Abstract
Objective
The aim of the study was to investigate treatment outcome of mandibular advancement devices (MADs) for positional and non-positional obstructive sleep apnea (OSA).
Study design
Forty-two positional (supine apnea-hypopnea index [AHI] ≥ 2x’s lateral AHI) and 30 non-positional (supine AHI < 2x’s lateral AHI) OSA patients performed two-nights of sleep study before and after insertion of MADs.
Results
The decreases in apnea severity based on a reduction in the overall and supine AHI values after MADs therapy were significantly greater for the positional OSA than non-positional OSA group. A multiple linear regression analysis showed that decrease in overall AHI was significantly associated with being in the positional group (standardized coefficient=0.505). Age, body mass index, gender, and time in supine position during sleep did not show significant associations with decrease in overall AHI after MAD therapy.
Conclusion
Our data suggest that MADs are more effective in positional OSA than non-positional OSA patients.
Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent episodes of obstruction in the upper airway during sleep. OSA has been identified as a major public health concern and has serious consequences such as daytime somnolence, systemic hypertension, and cardiovascular diseases.1–4 A number of risk factors including aging, gender, obesity, and craniofacial development have been found to be associated with OSA.5–7
Mandibular advancement devices (MADs) have become a common treatment for OSA and are used as a treatment alternative to positive airway pressure (PAP) devices.8–9 MADs are designed to protrude the mandible and increase the caliber of the airway during sleep. Many clinical studies have reported that MADs are less effective than PAP in reducing the sleep apnea but that oral devices are preferred by more patients and are more readily accepted than PAP.8 MADs have been most commonly prescribed for patients with mild to moderate, but not severe OSA.10–12 Previous reports have suggested that the effectiveness of MADs may be influenced by sleep position and that outcomes are improved in what they described as “supine-dependent” sleep apnea.13–15 In contrast, higher body mass index (BMI) and neck circumference negatively predict outcomes for MAD’s, but without consideration of sleep position.16 These findings raise the questions: “How does sleep position impact MAD outcome, independent of other factors?” And, “Can we obtain efficacious outcomes in positional OSA patients even though they have moderate to severe OSA?”
Patients with OSA usually have more obstructive events in the supine position than in the lateral position, and when this is the case, forcing a change to the non-supine position during sleep may be an effective treatment.17–20 Positional OSA patients have been defined as those who have a supine apnea-hypopnea index (AHI) that is at least two times higher than their lateral AHI, and non-positional patients those in whom the supine AHI is less than two times higher than the lateral AHI.18 Obviously, position restriction therapy is less logical for patients with non-positional OSA. The anthropomorphic studies that have been reported regarding patient differences between and risk factors for positional and non-positional OSA are few and conflicting. There are a limited number of studies describing positional apnea as a distinct risk factor for severity of disease or related co-morbidities. Some investigators have reported positional OSA patients to be younger and less obese, with less severe respiratory disturbance than non-positional patients.18,21 Others have found no differences in BMI between these groups, suggesting a cause other than weight gain.22 Although studies have consistently shown less severe respiratory disturbance in positional OSA patients, the anatomical and physiological mechanisms for this phenomenon have not been well explained.
We hypothesized that there may be factors other than age and BMI that influence the differences in sleep disordered breathing between positional and non-positional OSA patients and, if so, the treatment outcomes with MADs would likely be different between these two groups of OSA patients. We further speculate that identifying OSA patients as either positional or non-positional might have important therapeutic implications. The aim of the study was to investigate the treatment outcome of MADs for positional and non-positional OSA using multi-night in-home recordings.
METHODS
Subjects
Seventy-two consecutive OSA patients (51 men and 21 women) were recruited from three dental practices. The study was approved by an institutional review board (BioMed IRB, San Diego, CA, USA). After obtaining an informed consent patients completed a two-night pre-treatment in-home sleep study (described below). The inclusion criteria of the subjects were the patients who successfully completed a sleep study over two nights with a minimum of 4-hours of valid recording time each night, and had AHI ≥ 5.
Patient information including age, gender, height, weight, and their neck size were obtained and Epworth Sleepiness Scale (ESS) for daytime sleepiness was acquired at the time of both sleep studies.
Mandibular advancement devices (MADs)
All appliances used advanced the mandible and were custom made. The three types of MADs used in this study were the TAP II and III (Airway Management, Dallas, TX, USA), and a Modified Herbst appliance (Great Lakes Orthodontics, Ltd., Tonawanda, NY, USA). For the positional patients, 12 were fitted with the TAP II, 16 with TAP III, and 14 with the Modified Herbst appliance. For the non-positional patients, 8 were fitted with the TAP II, 12 with TAP III and 10 with the Herbst appliance. The appliances were made by the respective laboratories and the degree of mandibular advancement was set to 60% of the patient’s maximum protrusion using George Gauge measures taken at the time the impressions were made. After a titration period during which incremental anterior adjustments of the mandible were made until the maximum comfortable limit was reached, an additional sleep study was performed with the MAD to determine treatment efficacy.
Sleep study data
Two-night in-home recordings were performed for each pre- and post-treatment study with Apnea Risk Evaluation System (ARES) Unicorder (Advanced Brain Monitoring, Carlsbad, CA, USA). The ARES Unicorder measures oxygen saturation, pulse rate, airflow, respiratory effort, snoring levels, head movement, and head position from a wireless recorder self applied with a single strap to the forehead. Reflectance oximetry is used to obtain the SpO2 and pulse rate signals. Respiratory effort is derived from the measurement of changes in forehead venous pressure acquired using a combination of photoplethysmography and changes in surface pressure of the reflectance oximetry sensor, and head movement. Airflow is obtained via a nasal cannula and a pressure transducer. A calibrated acoustic microphone is used to acquire quantified snoring levels (dB). Accelerometers are used to measure head movement and derive head position. The recorder was designed to be easily affixed by the patient, and provide alerts during the study if poor quality airflow or SpO2 is detected so the device could be adjusted.
The description and validation of this device has been reported in two studies. The first had 284 valid comparisons of the in-laboratory simultaneous PSG and ARES and 187 valid comparisons of the in-laboratory PSG with a separate two nights unattended self-applied ARES Unicorder.23 The second study with 102 participants had 92 simultaneous in-laboratory comparisons and 86 in-home to in laboratory comparisons. Both studies showed that the ARES had high sensitivity and specificity.24
Automated scoring algorithms were applied off-line to detect sleep disordered breathing. The AHI was computed using a time-in-bed measure based on recording time with acceptable signal quality minus periods when the patient was upright or presumed to be awake based on actigraphy. Automated algorithms were used to detect apnea (based on a 10-s cessation of airflow) and hypopnea events (based on a 50% reduction and recovery in airflow, a minimum 3.5% reduction in SpO2 and at least a 1.0% recovery) for calculation of the apnea-hypopnea index (AHI). After the automated scoring was applied, the full disclosure recordings were visually inspected by a sleep medicine physician to confirm the accuracy of the automated scoring, and to reclassify as central and/or exclude auto-detected events if necessary. The physiological data including percentage of time in supine, total AHI, supine AHI, non-supine AHI values, and percentage time snoring greater than 40 dB were then calculated.
Data Stratification
The patients were divided into two groups as having positional or non-positional OSA following the criteria suggested by Cartwright et al.18 Specifically, Cartwright’s criteria state that positional OSA patients have a supine AHI at least two times higher than their lateral AHI, and non-positional patients have their supine AHI less than two times higher than their lateral AHI. Additionally, we revised these criteria to include two additional rules in order to improve sampling (1) all the patients should have at least 30 minute supine and 30 minutes non-supine positioned sleep, and (2) should have supine AHI more than 5. Forty-two positional OSA patients and 30 non-positional OSA patients were evaluated. Demographic data of the subjects are shown in Table I.
Table I.
Characteristics of study population at baseline
| Variables | Positional OSA (n=42) | Non-positional OSA (n=30) | P-value |
|---|---|---|---|
| Age (years) | 53.9 ± 10.4 | 50.0 ± 9.0 | 0.095 * |
| Gender (% of male) | 83.3 | 53.3 | 0.008 † |
| BMI | 27.6 ± 3.4 | 30.9 ± 5.9 | 0.004 * |
| Neck circumference (inch) | 16.3 ± 1.1 | 16.4 ± 1.5 | 0.758 * |
| Overall AHI | 21.79 ± 12.83 | 18.47 ± 16.24 | 0.378 * |
| Supine AHI | 39.62 ± 20.21 | 24.74 ± 22.78 | 0.006 * |
| Non-supine AHI | 6.15 ± 6.18 | 16.03 ± 16.47 | 0.001 ‡ |
| % time snoring | 22.30 ± 17.12 | 26.53 ± 19.03 | 0.328 * |
| % of time supine | 49.14 ± 23.56 | 46.10 ± 26.63 | 0.662 * |
| ESS | 10.93 ± 4.33 | 11.10 ± 4.90 | 0.876 * |
P-values were obtained from independent T-test
P-values were obtained from Chi-square test
P-values were obtained from Mann-Whitney test
Statistical analysis
The comparison of all measures of apnea severity and the effect of MAD between positional and non-positional OSA groups was performed by t-test for normal variables and Mann Whitney test for non-parametric. Normality of the variables was evaluated with the formal Kolmogorov-Smirnov test. The effect of MAD on AHIs was calculated from the percent changes of AHIs after MAD therapy compared to the pre-treatment AHI. A second measure of treatment efficacy assessed the percentage of positional and non-positional patients with post-treatment AHI < 5. The comparison of the second measure of treatment efficacy was performed by chi-square test.
The percent changes in supine AHI and in non-supine AHI were compared in each OSA group using paired t-test, and the relationships between percent change of overall AHI and the percentage of time in supine were evaluated in each group using Pearson’s correlation test.
To evaluate the relative influence of each independent variable (age, BMI, gender, positional OSA, and percent time of supine position) on overall AHI values, multiple linear regression analyses were performed. The linear regression assumptions of linearity, homoscedasticity and normality of the residuals were successfully evaluated.
The associations between treatment outcome (post-treatment AHI < 5) and each independent variable (age, BMI, gender, positional OSA, and percent time in supine position) were estimated by odds ratios (ORs) using the multiple logistic regression analysis. Each predictive value was stratified into two groups based on gender (men vs. women), group (positional vs. non-positional OSA), the median values for age (53 years), BMI (30 kg/m2), and percentage of time in supine (50%).
Results
Pre-treatment sleep study data
Pre-treatment sleep study data of the subjects are shown in Table I. There were no statistically significant differences in overall AHI, percentage of time in supine position, percentage time with snoring above 40 dB, and ESS between the two groups. Supine AHI was higher in positional OSA patients and non-supine AHI was higher in non-positional OSA patients.
After the titration period, the degree of mandibular advancement was close to 80% of the maximum protrusive distance in most cases and the advancement ranged from 5.6 mm to 9.6 mm. The amounts of the mandibular advancement were not significantly different between the two groups.
Comparison of the effects of MADs between positional and non-positional OSA patients
We performed the statistical analyses to investigate the differences in the effects (decreases and percent changes in overall, supine, and non-supine AHIs after MAD therapy) among three types of MADs used in this study, and confirmed that there were no significant differences in all treatment outcomes among three types of devices (all P values > 0.410 from one-way ANOVA).
Mean and standard deviation of percent change of overall AHI after MADs therapy in total subjects was 62.75 ± 30.17 %. Table II shows the descriptive data of the effects of MADs in each positional and non-positional OSA group. Mean difference and percent change of overall AHI (p=0.040, p<0.001), and mean difference and percent change of supine AHI (p=0.001, p<0.001) after MADs therapy were significantly higher in positional OSA patients than non-positional OSA patients. However, mean difference and percent change of non-supine AHI, percent time of snoring >40dB, and ESS after MADs therapy did not show any statistically significant difference between two groups.
Table II.
Changes in AHIs, percent time snoring, and ESS values after MAD therapy in positional and non-positional OSA patients
| Variables | Positional OSA | Non-positional OSA | P-value † | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Difference * | % change * | Pre | Post | Difference * | % change * | Difference | % change | |
| Overall AHI | 21.79 ± 12.83 | 5.17 ± 4.04 | 16.62 ± 11.55 | 74.69 ± 16.92 | 18.47 ± 16.23 | 7.93 ± 6.77 | 10.53 ± 12.95 | 46.03 ± 36.44 | 0.040 ‡ | <0.001 ‡ |
| Supine AHI | 39.62 ± 20.21 | 8.95 ± 7.55 | 30.67 ± 17.46 | 77.99 ± 14.89 | 24.74 ± 22.78 | 9.67 ± 8.38 | 15.07 ± 17.65 | 50.00 ± 39.14 | 0.001 ‡ | <0.001 § |
| Non-supine AHI | 6.15 ± 6.18 | 2.17 ± 2.46 | 3.98 ± 5.79 | 44.72 ± 81.48 | 16.03 ± 16.47 | 5.83 ± 6.47 | 10.20 ± 12.83 | 51.52 ± 41.46 | 0.058 § | 0.336 § |
| % time snoring | 22.30 ± 17.12 | 9.20 ± 12.16 | 13.10 ± 13.95 | 52.43 ± 64.42 | 26.53 ± 19.03 | 14.67 ±16.88 | 11.86 ± 17.11 | 40.03 ± 63.60 | 0.736 ‡ | 0.522 § |
| ESS | 10.93 ± 4.33 | 4.78 ± 4.29 | 5.67 ± 5.72 | 55.60 ± 36.82 | 11.10 ± 4.90 | 5.42 ± 4.26 | 5.55 ± 5.53 | 47.46 ± 36.30 | 0.774 § | 0.355 ‡ |
Means and standard deviations calculated from each subject’s score
Comparison between positional and non-positional OSA patients
P-values were obtained from independent T-test
P-values were obtained from Mann-Whitney test
Marklund et al.14 suggested other criteria for positional dependency of OSA patients. They reported that supine-dependent OSA was defined by a supine AHI ≥ 10, together with a lateral AHI < 10 and nonsupine-dependent OSA was considered in patients with a lateral AHI ≥ 10. When we used the Marklund’s criteria in our subjects, the results were very similar, and percent changes of overall AHI and supine AHI were also significantly higher in supine-dependent OSA than nonsupine-dependent OSA patients.
In the positional OSA group, percent change in supine AHI (77.32 ± 15.27 %) was significantly higher than percent change in non-supine AHI (44.72 ± 81.48 %, p=0.014), conversely, there was no significant differences in the non-positional group. Percent change of overall AHI showed significant correlation with the percentage of time in supine in the positional OSA patients (r=0.327, p=0.034), but not in the non-positional group.
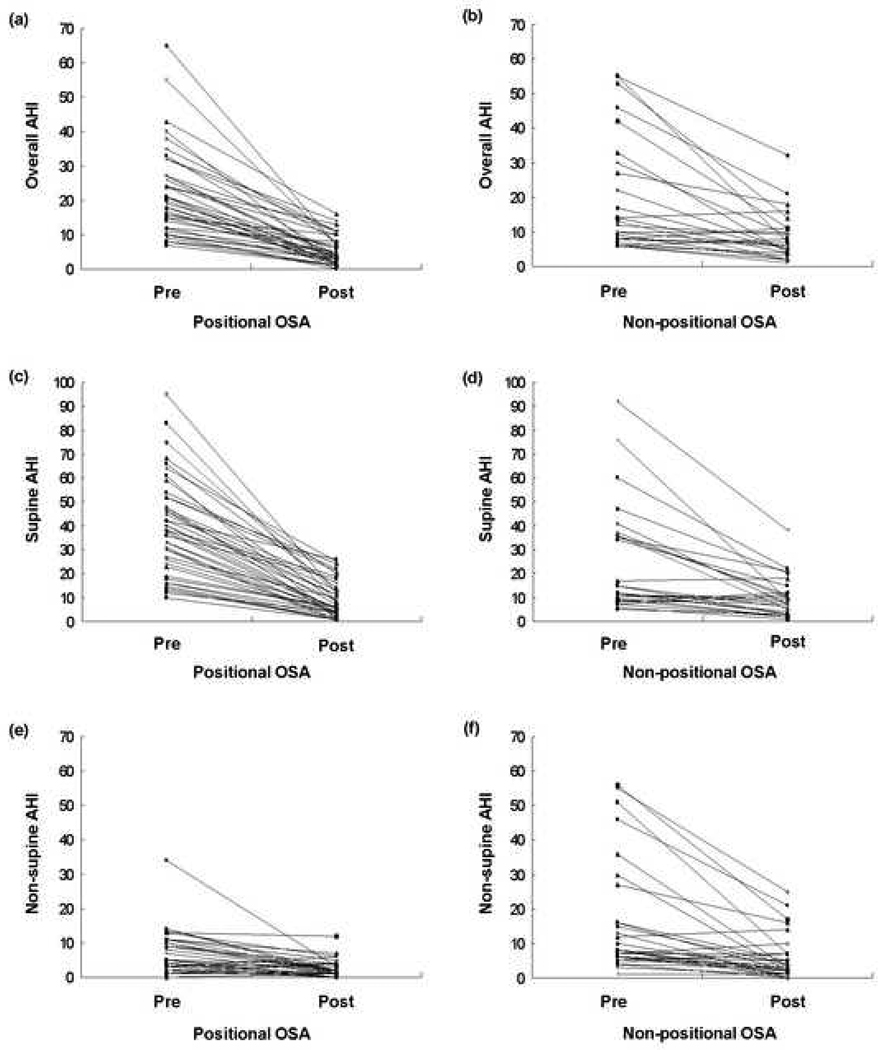
Fig. 1 shows pre- and post-treatment changes of overall AHI, supine AHI, and non-supine AHI in each patient of two groups. Percentages of the patients in whom the effect of MAD on overall AHI were above 50% were 92.9% (39/42) in the positional OSA group and 53.3% (16/30) in the non-positional OSA group (p<0.001).
Fig. 1.
These six graphs show the pre-post MAD changes for positional subjects (left side figures) and non-positional subjects (right side figures). The two top figures (a and b) show the overall AHI data, the middle two figures (c and d) show the supine only AHI data and the bottom two figures (e and f) show the non-supine AHI data.
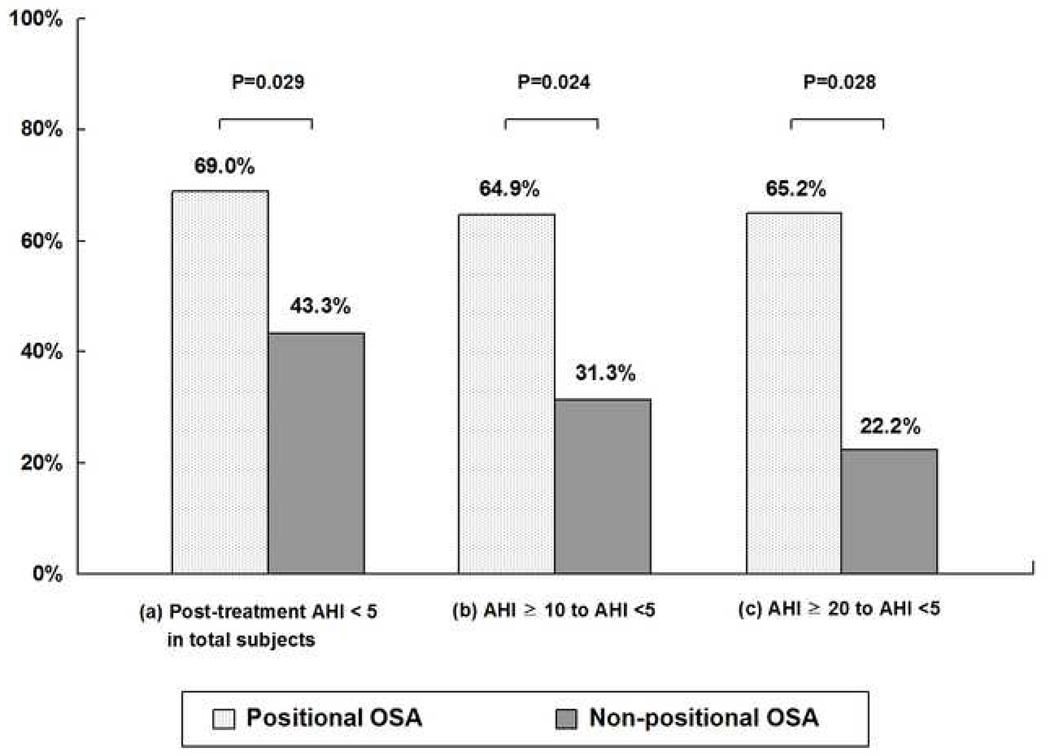
Fig. 2 shows the second measure of treatment efficacy. For overall patients, 69.0% (29/42) of positional patients were successfully treated with residual AHI less than 5 whereas 43.3% (13/30) of non-positional patients showed the same results (p=0.029). For the patients with pre-treatment clinical cut-offs of overall AHI ≥ 10, 64.9% (24/37) of positional patients were successfully treated such that residual AHIs were less than 5, whereas only 31.3% (5/16) of non-positional patients were treated with same level (p=0.024). For the patients with pretreatment overall AHI ≥ 20, 65.2% (15/23) of positional patients and 22.2% (2/9) of non-positional patients were treated with residual AHIs less than 5 (p=0.028). When we analyzed the severe OSA patients who had AHI ≥ 30 at baseline, 77.8% (7/9) of positional patients and 42.9% (3/7) of non-positional patients were treated with residual AHIs less than 10.
Fig. 2.
Treatment efficacy of MAD in positional and non-positional OSA patients.
Percentages of the patients (a) with post-treatment AHI < 5 in total patients (b) with pre-treatment clinical cut-offs of overall AHI ≥ 15 and post-treatment AHI less than 5, (c) with pre-treatment clinical cut-offs of overall AHI ≥ 20 and post-treatment AHI less than 5.
P-values were obtained from chi-square test.
Impacts of risk factors on the effect of MADs on overall AHI
In Table III we present the results of the multiple linear regression analysis. After adjusting for the explanatory variables in the model (age, BMI, gender, % of time in supine), the percent change of overall AHI was significantly associated only with being in the positional OSA group (standardized coefficient = 0.505, coefficient = 30.700, 95% CI = [16.104, 45.296]). Age, BMI, gender, and percent of time in supine did not show any significant associations with the percent change of overall AHI after MADs therapy.
Table III.
Multiple linear regression analysis of the risk factors on the effect of MAD on AHI (percent change of overall AHI)
| Explanatory variables | Standardized coefficient |
Coefficient | 95% CI | P-value |
|---|---|---|---|---|
| Age | −0.152 | −0.459 | −1.158, 0.239 | 0.194 |
| BMI | −0.152 | −0.780 | −2.215, 0.656 | 0.282 |
| Gender (Male) | 0.139 | 9.191 | −6.332, 24.713 | 0.241 |
| Positional OSA | 0.505 | 30.700 | 16.104, 45.296 | <0.001 |
| % of time in supine * | 0.007 | 0.009 | −0.258, 0.277 | 0.946 |
Average value in pre- and post-treatment sleep study
CI, confidence interval
Multivariate ANOVA F-test P=0.001, R2=0.259
Table IV shows the adjusted ORs of the risk factors on the treatment outcome with post-treatment AHI<5. Positional OSA patients were 4.1 times more likely to have successful treatment outcome with AHI<5 after MAD therapy than non-positional OSA patients (95% CI= 1.126–15.158). The ORs of other variables (age, gender, BMI, and % of time in supine) were not statistically significant.
Table IV.
Adjusted ORs of the risk factors on the effect of MAD on AHI (post-treatment AHI<5)
| Explanatory variables | Adjusted OR | 95% CI | P-value |
|---|---|---|---|
| Age (≥53 yrs) | 0.337 | 0.093, 1.220 | 0.098 |
| BMI (≥30) | 0.434 | 0.128, 1.465 | 0.179 |
| Gender (Male) | 2.758 | 0.678, 11.216 | 0.156 |
| Positional OSA | 4.132 | 1.126, 15.158 | 0.032 |
| % of time in supine * (≥50%) | 1.041 | 0.373, 2.908 | 0.939 |
Average value in pre- and post-treatment sleep study
CI, confidence interval
Model χ2=9.54, df=5, P=0.089, adjusted R2=0.167
DISCUSSION
Most of the previous studies assessing treatment outcomes with MADs focused on changes in the overall severity of obstructive sleep apnea and have not examined defined subgroups of OSA patients (e.g positional versus non-positional OSA patients). To date, MAD therapy has only been recommended as first line therapy in mild and moderated OSA patients. In our clinical experience, it is not uncommon for some patients with more severe OSA to have efficacious outcomes, but to date we are unable to predict when this might occur. This is one of the first studies to investigate the contribution of positional vs. non-positional OSA in predicting successful MAD outcomes, independent of OSA severity level.
In this study we found that patients with positional OSA had substantially better treatment outcomes than patients with non-positional OSA. When we used Marklund’s criteria, the results were very similar and supine-dependent OSA patients also showed better treatment efficacy than nonsupine-dependent OSA patients. Moreover, this effect cannot be explained by differences in OSA severity, as there were no significant differences in the overall AHI in the two groups at baseline. Because the BMI and percentage of females were significantly different between the two groups, we elected to perform the multiple linear regression analyses to evaluate the relative influence of each independent variable (age, BMI, gender, positional OSA, and percent time of supine position) on the effect of MAD therapy, and the percent change of overall AHI after MAD therapy was significantly associated only with being in the positional OSA group. These findings begin to make sense if we hypothesize the non-positional OSA group has a substantially more collapsible airway than our positional OSA group. We found that positional patients had significantly greater percentage changes in the supine AHI as a result of MAD therapy as compared to the non-positional group. Interestingly, in our positional OSA patients, MAD efficacy increased as the percentage of time in supine increased, and the percent reduction in supine AHI was significantly greater than the percent change in non-supine AHI. The lack of change in the lateral AHI may be due to a “floor effect” since the pretreatment lateral AHI in our positional group was low to begin with so less subject to change. A previous cephalometric study reported that positional OSA patients were found to have a larger posterior airway space, less elongated soft palate and somewhat more prominent retrognathia.25–26 More recently, pharyngeal magnetic resonance imaging and cephalometric radiography obtained during wakefulness found that positional OSA patients have wider airways in the lateral parts, lower facial height, and more backward position of the lower jaw, which may explain differences in the maintenance of pharyngeal airway patency in the lateral sleep position.27 Regardless of these putative anatomic changes, the ultimate determinant of the effectiveness of MADs therapy may be the degree of upper airway collapsibility during sleep. Since MADs are designed to protrude the mandible and thus the tongue and epiglottis during sleep, we were surprised to find the effects of gravity on the pharyngeal airway were not equally resolved by MAD therapy in our positional and non-positional OSA patients. Previously, Levendowski et al.16 found that BMI and neck circumference were predictors of patients who had less efficacious outcomes with MADs. Oksenberg et al.21 found that the positional OSA patients were younger and less obese than non-positional OSA patients. They speculated that age and BMI might explain the difference between the two groups and they also suggested that the two conditions (positional and non-positional OSA) are part of the same disorder with the difference being a progression in severity. However, in our subjects, there was no significant difference in age, neck circumference, and overall AHI severity between positional and non-positional OSA groups. And multiple linear regression analysis showed that the effect of MADs was significantly associated with being in the positional OSA group whereas age, BMI, gender, and percent of time in supine did not show any significant associations.
MAD therapy has been recommended and considered for the patient who has mild to moderate, but not severe OSA.10–12 However, our study showed that the positional OSA subjects who had severe degrees of AHIs also showed efficacious outcomes after MAD therapy.
Three limitations should be mentioned. First, the patients were treated at a limited number of dental sleep medicine practices so there were several dental therapists involved. Our perspective is that this makes our study more, not less generalized to private practice treatment of OSA patients. Of course a larger, multi-site study may be required to confirm these results. Second, we used three types of MADs in this study because we would suggest that using three different appliances adds to the value of the paper in that it gives the results more generalizability to the community of practitioners who might want to apply our results to their practice. Even though the mechanism of action of these devices is similar i.e. advancement of the mandible, we should consider that having three different appliance designs can be a possible confounder. However, we performed the statistical analyses to investigate the differences in the effects among the three devices, and confirmed that there were no significant differences in all treatment outcomes among three types of devices. Moreover, the distribution of three types of MADs was almost the same between positional and non-positional groups in this study. Third, we did not set the same amount of the mandibular advancement for each patient and the differences in the amounts of advancement can have an effect on the treatment outcome of MADs. However, we can state that the amounts of the mandibular advancement were not significantly different between positional and non-positional groups and did not influence the final results of our study.
In summary, positional OSA is based on a ratio of the supine AHI divided by non-supine AHI > 2.0, and it is important to know if the patient was classified to positional or non-positional group in order to predict the treatment outcome of MADs. We have shown that MADs are more effective in positional OSA patients than non-positional OSA patients. We speculate that the non-positional group may have an altered or more inherent pharyngeal airway collapsibility which distinctly worsens with obesity. Possibly, other factors beyond age and BMI are contributory to OSA in these subjects. Additional studies, such as evaluation of dynamic pharyngeal airway collapsibility, will be required to identify factors contributing to the differences in positional and non-positional OSA patients.
Acknowledgements
Dr. Jin Woo Chung was partially supported by the Overseas Research Program of Seoul National University Dental Hospital. Dr. Enciso was partially supported by NIDCR grant # K25DE016391. Mr. Levendowski, Dr. Westbrook, and Dr. Morgan were partially funded by NIDCR grant R44DE016772.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any financial conflict of interest.
REFERENCES
- 1.Arias MA, Sánchez AM. Obstructive sleep apnea and its relationship to cardiac arrhythmias. J Cardiovasc Electrophysiol. 2007;18:1006–1014. doi: 10.1111/j.1540-8167.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 2.Koskenvuo M, Kaprio J, Partinen M, Langinvainio H, Sarna S, Heikkilä K. Snoring as a risk factor for hypertension and angina pectoris. Lancet. 1985;1:893–895. doi: 10.1016/s0140-6736(85)91672-1. [DOI] [PubMed] [Google Scholar]
- 3.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkilä K, Sarna S. Snoring as a risk factor for ischemic heart disease and stroke in men. Br Med J. 1987;294:16–19. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton PG, Dunn EV. Snoring as a risk factor for disease: an epidemiological survey. Br Med J. 1985;291:630–632. doi: 10.1136/bmj.291.6496.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block AJ, Boysen PG, Wynne JW, Hunt LA. Sleep apnea, hypopnea and oxygen desaturation in normal subjects: a strong male predominance. N Engl J Med. 1979;300:513–517. doi: 10.1056/NEJM197903083001001. [DOI] [PubMed] [Google Scholar]
- 6.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–514. [PubMed] [Google Scholar]
- 7.Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J. 1996;9:117–124. doi: 10.1183/09031936.96.09010117. [DOI] [PubMed] [Google Scholar]
- 8.Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109:1477–1483. doi: 10.1378/chest.109.6.1477. [DOI] [PubMed] [Google Scholar]
- 9.Clark GT, Sohn JW, Hong CN. Treating obstructive sleep apnea and snoring: assessment of an anterior mandibular positioning device. J Am Dent Assoc. 2000;131:765–771. doi: 10.14219/jada.archive.2000.0275. [DOI] [PubMed] [Google Scholar]
- 10.Eveloff SE, Rosenberg CL, Carlisle CC, Millman RP. Efficacy of a Herbst mandibular advancement device in obstructive sleep apnea. Am J Respir Crit Care Med. 1994;149:905–909. doi: 10.1164/ajrccm.149.4.8143054. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson KA, Ono T, Lowe AA, Al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnea. Thorax. 1997;52:362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancer J, Al-Faift S, Al-Faift M, Hoffstein V. Evaluation of variable mandibular advancement appliance for treatment of snoring and sleep apnea. Chest. 1999;116:1511–1518. doi: 10.1378/chest.116.6.1511. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K. Influence of sleep posture on response to oral appliance therapy for sleep apnea syndrome. Sleep. 2001;24:538–544. doi: 10.1093/sleep/24.5.538. [DOI] [PubMed] [Google Scholar]
- 14.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–1635. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 15.Marklund M, Steunlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–1278. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 16.Levendowski DJ, Morgan TD, Patrickus JE, Westbrook PR, Berka C, Zavora T, Popovic D. In-home evaluation of efficacy and titration of a mandibular advancement device for obstructive sleep apnea. Sleep Breath. 2007;11:139–147. doi: 10.1007/s11325-006-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd SR, Cartwright RD. Physiologic basis of therapy for sleep apnea. Am Rev Respir Dis. 1987;136:525–526. doi: 10.1164/ajrccm/136.2.525b. [DOI] [PubMed] [Google Scholar]
- 18.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 19.Cartwright RD, Diaz F, Lloyd S. The effects of sleep posture and sleep stage on apnea frequency. Sleep. 1991;14:351–353. doi: 10.1093/sleep/14.4.351. [DOI] [PubMed] [Google Scholar]
- 20.Nakano H, Ikeda T, Hayashi M, Oshima E, Onizuka A. Effects of body position on snoring in apneic and nonapneic snorers. Sleep. 2003;26:169–172. doi: 10.1093/sleep/26.2.169. [DOI] [PubMed] [Google Scholar]
- 21.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 22.Pevernagie DA, Shepard JW. Relations between sleep stage, posture and effective nasal CPAP levels in OSA. Sleep. 1992;15:162–167. doi: 10.1093/sleep/15.2.162. [DOI] [PubMed] [Google Scholar]
- 23.Westbrook PR, Levendowski DJ, Cvetinovic M, Zavora T, Velimirovic V, Henninger D, Nicholson D. Description and validation of the Apnea Risk Evaluation System: A novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128:2166–2175. doi: 10.1378/chest.128.4.2166. [DOI] [PubMed] [Google Scholar]
- 24.Ayappa I, Norman RG, Seelall V, Rapoport DM. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4:26–37. [PMC free article] [PubMed] [Google Scholar]
- 25.Fleetham JA. Upper airway imaging in relation to obstructive sleep apnea. Clin Chest Med. 1992;3:399–416. [PubMed] [Google Scholar]
- 26.Hudgel DW. The role of upper airway anatomy and physiology in obstructive sleep apnea. Clin Chest Med. 1992;3:383–398. [PubMed] [Google Scholar]
- 27.Saigusa H, Suzuki M, Higurashi N, Kodera K. Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology. 2009;110:885–890. doi: 10.1097/ALN.0b013e31819b5d57. [DOI] [PubMed] [Google Scholar]