Abstract
Human umbilical cord blood (HUCB) cells have shown efficacy in rodent models of focal ischemia and in vitro systems that recapitulate stroke conditions. One potential mechanism of protection is through secretion of soluble factors that protect neurons and oligodendrocytes (OLs) from oxidative stress. To overcome practical issues with cellular therapies, identification of soluble factors released by HUCB and other stem cells may pave the way for treatment modalities that are safer for a larger percentage of stroke patients. Among these soluble factors is leukemia inhibitory factor (LIF), a cytokine that exerts pleiotropic effects on cell survival. Here, data show that LIF effectively reduced infarct volume, reduced white matter injury and improved functional outcomes when administered to rats following permanent middle cerebral artery occlusion. To further explore downstream signaling, primary oligodendrocyte cultures were exposed to oxygen glucose deprivation (OGD) to mimic stroke conditions. LIF significantly reduced lactate dehydrogenase release from OLs, reduced superoxide dismutase activity, and induced peroxiredoxin 4 (Prdx4) transcript. Additionally, the protective and antioxidant capacity of LIF was negated by both Akt inhibition and co-incubation with Prdx4 neutralizing antibodies, establishing a role for the Akt signaling pathway and Prdx4-mediated antioxidation in LIF protection.
Keywords: antioxidant, behavior, cell culture, CNS injury, stroke
Introduction
Human umbilical cord blood (HUCB) cell therapy has shown promise in experimental stroke models, with benefits that include enhanced cell viability, improved functional recovery (Vendrame et al., 2004; Vendrame et al., 2005; Hall et al., 2009a; Rowe et al., 2010), dampened apoptotic signaling, and increased expression of survival-associated proteins (Rowe et al., 2010). However, this and other cell therapies present difficulties in standardization, as well as additional health risks. Our laboratory has focused on identifying protective factors within the mononuclear HUCB cell fraction that may be administered as an alternative to cellular implantation.
Soluble factors secreted by these cells include cytokines, chemokines, metalloproteinase inhibitors, growth factors and interleukins (Neuhoff et al., 2007). Among the possible protective factors, we have been investigating leukemia inhibitory factor (LIF) as a potential player in HUCB cell protection. LIF is a 180 amino acid single 4-α-helix glycoprotein that binds to the LIF cell surface receptor complex, which includes the LIF receptor β (LIFR) and the gp130 receptor chain (Kurek, 2000; Metcalf, 2003). Following LIF binding, LIFR and gp130 dimerizes and activates the gp130 intracellular signaling cascade, thus activating several pathways including Janus kinase/signal transducer and activator for transcription (JAK/STAT) (Stahl et al., 1994), Ras mitogen activated (MAPK), and phosphatidylinositol 3 kinase (PI3/Akt) (Stahl et al., 1995; Oh et al., 1998).
Several lines of evidence indicate that LIF may play a key role in HUCB cell protection. In ischemic models, LIF protein expression was increased in neurons of rats subjected to middle cerebral artery occlusion (MCAO) and cultured astrocytes exposed to OGD (Slevin et al., 2008), suggesting a neuroprotective function in response to injury. In addition, intracerebral injections of LIF attenuated injury when administered after focal ischemia (Suzuki et al., 2005). Interestingly, LIF mRNA was upregulated in neural stem/precursor cells challenged with IFNγ (Laterza et al, 2013), a proinflammatory cytokine that we have identified as a major contributor to neurodegenerative injury following permanent focal ischemia (Seifert et al, 2012). Although no studies have yet investigated whether LIF is secreted by HUCB cells, these data support the notion that LIF signaling may account for the beneficial effects of this and other cellular therapies in reducing neurodegenerative injury following stroke.
In addition to neuronal damage, advances in imaging t echnology and an improved understanding of the complexity of stroke injury have sparked interest in the functional consequences of white matter damage. Of the glial cell types, oligodendrocytes (OLs) predominate the cerebral white matter and are most susceptible to ischemia (Lyons & Kettenmann, 1998). This inherent susceptibility has been linked to high metabolic rate, increased iron content, and reduced antioxidant capacity (Braughler et al., 1986; Connor & Menzies, 1996; Juurlink, 1997; Juurlink et al., 1998). The devastating effects of white matter injury on functional outcomes have been extensively documented in preterm and newborn infants exposed to hypoxic-ischemic events (Back, 2014; McQuillen & Ferriero, 2004), and white matter injury is now recognized as a hallmark of stroke pathology that must be evaluated when determining the efficacy of experimental therapeutics.
Based upon these findings and our previous work with HUCB cells, we sought to determine whether LIF might mediate the protective effects of this cellular therapy. By extension, we also investigated whether LIF-mediated protection of primary OL cultures from OGD is dependent upon AKT signaling, as we have previously shown that HUCB cells protect OLs from oxygen glucose deprivation (OGD) through the actions of secreted factors and a mechanism involving Akt signal transduction (Rowe et al., 2010).
Methods
Animal care
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Experiments were designed to minimize the number of animals required. Sprague-Dawley rats were purchased from Harlan Labs (Indianapolis, IN), maintained on a 12 h light/dark cycle (7 am - 7 pm) in a climate-controlled room, and allowed access to food and water ad libitum. Neonatal rats birthed from untimed-pregnant dams were used for in vitro experiments and 300-350 g male rats were used for in vivo experiments. All possible measures were taken to minimize pain and discomfort.
Permanent focal ischemia and LIF administration
Permanent focal ischemia was induced using the permanent middle cerebral artery occlusion (pMCAO) procedure, as previously described (Ajmo et al., 2006). Briefly, a 40 mm monofilament was introduced into the ECA, fed distally into the ICA, advanced approximately 25 mm to the base of the MCA, and secured in place to achieve permanent occlusion of the MCA. Blood flow was monitored throughout the procedure using a Laser Doppler monitoring system (Moore Lab Instruments, Farmington, CT). Exclusion criteria are set such that only animals with ≥ 60% blood flow reduction are included. Based upon this criteria, there were no animals excluded in the present study. For treatment, rats were administered either vehicle (PBS, pH 7.4) or LIF (25 or 125 μg/kg, i.v.) at 6, 24 and 48 h after pMCAO. Final injection volumes were weight-based (1.25 ml/kg), resulting in an average injection volume of approximately 0.4 ml. Rats were assessed for functional outcomes immediately before euthanizing at 72 h post-stroke.
Functional outcomes
Animals were assessed for functional outcomes immediately prior to being euthanized. Rats were evaluated for neurological/functional deficits using the following tests: Circling, Step Test, Paw Extension, and Elevated Body Swing Test (EBST). These tests provide reproducible measures of simple sensorimotor skills that are affected by stroke, and therefore are effective in identifying unilateral impairments. For circling, rats were placed in an open field and allowed to move freely. Circling behavior was recorded as “absence” or “presence.” For Step Test, each forepaw was dragged across a tabletop edge and the number of paw plants was recorded for each forepaw. For Paw Extension, rats were suspended by the tail and ability to extend the affected forelimb was recorded as “absence” or “presence.” For EBST, rats were suspended by the tail and the number of turns toward each side was recorded. Circling and Paw Extension data were expressed as the percentage of rats with “presence,” whereas EBST and Step Test data were expressed as the bias toward the affected side.
Fluoro-Jade histochemistry
For determination of infarct volume, Fluoro-Jade (Histochem, Jefferson, AR) staining was performed to label degenerating neurons. This method was adapted from that originally developed by Schmued et al. (Schmued et al., 1997) and has been subsequently detailed (Duckworth et al., 2005). Thaw-mounted sections were placed in 100% ethanol for 3 min followed by 70% ethanol and deionized water for 1 min each. Sections were then oxidized for 15 min in a 0.06% KMnO4, followed by three rinses in ddH2O for 1 min each. Sections were then stained in a 0.001% solution of Fluoro-Jade in 0.1% acetic acid for 30 min. Slides were again rinsed, dried at 45°C for 20 min, cleared with xylenes, and coverslipped using DPX mounting medium (Electron Microscopy Sciences, Ft. Washington, PA).
Mixed glial culture preparation and OL purification
All in vitro experiments were conducted using >95% pure OL cultures, as previously described (Hall et al., 2009a). Postnatal day 3 rat pups were used for all experiments. Rat cortices were collected, dissociated in a solution of 0.25% trypsin/2.21 mM EDTA, triturated, and pelleted. The pellet was re-suspended in DMEM (Mediatech, Manassas, VA) supplemented with 2.5% fetal bovine serum, 10% horse serum, and 1% antibiotic/antimycotic (DMEM+). Trypan Blue exclusion was used to assess cell viability. Cells were seeded (1.5 × 107) into poly-L-lysine-treated 75 cm2 tissue culture flasks. Media was changed with fresh DMEM+ the following day and cultures were incubated for 8 days at 37°C (Gottschall et al., 1995).
Mixed glial cultures were mechanically shaken for 1 h to separate microglial cells from the OL/astrocyte monolayer and media was discarded. Fresh DMEM+ was added and the flask was returned to the incubator for an additional 2 days at 37°C. OLs were purified from mixed glial preparations by shaking the preparations for 18 h to separate OLs and microglia from the astrocyte monolayer. The media was removed, the cells were pelleted and re-suspended in DMEM+. Viable cells were then counted using Trypan Blue exclusion. Microglia- and OL-containing media was added to 10 cm plastic tissue culture dishes at a density of 107 cells/dish and incubated for 15 min at 37°C (procedure repeated 3 times for microglial adherence to the plastic). After incubation, the dishes were gently swirled and media collected. The remaining suspension was pelleted, re-suspended in DMEM+, and plated on glass poly-L-lysine-treated coverslips at 3 × 105 cells/coverslip (McCarthy & de Vellis, 1980). The following day, media was changed to Neurobasal complete (Neurobasal supplemented with B-27, L-glutamine 0.5mM, and 10ng/ml PDGF AA) (Barres et al., 1993; Yang et al., 2005). OLs remained in Neurobasal complete and PDGFAA for 7 days to encourage proliferation. After the proliferation period, PDGF-AA was withdrawn for 5 days to induce OL differentiation into the mature phenotype (Yang et al., 2005). Experiments were conducted immediately following the 5 day PDGF-AA withdrawal.
Luxol fast blue myelin staining
Myelin disruption was assessed using Luxol fast blue staining with Cresyl violet counterstaining. Thaw mounted sections were dried, washed in ddH2O for 2 min, then 95% ethanol for 1 min. Slides were then incubated in Luxol fast blue solution overnight at 37°C. Slides were subsequently rinsed in 95% ethanol for 1 min followed by ddH2O for 2 min. Differentiation was achieved using a 0.05% lithium carbonate solution for 10 sec with agitation, followed by 60 sec in 70% ethanol and one ddH2O rinse. This sequence was performed twice, and tissues were then counterstained with a 0.1% cresyl violet solution for 1 min and washed in ddH2O. Tissues were then dehydrated in ethanol (70%, 95%, and 100% for 2 min each), cleared with xylenes, and coverslipped using DPX mounting media.
Oxygen glucose deprivation
Cells were subjected to normoxia or OGD as previously described (Hall et al., 2009a). Briefly, cells undergoing OGD were placed in an air-tight hypoxic chamber maintained at 37°C. The chamber was then flushed with hypoxic gas (95% N2, 4% CO2, 1% O2; Airgas, Tampa, FL) for 15 min and sealed for the duration of exposure, which was 24 h. Normoxic cells were maintained at 37°C in a standard tissue culture incubator. The media from each well was collected, clarified by centrifugation, and LDH analysis was performed immediately following OGD exposure.
Cell treatment
OLs were seeded onto glass coverslips and randomly assigned to OGD (DMEM without glucose) or normoxia (DMEM with glucose) conditions. Data were obtained from 3 independent cultures. LIF/GCSF (Millipore, Billerica, MA: 10-1000 ng/ml) or vehicle (DMEM containing 50mM sodium phosphate, 1mM DTT/10%glycerol, 250 mM Nacl, pH 7.4, and 0.02% Tween 20) was added to media as treatment required. Akt Inhibitor IV (10 μM/mL, EMD4Biosciences, Gibbstown, NJ) was added to media as treatment required. This compound inhibits Akt phosphorylation/activation by targeting the ATP binding site of a kinase upstream of Akt, but downstream of PI3K, therefore blocking Akt activity without affecting PI3K. All experimental groups received equal volumes of media. Negative controls of media and LIF working solution without cells were included to quantify media and buffer solution contribution to the LDH assay for each experimental condition. Akt Inhibitor IV was dissolved in DMSO and placed in appropriate media at a final concentration 10 μM. An equivalent volume of DMSO was added to media in experimental groups that did not receive Akt Inhibitor IV. Anti-Prdx4 (rabbit polyclonal, Abcam) was reconstituted as described for Akt Inhibitor IV and was added to selected cultures at a concentration of 10 μg/ml. The same concentration of normal rabbit IgG (Cell Signaling, Danvers, MA) was also used in parallel cultures exposed to the same conditions as a control for non-specific effects of antibody treatment.
Lactate dehydrogenase and superoxide dismutase
OL cell death in culture was determined using the lactate dehydrogenase (LDH) assay (Takara Bio, Inc., Madison, WI). Briefly, 100 μl of tissue culture media from each experimental group was added to a 96-well plate and 100 μl of LDH reagent was added to each well. Plates were incubated for 30 min at 25°C and absorbances were read on a microplate reader at a 548 nm wavelength. Media from LIF working solution without cells served as a control for buffer-derived, non-specific absorbance. Absorbances from negative control wells were subtracted from the total absorbance of the OL wells to remove background LDH activity from the analysis. A standard curve was used to extrapolate OL cell death from LDH levels after lysing cells, as previously described (Hall et al., 2009b), and data from LDH assays were represented as OL equivalents.
Superoxide dismutase (SOD) activity in OL cultures was determined using the SOD Assay (Kamiya Bomedical, Seattle, WA). Tissue culture media from each experimental group and supplied reagents were added to a 96-well plate as directed by the manufacturer. Plates were incubated for 25 min at 25°C and absorbances were read on a microplate reader at a 450 nm wavelength. SOD activity was determined from recorded absorbances using the equation provided by the manufacturer.
RNA collection and purification
All collection and purification steps were performed under nuclease-free conditions using DNAse/RNAse-free materials. For RNA lysate collection, 10 μl of β-mercaptoethanol (Pharmacia Biotech, Uppsala, Sweden) was added to 1 ml RTL buffer (Qiagen Inc., Valencia, CA) and 350 μl of the resulting mixture was added to each OL-containing well to lyse the cells. Cell lysates were then stored at −80°C prior to extracting the RNA. The Qiagen RNeasy Mini Kit was used to extract total RNA from each cell lysate using the optional Qiagen RNase-Free DNase set for DNase digestion (Qiagen Inc, Valencia, CA). Following the extraction, 1 μl of each RNA sample was tested in an Agilent 2100 Bio-analyzer to determine the purity and quantity of RNA present. The remaining samples were stored at −80°C for subsequent analysis.
Quantitative real time polymerase chain reaction
Primers for Prdx4 were purchased from SABiosciences (Frederick, MD; sequences are proprietary). Total RNA (10 ng/μl) from OL cultures was subjected to qRT-PCR. The RT reaction mixture consisted of 3 μl Oligo (dT) Primers, 10 μl cDNA Synthesis Master Mix (2X), 1 μl of Affinity Script RT/RNase Block enzyme mixture, and RNase-free water to a total volume of 20 μl (Stratagene, La Jolla, CA). The reaction was incubated at 25°C for 5 min to allow primer annealing, then incubated at 42°C for 45 min to allow cDNA synthesis followed by 5 min incubation at 95°C to terminate the cDNA synthesis reaction.
Complementary DNA from the RT reaction was added to a PCR reaction mix consisting of 1 μl cDNA, 12.5 μl 2X Brilliant 490 SYBR Green QPCR Master Mix (Stratagene), 2 μl primer, and nuclease-free PCR grade water to a total volume of 25 μl. The samples were amplified using a BioRad ICycler (Bio-Rad Laboratories, Hercules, CA) with the following protocol: heating to 95°C for 15 min, 40 cycles of 30 sec denaturation at 95°C, 30 sec of annealing at 55 °C, and 30 sec of elongation at 72°C. GADPH was used as a reference gene to calculate the mean normalized expression.
Image analyses
All analyses were performed by experimenters that were blinded to the experimental treatments. Coronal brain sections encompassing the corpus striatum (Bregma coordinates +1.7 through −0.3) were collected from each animal. Images were generated using a Zeiss Axioskop2 microscope controlled by Openlab software. Images were captured with a Zeiss Axiocam Color camera. The ImageJ 1.410 program was used to measure total area occupied by fluorescent signal, and the relative intensity ratios of ipsilateral vs. contralateral hemispheres were determined to control for non-specific Fluoro-Jade signal and ipsilateral brain swelling caused by edema. The mean area occupied by signal was calculated for each group and these numbers were extrapolated between selected Bregma points to calculate infarct volume.
Statistical analyses
Data from all experiments were quantified and analyzed using GraphPad Prism 4.0 (GraphPad, La Jola, CA) software. For all statistical tests, the threshold for significant differences between groups was set at P<0.05. For behavioral tests, Bartlett’s test for unequal variance showed no significant differences between groups. Based upon these statistics, significant effects of treatment were determined using unpaired, two-tailed t-tests with Welch’s Correction. For all other data, main effects were determined using one-way or two-way ANOVA followed by Fisher’s Least Significant Difference (LSD) test for pairwise comparisons between treatment groups.
Results
Delayed LIF administration reduces infarct volume at 72 hours
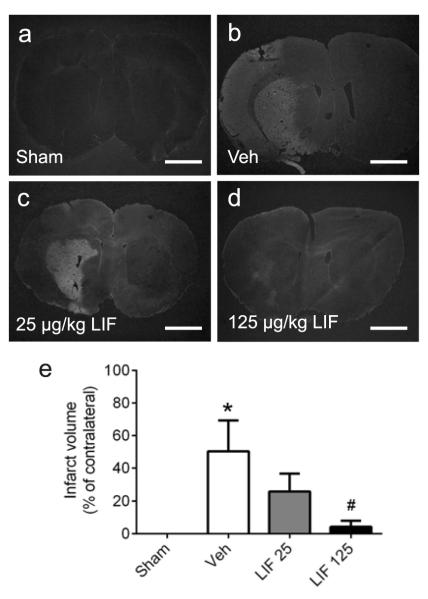
To first determine whether LIF provides efficacy in reducing infarct volume, rats were subjected to sham surgery or pMCAO and administered either vehicle or LIF (25 or 125 μg/kg, i.v.) at 6, 24 and 48 h after pMCAO. Rats were then euthanized at 72 h post-stroke for quantification of infarct volume using Fluoro-Jade histochemistry (Fig. 1). Rats subjected to sham surgery showed no Fluoro-Jade staining (Fig. 1a), indicating no regions of cerebral infarction which might have arisen from errant placement of the laser Doppler probe or other surgical errors. Rats that were subjected to pMCAO and administered vehicle showed large expanses of fluorescent staining (Fig. 1b), marking neurodegeneration that was largely limited to the corpus striatum and neighboring cortical areas. Rats administered 25 μg/kg LIF also showed large expanses of staining in the corpus striatum (Fig. 1c), similar to those from vehicle-treated rats, although there was less cortical staining in the 25 μg/kg group upon comparison. However, rats administered 125 μg/kg LIF showed very little fluorescent staining within the corpus striatum and cortical regions (Fig. 1d). Quantification (Fig. 1e) showed that infarct volume was significantly reduced in rats treated with 125 μg/kg LIF relative to vehicle controls (P=0.022, F3,15=4.3). Data were analyzed using one-way ANOVA followed by Fisher’s LSD test.
Fig 1. Delayed LIF administration reduces infarct volume at 72 hours.
Photomicrographs of Fluoro-Jade stained sections from rats subjected to pMCAO and administered vehicle or LIF. (a) Sham-operated control rat shows no fluorescent signal. (b) Vehicle-treated rat shows large regions of fluorescent staining, indicating severe infarction throughout the cortex and corpus striatum. (c) Rat treated with 25 μg/kg LIF shows fluorescent staining throughout the corpus striatum, but no cortical infarct. (d) Rat treated with 125 μg/kg LIF shows marked decrease in fluorescent signal within the corpus striatum, and no cortical infarction. (e) Quantification shows a significant increase in infarct volume for vehicle-treated (n=5) rats relative to sham-operated (n=5) controls (*P<0.05, significant from sham), whereas 125 μg/kg LIF (n=5) significantly reduced infarct volume relative to vehicle-treated rats (#P<0.05, significant from vehicle). Veh, vehicle. LIF, leukemia inhibitory factor. Scale bars = 500 μm.
Delayed LIF administration preserves white matter integrity at 72 hours
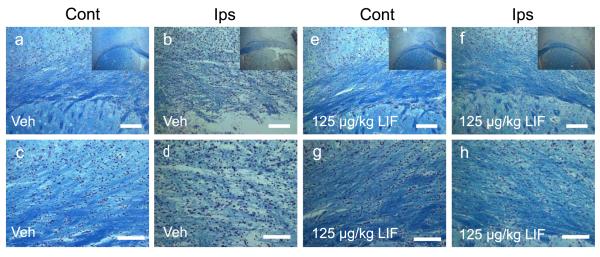
Since infarct volume was significantly reduced following treatment with 125 μg/kg LIF, adjacent sections were stained with Luxol fast blue and counterstained with Cresyl violet to determine whether LIF preserves white matter (Fig. 2). Results showed clear differences in white matter staining intensity between groups. Gross observations indicated a marked reduction in ipsilateral white matter staining after administration of vehicle compared to LIF treatment (Fig. 2, insets). This effect on white matter included the white matter-rich region of the external capsule and the striatal white matter bundles. A closer examination revealed substantial white matter degradation and disorganization of intrafascicular OL arrangement within the ipsilateral external capsule after treatment with vehicle (Fig. 2a-d). Additionally, labeled cells within the external capsule appeared larger compared to contralateral cells, which may reflect injury-induced swelling. In contrast, 125 μg/kg LIF treatment showed an ipsilateral staining intensity, OL arrangement and size that closely resembled the corresponding contralateral cells within this structure (Fig. 2e-h).
Fig. 2. Delayed LIF administration preserves white matter integrity at 72 hours.
Photomicrographs of sections stained with Luxol fast blue and counterstained with Cresyl violet from rats subjected to pMCAO and administered vehicle (a-d) or LIF (e-h) following stroke. Insets show gross effects of treatment at 2.5x magnification. Section from a vehicle-treated animal shows intense myelin staining in the contralateral external capsule and dorsal striatum (a) that was markedly reduced in the ipsilateral stroke hemisphere (b). Higher magnification (c,d) shows disruption of the intrafascicular OL arrangement within the ipsilateral external capsule. Section from an animal treated with 125 ug/kg LIF shows intense white matter staining (e,f) and preservation of intrafascicular OLs (g,h). Veh, vehicle. LIF, leukemia inhibitory factor. Scale bars = 100 μm.
Delayed LIF administration improves functional outcomes at 72 hours
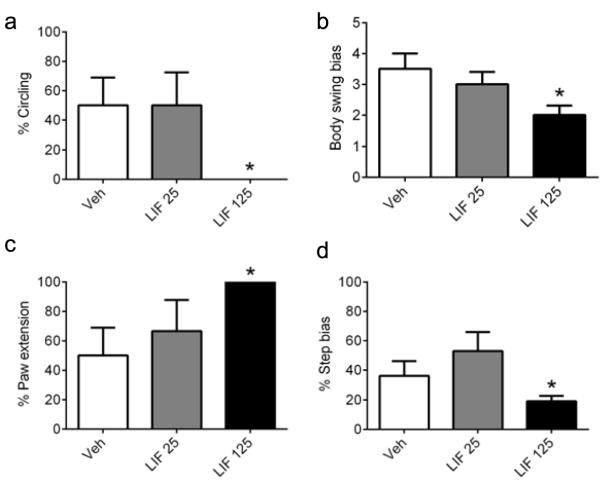
To determine whether histological assessments were associated with improved functional outcomes, animals subjected to sham surgery or pMCAO and administered vehicle or LIF, as described, were evaluated for functional deficits using four separate measures: circling, elevated body swing, paw extension, and step (Fig. 3). Treatment with 125 μg/kg LIF resulted in significant improvements for circling (P=0.033, t=2.5), elevated body swing (P=0.028, t=2.5), paw extension (P=0.033, t=2.6) and step (P=0.047, t=2.5), while the 25 μg/kg dose did not significantly improve performance in any of the assessments. The most striking result was in circling, in which circling was essentially eliminated in rats treated with 125 μg/kg LIF. These results were consistent with the infarct volume data, indicating that LIF was effective in reducing neurodegenerative injury and promoting functional recovery at the 125 μg/kg dose. Data were analyzed using unpaired, two-tailed t-test with Welch’s Correction.
Fig. 3. Delayed LIF administration improves functional outcomes at 72 hours.
Rats were evaluated for performance on a series of neurological/functional tests after being subjected to pMCAO and treated with vehicle or LIF. (a) Stroke-induced circling behavior was evident in rats treated with vehicle (n=5) or 25 μg/kg LIF (n=6), but was absent in rats treated with 125 μg/kg LIF (n=5) (*P<0.05, significant from vehicle and 25 μg/kg LIF). (b) EBST bias toward the affected side was significantly reduced in rats treated with 125 μg/kg LIF relative to vehicle-treated rats (*P<0.05), whereas the lower dose did not result in any improvement. (c) Stroke-induced impairments in paw extension were not evident in rats treated with 125 μg/kg LIF (*P<0.05), whereas there was no significant difference between the vehicle and 25 μg/kg LIF groups. (d) Step bias was not different between the vehicle and 25 μg/kg LIF groups, whereas treatment with 125 μg/kg LIF significantly reduced the step bias relative to vehicle-treated rats (*P<0.05). Veh, vehicle. LIF, leukemia inhibitory factor (25, 125 μg/kg).
Akt inhibition abolishes LIF protection in primary OL cultures subjected to OGD
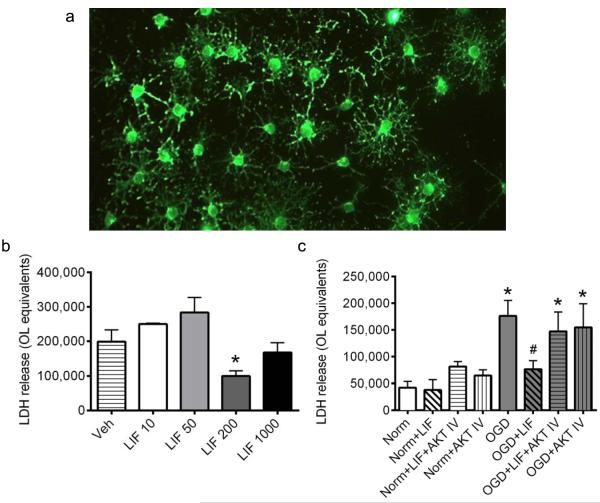
Primary rat OLs were cultured, proliferated and differentiated to the mature phenotype (Fig. 4a) prior to investigating LIF protection and signaling. Concentration-response experiments were first performed to determine whether LIF administration attenuates OGD-induced OL injury, and if so, to identify the optimal concentration for subsequent investigations. LIF was added to media at concentrations ranging from 10 ng/ml to 1000 ng/ml (Fig. 4b). Primary OL cultures were subjected to 24 h of OGD in the presence of vehicle or LIF, after which cellular cytotoxicity was assessed using the LDH assay. OL equivalents were derived from LDH values, as described in Methods. Co-incubation with 200 ng/ml LIF significantly reduced LDH levels in OLs subjected to OGD relative to OGD-only cultures (P=0.0047, F4,16=5.0). Based upon these data, the 200 ng/mL concentration was selected for all subsequent experiments examining the signaling mechanisms activated by LIF. Data were analyzed using one-way ANOVA followed by Fisher’s LSD test.
Fig. 4. Akt inhibition abolishes LIF protection in OLs during OGD.
Primary rat OL cultures were subjected to normoxia or OGD in the presence or absence of vehicle, LIF, or Akt IV. (a) Representative micrograph taken at 20x magnification shows mature OLs immunostained for myelin basic protein. (b) Concentration-response showing the effects of LIF on cellular injury, as measured by LDH release, during OGD. Co-incubation with 200 μg/ml LIF significantly reduced OGD-induced injury relative to vehicle controls (*P<0.05, significant from vehicle). (c) OLs subjected to normoxia or OGD were treated with 200 ng/ml LIF, Akt IV, or LIF+Akt IV. Co-incubation with LIF significantly reduced OGD-induced injury (#P<0.05, significant from OGD), while cultures treated with LIF in the presence of Akt IV showed no reduction in OGD-induced injury (*P<0.05, significant from normoxia). n=3 independent cultures. Veh, vehicle. LIF, leukemia inhibitory factor (10, 50, 200, 1000 ng/ml). Norm, normoxia. OGD, oxygen glucose deprivation. Akt IV, Akt Inhibitor IV.
Our previous studies have linked the efficacy of HUCB cells to Akt phosphorylation and anti-oxidant protein expression, both in vitro and when administered after focal ischemia (Rowe et al., 2012). To determine whether soluble LIF is involved in HUCB cell-mediated protection, primary OL cultures were used as a controlled system in which to selectively target this protective pathway. Cultures were exposed to 24 h OGD or normoxia and co-incubated with LIF in the presence or absence of Akt Inhibitor IV (Fig. 4c). The addition of 200 ng/ml LIF or 10 μM Akt Inhibitor IV to normoxic cultures, whether alone or in combination, had no significant effect on LDH release. As expected, cultures subjected to OGD showed significant elevations in LDH release relative to normoxic controls (P<0.00010, F7,56=6.1), indicating marked cellular injury. After co-incubation with LIF, OGD-exposed OLs showed a significant reduction in LDH release relative to OGD alone (P=0.032), and OGD+LIF levels were not significantly different from normoxic cells. Furthermore, LIF failed to protect cells subjected to Akt inhibition, as LDH release in cultures co-incubated with either LIF+Akt Inhibitor IV or Akt Inhibitor IV alone was significantly higher than normoxic cells (P=0.042) and was not significantly different from levels detected in the OGD-only group. Data were analyzed by two-way ANOVA followed by Fisher’s LSD test.
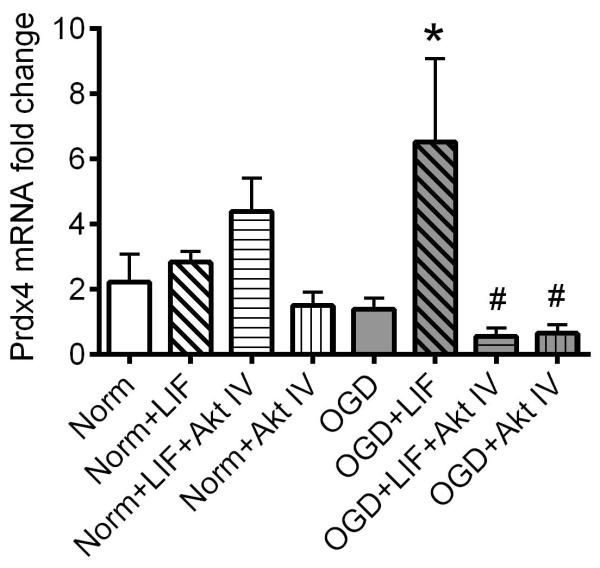
LIF induces Prdx4 mRNA expression through Akt signaling
Prdx4 was previously shown to be upregulated by HUCB cell treatment and is known to reduce reactive oxygen species (ROS) accumulation (Hozumi et al., 1998; Hofmann et al., 2002; Rowe et al., 2010). To determine whether Prdx4 is a downstream regulator of LIF protection, qRT-PCR was performed to analyze Prdx4 transcript in LIF-treated OL cultures subjected to normoxia or OGD. Additionally, the relative contribution of Akt signaling was evaluated by co-incubating LIF with Akt Inhibitor IV (Fig. 5). No significant differences in Prdx4 transcript were detected across normoxic groups, or between normoxic and OGD cells cultured in the absence of LIF or Akt inhibition. However, co-incubation with LIF or Akt Inhibitor IV resulted in significant alterations in Prdx4 transcript (P=0.029, F7,50=3.7). LIF treatment increased Prdx4 mRNA in OLs subjected to OGD relative to OGD alone (P=0.0081). Furthermore, Prdx4 transcript was significantly reduced in OGD-exposed cells co-incubated with Akt Inhibitor IV alone or Akt Inhibitor IV+LIF relative to the OGD+LIF group (P=0.0075). For both OGD groups co-incubated with Akt Inhibitor IV, Prdx4 mRNA levels were not significantly different from the OGD-only group, demonstrating that Prdx4 transcript induction in this culture system is Akt-dependent. Data were analyzed using two-way ANOVA followed by Fisher’s LSD test.
Fig. 5. LIF induces Prdx4 mRNA transcript in OLs during OGD.
Primary rat OL cultures were subjected to normoxia or OGD in the presence or absence of vehicle, 200 ng/ml LIF, or Akt IV. There were no significant differences in Prdx4 mRNA expression between any of the normoxic groups. Following OGD exposure, LIF-treated cells showed a significant increase in Prdx4 mRNA (*P<0.05, significant from OGD), and parallel cultures co-incubated with Akt IV showed Prdx4 mRNA levels that were significantly lower than cells treated with LIF alone (#P<0.05, significant from OGD+LIF). n=3 independent cultures. Norm, normoxia. OGD, oxygen glucose deprivation. LIF, leukemia inhibitory factor. Akt IV, Akt Inhibitor IV.
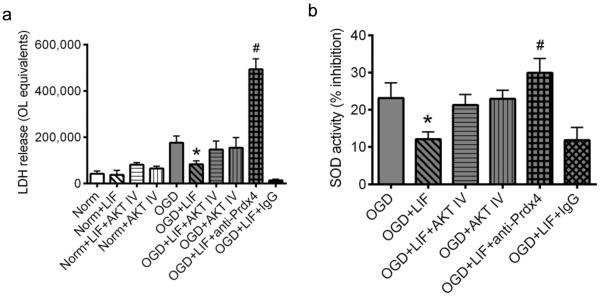
Prdx4 is required for Akt-mediated LIF protection in cultured OLs
Since Prdx4 transcript was upregulated following LIF treatment, and these effects were blocked by Akt inhibition, we sought to determine whether Prdx4 might be responsible, at least in part, for the protective effects of LIF. The same paradigm of OGD and LIF treatment was used to test whether the addition of a Prdx4 neutralizing antibody could block the protective effects of LIF (Fig. 6a). Data showed significant effects of treatment and alterations in LDH release that resulted from blocking Prdx4 activity (P=0.00010, F9,61=19) Consistent with previous results, co-incubation with LIF significantly attenuated OGD-induced cell death relative to OGD-only cultures (P=0.038). Additionally, OLs subjected to OGD and co-incubated with LIF+anti-Prdx4 were not protected, showing a marked increase in cellular injury relative to all other experimental groups (P=0.00016). Co-incubation of LIF-treated cells with anti-rabbit IgG did significantly alter the actions of LIF. Data were analyzed using two-way ANOVA followed by Fisher’s LSD test.
Fig. 6. Prdx4 is required for LIF-mediated OL protection during OGD.
Primary rat OL cultures were subjected to normoxia or OGD in the presence or absence of vehicle, 200 ng/ml LIF, Akt IV, anti-Prdx4 or anti-rabbit IgG. (a) Data from LDH assays shows no significant differences in cellular injury between any of the normoxic groups. Cells exposed to OGD and treated with LIF showed a significant reduction in cellular injury relative to OGD-only cultures (*P<0.05, significant from OGD), while LDH levels in parallel cultures co-incubated with Akt IV were not significantly different from OGD controls. Co-incubation of LIF-treated cells with the Prdx4 neutralizing antibody increased cellular injury to levels that were significantly higher than all other OGD cultures (#P<0.05, #significant from all other OGD groups). There were no significant differences in LDH release in LIF-treated cells co-incubated with anti-rabbit IgG. (b) Data from SOD assays shows significantly reduced SOD activity in media from LIF-treated cultures (*P<0.05, significant from OGD), whereas cultures co-incubated with Akt IV showed SOD activity levels that were not different from OGD-only controls. Co-incubation of LIF-treated cells with the Prdx4 neutralizing antibody resulted in significantly increased SOD activity levels relative to OGD+LIF cultures (#P<0.05, significant from OGD+LIF). Co-incubation of LIF-treated cultures with anti-rabbit IgG produced no significant effects on LIF-induced SOD activity. n=3 independent cultures. Norm, normoxia. OGD, oxygen glucose deprivation. LIF, leukemia inhibitory factor. Akt IV, Akt Inhibitor IV. anti-Prdx4, Prdx4 neutralizing antibody. IgG, anti-rabbit IgG.
Because the effects of the neutralizing antibody were so pronounced, a second series of experiments was conducted to validate these data and further explore the precise mechanism by which Prdx4 confers oligoprotection. Cultured OLs were subjected to the same normoxic or OGD conditions and extracellular oxidation was evaluated by measuring SOD activity in the media (Fig. 6b). Data showed significant effects from co-incubation with LIF and anti-Prdx4 (P=0.012, F4,23=3.716). SOD activity was significantly reduced in media from LIF-treated OLs relative to the OGD-only group (P=0.033). Furthermore, co-incubation of LIF with anti-Prdx4 resulted in significantly increased SOD activity levels relative to the OGD+LIF group (P=0.027), demonstrating that blocking Prdx4 function effectively negated LIF-mediated reductions in SOD activity. Data were analyzed using two-way ANOVA followed by Fisher’s LSD test.
Discussion
HUCB cellular therapy is effective in rodent stroke models by inhibiting the pro-inflammatory immune response and activating neural cell survival signaling. This protection is conferred, in part, through released soluble factors that activate the survival-associated protein kinase Akt (Rowe et al., 2012), leading to the increased expression of antioxidant genes. Although HUCB cell therapy demonstrates potential as a stroke therapeutic, identifying the factors that elicit these pro-survival actions will help develop safer, targeted approaches for standardized care. The present study investigated the therapeutic potential of LIF in improving functional outcomes and reducing cerebral infarction in rats subjected to permanent focal ischemia, while investigating the signaling pathways activated by LIF in primary OL cultures exposed to conditions similar to those which occur during stroke.
Previous studies have highlighted LIF protective effects in disease states including spinal cord injury and EAE (Kerr & Patterson, 2005; Azari et al., 2006; Butzkueven et al., 2006). Furthermore, LIF expression is upregulated in vitro in cells exposed to OGD, and is rapidly expressed in the infarct boundary post-stroke (Slevin et al., 2008). Thus, upregulation of LIF in disease states appears to have a protective effect. Data here document the protective efficacy of LIF when administered following permanent focal ischemia, providing proof of concept that LIF treatment is a viable therapeutic option for the treatment of stroke.
Additionally, our results support the idea that LIF acts through similar mechanisms as those previously identified for HUCB cell therapy. Experiments with primary OL cultures demonstrated a role for Akt signaling and the antioxidant actions of Prdx4, consistent with our previous work in the same culture system (Rowe et al., 2012). These findings are also consistent with LIF protection in other injury models, as well as its capacity in eliciting these downstream responses. For example, LIF was shown to preserve white matter integrity through STAT3 and PI3/Akt activation following spinal cord injury (Metcalf & Gearing, 1989b; Azari et al., 2006). In the experimental autoimmune encephalitis (EAE) model of multiple sclerosis, LIF administration promoted OL viability, while administration of LIF blocking antibodies enhanced disease pathology (Metcalf & Gearing, 1989b; 1989a; Butzkueven et al., 2002). Another report demonstrated that LIF rescued mature OLs from interferon gamma- (IFNγ) and tumor necrosis factor alpha- (TNFα) induced cell death through the activation of the JAK/STAT and PI3/Akt pathways (Slaets et al., 2008).
Interestingly, a recent, detailed study showed that LIF transcript was upregulated in neural stem/precursor cells stimulated with IFNγ and TNFα, and transplanted but undifferentiated cells improved outcomes in EAE mice (Laterza et al., 2013). The fact that undifferentiated cells exerted such effects is indirect evidence that soluble LIF may be responsible, at least in part, for the protection afforded by stem cell therapy. Interestingly, we have previously documented an important role for IFNγ in stroke-induced inflammatory signaling and neural injury in the present model of focal ischemia (Seifert et. al, 2012). Since LIF induction reflects a response to IFNγ and other stimuli that are well-known to facilitate the pro-inflammatory response following stroke (Amor et al., 2013; Siniscalchi et al., 2014), it is conceivable that the neuroprotective effects of HUCB cell therapy result from the actions of LIF. Indeed, a previous report showed that LIF inhibits the production of pro inflammatory mediators, including TNFα and macrophage-derived ROS (Hendriks et al., 2008). Taken together, these data suggest that LIF is upregulated in response to proinflammatory signals, such as IFNγ, as means of protecting the brain from additional injurious cascades. However, LIF also exerts both proliferative and protective effects on glial cells, which are critically important in preserving neuronal cell function and the integrity of the neurovascular unit. For example, LIF stimulated GFAP expression in astrocytic progenitors, whereas in oligodendrocyte progenitor cultures, LIF enhanced myelin basic protein expression, survival, maturation and generation (Mayer et al., 1994; Nakagaito et al., 1995). Therefore, it is possible that the protective effects of LIF in the MCAO model are due to one or more of these actions.
One important finding, which was documented with HUCB cell therapy and confirmed in the present study, is that antioxidant protein function is critical in the protection of OLs exposed to OGD conditions. Following ischemic stroke, reductions in cerebral blood flow induce ROS production and lead to mitochondrial dysfunction, which is attenuated by PI3/Akt activation (Zhao et al., 2006). In regards to white matter injury, relatively low antioxidant content renders OLs vulnerable to excessive ROS and reactive nitrogen species (RNS) accumulation (Juurlink, 1997; Juurlink et al., 1998). In addition, iron is an important myelin cofactor that is highly reactive and conducive to ROS/RNS generation, free radical formation and lipid peroxidation following stroke (Braughler et al., 1986; Connor & Menzies, 1996; McTigue & Tripathi, 2008; Allen & Bayraktutan, 2009). Among the reactive species that facilitate cellular injury, superoxide is the primary ROS from which other ROS are generated (Allen & Bayraktutan, 2009). Here, LIF treatment increased Prdx4 mRNA expression in cultured OLs exposed to OGD, and either neutralizing Prdx4 activity or blocking Akt signaling negated protection while increasing SOD activity. In response to excessive oxidative stress, SOD is upregulated to prevent cellular injury (Allen & Bayraktutan, 2009), which is consistent with the observed elevations in SOD upon inhibition of Prdx4 extracellular antioxidant activity.
Due to the nature of this response and the present findings, it is tempting to attribute the protective effects and increased SOD activity to the antioxidant activity of Prdx4. However, Prdx4 exhibits multiple functions, serving as a free radical scavenger through its peroxidase activity, detoxifying a range of free radical-forming organic hydroperoxides (Hofmann et al., 2002), and engaging in chaperone activity (Jang et al., 2004) that prevents free radical-induced aggregation of cytosolic proteins (Jang et al., 2004; Kang et al., 2005). Although we did not investigate cytosolic protein aggregation in the present study, it is possible that this chaperone activity contributes to the protection afforded by Prdx4 in culture. Future investigations into this mechanism are necessary and should be conducted to further explore the therapeutic potential and identify new treatment options for focal ischemia.
Acknowledgements
This work was supported in part by the National Institutes of Health (R01 NS052839 and R21NS078517), and the University of South Florida Department of Molecular Pharmacology and Physiology.
Footnotes
Disclosure A.E. Willing is a consultant to Saneron CCEL Therapeutics, Inc. and is an inventor on cord blood-related patents.
References
- Ajmo CT, Jr., Vernon DO, Collier L, Pennypacker KR, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM. Inflammation in neurodegenerative diseases - an update. Immunology. 2013;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari MF, Profyris C, Karnezis T, Bernard CC, Small DH, Cheema SS, Ozturk E, Hatzinisiriou I, Petratos S. Leukemia inhibitory factor arrests oligodendrocyte death and demyelination in spinal cord injury. J Neuropathol Exp Neurol. 2006;65:914–929. doi: 10.1097/01.jnen.0000235855.77716.25. [DOI] [PubMed] [Google Scholar]
- Back SA. Cerebral White and Gray Matter Injury in Newborns: New Insights into Pathophysiology and Management. Clin Perinatol. 2014;41:1–24. doi: 10.1016/j.clp.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem. 1986;261:10282–10289. [PubMed] [Google Scholar]
- Butzkueven H, Emery B, Cipriani T, Marriott MP, Kilpatrick TJ. Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia. 2006;53:696–703. doi: 10.1002/glia.20321. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR. Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042:29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res. 1995;42:335–342. doi: 10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- Hall AA, Guyer AG, Leonardo CC, Ajmo CT, Jr., Collier LA, Willing AE, Pennypacker KR. Human umbilical cord blood cells directly suppress ischemic oligodendrocyte cell death. J Neurosci Res. 2009a;87:333–341. doi: 10.1002/jnr.21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AA, Leonardo CC, Collier LA, Rowe DD, Willing AE, Pennypacker KR. Delayed treatments for stroke influence neuronal death in rat organotypic slice cultures subjected to oxygen glucose deprivation. Neuroscience. 2009b;164:470–477. doi: 10.1016/j.neuroscience.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JJ, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, Hellings N. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol. 2008;204:52–57. doi: 10.1016/j.jneuroim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- Hozumi I, Inuzuka T, Tsuji S. Brain injury and growth inhibitory factor (GIF)--a minireview. Neurochem Res. 1998;23:319–328. doi: 10.1023/a:1022401315721. [DOI] [PubMed] [Google Scholar]
- Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW, Choi YO, Kim WY, Kang JS, Cheong GW, Yun DJ, Rhee SG, Cho MJ, Lee SY. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Juurlink BH. Response of glial cells to ischemia: roles of reactive oxygen species and glutathione. Neurosci Biobehav Rev. 1997;21:151–166. doi: 10.1016/s0149-7634(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, Patterson PH. Leukemia inhibitory factor promotes oligodendrocyte survival after spinal cord injury. Glia. 2005;51:73–79. doi: 10.1002/glia.20177. [DOI] [PubMed] [Google Scholar]
- Kurek J. AM424: history of a novel drug candidate. Clin Exp Pharmacol Physiol. 2000;27:553–557. doi: 10.1046/j.1440-1681.2000.03289.x. [DOI] [PubMed] [Google Scholar]
- Laterza C, Merlini A, De Feo D, Ruffini F, Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, Quattrini A, Taveggia C, Farina C, Cattaneo E, Martino G. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun. 2013;4:2597. doi: 10.1038/ncomms3597. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The unsolved enigmas of leukemia inhibitory factor. Stem Cells. 2003;21:5–14. doi: 10.1634/stemcells.21-1-5. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Gearing DP. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1989a;86:5948–5952. doi: 10.1073/pnas.86.15.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, Gearing DP. A myelosclerotic syndrome in mice engrafted with cells producing high levels of leukemia inhibitory factor (LIF) Leukemia. 1989b;3:847–852. [PubMed] [Google Scholar]
- Nakagaito Y, Yoshida T, Satoh M, Takeuchi M. Effects of leukemia inhibitory factor on the differentiation of astrocyte progenitor cells from embryonic mouse cerebral hemispheres. Brain Res Dev Brain Res. 1995;87:220–223. doi: 10.1016/0165-3806(95)00063-j. [DOI] [PubMed] [Google Scholar]
- Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, Meier C. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007;35:1119–1131. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem. 1998;273:9703–9710. doi: 10.1074/jbc.273.16.9703. [DOI] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Hall AA, Shahaduzzaman MD, Collier LA, Willing AE, Pennypacker KR. Cord blood administration induces oligodendrocyte survival through alterations in gene expression. Brain Res. 2010;1366:172–188. doi: 10.1016/j.brainres.2010.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DD, Leonardo CC, Recio JA, Collier LA, Willing AE, Pennypacker KR. Human umbilical cord blood cells protect oligodendrocytes from brain ischemia through Akt signal transduction. J Biol Chem. 2012;287:4177–4187. doi: 10.1074/jbc.M111.296434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, De Sarro G. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol. 2014;25:131–137. doi: 10.1515/jbcpp-2013-0121. [DOI] [PubMed] [Google Scholar]
- Slaets H, Dumont D, Vanderlocht J, Noben JP, Leprince P, Robben J, Hendriks J, Stinissen P, Hellings N. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Aktphosphorylation and up-regulation of 14-3-3. Proteomics. 2008;8:1237–1247. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Mitsios N, Perikleous C, Cuadrado E, Montaner J, Sanfeliu C, Luque A, Kumar S, Kumar P, Gaffney J. Leukaemia inhibitory factor is over-expressed by ischaemic brain tissue concomitant with reduced plasma expression following acute stroke. Eur J Neurol. 2008;15:29–37. doi: 10.1111/j.1468-1331.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr., Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, Suzuki N. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:685–693. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, De Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Yang Z, Watanabe M, Nishiyama A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 2005;149:50–56. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]