bars). Time course analysis of HIF-1α expression in the presence of asTF (10nM) shows a plateau at 6 hours (right). B. asTF enhanced protein synthesis of HIF-1α with no effect on degradation. C. Representative immunoblot (upper panel) shows inhibition of asTF-induced HIF-1α by integrin blockade. Bars (lower panel) show quantification of three separate experiments. *P<0.01 vs. vehicle. **P<0.01 vs. asTF. D. FAK activation by asTF was detected in endothelial cells stimulated with asTF. E. FAK inhibition by PP2 resulted in partial down-regulation of asTF-induced HIF-1α. F HIF-1α expression was inhibited in cells transduced with an adenovirus encoding dominant negative Akt (Ad.dnAkt) but not with Ad.βgal. Briefly, 24 hours following Ad.dnAkt or Ad.βgal (100 MOI) human aortic endothelial cells were treated with asTF (10nM) for 8 hours and HIF-1α expression measured by western blot. Data are the average of triplicates from single experiments that were independently repeated 3 times.
To investigate whether asTF affects HIF-1α degradation, endothelial cells treated with asTF were incubated with the proteasome inhibitor MG132 and the protein-synthesis inhibitor cycloheximide. Cycloheximide treatment abolished asTF-induced HIF-1α expression and, as expected, MG132 alone increased HIF-1α accumulation as a result of reduced proteasomal degradation. In agreement with this interpretation, the addition of MG132 to asTF-treated cells resulted in a drastic accumulation of polyubiquinated forms of HIF-1α (Figure 5B).
HIF-1α induction by asTF occurs via integrin-mediated activation of MAPK and PI3K/Akt signal pathways
Next we investigated whether the induction of HIF-1α depends on integrins. The blockade of α6-, β3- and β1-integrins significantly attenuated the up-regulation of HIF-1α by asTF (Figure 5C) yet, no significant effect was observed following αv integrin inhibition. In vivo results showed that when asTF was co-administered with α6-, αv, β3- or β1-integrin neutralizing antibodies neovessel formation was significantly reduced (Supplemental Figure 6).
Furthermore, lower HIF-1α+ cell density in the plugs was observed when asTF was co-administered with antibodies against α6- and β3-integrins. A trend (P=0.05) towards a reduced HIF-1α+ cell density was also observed when β1- neutralizing antibody was co-administered with asTF. In line with the in vitro data, no significant reduction of HIF-1α induction by asTF was observed in the presence of αv-integrin blockade (Figure 4). In addition, endostatin alone, which was used as positive control, showed no effect on either neovessel formation or HIF-1α basal expression (Figure 4 and Supplemental Figure 6). When endostatin was co-administered with asTF a significant inhibition of asTF-induced angiogenesis and HIF-1α up-regulation in vivo was observed (Figure 4 and Supplemental Figure 6). To determine the contribution of FAK to angiogenesis and HIF-1α expression by asTF, we first analyzed the phosphorylation status of effect of FAK by immunoblotting and found a 3.5-fold increase of FAK Y397 phosphorylation (p=0.01 vs. baseline) in endothelial cells treated with asTF (Figure 5D). When asTF was co-administered with FAK inhibitor neovessel formation in vivo was significantly reduced (Supplemental Figure 6); however, FAK inhibition did not significantly inhibit asTF-induced HIF-1α expression under our in vitro (Figure 5E) and in vivo (Figure 4) experimental conditions.
The contribution of MAPK and PI3K-Akt signaling pathways to HIF-1α induced by asTF was also investigated. To elucidate the role of MAPK pathways, extracellular-signal-regulated kinases½ (ERK½) and mitogen-activated protein kinases½ (MEK½) inhibitors were used. Inhibitors alone did not significantly affect either neovessel formation or HIF-1α expression (Figure 4 and Supplemental Figure 6) but inhibitors significantly inhibited asTF-induced angiogenesis and HIF-1α up-regulation when co-administered with asTF (Figure 4 and Supplemental Figure 6). To determine whether Akt activation plays a role in the control HIF-1α expression induced by asTF, endothelial cells were first transduced with an adenovirus encoding a dominant-negative Akt mutant (dn-Akt) and then treated with asTF. Of note, HIF-1α expression was significantly reduced in dn-Akt transduced cells vs. Ad.βGal (Figure 5F), suggesting a role of Akt pathway in HIF-1α up-regulation.
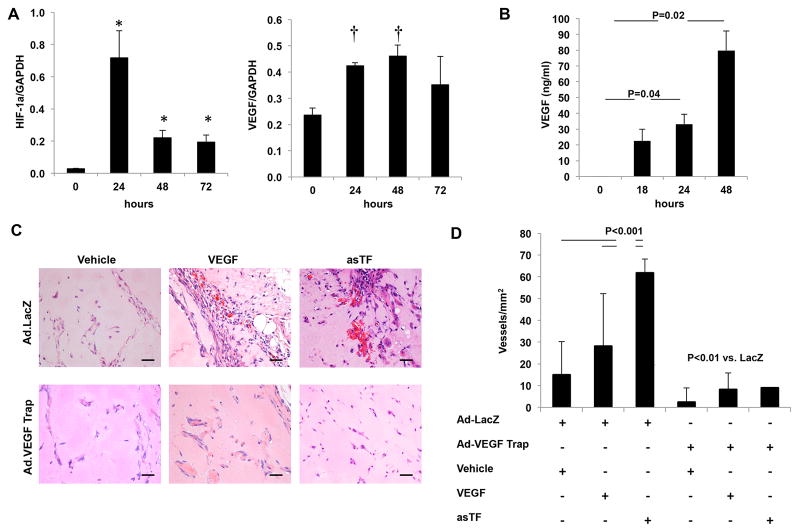
asTF angiogenic activity is mediated via the expression of vascular endothelial growth factor
Next we tested the hypothesis that HIF-1α up-regulation by asTF results in transactivation of VEGF. To this end, we investigated the effect of asTF on the gene expression of VEGF-A isoforms. The results of these experiments confirmed a significant increase of HIF-1α mRNA and revealed that the level of VEGF165 isoform was significantly increased in endothelial cells that were stimulated with asTF (Figure 6A). A significant increase of the soluble VEGF165 protein was also observed in the conditioned media of cells treated with asTF (Figure 6B). The contribution of VEGF to the angiogenic activity of asTF was investigated in vivo using an adenovirus vector-encoding the receptor chimera VEGF-Trap R1R2. As expected, the angiogenic activity of VEGF was completely abolished in mice overexpressing VEGF-Trap vs. LacZ controls. Remarkably, the angiogenic activity of asTF was inhibited in mice overexpressing VEGF-Trap (Figure 6C, D) confirming the role of VEGF in asTF-induced neoangiogenesis.
Figure 6.
The angiogenic effect of asTF is mediated via HIF-1 mediated activation of VEGF. A. Time course of the expression of HIF-1α and the VEGF-A isoform 165 in endothelial cells treated with asTF (10nM). No significant up-regulation of the other VEGF-A isoforms (121, 189, 206) was observed (data not shown). B. A significant increase in expression of VEGF-A isoform 165 was observed in conditioned media from cells treated with asTF (10nM) for up to 72 hours by ELISA.. C. H&E stained sections of Matrigel plugs supplemented with asTF (10nM), VEGF (50 ng/ml) or PBS (vehicle). Twenty-four hours prior to Matrigel injection, mice were systemically administered either Ad.VEGF-TrapR1R2 (1011 vp) or Ad.LacZ (1011 vp). Magnification 400x. Scale Bar=20 μm. D. As expected, quantitative analysis showed a significant inhibition of VEGF-induced angiogenesis. A similar inhibition of was observed in when the angiogenic effect of asTF was tested in mice injected with Ad.VEGF-TrapR1R2 vs. Ad.Lacz. *P=0.03; †P<0.02. A–B: data are the average of triplicates from single experiments that were independently repeated 3 times. C, D: n=2 plugs/animal; n=5 animals/group).
asTF overexpression promotes neointima formation and angiogenesis in response to arterial injury in ApoE−/−
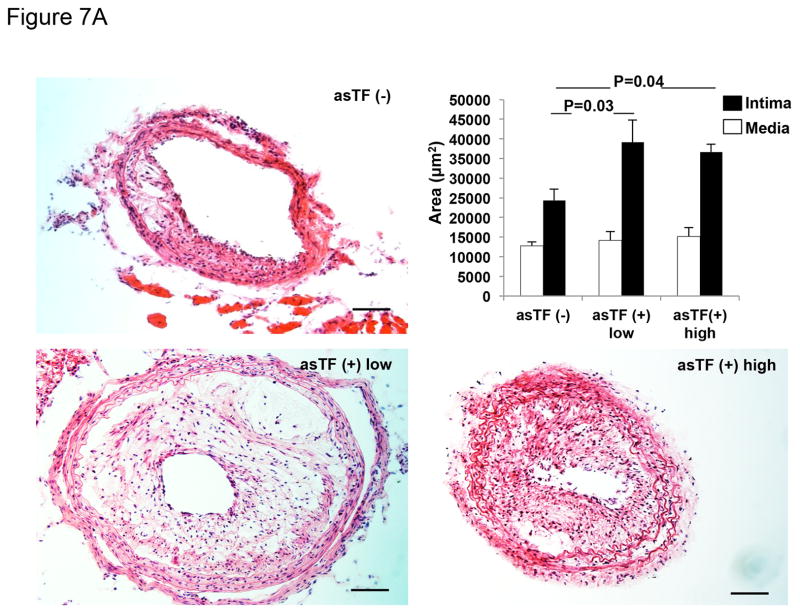
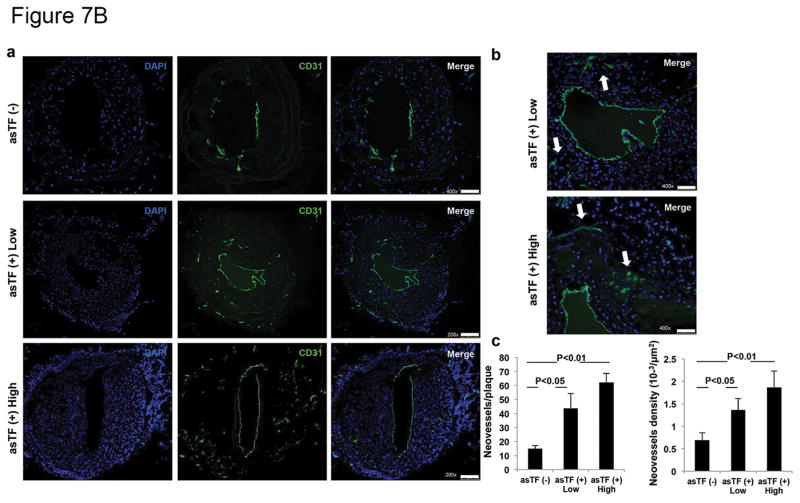
To test the hypothesis that asTF contributes to the progression of atherosclerosis and to plaque angiogenesis, left carotid artery injury, with or without asTF gene transfer, was performed in ApoE−/− kept on a Western-type starting 2 weeks before surgery. As expected, 4 weeks after arterial wire injury, histological analysis revealed the presence of complex neointima lesions in all injured arteries. Results showing efficient transduction of HEK293T cells with generated lentivirus encoding for GFP or asTF-GFP are presented in Supplemental Figure 7A (see Supplemental Figure 7B for details on asTF and spliced sites of fl-TF exons). Efficient transduction was confirmed in vivo by the expression of GFP in the injured carotids transduced with lentivirus (Supplemental Figure 8). Remarkably, neointima thickness was significantly greater in asTF-transduced/injured carotid arteries. No significant difference in medial area was observed between groups (Figure 7A). Importantly, an increased number of neovessels/plaque and increased neovessel density were observed in asTF-transduced lesions vs. not-transduced ones (Figure 7B).
Figure 7.
Effect of asTF on neointima formation and neovascularization in an experimental model of accelerated atherosclerosis. A. Bright-field photomicrograph of cross sections of left common carotid artery (LCCA) harvested 4 weeks after injury and stained with hematoxylin-eosin (H&E) from ApoE−/− mice. LCCA were infected with a lentivirus encoding asTF; asTF(+) low (n=4) and asTF (+) high (n=4) doses. Injured but non-transduced LCCA, asTF (−), were used as controls (n=5). Magnification 200x. Scale Bar: 40 μm. Quantitative analysis of neointima (black bars) and media (white bars) formation in the injured LCCA of ApoE−/− mice overexpressing asTF (asTF+ Low and –High; 2×106 and 8×106 IU, respectively) showed increased neointima formation vs. controls (asTF-). No difference was observed in media formation among groups. B. a. Representative pictures of LCCA cross sections immunostained for CD31 (Alexa 488). Pictures clearly show increased neovascularization in arteries injured and transduced with asTF (asTF+ low and High) vs. non-transduced controls (asTF-). Magnification for asTF (−) is 400x (scale bar 50 μm) while for asTF+ is 200x (scale bar 100 μm). Higher magnification (400x) of asTF+ low and high is shown for comparison with asTF (−). b. Quantification of neovessel formation shown as neovessels/plaque (left) and neovessel density (right).
Discussion
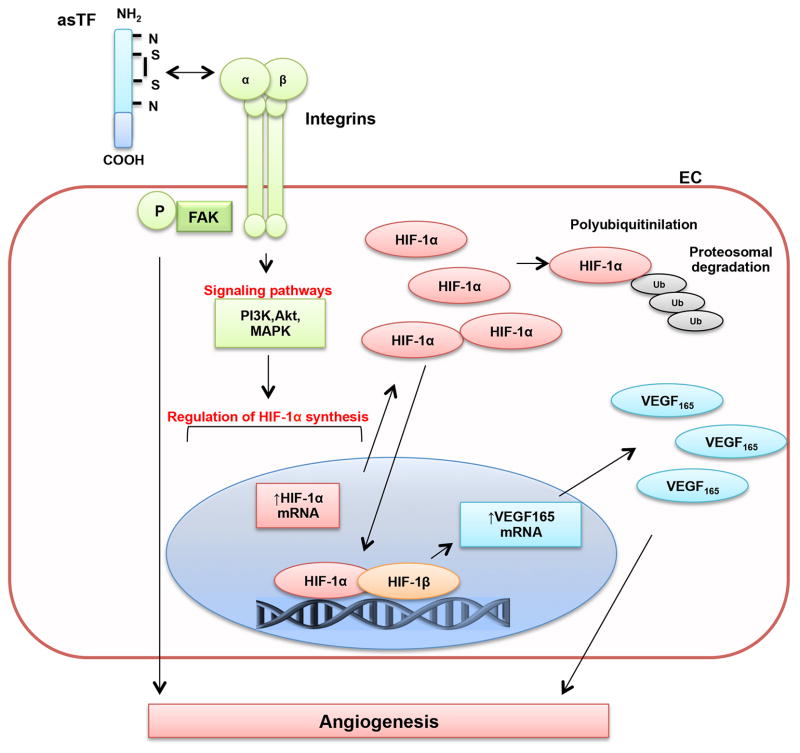
The results of this study provide strong evidence that asTF promotes neointima formation and angiogenesis in an experimental model of accelerated atherosclerosis. Herein, we describe that the angiogenic effect of asTF is mediated via the activation of the HIF-1/VEGF signaling. HIF-1α up-regulation occurs through integrin and activation of MAPK and PI3K-Akt pathways (Figure 8). This mechanism may be relevant to neovascularization, progression and complications of human atherosclerosis as suggested by the higher expression of asTF in complicated vs. uncomplicated human carotid and coronary plaques.
Figure 8.
Schematic representation of asTF signaling pathway. EC indicates endothelial cell; P-FAK, phosphorylated focal adhesion kinase; PI3K, phosphatidylinositol 3-kinase; Akt; MEK, mitogen-activated protein kinases; ERK, extracellular-signal-regulated kinases; HIF-1α, hypoxia-inducible factor-1α (inducible); HIF-1β, hypoxia-inducible factor-1β (constitutive); Ub, ubiquitin; VEGF165, vascular endothelial growth factor isoform 165 (soluble).
Cellular distribution analysis of human plaques revealed that asTF was highly expressed within macrophage-rich and highly vascularized areas of complicated coronary and carotid lesions. Since monocytes/macrophage infiltration has been implicated in plaque angiogenesis 38 as well as vulnerability39 and asTF appear to increase monocytes adhesion to endothelial cells 26, it was not surprising to observe that the number of asTF+/CD68+ and asTF+/CD31+ cells was higher in complicated vs. uncomplicated plaques. Particularly, asTF appeared to be expressed by specific subsets of macrophages and endothelial cells identified in complicated lesions.
These observations are consistent with the involvement of asTF in the angiogenic switch of unstable atherosclerotic plaques. In light of the observed higher expression of asTF in plaques from patients with acute coronary events vs. lesions from patients with stable angina, it is logical to speculate that asTF may contribute to the onset of coronary events. Remarkably, asTF influences all critical phases involved in the formation of new blood vessels such as endothelial cells’ migration, proliferation and differentiation into capillaries 40. Increased proliferation of endothelial cells treated with asTF involves the expression Cyclin D1 showing an early up-regulation followed by down-regulation, reflecting the typical Cyclin D1 rise early in G1 followed by its rapid decline in G1/S-phase when DNA replication occurs41. Co-localization of asTF with the proliferation marker Ki67 was observed in complicated plaques highly expressing asTF, suggesting that asTF promotes cell proliferation in human atherosclerosis.
asTF increases endothelial cells migration via both chemotaxis and haptotaxis as demonstrated by directional migration towards a gradient of soluble asTF, an effect that was further increased when inserts were coated with asTF.
Others could only observe the haptotatic activity and could not detect any proliferative effect of asTF on endothelial cells19; however, in these studies different approaches and higher concentrations of asTF (100nM), which we found to be cytotoxic, were used.
We observed a greater angiogenic potency of asTF vs. fl-TF in vivo. Of note, this effect was observed using equipotent concentrations of tissue factor isoforms and VEGF when their activities were tested in vitro. Although suggestive of a greater angiogenic potency of asTF vs. fl-TF, the pathophysiological relevance of these findings is difficult to extrapolate to atherosclerotic disease. In fact, the relative expression of fl-TF and asTF is in favor of fl-TF in human atherosclerotic plaques and it remains undetermined how much asTF is expressed in vivo. Moreover, in our studies we used re-lipidated fl-TF to mimic the effect of circulating fl-TF42, 43, an approach that does not reflect the proteome complexity of circulating microparticles carrying fl-TF in humans.
Despite these limitations, our findings highlight the possibility that asTF activates specific signaling pathways. In separate experiments we observed that asTF but not fl-TF induces a significant up-regulation of HIF-1α. A positive correlation between HIF-1α+ cells and neovessel density in vivo further confirmed the hypothesis that the angiogenic activity of asTF is mediated via HIF-1α activation. In light of the observation that HIF-1α protein up-regulation occurs only after 6 hours of asTF stimulation, it was not surprising that we did not detect a significant difference in the angiogenic activity of asTF vs. fl-TF in vitro. In fact, capillary formation in vitro was measured at 6 hours following treatments while the in vivo Matrigel plug assay was examined after 10 days.
The observed significant dose dependent up-regulation of HIF-1α mRNA suggests that asTF regulates HIF-1α at the transcriptional level. In fact, regulation of HIF-1α protein degradation, which under normoxic conditions occurs via its hydroxylation, binding to pVHL, poly-ubiquitylation and ultimately proteasomal degradation, is the most recognized mechanism for HIF-1α stabilization resulting in HIF-1 heterodimer formation44. Interestingly, the detection of polyubiquinated forms of HIF-1α demonstrates that asTF does not interfere with polyubiquitination, a required steps for proteasomal degradation. Of note, the inhibition of HIF-1α protein synthesis by cycloheximide suggests that asTF may increase de-novo protein synthesis via increased HIF-1α mRNA transcription.
In previous studies, asTF was found to oligomerize integrins and activate downstream signaling to promote endothelial cell migration and capillary formation in vitro19. However, in vitro conditions may not reflect the complexity of physiological interactions (i.e. blood flow, interactions to cells and matrices) occurring in vivo. Here, we report that the downstream signaling events activated by asTF through integrin activation are relevant for the angiogenic activity of asTF in vivo. A critical role of β3-, α6- and β1-integrins in mediating the angiogenic activity of asTF was demonstrated by the significant reduction of neovessels formation induced by asTF in vivo upon integrin inhibition. We have also demonstrated that HIF-1α up-regulation induced by asTF is mediated via integrin activation. Specifically, it was observed that β3, β1 and α6-integrin blockade was associated with significant reduction of HIF-1α up-regulation by asTF. In contrast, no effect was observed in the presence of αv blockade.
In support of the validity of our experimental conditions, the formation of large vessels with anti-β3 neutralizing antibody alone was expected and in accord with previous findings revealing paradoxically increased angiogenesis via VEGFR-2 up-regulation45.
Analysis of the downstream signaling of integrins confirmed the activation of FAK induced by asTF19. However, whereas FAK inhibition abolished neovessel formation in vivo, it only partially inhibited HIF-1α induction by asTF. Taken together, these findings suggest that, although required for asTF-induced angiogenesis, FAK activation is not essential for asTF-induced HIF-1α expression. A possible explanation is the essential role of FAK in modulating early, essential steps (i.e. endothelial cells migration and proliferation) for the sprouting of new capillaries46, 47 such that residual HIF-1α expression cannot balance under our experimental conditions.
Since integrins lack kinase activity, it is possible that signaling pathways other than FAK are involved. Our results revealed that MAPK and PI3K-Akt signaling pathways modulate HIF-1α up-regulation following asTF stimulation. Integrin-mediated activation of MAPK and PI3K-Akt independent of FAK has been extensively described48 and our results suggest that, as in cancer36, stimuli other than hypoxia, such asTF, may activate HIF-1 in atherosclerosis possibly contributing to disease progression.
Increased expression of HIF-1α was detected in complicated plaques with high levels of asTF, findings that support the pathophysiological importance of the proposed mechanism in the progression and complications of atherosclerotic disease.
Upon asTF stimulation of endothelial cells, HIF-1α translocates from the cytoplasm to the nucleus. Here HIF-1α dimerizes with HIF-1β becoming transcriptionally active directly activating the expression of a number of proangiogenic factors, the best characterized of which is VEGF, thereby promoting the formation of new vessels49. Our results demonstrate that asTF induces gene and expression of the secreted VEGF-A isoform 165. Of note, the angiogenic effect of asTF was reduced when VEGF signaling was inhibited by gene transfer of VEGF-TrapR1R2 in vivo. These observations suggest that the angiogenic activity of asTF is mediated via the activation of HIF-1/VEGF signaling.
Finally, we demonstrated for the first time that asTF plays a critical role in the progression of atherosclerosis and plaque angiogenesis. Our results show a significantly greater neointima thickness in injured carotid arteries that have been transduced with lentivirus encoding asTF compared to injured but untransduced carotid arteries of ApoE−/− on a Western-type diet. Remarkably, plaque neovascularization was significantly higher in injured and asTF-transduced arterial vessels.
In previous studies it was determined that a 50% reduction of TF, in all cells or in hematopoietic cells only in ApoE−/− and LDLR−/− mice, respectively, does not affect atherosclerosis development50. In our study we found that asTF overexpression increase neointima formation and lesion’s neovascularization following arterial injury in ApoE−/− mice fed a Western-type diet. In line with the possibility of a pathophysiological effect of asTF on atherosclerosis progression and complications is the observation that asTF is highly expressed within advanced human carotid and coronary plaques complicated by hemorrhage and/or disruption.
Conclusions
We have demonstrated for the first time that asTF promotes neointima formation and neovascularization in a model of accelerated atherosclerosis. The angiogenic activity of asTF is mediated via the activation of HIF-1/VEGF signaling through the integrin-mediated activation of MAPK and PI3K-Akt signaling pathways. The potential role of asTF in plaque neovascularization and to the progression and complications of atherosclerosis is further supported by the by the increased expression of asTF in complicated coronary plaques from patients with acute coronary events. The identification of asTF as a potent angiogenic factor, acting via the non-hypoxic HIF-1α up-regulation in atherosclerosis, makes it an attractive marker of plaque vulnerability and a potential therapeutic target for plaques stabilization and prevention of cardiovascular clinical events.
Supplementary Material
Acknowledgments
The authors thank Rolando Nolasco for immunohistochemistry studies. We acknowledge Aesha Patel (RPA-C), the Institutional Biorepository/Biospecimen Bank Shared Resource Facility (SRF) and the Microscopy (SRF) of the Mount Sinai Medical Center. The pTriEx3-Neo -asTF expression vector was a gift from Dr. V.Y. Bogdanov to Dr. G.A. Soff. Ad.VEGF-Trap was a generous gift from Regeneron to R. Hutter and J. J. Badimon.
Funding Sources: This work was supported by the NIH-National Heart, Lung, and Blood Institute grants K23HL111339, 5T32HL 7824-15 (Dr. Giannarelli), R01 HL093183, HL088434 (Dr. Hajjar), R01HL071021 (Dr. Fayad), K08HL111330 (Dr. Kovacic). We acknowledge research support from The Leducq Foundation (Transatlantic Network of Excellence Award) to Drs. Hajjar and Kovacic and AstraZeneca (Dr. Kovacic).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the u.S. From 1980 through 2002: Concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 3.Giannarelli C, Zafar MU, Badimon JJ. Prostanoid and tp-receptors in atherothrombosis: Is there a role for their antagonism? Thromb Haemost. 2010;104:949–954. doi: 10.1160/TH10-03-0195. [DOI] [PubMed] [Google Scholar]
- 4.Laslett LJ, Alagona P, Jr, Clark BA, 3rd, Drozda JP, Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the american college of cardiology. J Am Coll Cardiol. 2012;60:S1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 6.Milei J, Parodi JC, Alonso GF, Barone A, Grana D, Matturri L. Carotid rupture and intraplaque hemorrhage: Immunophenotype and role of cells involved. Am Heart J. 1998;136:1096–1105. doi: 10.1016/s0002-8703(98)70169-3. [DOI] [PubMed] [Google Scholar]
- 7.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The oxford plaque study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 8.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 9.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 10.Kolodgie FD, Narula J, Yuan C, Burke AP, Finn AV, Virmani R. Elimination of neoangiogenesis for plaque stabilization: Is there a role for local drug therapy? J Am Coll Cardiol. 2007;49:2093–2101. doi: 10.1016/j.jacc.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 11.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 12.Granada JF, Feinstein SB. Imaging of the vasa vasorum. Nat Clin Pract Cardiovasc Med. 2008;5 (Suppl 2):S18–25. doi: 10.1038/ncpcardio1157. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 14.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–1550. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 15.Ruf W, Disse J, Carneiro-Lobo TC, Yokota N, Schaffner F. Tissue factor and cell signalling in cancer progression and thrombosis. J Thromb Haemost. 2011;9 (Suppl 1):306–315. doi: 10.1111/j.1538-7836.2011.04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernandez-Ortiz A, Chesebro JH, Badimon L, Nemerson Y, Fuster V, Badimon JJ. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation. 1997;95:594–599. doi: 10.1161/01.cir.95.3.594. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: A circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 18.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125 (Suppl 2):S36–38. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, Spek CA, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106:19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Signaevsky M, Hobbs J, Doll J, Liu N, Soff GA. Role of alternatively spliced tissue factor in pancreatic cancer growth and angiogenesis. Semin Thromb Hemost. 2008;34:161–169. doi: 10.1055/s-2008-1079256. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs JE, Zakarija A, Cundiff DL, Doll JA, Hymen E, Cornwell M, Crawford SE, Liu N, Signaevsky M, Soff GA. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120 (Suppl 2):S13–21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- 22.Chand HS, Ness SA, Kisiel W. Identification of a novel human tissue factor splice variant that is upregulated in tumor cells. Int J Cancer. 2006;118:1713–1720. doi: 10.1002/ijc.21550. [DOI] [PubMed] [Google Scholar]
- 23.Haas SL, Jesnowski R, Steiner M, Hummel F, Ringel J, Burstein C, Nizze H, Liebe S, Lohr JM. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J Gastroenterol. 2006;12:4843–4849. doi: 10.3748/wjg.v12.i30.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollin J, Regina S, Gruel Y. Tumor expression of alternatively spliced tissue factor is a prognostic marker in non-small cell lung cancer. J Thromb Haemost. 2010;8:607–610. doi: 10.1111/j.1538-7836.2009.03713.x. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg YW, Versteeg HH. Alternatively spliced tissue factor. A crippled protein in coagulation or a key player in non-haemostatic processes? Hamostaseologie. 2010;30:144–149. [PubMed] [Google Scholar]
- 26.Srinivasan R, Ozhegov E, van den Berg YW, Aronow BJ, Franco RS, Palascak MB, Fallon JT, Ruf W, Versteeg HH, Bogdanov VY. Splice variants of tissue factor promote monocyte-endothelial interactions by triggering the expression of cell adhesion molecules via integrin-mediated signaling. J Thromb Haemost. 2011;9:2087–2096. doi: 10.1111/j.1538-7836.2011.04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandradas S, Deikus G, Tardos JG, Bogdanov VY. Antagonistic roles of four sr proteins in the biosynthesis of alternatively spliced tissue factor transcripts in monocytic cells. J Leukoc Biol. 2010;87:147–152. doi: 10.1189/jlb.0409252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann J, Mannheim D, Wohlert C, Versari D, Meyer FB, McConnell JP, Gossl M, Lerman LO, Lerman A. Expression of lipoprotein-associated phospholipase a(2) in carotid artery plaques predicts long-term cardiac outcome. Eur Heart J. 2009;30:2930–2938. doi: 10.1093/eurheartj/ehp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, Lerman LO, Lerman A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2132–2139. doi: 10.1161/01.ATV.0000232501.08576.73. [DOI] [PubMed] [Google Scholar]
- 30.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 31.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 33.Depre C, Wijns W, Robert AM, Renkin JP, Havaux X. Pathology of unstable plaque: Correlation with the clinical severity of acute coronary syndromes. J Am Coll Cardiol. 1997;30:694–702. doi: 10.1016/s0735-1097(97)00213-1. [DOI] [PubMed] [Google Scholar]
- 34.Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, Fuster V, Badimon JJ, Sauter BV. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004;110:2430–2435. doi: 10.1161/01.CIR.0000145120.37891.8A. [DOI] [PubMed] [Google Scholar]
- 35.Lipskaia L, Bobe R, Chen J, Turnbull IC, Lopez JJ, Merlet E, Jeong D, Karakikes I, Ross AS, Liang L, Mougenot N, Atassi F, Lompre AM, Tarzami ST, Kovacic JC, Kranias E, Hajjar RJ, Hadri L. Synergistic role of protein phosphatase inhibitor 1 and sarco/endoplasmic reticulum ca2+-atpase in the acquisition of the contractile phenotype of arterial smooth muscle cells. Circulation. 2014;129:773–785. doi: 10.1161/CIRCULATIONAHA.113.002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenza GL. Hif-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-fak-rhob: A novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 38.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 40.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 41.Bartek J, Lukas J. DNA repair: Cyclin d1 multitasks. Nature. 2011;474:171–172. doi: 10.1038/474171a. [DOI] [PubMed] [Google Scholar]
- 42.Sambola A, Osende J, Hathcock J, Degen M, Nemerson Y, Fuster V, Crandall J, Badimon JJ. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–977. doi: 10.1161/01.cir.0000050621.67499.7d. [DOI] [PubMed] [Google Scholar]
- 43.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: Limitations of a commercial assay. J Thromb Haemost. 2009;7:894–897. doi: 10.1111/j.1538-7836.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL. Hydroxylation of hif-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 46.Tavora B, Batista S, Reynolds LE, Jadeja S, Robinson S, Kostourou V, Hart I, Fruttiger M, Parsons M, Hodivala-Dilke KM. Endothelial fak is required for tumour angiogenesis. EMBO Mol Med. 2010;2:516–528. doi: 10.1002/emmm.201000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226:404–412. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- 48.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 49.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 50.Tilley RE, Pedersen B, Pawlinski R, Sato Y, Erlich JH, Shen Y, Day S, Huang Y, Eitzman DT, Boisvert WA, Curtiss LK, Fay WP, Mackman N. Atherosclerosis in mice is not affected by a reduction in tissue factor expression. Arterioscler Thromb Vasc Biol. 2006;26:555–562. doi: 10.1161/01.ATV.0000202028.62414.3c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.