Abstract
Neuronal migration and subsequent differentiation play critical roles for establishing functional neural circuitry in the developing brain. However, the molecular mechanisms that regulate these processes are poorly understood. Here, we show that microtubule actin crosslinking factor 1 (MACF1) determines neuronal positioning by regulating microtubule dynamics and mediating GSK-3 signaling during brain development. First, using MACF1 floxed allele mice and in utero gene manipulation, we find that MACF1 deletion suppresses migration of cortical pyramidal neurons and results in aberrant neuronal positioning in the developing brain. The cell autonomous deficit in migration is associated with abnormal dynamics of leading processes and centrosomes. Furthermore, microtubule stability is severely damaged in neurons lacking MACF1, resulting in abnormal microtubule dynamics. Finally, MACF1 interacts with and mediates GSK-3 signaling in developing neurons. Our findings establish a cellular mechanism underlying neuronal migration and provide insights into the regulation of cytoskeleton dynamics in developing neurons.
Keywords: MACF1, neuronal migration, cytoskeleton, microtubule, GSK-3
Introduction
Excitatory pyramidal neurons are generated in the cortical ventricular zone and migrate into the cortical plate alongside the radial glial processes (Chanas-Sacre et al., 2000; Hartfuss et al., 2001; Noctor et al., 2001; Rakic, 1972; Tan et al., 1998). Neuronal migration determines the positioning of developing neurons into cortical layers and is therefore important in generating lamina-specific neural circuits. Once neurons reach their destination, they further differentiate by creating extensive dendritic branching and forming spines to establish functional connectivity (Jan and Jan, 2010). Thus, correct positioning of neurons are critical determinants of neural circuit formation during brain development. Accordingly, problems in neuron migration during development cause brain malformations and are associated with a variety of neurological diseases such as mental retardation, autism, and schizophrenia (Gleeson and Walsh, 2000; Jan and Jan, 2010; Kaufmann and Moser, 2000; Wegiel et al., 2010). However, the molecular mechanisms of developing neuron migration are not fully understood.
Microtubule actin crosslinking factor 1 (MACF1) is a protein that belongs to the plakin family of cytoskeletal linker proteins (Fuchs and Karakesisoglou, 2001; Jefferson et al., 2004; Kodama et al., 2003; Roper et al., 2002). The protein bridges microtubules and actins through its corresponding domains at N-and C-terminals. Mutations in shot, the Drosophila homolog of MACF1, induce abnormal neurite outgrowth and guidance (Gao et al., 1999; Jan and Jan, 2001; Kolodziej et al., 1995; Lee et al., 2000a; Lee and Luo, 1999; Prokop et al., 1998). However, the functions and mechanisms of MACF1 in neuronal differentiation in the developing mammalian brain have not been clearly determined. Interestingly, MACF1 is identified as a part of an interactome of DISC1, a schizophrenia and autism susceptibility factor (Camargo et al., 2007). In a separate study, MACF1 is shown to be associated with glycogen synthase kinase-3 (GSK-3) signaling (Wu et al., 2011). It is noted that DISC1 and GSK-3 bind to each other and coordinate neuronal migration in the developing brain (Ishizuka et al., 2011; Singh et al., 2010). Thus, these findings suggest a potential interplay among MACF1, GSK-3, and DISC1 in neuronal development. We hypothesize that MACF1 plays critical roles in neuronal migration in the developing brain by interacting with GSK-3 signaling and controlling microtubule stability.
Developing cortical and hippocampal neurons in mice actively migrate and differentiate between embryonic day 13 (E13) and 20. MACF1 null mice die before E11 (Chen et al., 2006), precluding the use of MACF1 null mice in the analysis of MACF1 in neuronal migration and further differentiation. To investigate the functions and mechanisms of MACF1 in neuronal development in vivo, we used conditional knockout strategies to target MACF1 specifically in developing neurons. Here, we show that MACF regulates pyramidal neuronal migration via microtubule dynamics and GSK-3 signaling in the developing brain. Our findings demonstrate the mechanisms of MACF1-regulated positioning of developing pyramidal neurons.
Results
Neural expression of MACF1
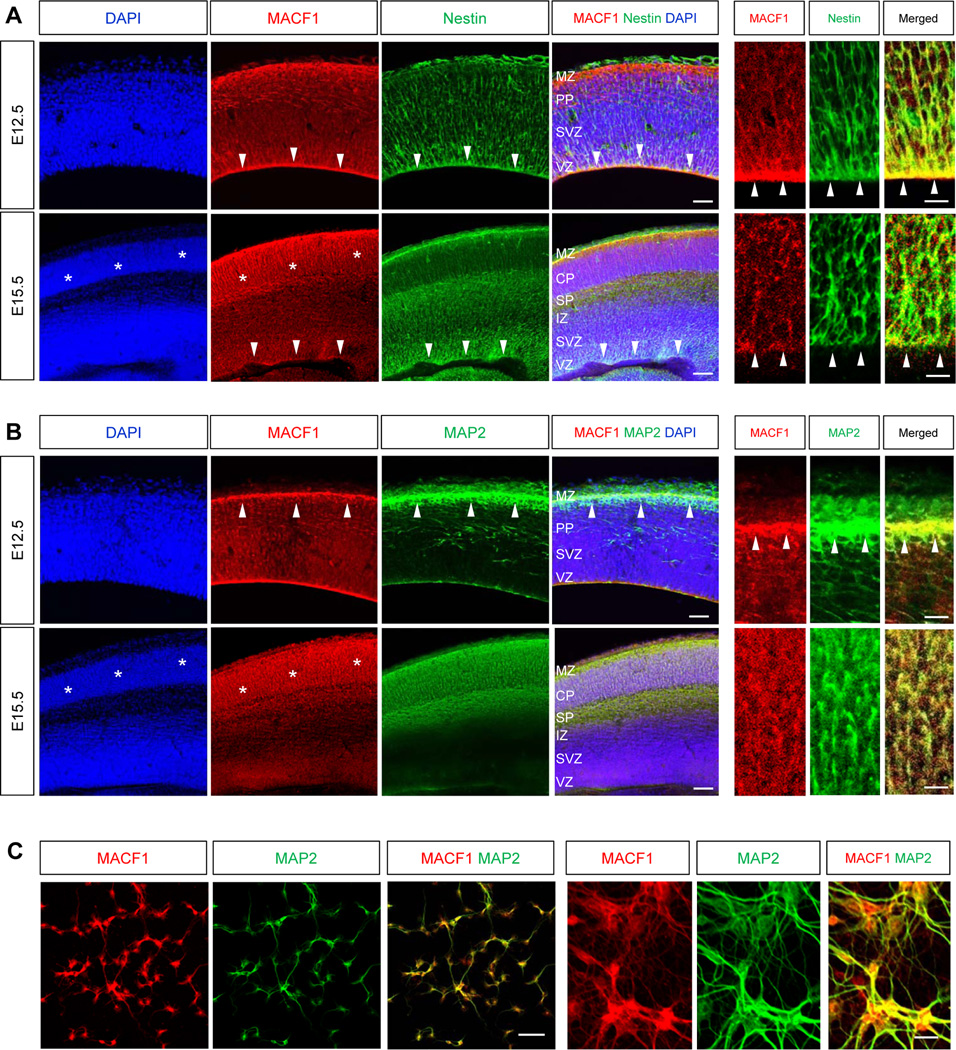
To begin to explore the role of MACF1 in neuronal development, we first assessed the expression patterns of MACF1 in the developing brain with immunohistochemistry. The antibody specificity was determined by Western blotting using brain lysates from control and MACF1 knockout mice (Supplemental Fig. 1). MACF1 was broadly expressed in the developing cerebral cortex at E12.5, with higher levels in the ventricular zone and upper cortical areas near the marginal zone (Fig. 1A, top panels). Ventricular labeling was associated with nestin-positive radial glial neural progenitors (Fig. 1A, arrow heads). At E15.5, MACF1 expression at the ventricular zone was reduced compared to the expression pattern at E12.5 (Fig. 1A, bottom panels, arrow heads). Instead, MACF1 levels were higher in the cortical plate where postmitotic neurons were positioned (Fig. 1A, bottom panels, stars). The neuronal localization of MACF1 in the cortical plate and the marginal zone was shown by double immunostaining with a MAP2 antibody (Fig. 1B). At E12.5, MACF1 was accumulated in the neurons at the marginal zone layer (Fig. 1B, top panels, arrow heads). The expression was expanded throughout the cortical plate by E15.5 (Fig. 1B, bottom panels, stars), suggesting a role for MACF1 in neuronal differentiation. Immunostaining of cultured cortical neurons confirmed the neuronal expression of MACF1 (Fig. 1C). MACF1 proteins were found in cortical neurites as well as somata.
Figure 1. Expression of MACF1 in the developing cortex.
(A) Top panels: Immunostaining for MACF1 showed that the protein was broadly expressed in the developing mouse brain at E12.5. Notably, MACF1 was accumulated in the VZ where nestin-positive radial glial neural progenitors were present (arrow heads). Scale bar, 25 µm. Right three panels are higher magnification images showing co-localization of MACF1 and nestin. Scale bar, 10 µm. Bottom panels: At E15.5 stage, higher expression of MACF1 was found in the CP (stars), while the expression was reduced in radial glial progenitors at the VZ and the SVZ (arrow heads). Scale bar, 50 µm. Right three panels are higher magnification images. (B) Top panels: MACF1 protein was present in MAP2-positive cortical neurons in the MZ at E12.5 (arrow heads). Scale bar, 25 µm. Bottom panels: MACF1 was strongly expressed in the neurons of the CP and the SP at E15.5 (stars). Scale bar, 50 µm. Right three columns represent higher magnification images of upper cortex containing the MZ and PP (top), and the CP (bottom). Scale bar, 10 µm. MZ: marginal zone. PP: pre-plate. CP: cortical plate. SP: sub-plate. IZ: intermediate zone. SVZ: subventricular zone. VZ: ventricular zone. (C) MACF1 is expressed in neurites and somas of cortical neurons. Cortical neurons from E14.5 mice were cultured and immunostained with a MAP2 antibody. Scale bar, 50 µm. Right three panels are higher magnification images showing co-localization of MACF1 and MAP2. Scale bar, 25 µm.
Cell-autonomous role of MACF1 in positioning and migration of cortical pyramidal neurons
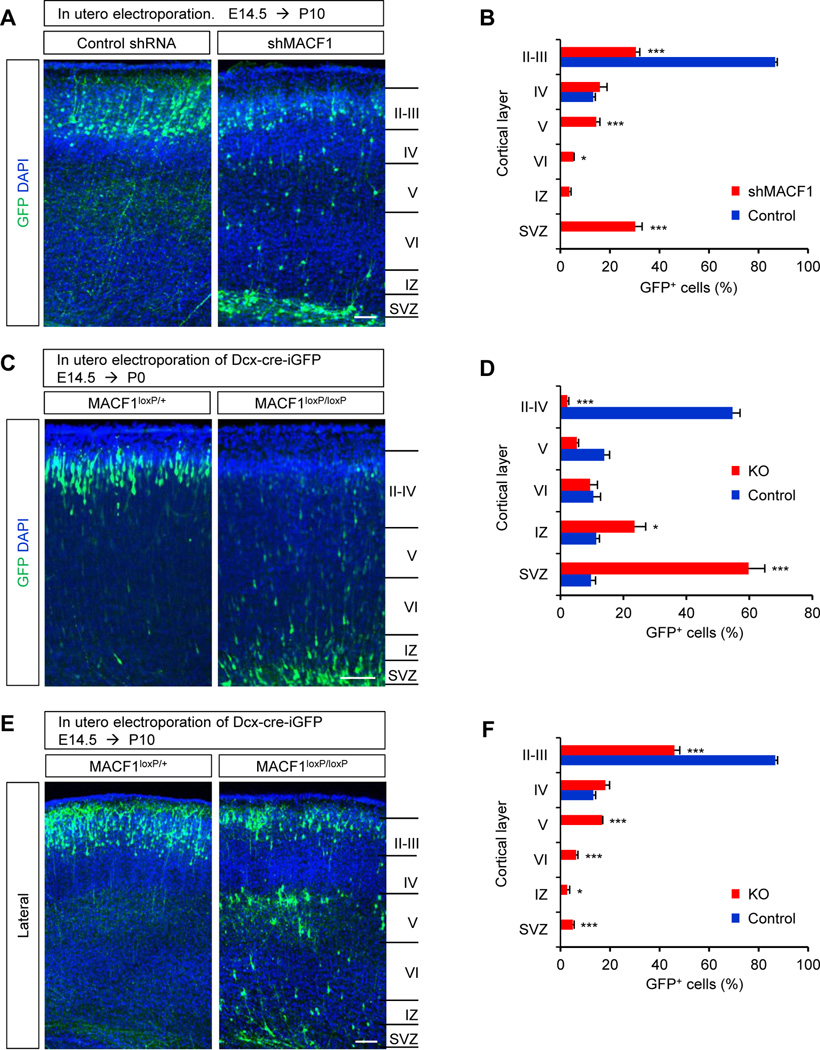
To investigate potential roles of MACF1 in neuron development, we first examined radial neuron migration in the developing cortex using an shRNA to MACF1 (shMACF1). The knockdown efficiency of shMACF1 was checked by measuring the level of endogenous MACF1 in cultured cortical cells (Supplemental Fig. 2A). We used an in utero electroporation of shMACF1 to delete MACF1 transcripts and trace radial migration of newly-born neurons (Supplemental Fig. 2B). shMACF1 encodes GFP in a separate reading frame of an shRNA sequence, thus GFP expression marks the cells transfected with the shRNA. We electroporated in utero either a plasmid encoding non-silencing shRNA (control) or an shMACF1 into the ventricles of E14.5 brains. Then, we sacrificed the mice and collected brain samples at P10. The electroporation targeted similar regions of the cerebral cortex in control and shMACF1-injected brains (Supplemental Fig. 2C). Most GFP-labeled neurons were found in the cortical plate in control brain sections (Fig. 2A, 2B). However, neurons expressing shMACF1 were localized throughout the cerebral cortex with the highest numbers within ventricular/subventricular zones and upper layers of the cortical plate. At E18.5, GFP-labeled neurons were mostly retained within the ventricular/subventricular zones (Supplemental Fig. 2D). These results suggest a critical role of MACF1 in radial neuronal migration during brain development.
Figure 2. MACF1 regulates radial neuron migration in the developing brain.
(A) shRNA-mediated MACF1 deletion induced abnormal localization of electroporated cells in the brain. E14.5 mouse brains were electroporated in utero with non-silencing shRNA (control) or shMACF1 construct. shRNAs encode GFP in a separate reading frame for a labeling purpose. The electroporated mice were then sacrificed at age P10 and the brain samples were collected. GFP-positive cells were visualized in the lateral cerebral cortex. Scale bar: 50 µm. (B) Quantification of neuron positions throughout the cerebral cortex. n = 5 mice for each condition; cell counts = 3605 cells for control and 3912 cells for shMACF1. Statistical significance was determined by multiple t-tests with Bonferonni correction test. Data shown are mean ±SEM. Stars indicate significant difference when compared with controls. * p<0.05, ** P<0.01, *** p<0.001. (C) Neuron-specific deletion of MACF1 leads to abnormal neuron migration in the developing cerebral cortex. Control (MACF1loxP/+) or MACF1loxP/loxP embryos were electroporated in utero with Dcx-cre-iGFP at E14.5 to target radially-migrating neurons. The electroporated brains were collected at P0 and neurons expressing GFP were visualized in the lateral cerebral cortex. Scale bar: 50 µm. (D) Quantification of neuron positioning in the brain. Control: MACF1loxP/+; Dcx-cre-iGFP. KO: MACF1loxP/loxP; Dcx-cre-iGFP. n = 5 mice for each condition; cell counts = 2983 cells for control and 3318 cells for KO. Statistical significance was determined by multiple t-tests with Bonferonni correction test. * p<0.05, ** P<0.01, *** p<0.001. (E) Same experiments were performed as (C), but brain samples were collected at P10 to examine postnatal migration patterns. Scale bar: 50 µm. (F) Quantification of (E). Positioning of GFP-positive neurons in the lateral cortex. Control: MACF1loxP/+; Dcx-cre-iGFP. KO: MACF1loxP/loxP; Dcx-cre-iGFP. n = 5 mice for each condition; cell counts = 2776 cells for control and 2454 cells for KO. Statistical significance was determined by multiple t-tests with Bonferonni correction test. * p<0.05, ** P<0.01, *** p<0.001.
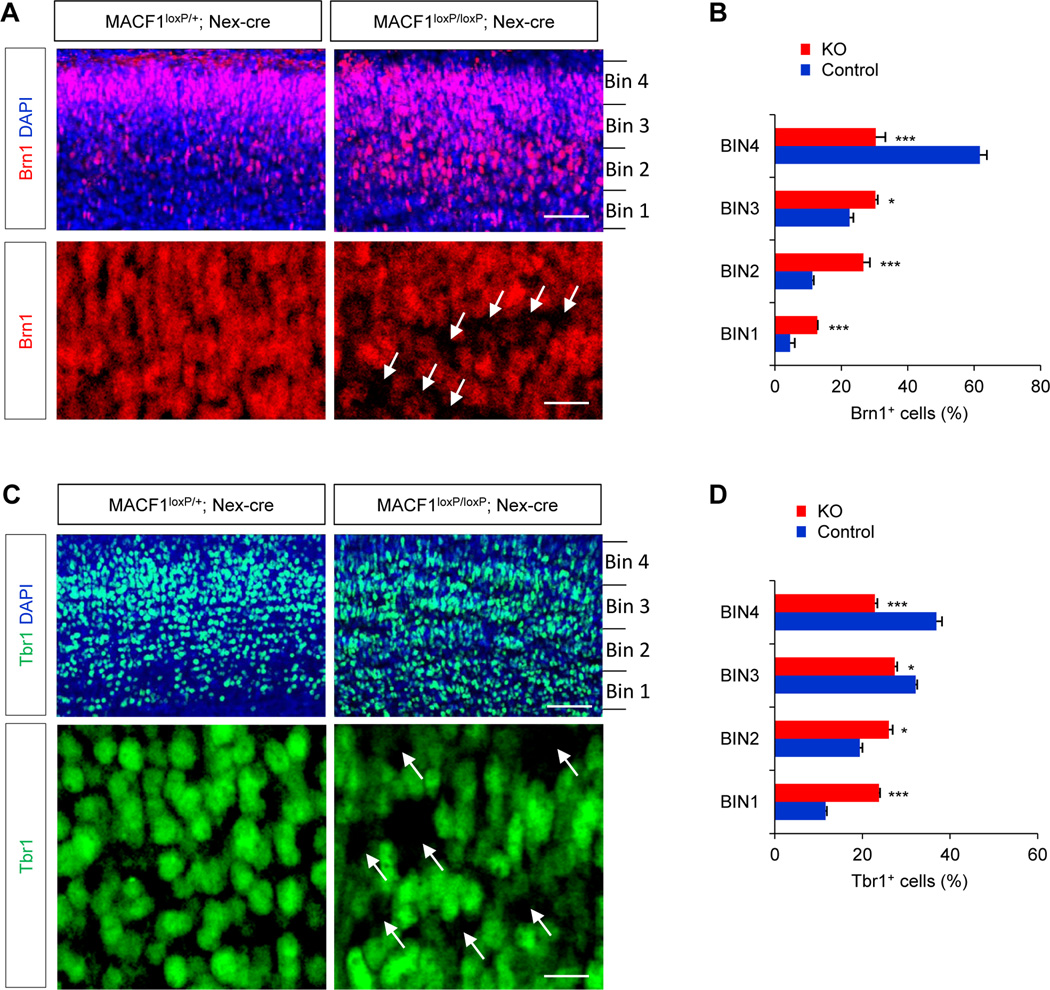
Electroporation of shRNA into the brain ventricles targets radial glial neural progenitors at the ventricular zone. Thus, there is a possibility that the migration defects with shMACF1 might indirectly result from disrupted regulation of radial neural progenitors. Furthermore, it is difficult to assess cell autonomous effects of some genes as the radial glial scaffold contributes to neuronal migration in the developing brain. Defects in the radial platform could secondarily influence migration phenotypes. These issues need to be resolved to define the role of MACF1 in neuronal migration. Thus, we deleted MACF1 in developing neurons by performing in utero electroporation of E14.5 MACF1loxP/loxP mice with Dcx-cre-iGFP plasmid. The Dcx-cre-iGFP construct expresses Cre recombinase only in neuronal populations under the Dcx promoter, not in radial neural progenitors (Franco et al., 2011). Thus, MACF1 is knocked out selectively in neuronal population transfected with DCX-cre-iGFP. After electroporation, we collected brain tissues at P0 and P10 and assessed neuron migration patterns. Control (MACF1loxP/+; Dcx-cre-iGFP) neurons were positioned normally in the cortical plate at P0 (Fig. 2C, 2D). In striking contrast, MACF1loxP/loxP; Dcx-cre-iGFP neurons were mostly found in the ventricular/subventricular zone. At P10 stage after the electroporation, MACF1loxP/loxP; Dcx-cre-iGFP neurons were found throughout the cerebral cortex while control neurons were confined in the cortical plate (Fig. 2E, 2F, top panels). The increased proportion of MACF1-deleted neurons in the cortical plate at P10 compared to P0 samples suggests a migration delay (Fig. 2D, 2F). It is important to note that only 5% of MACF1loxP/loxP; DCX-cre-iGFP neurons were found in the ventricular/subventricular zone whereas approximately 35% shMACF1-transfected cells were localized in the area at P10 stage, indicating the importance of neuron-specific gene deletion. Next, we confirmed these results with another strategy to delete MACF1 in neuronal populations in vivo using a Nex-cre mouse line (Goebbels et al., 2006; Wu et al., 2005). The Nex-cre line expresses Cre recombinase exclusively in neurons but not in dividing neural progenitors in the developing cerebral cortex. We generated control (MACF1loxP/+; Nex-cre) and mutant (MACF1loxP/loxP; Nex-cre) mice. Western blots showed that MACF1 was eliminated in the mutant brain (Supplemental Fig. 3). Then, we examined Brn1-positive upper layer neurons and Tbr1-positive deeper layer neurons in control and MACF1loxP/loxP; Nex-cre brains (Fig. 3). Brn1-positive neurons in MACF1loxP/loxP; Nex-cre mice were found in both higher bins (3, 4) and lower bins (1, 2) of the cortical plate while control Brn1-positive neurons were relatively accumulated in higher bins (Fig. 3A, 3B). Similar patterns were observed with Tbr1 immunostaining. Tbr1-positive neurons in MACF1loxP/loxP; Nex-cre mice were spread out evenly throughout the cortical bins compared to controls (Fig. 3C, 3D). Notably, both Brn1- and Tbr1-positive neurons were appeared to be abnormally spaced in the MACF1loxP/loxP; Nex-cre cortical plate (arrows), suggesting that MACF1 plays a role in neuronal contact and organization. These phenotypes of neuron positioning in MACF1loxP/loxP; Nex-cre brains are not associated with cell death because there was no change in the level of cleaved caspase-3 in the mutant brain tissues (Supplemental Fig. 4).
Figure 3. Neuronal placement in MACF1loxP/loxP; Nex-cre brains.
(A) MACF1 deletion disrupted cortical neuron placement in the developing brain. Brn1 immunostaining of P10 control (MACF1loxP/+; Nex-cre) and MACF1loxP/loxP; Nex-cre brains. Top panels: The distinct localization pattern of Brn1-positive neurons was not seen in MACF1loxP/loxP; Nex-cre brain sections. Cells were counterstained by DAPI. Scale bar: 50 µm. Bottom panels: Higher magnification images. Arrows indicate noticeable empty spaces. Scale bar: 10 µm. (B) Quantification of the positioning patterns of Brn1-positive neurons in the cortical plate. The graph indicates the distribution of Tbr1-positive neurons in the 4 bins dividing the thickness of the cortical plate as indicated in (A) in each genotype. Brn1-positive neurons were distributed relatively evenly in the cortical plate of MACF1-deficient brains while they were relatively accumulated in higher bins in controls. Control: MACF1loxP/+; Nex-cre. KO: MACF1loxP/loxP; Nex-cre. n = 5 mice for each condition; cell counts = 11878 cells for control and 10266 cells for KO. Statistical significance was determined by multiple t-tests with Bonferonni correction test. * p<0.05, ** P<0.01, *** p<0.001. (C) Tbr1 immunostaining patterns of control and MACF1-deficient samples. Top panels: Brain sections from P10 control and MACF1loxP/loxP; Nex-cre mice were immunostained with Tbr1 antibody. Scale bar: 50 µm. Bottom panels: Higher magnification images. Arrows indicate noticeable empty spaces. Scale bar: 10 µm. (D) The distribution of Tbr1-positive neurons was quantified. Control: MACF1loxP/+; Nex-cre. KO: MACF1loxP/loxP; Nex-cre. n = 5 mice for each condition; cell counts = 16724 cells for control and 14935 cells for KO. Statistical significance was determined by multiple t-tests with Bonferonni correction test. * p<0.05, ** P<0.01, *** p<0.001.
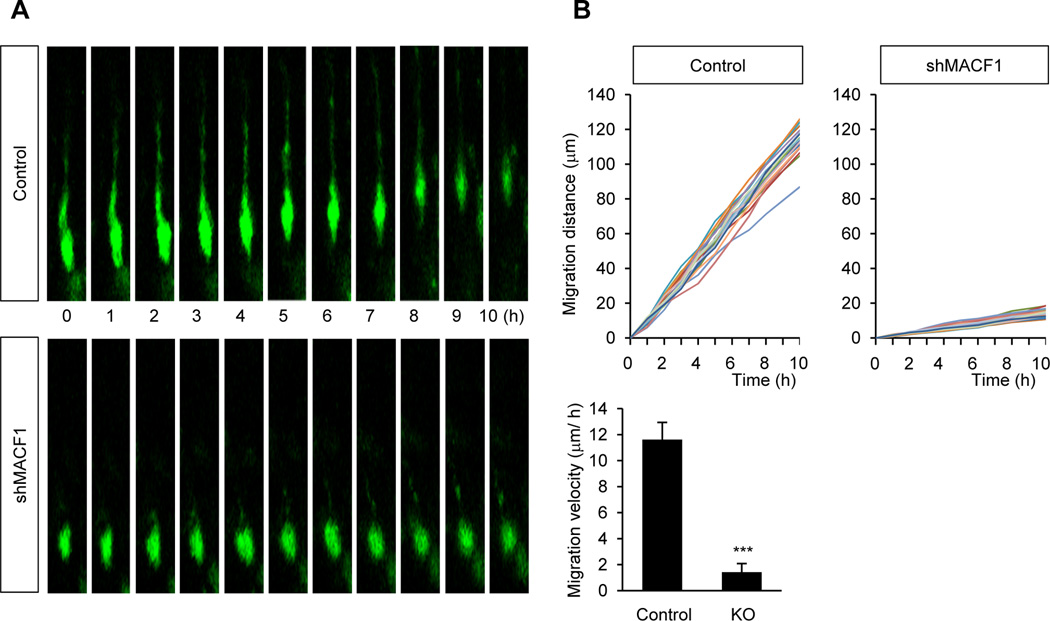
We examined movement of migrating neurons in control and MACF1-deleted neurons using time-lapse imaging on cortical slice cultures. Abnormal positioning of cortical neurons found above (Fig. 2, Fig. 3) still raises a possibility that MACF1-deleted neurons might migrate at rates similar to control neurons, but forward-and-backward movement could lead to the aberrant positioning. Time-lapse imaging can clarify this issue. Control neurons developed a leading process toward the cortical plate and the soma moved following the process (Fig. 4A, 4B). In contrast, the movement of MACF1-deficient neurons was minimal, indicating MACF1 is required for neuron migration in the developing brain. Taken together, our results demonstrate that MACF1 cell-autonomously determines the positions of cortical pyramidal neurons by controlling neuronal migration.
Figure 4. Time-lapse imaging of MACF1-deleted neurons.
(A) MACF1 deletion suppressed radial neuron migration. E14.5 mouse brains were electroporated in utero with a non-silencing control or shMACF1. After two days, brains were collected and subjected to slice cultures. Then, radial movement of migrating neurons in the slices was traced by using fluorescence live-cell imaging. (B) Neuron migration was quantified. Top graphs: The somal movements were traced in control and MACF1-deficient neurons. Each colored line represents the movement of each neuron. Bottom graph: Migration distances per hour were measured. n = 21 cells from 3 mice for control, and 20 cells from 3 mice for shMACF1. Statistical significance was determined by two-tailed Student’s t-test. *** p<0.001.
MACF1 functions in cortical neuron migration strongly suggest that the protein carries similar roles during hippocampal development. To test this idea, we manipulated genes in the developing hippocampus using an in utero electroporation with a modified orientation of the electrodes (Supplemental Fig. 5A). Using this method, we targeted Dcx-cre-iGFP into the lower part of the medial cortical region around the cortical hem at E14.5, which forms hippocampus at later developmental stages (Grove and Tole, 1999; Grove et al., 1998; Lee et al., 2000b; Mangale et al., 2008; Monuki et al., 2001). Control hippocampal neurons (MACF1loxP/+; Dcx-cre-iGFP) were found exclusively within the pyramidal layer of the hippocampus at P10 (Supplemental Fig. 5B, 5C). However, MACF1loxP/loxP; Dcx-cre-iGFP neurons were mostly located in the alveus and oriens layers that are supposed to contain axons and basal dendrites of pyramidal neurons in a normal hippocampus. The proportion of MACF1-deleted cells in the pyramidal layer was sharply decreased, compared to controls. These findings indicate MACF1 regulates the placement of hippocampal pyramidal neurons.
Elimination of MACF1 disrupts the formation of leading processes
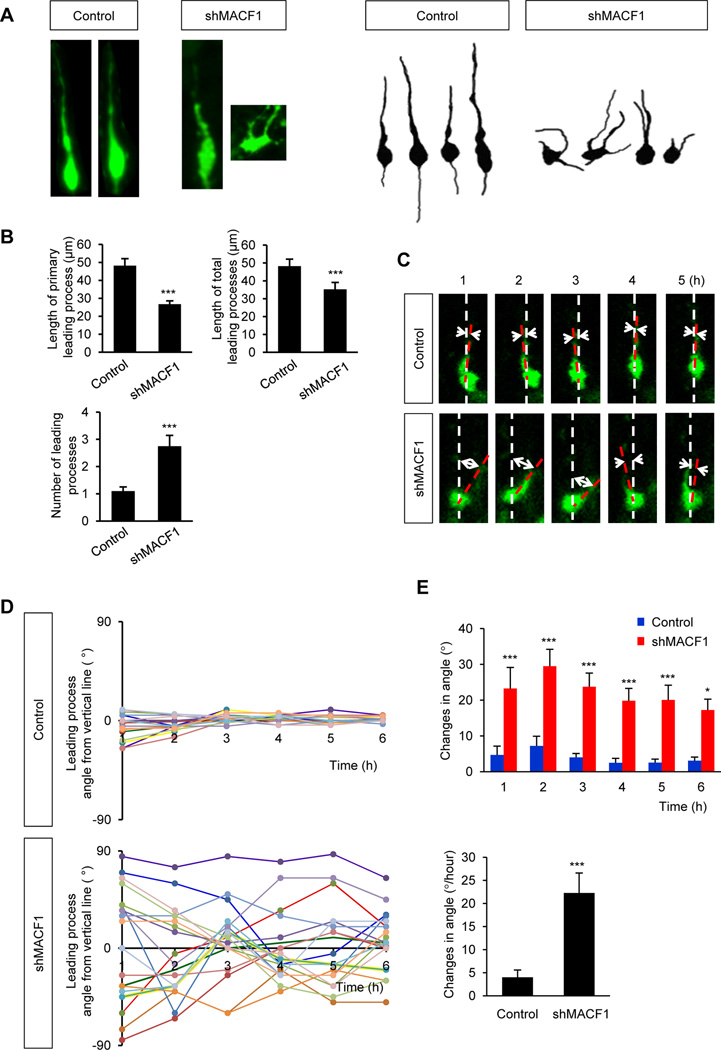
Extension of a leading process is important for neuronal migration in the cerebral cortex because somal movement is coupled to specific dynamics of leading processes. Migrating neurons first extend a leading process, and then the soma translocates into the leading process (Marin et al., 2010). Thus, we investigated leading process development in MACF1-deficient neurons. Most control neurons formed a single leading process which was aligned vertically (Fig. 5A). However, MACF1-deleted neurons often developed short multiple leading processes. The lengths of total and primary leading processes were decreased in MACF1-deficient neurons, while the number of processes was increased (Fig. 5B). Furthermore, we examined the dynamics of leading process by measuring angles between a leading process and the midline of the cell by live-cell imaging. Control leading process showed small differences in angle changes (Fig. 5C, 5D, 5E). However, leading processes of MACF1-deleted neurons were actively swinging around the midline of the cells. These results revealed an important role of MACF1 in leading process morphogenesis and dynamics.
Figure 5. Abnormal morphogenesis of leading processes in MACF1-deficient migrating neurons.
(A) MACF1 deletion induced multiple shorter leading processes in radially migrating neurons. Left panels: A construct encoding either a GFP (control) or shMACF1 was electroporated in utero into E14.5 embryos. The electroporated brains were collected at E16.5 and migrating neurons expressing GFP were visualized. Right panels: Representative control and shMACF1-expressing neurons with their leading processes. (B) Quantification of lengths and numbers of leading processes in control and MACF1-deficient neurons. MACF1-deficient neurons exhibited decreased lengths, but increased numbers of leading processes. n = 56 cells from 5 mice for control, and 61 cells from 5 mice for shMACF1. Statistical significance was determined by two-tailed Student’s t-test. *** p<0.001. (C) MACF1-deficient neurons did not maintain directionality of leading processes. E14.5 mouse brains were electroporated with a GFP construct or shMACF1. Brain slices were prepared two days later and cultured for examining movement of leading processes with live-cell imaging. Dashed white lines indicate vertical midlines of migrating neurons. Dashed red lines follow leading processes and show the directionality of the processes. MACF1-deficient neurons changed the leading process direction noticeably more than controls. (D) Graphs indicate the representative tracings of the directional movement of the leading processes in control (top graph) and MACF1-deficient cells (bottom graph). The angles between white lines and red lines shown in (C) were measured every hour. Each colored line represents the changes of leading process angles in each neuron. (E) Quantification of leading process directionality. Changes in the leading process angles were quantified at each time point (top graph), and the angle changes per hour were calculated (bottom graph). n = 20 cells from 3 mice for each condition. Statistical significance was determined by multiple t-tests with Bonferonni correction test (top graph) or by two-tailed Student’s t-test (bottom graph). * p<0.05, ** P<0.01, *** p<0.001.
MACF1 regulates centrosome movement in migrating neurons
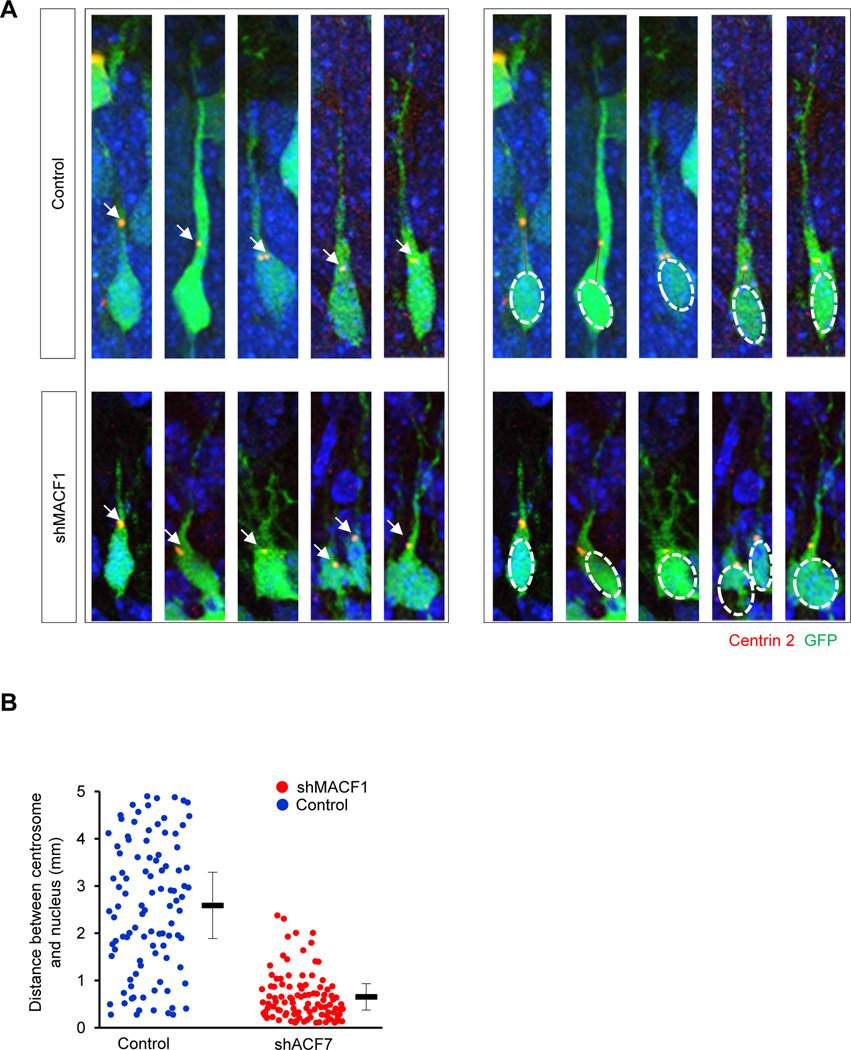
Centrosomes are crucial for coordinating neuronal migration (Kuijpers and Hoogenraad, 2011; Sakakibara et al., 2014). Centrosomes in a migrating neuron orient and move toward the tip of leading process, and the nucleus follows the movement. We examined whether MACF1 plays a role in centrosome dynamics in radially migrating neurons. We electroporated a plasmid (dsRed-cent2) encoding Centrin-2, a centrosome marker, tagged with a red fluorescent protein into the developing brain in utero. Then, we assessed the location of centrosomes in control and MACF1-deficient brains. Fig. 6A shows migrating neurons expressing dsRed-cent2. The locations of centrosomes in control neurons varied along the leading processes (Fig. 6A). In contrast, most centrosomes in MACF1-deficient neurons were localized close to somas. The distances between centrosomes and nuclei of MACF1-deficient neurons mostly ranged from 1 to 2 µm while control neurons showed wider distribution patterns (Fig. 6B). These results revealed a novel function of MACF1, i.e. a determinant of centrosome movement in migrating neurons. Along with the results presented in Fig. 5, these data suggest that migrating neurons require MACF1 activity to properly coordinate dynamics of leading process development and centrosomal movement.
Figure 6. Elimination of MACF1 leads to disrupted positions of centrosomes.
(A) Centrosomes were localized closer to somas in MACF1-deleted neurons compared to control cells. Left panels: E14.5 mouse were electroporated with either a GFP (control) and dsRed-cent2 constructs or shMACF1 and dsRed-cent2 constructs. Brain samples were collected two days later and red-fluorescent centrin 2 was visualized within GFP-positive cells. Arrows indicate dsRed-cent2-positive centrosomes. Cells were counterstained by DAPI. Right panels: Same images as left panels. DAPI-stained nuclei were marked with white ovals. Red lines indicate distances between centrosomes and nuclei. (B) The distances shown in right panels of (A) were quantified. Each dot represents the distance in a cell. n = 75 cells from 5 mice for each condition.
MACF1 controls microtubule stability and dynamics in cortical neurons
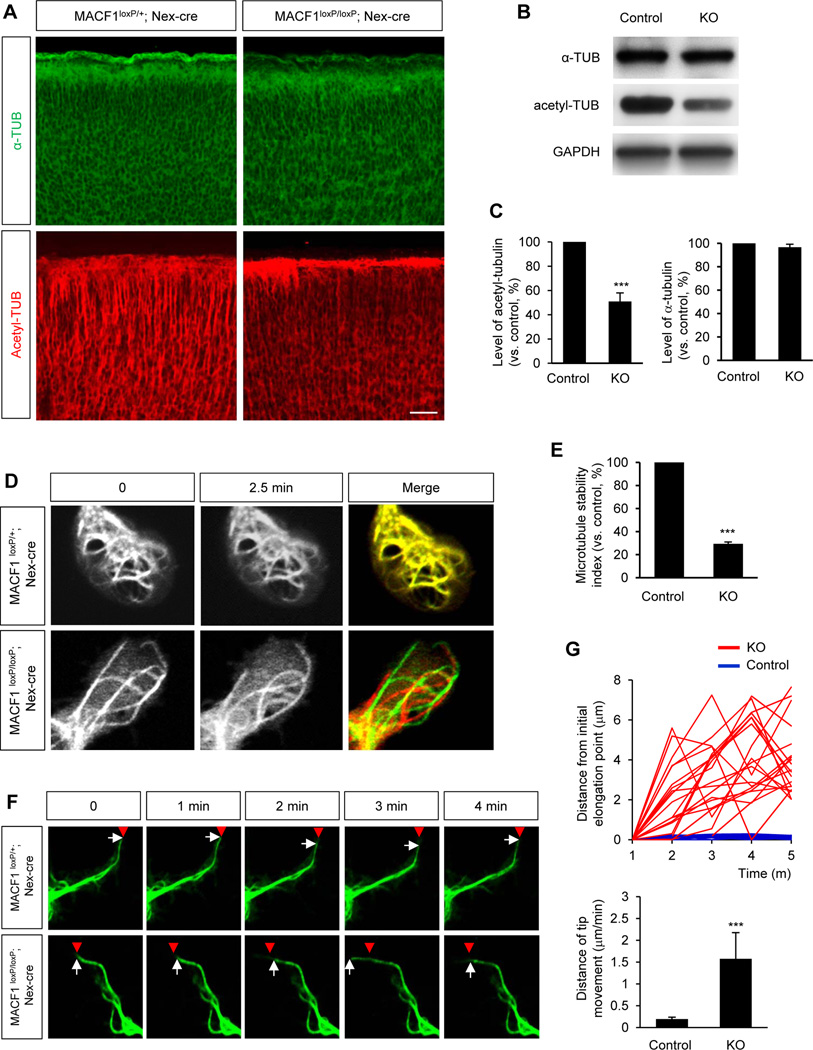
Cytoskeletal components regulate the migration of developing neurons. This raises a question of whether MACF1 plays roles in microtubule dynamics in developing neurons. Thus, we assessed microtubule stability in control and MACF1-deleted neurons. First, we examined the levels of total and acetylated-tubulin by immunostaining of E14.5 control and MACF1loxP/loxP; Nex-cre brain tissues. When acetylated, microtubules are stabilized (Westermann and Weber, 2003). The level of acetylated-tubulin was decreased in MACF1loxP/loxP; Nex-cre brains compared to control samples while the level of total tubulin was not changed (Fig. 7A). Western blotting confirmed the immunostaining results (Fig. 7B, 7C).
Figure 7. Deletion of MACF1 disrupt microtubule stability.
(A) Microtubule stability was reduced in MACF1loxP/loxP; Nex-cre brains. Brain sections of control (MACF1loxP/+; Nex-cre) and MACF1loxP/loxP; Nex-cre mice at E14.5 were immunostained with an α-tubulin (top panels) or an acetylated-tubulin antibody (bottom panels). Scale bar: 50 µm. (B) Western blotting was performed to measure levels of α-tubulin or acetylated-tubulin using E14.5 control and MACF1loxP/loxP; Nex-cre brain lysates. Control: MACF1loxP/+; Nex-cre. KO: MACF1loxP/loxP; Nex-cre. (C) The levels of tubulins as shown in (B) were quantified. Data were shown as relative changes vs. control. n = 3 independent experiments using 3 mice for each condition. Statistical significance was determined by two-tailed Student’s t-test. *** p<0.001. (D) MACF1-deleted neurons showed faster dynamics of microtubule structures. A plasmid encoding EMTB-3XGFP was transfected into control and MACF1loxP/loxP; Nex-cre neurons. Then, microtubule structures labeled by EMTB-3XGFP were traced by live-cell imaging at 2.5 minute intervals. Images from adjacent time intervals were superimposed to assess the structural changes of microtubule cytoskeleton between different time points of observation. The more overlap of microtubule cytoskeleton between different time points indicates the more stability of microtubules and less overall dynamic changes in microtubule structures. (E) Microtubule stability index was calculated as indicated in (D). Data were shown as relative changes vs. control. Control: MACF1loxP/+; Nex-cre. KO: MACF1loxP/loxP; Nex-cre. n = 30 cells from 5 mice for each condition. Statistical significance was determined by two-tailed Student’s t-test. *** p<0.001. (F) Microtubule polymerization and depolymerization were traced at neurite tips after EMTB-3XGFP transfection into control and MACF1loxP/loxP; Nex-cre neurons. Neurons labeled with EMTB-3XGFP were imaged at 1 minute intervals. Distances between the neurite tip (arrows) and the initial elongation point (red arrow heads) were assessed. (G) Quantification of (F). Top graph: Each line represents a neuron. Control: MACF1loxP/+; Nex-cre. KO: MACF1loxP/loxP; Nex-cre. n = 20 cells from 5 mice for each condition. Bottom graph: Distances of neurite tip movement per minute were quantified. Statistical significance was determined by two-tailed Student’s t-test. *** p<0.001.
Next, we examined microtubule dynamics by tracing cellular microtubule structures by transfecting control and MACF1loxP/loxP; Nex-cre neurons with a plasmid construct encoding EMTB-3XGFP. Expression of EMTB-3XGFP construct labels polymerized microtubules (Miller and Bement, 2009). The extent of microtubule rearrangement and stability can be measured by comparing microtubule cytoskeleton from adjacent time points of observation. Dynamically unstable microtubule cytoskeletons continuously remodel their structures. Thus, the levels of overlap of microtubule cytoskeletons between neighboring time points indicate the extent of stability and dynamic changes in microtubule cytoskeleton (Reilein et al., 2005; Yokota et al., 2009). We visualized EMTB-3XGFP expression patterns with time-lapse imaging. Images were taken at 2.5 minute intervals, and the images of adjacent time frames were superimposed to measure microtubule stability. Microtubules in control neurons were relatively stable within the time frames (Fig. 7D, 7E). However, microtubules in MACF1-deficient neurons frequently changed their structures within the short time frames. Additionally, we traced microtubules at neurite tips. Microtubules at the tip of MACF1-deleted neurites underwent more dynamics of polymerization and depolymerization within the defined time compared to microtubules in control cells (Fig. 7F, 7G). These findings demonstrate that MACF1 controls microtubule stability in the developing neurons and suggest that MACF1-mediated regulation of microtubule stability contributes to neuronal migration and differentiation.
Association of MACF1 with GSK-3 signaling in cortical neuron migration
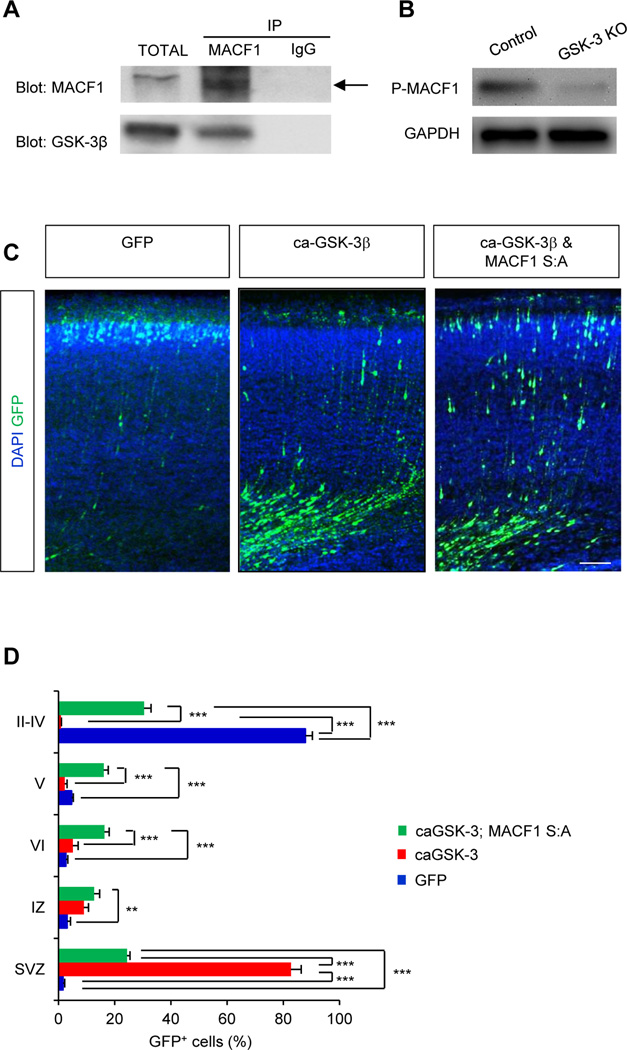
MACF1 is implicated in GKS-3 signaling in skin stem cells (Wu et al., 2011), and GSK-3 is shown to regulate cortical placement and radial migration of pyramidal neurons (Asada and Sanada, 2010; Yokota et al., 2009). We wondered whether MACF1 interacts with GSK-3 in the developing brain. E14.5 brain lysates were coimmunoprecipitated with GSK-3β or MACF1 antibody, and subsequently immunoblotted with the antibodies. We found that MACF1 was indeed physically bound to GSK-3 in the developing brain (Fig. 8A). Interestingly, a recent study showed that MACF1 has potential GSK-3 phosphorylation target motifs (Wu et al., 2011). Thus, we assessed the levels of phosphorylated MACF1 in wild type control and GSK-3 knockout brain samples. Phosphorylation of MACF1 was suppressed in GSK-3 knockout brain lysates (Fig. 8B) indicating GSK-3 phosphorylates MACF1. When phosphorylated by GSK-3, MACF1 is inactivated in skin cells (Wu et al., 2011), suggesting a potential interplay between GSK-3 and MACF1 in neuronal migration. Thus, we examined the role of GSK-3 phosphorylation of MACF1 in migrating neurons by electroporating a control GFP, a constitutively active GSK-3β (ca-GSK-3β), or ca-GSK-3β and MACF1 S:A in utero into the developing brain. There are MACF1 plasmid constructs containing point mutations that convert GSK-3 phosphorylation sites to a kinase-refractile version harboring Ser/Ala mutations (MACF1 S:A). Expression of ca-GSK-3β suppressed radial neuron migration (Fig. 8C, 8D). Importantly, co-expression of MACF1 S:A partially rescued the inhibitory effects of ca-GSK-3β in radial neuronal migration. These findings suggest that GSK-3-mediated phosphorylation is an important mechanism for MACF1 function in neuron migration.
Figure 8. MACF1 interacts with GSK-3 signaling in the developing brain.
(A) MACF1 bound to GSK-3. E14.5 brain lysates were immunoprecipitated with a MACF1 antibody and subsequently subjected to Western blotting using either a GSK-3β or a MACF1 antibody. (B) GSK-3 deletion inhibited phosphorylation of MACF1in the developing brain. Phosphorylation of MACF1 was measured by Western blotting using brain lysates from control and GSK-3 knockout mice. (C) Suppression of GSK-3 phosphorylation of MACF1 partially restored the inhibitory effects of GSK-3 in neuronal migration. E14.5 mice were electroporated in utero with a GFP, ca-GSK-3β-GFP, or ca-GSK-3β-GFP and MACF1 S:A-GFP construct. Brain sections were prepared at P10 to assess neuronal positioning. The overexpression of ca-GSK-3β-GFP inhibited neuron migration. However, the defective migration was partially rescued by co-overexpression of ca-GSK-3β-GFP with MACF1 S:A-GFP construct. Scale bar: 50 µm. (D) Quantification of (C). n = 5 mice for each condition; cell counts = 1893 cells for control, 2026 cells for ca-GSK-3β and 1807 cells for ca-GSK-3pβ MACF1 S:A. Statistical significance was determined by one-way ANOVA with Bonferonni correction test. * p<0.05, ** P<0.01, *** p<0.001.
Discussion
Here, we have defined the role and mechanisms of MACF1 in pyramidal neuronal migration in the mammalian developing brain. Inactivation of MACF1 gene leads to disrupted migration of cortical and hippocampal pyramidal neurons, and subsequent mis-positioning in neuronal layers. The defective migration in MACF1-deficient neurons is caused by unstable microtubules and static centrosomes. Furthermore, MACF1 mediates GSK-3 signaling for correct positioning of migrating neurons.
MACF1 in pyramidal neuron migration in the developing brain
Studies have revealed that MACF1 is expressed in the nervous system during development (Chen et al., 2006; Leung et al., 1999). Consistently, we found MACF1 expression in the developing cerebral cortex. A recent study reported that Tbr1- and Citp2-positive cortical layers are partially mixed in MACF1 conditional knockout brains induced by a Nestin-cre driver (Goryunov et al., 2010), suggesting a potential role of MACF1 in cortical neuron migration. However, the role of MACF1 in neuronal migration was unclear in the previous study due to the use of the Nestin-cre driver that expresses Cre recombinase in radial glial neural progenitors at E9 (Tronche et al., 1999). Neural progenitors mainly maintain their pools by self-renewal at early stages of development and actively generate neurons by asymmetric division at later stages (Fietz and Huttner, 2011; Gotz and Huttner, 2005; Shitamukai and Matsuzaki, 2012). Thus, changes in neural progenitor development such as defective neural progenitor self-renewal, cell cycle progression, or neurogenesis can indirectly lead to cortical neuron misplacement. Additionally, the mixed cortical layers found in the study could be due to a delay in neural progenitor fate determination. Furthermore, correct formation of the radial glial scaffold is necessary for radial neuronal migration. Migrating projection neurons in the developing cortex follow a trajectory that is perpendicular to the ventricular surface, moving alongside the radial glial scaffold (Hatten, 1999; Marin et al., 2010). Abnormal formation of the radial glial scaffold due to defective development of radial neural progenitors could result in neuronal disorganization. In this regard, to define the cell-autonomous effect of MACF1 in neuronal migration, it is important to eliminate MACF1 selectively in migrating neurons, but not in neural progenitors in the developing brain. In our study, we eliminated MACF1 only in neuronal populations, but not in neural progenitors using in utero electroporation with Dcx-cre-iGFP. We also analyzed neuronal migration in MACF1loxP/loxP; Nex-cre brains in which MACF1 is exclusively deleted in neurons of dorsal telencephalon. Using these strategies, we conclusively showed that MACF1 is required for migrating neurons to be positioned correctly in the cortical plate. MACF1 deletion in migrating neurons mediated by Dcx-cre-iGFP expression had no effects on radial glial scaffold formation. Additionally, we observed that MACF1loxP/loxP; Nex-cre brains develop radial glial fibers normally. Thus, the abnormal migration phenotype induced by MACF1 deficiency appears to be cell-autonomous. However, due to the timing of Nex-cre expression, we cannot exclude the possibility that radial glial platform can influence neuronal migration and subsequent positioning non-autonomously in MACF1-deficient brains. Although knocking down of MACF1 suppresses radial migration of cortical neurons, a substantial number of cells are still capable of positioning normally in the cortex. This result suggests that MACF1 may be necessary, but not be sufficient for neuronal migration. However, the recombination efficiency of the MACF1 gene should also be considered. Whether some neurons can truly migrate independently of MACF1 or whether the normally-migrating neurons represent a population of pyramidal neurons that still express some MACF1 due to either late or incomplete deletion of MACF1 remains to be determined.
For functional circuitry, developing hippocampal neurons must migrate into the correct positions and differentiate appropriately. Neuronal positioning and subsequent hippocampal development are the main components of neural circuits and therefore are considered to be important in learning and memory processes. We found that MACF deletion suppressed integration of hippocampal pyramidal neurons into appropriate cell layers. Our data demonstrate the requirement of MACF1 for hippocampal neuron positioning and further differentiation in the developing hippocampus.
Cytoskeletal regulation by MACF1
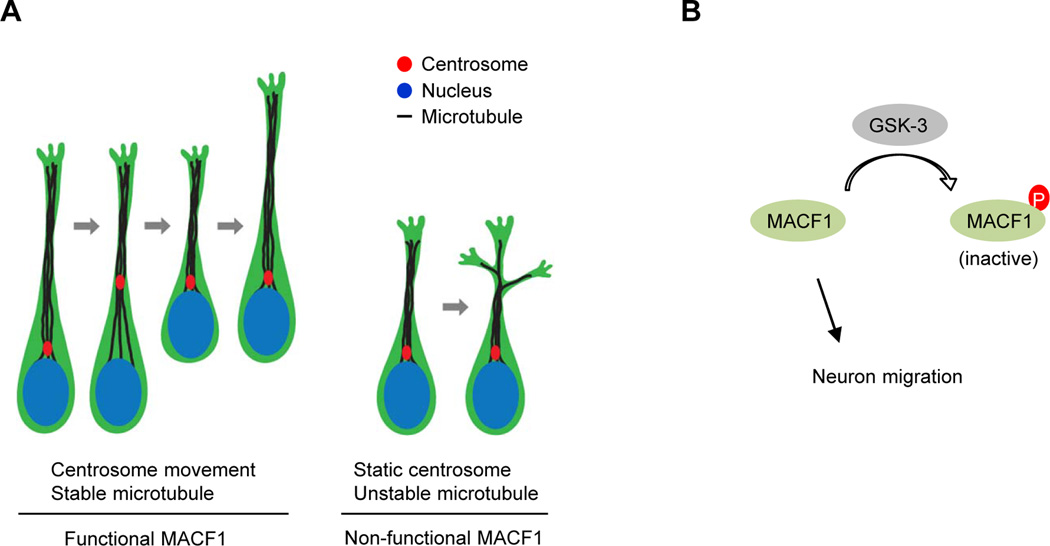
We found that deletion of MACF1 reduces the levels of stable microtubules and destabilizes polymerized microtubules in developing neurons. This microtubule instability results in excessive dynamics in microtubule localization in MACF1-deficient neurons. The relatively unstable leading processes of MACF1-deficient migrating neurons may be attributed to the lack of MACF1 functions in microtubule stabilization. Likewise, the immobilized centrosomes in MACF1-deleted migrating neurons also appear to be caused by microtubule instability. Consistent with an important function in mammalian cells, the role of MACF1 in maintaining microtubule stability is conserved in non-mammalian systems too. For example, mutations in the MACF1 gene cause a loss of stable microtubule localization to the periphery of the zebrafish oocyte (Gupta et al., 2010). A model of how centrosomes move in the absence or presence of MACF1 is presented in Fig. 9A. MACF1 appears to stabilize microtubules along the leading process, which allows leading process extension and centrosome movements followed by somal translocation. Additionally, soma morphology is more roundish in MACF1-deficient neurons, suggesting that MACF1 functions more globally as a microtubule regulator than just locally in centrosome positioning.
Figure 9. A model for a role of MACF1 in neuronal migration.
(A) The regulation of neuron migration by MACF1. In control migrating neurons, MACF1 stabilizes polymerized microtubules, which enables them to elongate single long leading process toward the pia in the developing brain. Then, centrosomes move forward to the tip of the leading process, followed by somal translocation of migrating neurons. In contrast, MACF1 deletion renders instability of microtubules in leading processes and suppresses elongation of the processes. Instead, MACF1-deficient neurons form multiple leading processes that have problems in maintaining directionality. Centrosomes also have no movement, resulting in disrupted migration. (B) GSK-3 regulation of MACF1. Unphosphorylated MACF1 is required for the migration of pyramidal neurons in the developing brain. However, when phosphorylated by GSK-3, MACF1 is inactivated, resulting in migration abnormalities.
Shot organizes the microtubule network and promotes microtubule assembly by forming a complex with the microtubule binding proteins adenomatous polyposis coli (APC) and end-binding 1 (EB1) (Subramanian et al., 2003). Recently, APC and EB are shown to play important roles in neuronal placement (Alves-Silva et al., 2012; Chen et al., 2011; Mattie et al., 2010; Yokota et al., 2009). It will be interesting to see whether MACF1 determines the localization of APC and EB1 in mammalian neuronal cells. Along with previous findings, our data strongly suggest that MACF1 plays an essential role in neuronal migration via microtubule stabilization.
Interestingly, MACF1 contains EF-hand calcium-binding motif at C-terminal (Jefferson et al., 2004; Roper et al., 2002). Calcium is a ubiquitous second messenger and is important in the control of neuronal migration and neurite development (Komuro and Kumada, 2005; Zheng and Poo, 2007). Studies have shown that EF-hand proteins such as MACF1, caltubin, and calmyrin1 regulate cytoskeletal components through EF-hand motifs during neurite outgrowth (Nejatbakhsh et al., 2011; Sanchez-Soriano et al., 2009; Sobczak et al., 2011), suggesting potential roles of EF-hand motif in MACF1 activity during neuronal development. Future studies would address whether the actin-microtubule bridging property of MACF1 is dependent on calcium binding in the EF-hand motif.
MACF1 in GSK-3 signaling
GSK-3, a major downstream of Wnt pathway, is involved in neuronal migration in the developing cortex. Radial migration and placement of cortical neurons were aberrant in GSK-3-deleted brains (Yokota et al., 2010). A separate study showed that in utero electroporation with active GSK-3β plasmid into cortical ventricular zone elicited neuronal migration defects (Asada and Sanada, 2010). MACF1 is associated with the canonical Wnt pathway. By binding to the Wnt-mediated destruction complex, MACF1 modulates cellular β-catenin levels (Chen et al., 2006). GSK-3, a primary mediator of Wnt signaling, is responsible for regulating cellular β-catenin levels (Doble and Woodgett, 2003). These findings suggest that MACF1 interacts with GSK-3 signaling. Indeed, a recent study showed that GSK-3 phosphorylates C-terminal domain of MACF1 and that the phosphorylation controls MACF1’s microtubule-binding capacity and migration potential in skin stem cells (Wu et al., 2011). Consistent with this, our study found that GSK-3 physically binds to and phosphorylates MACF1. Furthermore, overexpression of MACF1 S:A partially rescued ca-GSK-3 effects in the developing brain. Thus, MACF1 phosphorylation by GSK-3 appears to inhibit its role in neuronal migration (Fig. 9B). These findings suggest MACF1 is a downstream target of GSK-3 signaling in migrating neurons.
GSK-3 regulates centrosome reorientation and microtubule spindle formation during cell division (Cheng et al., 2008; Izumi et al., 2008; Kim and Snider, 2011; Wakefield et al., 2003). Phospho-GSK-3 (serine 9; inactive GSK-3) is abundant at the centrosome and spindle microtubules (Wakefield et al., 2003), suggesting its role in stabilization of microtubules in the cell. This would allow centrosomes to be an important site of microtubule growth. To the best of our knowledge, centrosome positioning in GSK-3 knockout mice is not known. However, a mouse model that generated by in utero electroporation of GSK-3beta mutant plasmid showed that GSK-3beta is required for centrosomal forward movement in the leading process of migrating neurons (Asada and Sanada, 2010). Overexpression of a GSK-3beta mutant plasmid that cannot be phosphorylated at serine 9 leads to abnormal centrosome positioning and leading process extension in migrating neurons. Furthermore, microtubules at the leading process are unstable in these neurons (Asada and Sanada, 2010). GSK-3 is well known for transducing polarity signals into microtubule stabilization (Hur and Zhou, 2010). GSK-3 can phosphorylate many microtubule binding proteins such as APC, EB proteins, CLIP-associating proteins, Tau, and collapsin response-associated proteins (Akhmanova et al., 2001; Cole et al., 2004; Hur and Zhou, 2010; Zumbrunn et al., 2001). Phosphorylation of these proteins by GSK-3 inhibits their ability to bind to microtubules, thus destabilizing microtubules (Akhmanova et al., 2001; Watanabe et al., 2009; Zumbrunn et al., 2001). For example, binding of APC to the microtubule is negatively regulated by GSK3-mediated phosphorylation (Zhou et al., 2004; Zumbrunn et al., 2001). APC is required for centrosomal movement and neuronal migration in the developing brain (Asada and Sanada, 2010). Like APC, MACF1 is negatively regulated by GSK-3 (Wu et al., 2011). MACF1 appears to mediate GSK-3 signal in microtubule stabilization during neuronal migration. Thus, these findings suggest that GSK-3 regulation of microtubule binding proteins such as MACF1 is a key mechanism of microtubule stabilization and neuronal migration during brain development.
Disc1, an autism and schizophrenia susceptibility factor, is well known molecule for the migration control of excitatory and inhibitory neurons in the developing cortex and the hippocampus (Brandon et al., 2009; Kamiya et al., 2005; Steinecke et al., 2012; Tomita et al., 2011). Importantly, MACF1 has been identified as an interactome of Disc1 (Camargo et al., 2007). Furthermore, Disc1 interacts with GSK-3 in developing neural cells and the interaction has been implicated in control of cortical neuronal migration (Ishizuka et al., 2011; Singh et al., 2010). Our data provide evidence for the interplay between MACF1 and GSK-3 in migrating neurons. Interestingly, our results and previous studies have revealed that MACF1, DSC1, and GSK-3 are involved in centrosome localization and functions (Asada and Sanada, 2010; Kamiya et al., 2005). These findings suggest that the interaction of DISC1/GSK-3/MACF1 coordinately plays critical roles in cortical neuron migration and neuronal connectivity. Given that neuronal hypo- or hyper-connectivity is increasingly implicated with neurodevelopmental disorders (Geschwind and Levitt, 2007; Uddin et al., 2013), abnormal activities of these molecules may result in pathophysiological symptoms of neurodevelopmental disorders.
Materials and Methods
Plasmids
Constitutively-active GSK-3β (S9A) plasmid was generously provided by Dr. James Woodgett (Samuel Lunenfeld Research Institute). Dcx-cre-iGFP was described previously (Franco et al., 2011). To generate shMACF1, we targeted a sequence (5’-GCAGAGATGTATCATCCATCA-3’) and its complement, and then cloned them into a modified pSuper-Basic vector as previously described (Kim et al., 2006). For control, non-silencing shRNAs were generated using scrambled targeting sequences (5’ -GATTAACCGACGCTTCAGATA- 3’ and 5’ - GTCCAGTCTACGATCTAAAGA - 3’). MACF1-GFP S:A plasmid was a generous gift from Dr. Elaine Fuchs (Howard Hughes Medical Institute, The Rockefeller University). EMTB-3XGFP and dsRed-cent2 plasmids were purchased from Addgene.
Mice
Mice were handled according to our animal protocol approved by the University of Nebraska Medical Center. MACF1 floxed mouse (Wu et al., 2011) was described previously. Nex-cre mouse (Goebbels et al., 2006) was used to generate conditional MACF1 knockout mice (MACF1loxP/loxP; Nex-cre). Nestin-cre mouse (#003771) was purchased from Jackson Laboratory.
Immunohistochemistry
Immunohistochemical labeling of embryonic brain sections or dissociated neural cells was performed as described previously (Kim et al., 2009). The following primary antibodies were used: rabbit anti-MACF1 (Wu et al., 2011), rabbit anti-MACF1 (Santa Cruz), rabbit anti-phospho-MACF1 (Wu et al., 2011), chicken anti-nestin (Neuromics), mouse anti-MAP2 (Covance), rabbit anti-Tbr1 (Chemicon), rabbit anti-Cux1 (Santa Cruz), goat anti-Brn1 (Novus Biologicals), rabbit anti-acetyl-α-tubulin (Cell Signaling), mouse anti-α-tubulin (Sigma), and chicken anti-actin (Millipore). Appropriate secondary antibodies conjugated with Alexa Fluor dyes (Invitrogen) were used to detect primary antibodies.
In utero electroporation
Timed pregnant female mice from E14.5 day of gestation were deeply anesthetized and the uterine horns were gently exposed. The lateral ventricles of an embryonic brain were injected with plasmid DNA (2 µg/µl) and 0.001% fast green using a Picospritzer II (Parker Inc.). Electroporation was achieved by placing two sterile forceps-type electrodes on opposing sides of the uterine sac around the embryonic head and applying a series of short electrical pulses using BTX ECM830 elecroporator (5 pulses with 100 ms length separated by 900 ms intervals were applied at 45V). The small electrical pulses drive charged DNA constructs into surrounding cells in the embryonic brain. Embryos were allowed to develop in utero for the indicated time. For hippocampal gene delivery, one lateral ventricle of E14.5 brain was injected with a DNA mixture. The electrodes were placed at an angle to the opposite way of cortical targeting.
Morphometry
For the quantification of lengths, numbers, or thickness of leading processes, images of 20 different brain sections at periodic distances along the rostro-caudal axis were taken with Zeiss LSM510 and LSM710 confocal microscopes and a Nikon Eclipse epifluorescence microscope attached with a QImaging CCD camera. The images were analyzed by using ZEN (Zeiss), LSM image browser (Zeiss), QCapture software (QImaging), and ImageJ (NIH). The calculated values were averaged, and some results were recalculated as relative changes versus control.
For cell counts, numbers of neurons positive to Tbr1, Cux1, Brn1, GFP, or DAPI were obtained as described previously (Cappello et al., 2006). Ten mice for each experiment (control mice, n=5; mutant mice, n=5) were used. Cell counts were described in Figure legends. More than 20 coronal tissue sections alongside rostro-caudal axis from each embryonic brain were examined. For analyzing cultured cells, more than 20 fields scanned horizontally and vertically were analyzed in each condition. Cell numbers examined were described in Figure legends.
The analysis of microtubule dynamics in neurons was performed as described in a previous paper with some modifications (Yokota et al., 2009). EMTB-3XGFP plasmid was transfected into E14.5 control and MACF1-deleted neurons. EMTB-3XGFP-positive neurons were repeatedly imaged at 2.5 minute intervals, and changes in EMTB-3XGFP-positive microtubule organization within the soma were quantified using Zeiss LSM image browser. Images from adjacent time points of observation were superimposed, and a 20µm length line scan on neurons was used to quantify the number of spots of microtubule co-localization at three different locations and used as microtubule stability index.
Western blotting
Lysates from E14.5 telencephalon were prepared using RIPA buffer and the protein content was determined by a Bio-Rad Protein Assay system. Proteins were separated on 3%-8% or 4%-12% SDS-PAGE gradient gel and transferred onto nitrocellulose membrane. Then the membrane was incubated with rabbit anti-MACF1 (Wu et al., 2011), rabbit anti-MACF1 (Santa Cruz), rabbit anti-phospho-MACF1 (Wu et al., 2011), mouse anti-GSK-3β (BD Biosciences), rabbit anti-acetyl-α-tubulin (Cell Signaling), mouse anti-α-tubulin (Sigma), or rabbit anti-GAPDH (Cell Signaling) at 4 °C overnight. Appropriate secondary antibodies conjugated to HRP were used (Cell Signaling) and the ECL reagents (Amersham) were used for immunodetection.
For quantification of band intensity, blots from 3 independent experiments for each molecule of interest were used. Signals were measured using ImageJ software and represented by relative intensity versus control. GAPDH was used as an internal control to normalize band intensity.
Primary neuron cultures
Primary neuronal culture was described previously (Kim et al., 2006). Briefly, cerebral cortices or hippocampi from E13.5–16.5 mice were isolated and dissociated with trituration after trypsin/EDTA treatment. Then, the cells were plated onto poly-D-lysine/ laminin-coated coverslips and cultured in the medium containing neurobasal medium, 5% serum, B27 and N2 supplements.
Time-lapse experiments
Organotypic brain slices were prepared from E14.5 mice as described previously (Polleux and Ghosh, 2002). Briefly, E14.5 mice were electroporated as described above and the brains were collected two days later. The brains were embedded in 3% low melting point agarose and coronal brain slices at 250 µm thickness were prepared using a LEICA VT1000S vibratome. The slices were then placed on poly-lysine/ laminin-coated transwell inserts and cultured in neurobasal media organotypically using an air interface protocol until imaging.
For time-lapse imaging, a LSM 710 inverted confocal microscope (Zeiss) equipped with a CO2 incubator chamber (5% CO2, 37°C) was used. Multiple Z-stacks with the options of 10–20 successive ‘z’ optical planes spanning 50–70 µm were acquired on pre-selected positions of electroporated slices. Repetitive imaging was performed every 15 minutes for up to 11 hours. Mean velocity of migrating cells was obtained using the Image J plugin Manual tracking.
Cell transfection
Mouse cortical or hippocampal neurons were transfected with various plasmids as described in a previous paper (Kim et al., 2006). Briefly, embryonic cortices or hippocampi were dissociated and suspended in 100 µl of Amaxa electroporation buffer with 1–10 µg of plasmid DNA. Then, suspended cells were transferred to Amaxa electroporation cuvette and electroporated with an Amaxa Nucleofector apparatus.
After electroporation, cells were plated onto coated coverslips and the medium was changed 4 hours later to remove the remnant transfection buffer.
Statistical analysis
Normal distribution was tested using Kolmogorov-Smirnov test and variance was compared. Unless otherwise stated, statistical significance was determined by two-tailed unpaired Student’s t-test for two-population comparison or one-way ANOVA with Bonferonni correction test for multiple comparisons. Data were analyzed using GraphPad Prism and presented as mean (+/−) SEM.
Supplementary Material
MACF1 is required for cortical neuron migration and positioning.
Elimination of MACF1 disrupts the formation of leading processes
MACF1 regulates centrosome movement in migrating neurons
MACF1 controls microtubule stability and dynamics in cortical neurons
MACF1 is associated with GSK-3 signaling in cortical neuron migration
Acknowledgements
We are thankful to Drs. Klaus-Armin Nave (Max Planck Institute) and Dr. Elaine Fuchs for their generous gifts of Nex-cre mouse, and MACF1 plasmids and antibodies, respectively. We thank Drs. Robert Norgren, Anna Dunaevsky, and Shelley Smith for valuable advice and comments on the manuscript. We are also grateful to Raquel Telfer and Matt Latner for animal care. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103471, a grant from NE DHHS (Stem Cell 2012–05), and a grant from Alzheimer’s Association (NIRP-12–258440) to WYK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
W.K. conceived and supervised the study. M.K. and W.K. designed, performed and analyzed the experiments. E.J. performed the experiments. U.M. analyzed and edited the paper. W.K. wrote the paper.
References
- Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, Galjart N. Clasps are CLIP-115 and −170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Alves-Silva J, Sanchez-Soriano N, Beaven R, Klein M, Parkin J, Millard TH, Bellen HJ, Venken KJ, Ballestrem C, Kammerer RA, Prokop A. Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins) The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9143–9158. doi: 10.1523/JNEUROSCI.0416-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada N, Sanada K. LKB1-mediated spatial control of GSK3beta and adenomatous polyposis coli contributes to centrosomal forward movement and neuronal migration in the developing neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8852–8865. doi: 10.1523/JNEUROSCI.6140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12768–12775. doi: 10.1523/JNEUROSCI.3355-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Molecular psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nature neuroscience. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Chanas-Sacre G, Rogister B, Moonen G, Leprince P. Radial glia phenotype: origin, regulation, and transdifferentiation. Journal of neuroscience research. 2000;61:357–363. doi: 10.1002/1097-4547(20000815)61:4<357::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RK. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes & development. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tian X, Kim WY, Snider WD. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PloS one. 2011;6:e24335. doi: 10.1371/journal.pone.0024335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TS, Hsiao YL, Lin CC, Yu CT, Hsu CM, Chang MS, Lee CI, Huang CY, Howng SL, Hong YR. Glycogen synthase kinase 3beta interacts with and phosphorylates the spindle-associated protein astrin. The Journal of biological chemistry. 2008;283:2454–2464. doi: 10.1074/jbc.M706794200. [DOI] [PubMed] [Google Scholar]
- Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. The Journal of biological chemistry. 2004;279:50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of cell science. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Current opinion in neurobiology. 2011;21:23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes & development. 2001;15:1–14. doi: 10.1101/gad.861501. [DOI] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes & development. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current opinion in neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gil-Sanz C, Franco SJ, Martinez-Garay I, Espinosa A, Harkins-Perry S, Muller U. Cajal-Retzius Cells Instruct Neuronal Migration by Coincidence Signaling between Secreted and Contact-Dependent Guidance Cues. Neuron. 2013;79:461–477. doi: 10.1016/j.neuron.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends in neurosciences. 2000;23:352–359. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Goryunov D, He CZ, Lin CS, Leung CL, Liem RK. Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain. Molecular and cellular neurosciences. 2010;44:1–14. doi: 10.1016/j.mcn.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nature reviews. Molecular cell biology. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S. Patterning events and specification signals in the developing hippocampus. Cerebral cortex. 1999;9:551–561. doi: 10.1093/cercor/9.6.551. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Gupta T, Marlow FL, Ferriola D, Mackiewicz K, Dapprich J, Monos D, Mullins MC. Microtubule actin crosslinking factor 1 regulates the Balbiani body and animal-vegetal polarity of the zebrafish oocyte. PLoS genetics. 2010;6:e1001073. doi: 10.1371/journal.pgen.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Developmental biology. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annual review of neuroscience. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nature reviews. Neuroscience. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, Murdoch H, Dunlop AJ, Kubo K, Furukori K, Huang B, Zeledon M, Hayashi-Takagi A, Okano H, Nakajima K, Houslay MD, Katsanis N, Sawa A. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473:92–96. doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N, Fumoto K, Izumi S, Kikuchi A. GSK-3beta regulates proper mitotic spindle formation in cooperation with a component of the gamma-tubulin ring complex, GCP5. The Journal of biological chemistry. 2008;283:12981–12991. doi: 10.1074/jbc.M710282200. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Dendrites. Genes & development. 2001;15:2627–2641. doi: 10.1101/gad.916501. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nature reviews. Neuroscience. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, Liem RK. Plakins: goliaths that link cell junctions and the cytoskeleton. Nature reviews. Molecular cell biology. 2004;5:542–553. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nature cell biology. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kim WY, Snider WD. Functions of GSK-3 Signaling in Development of the Nervous System. Frontiers in molecular neuroscience. 2011;4:44. doi: 10.3389/fnmol.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nature neuroscience. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD. Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron. 2006;52:981–996. doi: 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E. ACF7: an essential integrator of microtubule dynamics. Cell. 2003;115:343–354. doi: 10.1016/s0092-8674(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Jan LY, Jan YN. Mutations that affect the length, fasciculation, or ventral orientation of specific sensory axons in the Drosophila embryo. Neuron. 1995;15:273–286. doi: 10.1016/0896-6273(95)90033-0. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Molecular and cellular neurosciences. 2011;48:349–358. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Lee S, Harris KL, Whitington PM, Kolodziej PA. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000a;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000b;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. The Journal of cell biology. 1999;147:1275–1286. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, Li Y, Flanagan LA, Tole S, Monuki ES. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harbor perspectives in biology. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattie FJ, Stackpole MM, Stone MC, Clippard JR, Rudnick DA, Qiu Y, Tao J, Allender DL, Parmar M, Rolls MM. Directed microtubule growth, +TIPs, and kinesin-2 are required for uniform microtubule polarity in dendrites. Current biology : CB. 2010;20:2169–2177. doi: 10.1016/j.cub.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nature cell biology. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Nejatbakhsh N, Guo CH, Lu TZ, Pei L, Smit AB, Sun HS, van Kesteren RE, Feng ZP. Caltubin, a novel molluscan tubulin-interacting protein, promotes axonal growth and attenuates axonal degeneration of rodent neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15231–15244. doi: 10.1523/JNEUROSCI.2516-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Polleux F, Ghosh A. The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal development. Science's STKE : signal transduction knowledge environment. 2002;2002:pl9. doi: 10.1126/stke.2002.136.pl9. [DOI] [PubMed] [Google Scholar]
- Prokop A, Uhler J, Roote J, Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. The Journal of cell biology. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. The Journal of comparative neurology. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. The Journal of cell biology. 2005;171:845–855. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K, Gregory SL, Brown NH. The 'spectraplakins': cytoskeletal giants with characteristics of both spectrin and plakin families. Journal of cell science. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Sato T, Ando R, Noguchi N, Masaoka M, Miyata T. Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cerebral cortex. 2014;24:1301–1310. doi: 10.1093/cercor/bhs411. [DOI] [PubMed] [Google Scholar]
- Sanchez-Soriano N, Travis M, Dajas-Bailador F, Goncalves-Pimentel C, Whitmarsh AJ, Prokop A. Mouse ACF7 and drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. Journal of cell science. 2009;122:2534–2542. doi: 10.1242/jcs.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. Development, growth & differentiation. 2012;54:277–286. doi: 10.1111/j.1440-169X.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak A, Debowska K, Blazejczyk M, Kreutz MR, Kuznicki J, Wojda U. Calmyrin1 binds to SCG10 protein (stathmin2) to modulate neurite outgrowth. Biochimica et biophysica acta. 2011;1813:1025–1037. doi: 10.1016/j.bbamcr.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:738–745. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Prokop A, Yamamoto M, Sugimura K, Uemura T, Betschinger J, Knoblich JA, Volk T. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Current biology : CB. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- Tan SS, Kalloniatis M, Sturm K, Tam PP, Reese BE, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- Tomita K, Kubo K, Ishii K, Nakajima K. Disrupted-in-Schizophrenia-1 (Disc1) is necessary for migration of the pyramidal neurons during mouse hippocampal development. Human molecular genetics. 2011;20:2834–2845. doi: 10.1093/hmg/ddr194. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature genetics. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in human neuroscience. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. Journal of cell science. 2003;116:637–646. doi: 10.1242/jcs.00273. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, Galjart N, Kaibuchi K. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. Journal of cell science. 2009;122:2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta neuropathologica. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nature reviews. Molecular cell biology. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, Nakamura K, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Eom TY, Stanco A, Kim WY, Rao S, Snider WD, Anton ES. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–4110. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, Anton ES. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annual review of cell and developmental biology. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zumbrunn J, Kinoshita K, Hyman AA, Nathke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Current biology : CB. 2001;11:44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.