Abstract
In this work, cassava starch was modified by treatment with sodium hypochlorite (NaClO) at different concentrations (0.8, 2.0 and 5.0 % of active chlorine) and selected physicochemical properties of the oxidized starches were investigated. The native and modified samples were evaluated considering moisture, carboxyl content, apparent viscosity, susceptibility to syneresis, mid-infrared spectroscopy and crystallinity index. The treatment with NaClO resulted in alterations in carboxyl content of the oxidized starches that increased with increasing concentration of the oxidant. Oxidized starches also showed higher susceptibility to syneresis, as assessed by the release of liquid during freezing and thawing. Apparent viscosity analysis showed decrease in peak viscosity of the oxidized starches. X-ray diffractograms showed that the oxidation influenced the extent of cassava starch relative crystallinity found to lie between 34.4 % (native) and 39.9 % (2.0 % active chlorine). The infrared spectra are sensitive to structural changes on starch macromolecules and presented characteristic peaks as C-O-C of the six carbon glucose ring absorbs at 1,150–1,085 cm−1 and due to axial deformation these bands changed with the crystal structure of the starch samples.
Keywords: Manihot esculenta, Oxidation, Active chlorine, Carboxyl content, Apparent viscosity
Introduction
Starch is a natural polymer made of amylose and amylopectin. It is extracted from several sources as semi-crystalline granules with different shapes and diameters (Wurzburg 1989). Starches are of great value for the food industry, but have some limitations in the native form. Some of these constraints are the insolubility in cold water, low stability to freeze-thawing, syneresis that in some cases make difficult their utilization (Takizawa et al. 2004; Silva et al. 2008).
The cassava starch has special technological properties that allow its utilization in many industrial applications. Among these properties are the absence of the typical “cereal flavor” of corn and other cereal starches, its ability of higher swelling degree during cooking, and its lower pasting temperature, if compared again with cereal starches. Its low protein and lipid contents must also be valued contributing to its neutral flavor and white color.
The chemical reactions in the starch granules depend on their large amount of hydroxyl groups that may react with different reagents. These reactions may happen as esterification, etherification, oxidation and acid (Wurzburg 1989) or enzymatic hydrolysis (Sumerly et al. 2003). In general, modifications of cassava starch produce pastes of smooth, creamy and soft texture very important for dairy desserts and yoghurts, soups and salad dressings (Cereda et al. 2003).
Starches from different sources are modified by chemical, physical, enzymatic and combined techniques for producing specific rheological behavior needed by the industry for developing new products as well as for improving traditional products and processes (Cereda et al. 2003; Demiate and Kotovicz 2011; Takizawa et al. 2004).
In the case of oxidized starches, they present lower hot paste viscosity due to partial degradation of the macromolecules, lower tendency to syneresis due to the presence of bulk carbonyl and carboxyl groups, and whiter granules as well as more transparent pastes (Cereda et al. 2003).
Starches may be oxidized by many chemicals as sodium hypochlorite (Kuakpetoon and Wang 2006), bromine (Muhrbeck et al. 1990), potassium and ammonia persulfate (Harmon et al. 1971), potassium permanganate (Takizawa et al. 2004) and H2O2 (Demiate et al. 2000).
In the oxidation reaction, the hydroxyl groups of some α- D-glucose units are oxidized to carboxyl groups, in a random way. The glucose rings are disrupted resulting in carboxyl (-COOH) and carbonyl (-C=O) radicals, and at the same time depolymerization occurs (Wurzburg 1989). Depending on the oxidizing agent and on the reaction conditions, acid, aldehyde and ketone functions may appear. The importance of each kind of reaction varies depending on the desired end product’s properties.
The oxidation using sodium hypochlorite is a well known process for starch modification. As related by Dias (2001), the first time this chemical process was used is registered in an industrial patent of Samuel Hall in 1882. The oxidant hypochlorite is the most common method for the production of oxidized starches in an industrial scale. Starch modified by oxidizing agent presents changes in its molecular structure resulting in a raw material with different characteristics. The concentration of sodium hypochlorite employed for the oxidation of starch depends on the desired degree of modification. Kuakpetoon and Wang (2006) oxidized corn starches with NaClO at three concentrations (0.8, 2.0 and 5.0 %). Botanical origin of native starch, the type of oxidizing agent and the reaction conditions influences the changes on the structural and physicochemical properties of the modified starch. The level of chlorine will result in a bleached or an oxidized starch. For producing bleached starches the chlorine concentration must be low and the starch carboxyl contents will be lower than 0.1 %. Starches treated with higher concentrations of chlorine are classified as oxidized and present more than 0.1 % of carboxyl groups (Taggart 2004).
The objective of this work was to modify cassava starch using low, medium and high concentrations of sodium hypochlorite (0.8, 2.0 and 5.0 %) and evaluate selected physicochemical properties of the modified starches.
Materials and methods
Starch and reagents
The cassava native starch used in this work was received from a processing industry of Paraná State (Brazil). The sodium hypochlorite with around 11.5 % of active chlorine was analyzed previously to use for knowing the exact chlorine level. The sodium hypochlorite was properly diluted and a quote of 10 mL was transferred to an Erlenmeyer, added of 30 mL of potassium iodide (10 %, w/v) and 30 mL of acetic acid 1:2 (v/v). This solution was titrated with 0.1 M sodium thiosulphate to a light yellow color, when five drops of a 0.5 % (w/v) starch solution were added an the titration followed until the solution became colorless. Active chlorine content was calculated by using the Eq. 1 (ABNT 2004).
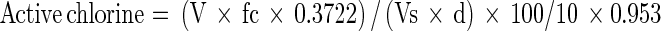 |
where:
V=0.1 M thiosulphate solution volume (mL)
fc=correction factor of the 0.1 M thiosulphate solution
Vs=Volume of the sample in mL
d=density of the sample in g L−1
Commercial grade sodium hypochlorite solution (bleach) presents around 2.0 to 2.5 % of active chlorine and is commercialized in supermarkets, but the product used in the present paper was a concentrated solution bought from a specialized dealer.
All other chemicals were of reagent grade. The moisture content of the starch samples was gravimetrically measured after oven drying at 105 °C until reaching constant mass. The analysis was made in triplicate.
Oxidized starch preparation
The oxidized starch samples were produced as described by Kuakpetoon and Wang (2006) with few modifications. The 40.0 % native starch slurry was made with deionized water and kept at 35 °C. The pH was adjusted to 9.5 with 2.0 M NaOH and different amounts of concentrate sodium hypochlorite solution were added slowly with a burette, under stirring for 30 min, until reaching different final concentrations (0.8, 2.0 and 5.0 g of active chlorine for 100 g of starch). During the reaction the pH was kept at 9.5 by adding 2.0 M H2SO4 solution. After that, sodium metabisulphite was added to stop the reaction and the pH was brought to 7.0 by adding 1.0 M H2SO4 solution. The starch was recovered by filtration, in qualitative paper being washed several times with distilled water until being free of chlorine (qualitative test with AgNO3) and dried at 45 °C/48 h in air circulating oven.
Carboxyl group content
The carboxyl content was evaluated by the method described by Lawal et al. (2005) with modifications. Around 2 g of modified starch was dispersed in 25 mL of 0.1 M HCl for 30 min. After filtration and chlorine elimination, the starch slurry was quantitatively transferred to an Erlenmeyer flask and heated to boiling with continuous stirring to be gelatinized and then was titrated with a standardized 0.01 M NaOH solution to pH 8.3. A blank was made using the native starch. The analysis was made in triplicate.
Apparent viscosity (expressed as torque) of the starch pastes
The viscographic pattern of the starches (heating, cooking and cooling under stirring) was evaluated by using a Brookfield® RVDV II+PRO rotary viscometer (Middleboro, MA, USA) with maximum torque of 7,187.10−4 N-m, equipped with a SC4-13RPY Small Sample Adaptor and a MVS1Y-Flag Impeller Spindle, attached to a Brookfield TC-112P water bath (Middleboro, MA, USA) (Fig. 1), a method adapted from Anderson and Naujoks (1994). This is a low cost alternative to the Brabender viscoamylograph (Duisburg, Germany) and also to the RVA amylograph (Newport Scientific Pty. Ltd., Warriewood, Australia), that are the standard equipments for evaluating starch paste properties (Steffe et al. 1989). Starch slurries with 6.67, 10.0 and 20.0 % (m/m, dry weight) were analyzed, for the modified starches, oxidized with 0.8, 2.0 and 5.0 % of active chlorine, respectively. The native starch was analyzed in the same concentration as the starch oxidized with 0.8 % of chlorine. The starch slurries were stirred at 160 rpm kept at 50 °C until equilibrium and heated as fast as possible to 95 °C (approximately 4 °C/min), staying at this temperature for 7 min. After this period, the starch slurry was cooled as fast as possible to 49 °C (approximately 4 °C/min) and kept at this temperature for 2 min. The starch paste properties were evaluated employing the Brokfield’s software Rheocalc® (Zortéa et al. 2011) and the consistency (apparent viscosity) expressed as percentage of the maximum torque of the viscometer. Although in the present paper the results of apparent viscosity are expressed as torque they are proportional and interchangeable with the RVA-4 data (Demiate and Kotovicz 2011; Zortéa et al. 2011).
Fig. 1.
Brookfield rotary viscometer (a) and small sample adaptor with flag impeller spindle (b) employed for evaluating the cooking profile of the starch samples
Syneresis
The starches were dispersed in deionized water at 10.0 % (w/w) and the slurries boiled for 10 min. A starch slurry with 30.0 % of modified starch was also prepared for making it possible to analyze the starch oxidized with the higher level of chlorine, i.e., 5.0 %. When this modified starch sample was analyzed at the same condition of the others, it produced an extremely fluid solution and even after thawing that did not allow filtration with liquid recovery as all the solution passed through the paper filter. The pastes produced after boiling were divided in three portions and frozen at −18 °C in hermetic plastic bags, being submitted to three freeze-thaw cycles. Each freeze cycle took 72 h. The amount of liquid that came out of the pastes under vacuum filtration (−690 mmHg) was weighed and the results were registered as water percentage in relation to initial mass (Karim et al. 2000). The analysis was made in triplicate.
Mid-infrared spectroscopy (mid-FTIR) and chemometric analysis
The spectroscopic analysis of the modified starches was made using a Shimadzu 8400 FTIR spectrophotometer (Kyoto, Japan), with 4 cm−1 resolution. The spectra were collected by using KBr pellets made with a homogenous mixture of 2 mg of starch (dry basis) and 100 mg of dry KBr. The absorbance and transmittance spectra were collected in the region 4,000–400 cm−1. A pure KBr pellet was used for the background. The chemometric analysis of the mid-FTIR spectra was made by using the software Pirouette v. 3.11 (Infometrix, Bothell, WA, USA) and the principal component analysis (PCA) was carried out.
X-ray diffractometry
X-ray diffractometry was carried out with a Siemens D-5,000 X-ray diffractometer (Munich, Germany) under the following conditions: X-ray tube Cu Kα (λ = 1.544 Å); voltage 40 kV; current 20 mA. The crystallinity index (CI) was estimated quantitatively, where the CI was defined as the ratio between the crystalline area and the total area covered by the curve, consisting of crystalline area and amorphous area of the region, according to the Eq. 1:
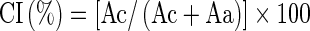 |
1 |
Where: Ac=crystalline area; Aa=amorphous area in the diffractogram.
The crystallinity index (CI) was calculated as proposed by Hayakawa et al. (1997) and employed by Rocha et al. (2008).
Statistical analysis
Statistical analysis (ANOVA and Tukey test, at 5 % significance level) was carried out only for the carboxyl content of the starches due to the fact that for other measurements it was necessary to change starch concentrations for obtaining experimental results and the results should be compared qualitatively.
Results and discussion
The oxidative treatment was carried out as already described and the chlorine concentration in starch slurries may not exceed levels of 5.0 to 6.0 %. The oxidative media must be alkaline due to the fact that acidic or neutral conditions and high temperatures accelerate conversion of hypochlorite to chlorate that is not efficient in promoting oxidation (Wurzburg 2006).
The yield of recovering the oxidized starches decreased from 80.7 % to 76.7 % with increasing concentration of chlorine from 0.8 % to 5.0 % (Table 1). The decreasing yields are probably related with partial solubilization of the starch granules caused by the oxidative reaction. These results are in accordance with those reported by Forssell et al. (1995) that found decreasing starch recovery (yield) in the oxidation of barley and potato starches when the chlorine concentration increased (1.0 to 4.0 % of chlorine). The effect of oxidation includes depolymerization and weakening of the starch granular structure, what makes the starch more soluble (Lawal et al. 2005).
Table 1.
Yield and selected physicochemical characteristics of cassava starches
| Starch/Chlorine % | Yield | Moisture±SD | Carboxyl content±SD* |
|---|---|---|---|
| % | |||
| Native | --- | 12.6 ± 0.22 | 0a |
| 0.8 | 80.7 | 13.1 ± 0.85 | 0.07b ± 0.002 |
| 2.0 | 81.2 | 11.5 ± 0.98 | 0.44c ± 0.006 |
| 5.0 | 76.7 | 11.6 ± 0.99 | 1.08d ± 0.017 |
*For carboxyl content column, different letters mean statistical difference (p < 0.05)
The moisture contents of the native and modified cassava starches were lower than 14.0 % (Table 1), being a safe moisture according to the Brazilian legislation (Brasil 2005).
The carboxyl group contents of the oxidized cassava starches are shown in Table 1. It is possible to note the carboxyl group content increased with the concentration of NaClO (p < 0.05), in accordance with the results of other papers for oxidized potato, corn and rice starches (Kuakpetoon and Wang 2001; 2006; Martinez-Bustos et al. 2007), regular and waxy corn starches (Wang and Wang 2003) and of banana starch (Sánchez-Rivera et al. 2005).
As cited by Taggart (2004), the bleached starches have less than 0.1 % of carboxyl groups, being considered as slightly oxidized. According to this criterion, the starch oxidized with 0.8 % of chlorine may be considered as bleached.
In the present paper, the reaction pH was kept at 9.5 and the reaction time was of 50 min after hypochlorite addition. Sangseethong et al. (2006) found maximum carboxyl content (0.75 to 0.85 %) in the oxidation of cassava starch with NaClO (3.0 % of chlorine/100 g of starch), pH between 8.0 and 9.0 and reaction time between 30 and 60 min. Kuakpetoon and Wang (2006) also reported similar results for corn starch. During the oxidative reaction, the pH decrease may be explained by the formation of carboxyl groups along the starch polysaccharides; an expected partial dissociation of those groups results in a more acidic starch (Lawal et al. 2005).
The pasting properties were also evaluated using a rotary viscometer, considering selected points of the starch cooking process. The results are expressed in torque percentage (100 % torque = 7,187.10−4 N-m) and are shown in the Table 2.
Table 2.
Pasting properties and crystallinity index (CI) of the native and modified starches
| Sample | Torque at viscosity peak (%)a | Temperature of peak (°C) | Pasting temperature (°C) | Torque of the hot paste (%)a | Breakdown (torque %)c | Setback (torque %)b | Torque at 50 °Ca (%) | CI % |
|---|---|---|---|---|---|---|---|---|
| Natived | 50.1 | 86.1 | 70.0 | 36.0 | 26.8 | 11.7 | 35.0 | 34.4 |
| 0.8d | 22.3 | 80.3 | 73.2 | 18.6 | 11.4 | 0.2 | 11.1 | 35.3 |
| 2.0e | 58.4 | 77.2 | 69.0 | 22.2 | 51.1 | 0.5 | 7.8 | 39.9 |
| 5.0f | 29.7 | 67.2 | 67.0 | 0.4 | 29.7 | 0.0 | 0.0 | 37.5 |
aTorque 100 % corresponds to 7,187.10−4 N-m that is roughly 2,000 cP in the RVA viscoamylogram (Demiate and Kotovicz 2011; Zortéa et al. 2011).
bDifference from final torque to through (minimum torque after peak) (Pongsawatmanit and Srijunthongsiri 2008; Zaidul et al. 2007)
cDifference from peak to through (Pongsawatmanit and Srijunthongsiri 2008; Zaidul et al. 2007)
dstarch dispersion at 6.67 %;
estarch dispersion at 10.0 %;
fstarch dispersion at 20.0 %
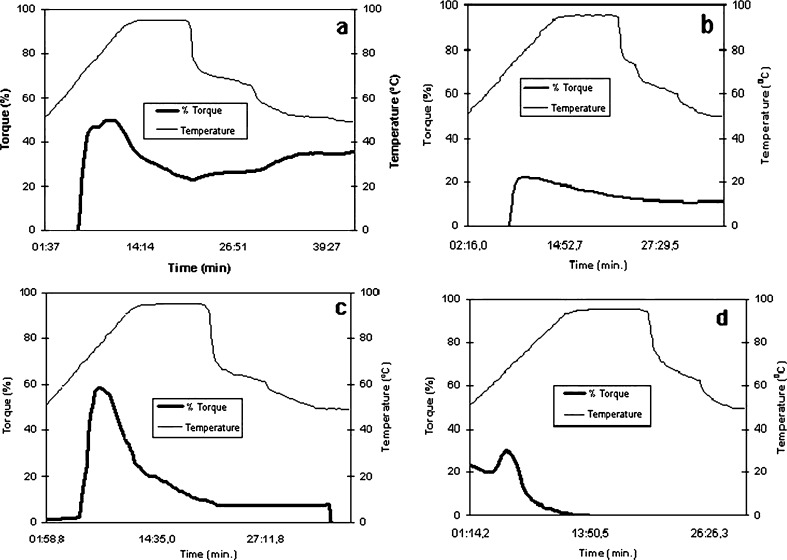
For the native and slightly oxidized (0.8 % of chlorine) starches, the slurries were produced at 6.67 %, whereas for the 2.0 and 5.0 % chlorine oxidized starches the slurries were prepared at 10 and 20 %, respectively. These different concentrations were necessary for making it possible to fit the viscosity range of the employed viscometer that would also happen with the conventional RVA and Brabender viscoamylograph. As the starch slurries were analyzed with different concentrations, it is necessary to look at the results with special attention. It is very important to report that in previous experiments by using the same starch concentration for analyzing all samples of this work resulted in absence of detectable viscosity for the most oxidized starches (2.0 and 5.0 % of chlorine) and, on the other way, when increasing the starch concentration for all samples, the apparent viscosities of the native and the starch oxidized with the lower chlorine concentration (0.8 % of chlorine) were higher than the viscometer torque range making it impossible to have the readings. The oxidizing treatments produce thin boiling starches, with decreased apparent viscosities. By heating starch slurry, the granules swell and leaching of some fractions occurs. There is also granular disintegration and starch viscous pastes are formed (Franco et al. 2001).
In the present paper the oxidized starch with higher carboxyl content (5.0 % chlorine) produced very fluid paste, being necessary to increase the starch slurry concentration to detect any viscosity; another important aspect to be considered is the low final viscosity of the modified starches indicating macromolecular degradation during starch paste cooking that took place in the viscographic analysis (Fig. 2).
Fig. 2.
Viscoamylograms of the starches (a) native (slurry at 6.67 %), (b) 0.8 % (slurry at 6.67 %), (c) 2.0 % (slurry at 10.0 %) and (d) 5.0 % (slurry at 20.0 %)
The apparent viscosities, represented by the torque values, of the oxidized starches decreased as the chlorine concentration increased. For the sample produced by treatment with chlorine at 5.0 % this effect was more pronounced (at 67.2 °C the torque % was of 29.7), for a high concentration starch slurry (20 %). This starch also presented cold swelling, with detectable viscosity (around 20 % of torque) at the beginning of the analysis (50 °C), as shown in the Fig. 2d. These results are in agreement with those of Kuakpetoon and Wang (2006) that found lower pasting temperatures for different corn starches when oxidized, mainly at the concentration of 5.0 % of NaClO, related with structural weakening of the starch granules.
The oxidative reaction promoted by NaClO results in partial glycosidic bonds disruption, lowering molecular weight that is reflected by starch paste viscosity decrease. The presence of bulky carboxyl groups, if compared with hydroxyl groups, weakens the granular structure of starch and contributes to lowering the paste viscosity (Kuakpetoon and Wang 2001; Lawal et al. 2005).
When the starch pastes are cooled, the starch macromolecules tend to re-associate due to hydrogen bonds formed between hydroxyls and as a result, the viscosity increase. In the case of oxidized starch, this effect is restricted due to steric hindrance and also to electrostatic effect of the negative charges that may be present (Wurzburg 1989). As shown in the Fig. 2, the oxidized starches differed from the native, presenting a lower viscosity peak and also lower final viscosity, with absence of retrogradation (Fig. 2a). It is important to explain that using the alternative Brookfield viscometer coupled with a water bath the results are expressed in torque, that is also the way of expressing the Brabender viscoamylograph results, in arbitrary Brabender units (BU) or as torque in cm-g (Suh and Jane 2003). Zortéa et al. (2011) reported similar values for starch samples evaluated both by this Brookfield system and by the Newport Scientific RVA4.
The syneresis tendency of the starches was expressed as liquid liberated from their pastes after three freeze-thaw cycles (Table 3).
Table 3.
Syneresis of starch pastes (%) after the freeze-thaw cycles
| Samples | 1st cyclea (%) | 2nd cyclea (%) | 3rd cyclea (%) |
|---|---|---|---|
| Native (a) | 0.45 ± 0.12 | 0.5 ± 0.07 | 0.7 ± 0.17 |
| Native (b) | 0 | 0.5 ± 0.15 | 0.2 ± 0.05 |
| 0.8 (b) | 0 | 0 | 0.5 ± 0.01 |
| 2.0 (b) | 0 | 1.5 ± 0.01 | 2.0 ± 0.14 |
| 5.0 (c) | b | 0 | 0 |
aaverage value±standard deviation
bResidue very fluid; (a) slurry at 10 %, (b) slurry at 18 % and (c) slurry at 30 % of starch in water
For evaluating the syneresis by freeze-thaw cycles, it was necessary to increase the starch concentration for producing the pastes and comparing the results with the native starch (10 % dry basis). For the starch modified with the treatment with 5.0 %, the starch concentration for producing the paste was 30 % (dry basis) and for the starches treated with both 0.8 and 2.0 %, the concentration was of 18 % (dry basis). The starch modified with the higher concentration of oxidative reactant presented a different behavior.
The pasting of the starch modified with 5.0 % of chlorine (starch slurry with 30 % on dry basis) produced a very fluid paste. During the filtration for recovering the water liberated from the paste after the first freeze-thaw cycle, everything passed through the filter paper. In the next cycles (2nd and 3rd) the pastes neither pass through the filter nor liberate water. This result indicates that the greater the freezing time, the lower retrogradation occurs at this concentration of starch. In the case of previous tests with concentrations of 18 and 10 %, the pastes were very fluid and it was not possible to evaluate the water liberation after three freeze-thaw cycles, because all paste passed through the filter paper (not shown in Table 3). In previous tests, considering the same starch concentration for all samples, native or oxidized, the results were not reached as the oxidized samples produced very fluid pastes. Analyzing the samples with different starch concentrations was an alternative for trying to measure the liquid liberation from the pastes, as this behavior is important for this type of converted starch and differentiate it from the most common acid-modified ones.
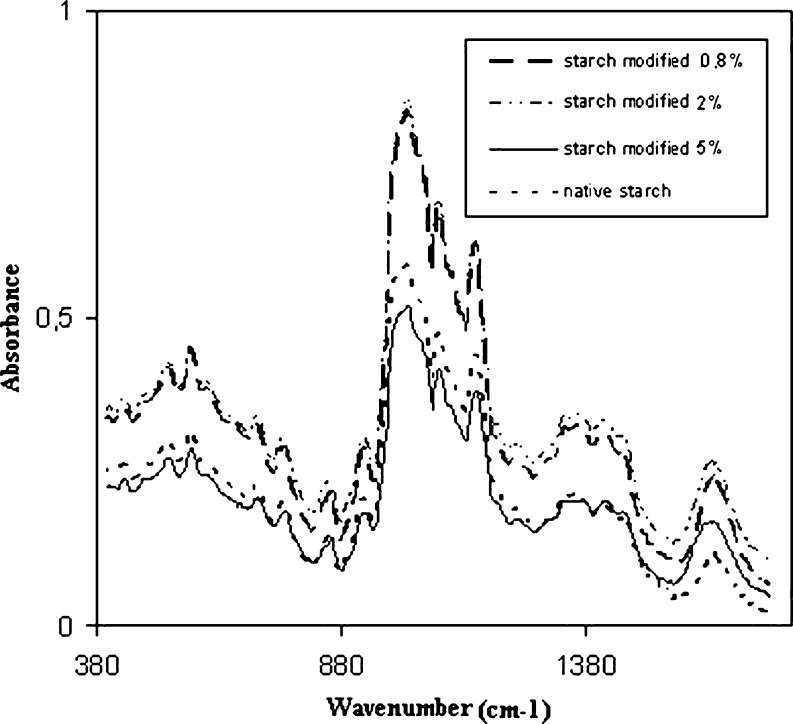
The infrared spectroscopy is sensitive to structural changes on starch macromolecules, including helicoidal chain conformation, crystallinity, retrogradation and water content. The infrared spectra of native starches (potato, wheat, regular or waxy corn) present bands in the region 2,900–3,000 cm−1 (corresponding to C-H stretching), in 1,163, 1,150, 1,124 and 1,103 cm−1, that correspond to C–O and C–C stretching with some contribution of the C-OH stretching. The regions 1,077, 1,067, 1,047, 1,022, 994 and 928 cm−1 are attributed to C-OH and CH2 deformations (Kizil et al. 2002 e van Soest et al. 1995). The C-O-C (ether) present in a six carbon glucose ring absorbs at 1,150–1,085 cm−1 and due to axial deformation (symmetric or asymmetric) these bands will change with the crystal structure of the starch samples. As showed in the Fig. 3, the FTIR spectra of all starch samples exhibit these bands. The oxidation caused structural changes on the starch macromolecules but they are very discrete to be revealed by the FTIR spectroscopy due to overlapping. The region between 800 and 1,500 cm−1 typically shows intense overlapping making the assignment of individual bands difficult (Kizil et al. 2002). These infrared data were explored by using chemometrics and a principal component analysis (PCA) was carried out. The PCA grouped the oxidized samples (data not shown) but it was not possible to relate with any spectral region. Similar results, with oxidized starch grouping, were already published by Takizawa et al. (2004).
Fig. 3.
Infrared spectra of cassava starch
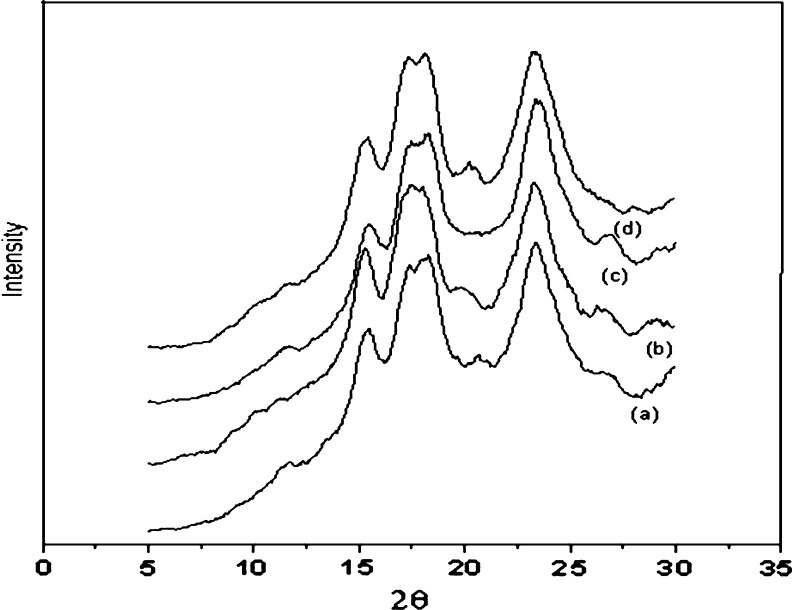
X-ray diffraction studies proved that the modified starches suffered change in crystallinity, as showed in the Fig. 4. Starches tend to present pertinent crystalline arrangements depending on their botanical origin (Cereda and Vilpoux 2003). Native cassava starch had a C-type of X-ray diffraction pattern. Tubers pattern are recognized by the intensity of the corresponding band to one small peak at 5 and 6° (2 θ), peaks at 17° and 18° (2 θ) and at 22 and 24° (2 θ). X-ray diffraction patterns of cassava starch are shown in the Fig. 4. It was possible to observe the major peaks intensities modifications. In addition, after modification, there was a more evident peak at 22 ° (2 θ), suggesting change in crystallinity after the treatment. As already shown the amount of active chlorine also influenced the crystallinity of cassava starch. An increase in the crystallinity index was observed until 2.0 % of chlorine active (34.4 to 39.9 %, native and 2.0 % active chlorine, respectively). It is possible that sodium hypochlorite caused a formation of carboxyl groups in the amorphous part of cassava granules, which resulted in partial reorganization of these regions. The loss of crystallinity observed when more concentrated hypochlorite sodium (5.0 % active chlorine) was used could be caused by depolymerization of crystalline clusters present in amylopectin (Kuakpetoon and Wang 2006). As already shown by Kuakpetoon and Wang (2006), the changes in crystallinity of the starches are not evident if only the diffractograms are observed as pictures.
Fig. 4.
X-ray diffraction powder patterns (a) native starch granules; (b) modified starch 0.8 %; (c) modified starch 2.0 %; (d) modified starch 5.0 %
Conclusion
The physicochemical properties of the oxidized cassava starches were influenced by the presence of carboxyl groups and by the partial degradation resulting from the oxidizing treatment as shown by the rheological analysis. The results were dependent on the chlorine concentrations employed on starch treatment: low, medium or high (0.8, 2.0 and 5.0 %, respectively). The physicochemical properties of the oxidized cassava satarches were different from those of its native counterpart with increased carboxyl content and paste clarity as well as changes in its molecular integrity resulting in more fluid pastes. By analyzing the paste properties in a rotary viscometer the retrogradation was not observed as well as the syneresis. The oxidized starches presented low viscosity peaks as well as low hot viscosity values. The X-ray diffraction analysis showed a tuber pattern, and after oxidizing treatment showed changes in crystallinity index.
Acknowledgements
The authors are grateful to the Brazilian Ministry of Education/Coordination for Higher Education Staff (MEC/CAPES) and to the National Scientific and Technological Research Council (CNPq) for financial support.
References
- Associação Brasileira de Normas Técnicas—ABNT (2004) Solução de hipoclorito de sódio comercial—Determinação do teor de cloro ativo pelo método volumétrico: NBR 9425. ABNT: Rio de Janeiro, pp 1–3
- Anderson K, Naujoks B. The SSB viscometer system: starch applications. Cedar Rapids: Cargill; 1994. pp. 1–3. [Google Scholar]
- Brasil (2005) Resolução RDC ANVISA/MS nº. 263, de 22 de setembro de 2005. Regulamento Técnico para Produtos de Cereais, Amidos, Farinhas e Farelos. Diário Oficial da União, Brasília, DF. Seção 1
- Cereda MP, Vilpoux OF. Tecnologia, usos e potencialidades de tuberosas amiláceas latino americanas. São Paulo: Fundação Cargill; 2003. p. 711. [Google Scholar]
- Cereda MP, Vilpoux O, Demiate IM. Amidos modificados. In: Cereda MP, Vilpoux OF, editors. Tecnologia, usos e potencialidades de tuberosas amiláceas latino americanas. São Paulo: Fundação Cargill; 2003. pp. 246–332. [Google Scholar]
- Demiate IM, Kotovicz V. Cassava starch in the Brazilian food industry. Cienc Tecnol Alim. 2011;31:388–397. doi: 10.1590/S0101-20612011000200017. [DOI] [Google Scholar]
- Demiate IM, Dupuy N, Huvenne JP, Cereda MP, Wosiacki G. Relationship between baking behavior of modified cassava starches and starch chemical structure determined by FTIR spectroscopy. Carbohydr Polym. 2000;42:149–158. doi: 10.1016/S0144-8617(99)00152-6. [DOI] [Google Scholar]
- Dias ARG (2001) Efeito de oxidantes, de ácidos orgânicos e da fração solúvel em água na propriedade de expansão do amido de mandioca fermentado. 2001.pp 183. Tese (Doutorado em Tecnologia de Alimentos)—Universidade Estadual de Campinas, Campinas
- Forssell P, Hamunen A, Autio K, Suortti T, Poutanen K. Oxidation of potato starch by hydrogen peroxide. Starch-Starke. 1995;47:371–377. doi: 10.1002/star.19950471002. [DOI] [Google Scholar]
- Franco CML, Daiuto ER, Demiate IM, Carvalho LJCB, Leonel M, Cereda MP, Vilpoux OF, Sarmento SBS. Propriedades gerais do amido. Campinas: Fundação Cargill; 2001. p. 221. [Google Scholar]
- Harmon RE, Gupta SK, Johnson J. Oxidation of starch catalyzed by persulfate. Starch-Starke. 1971;23:197–199. doi: 10.1002/star.19710230603. [DOI] [Google Scholar]
- Hayakawa K, Tanaka K, Nakamura T, Endo S, Hoshino T. Quality characteristics of waxy hexaploid wheat (Triticum aestivum L.): properties of starch gelatinization and retrogradation. Cereal Chem. 1997;74:576–580. doi: 10.1094/CCHEM.1997.74.5.576. [DOI] [Google Scholar]
- Karim AA, Norziah MH, Seow CC. Methods for the study of starch retrogradation. Food Chem. 2000;71:9–36. doi: 10.1016/S0308-8146(00)00130-8. [DOI] [Google Scholar]
- Kizil R, Irudayaraj J, Seetharaman K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J Agric Food Chem. 2002;50:3912–3918. doi: 10.1021/jf011652p. [DOI] [PubMed] [Google Scholar]
- Kuakpetoon D, Wang YJ. Characterization of different starches oxidized by hypochlorite. Starch/Starke. 2001;53:211–218. doi: 10.1002/1521-379X(200105)53:5<211::AID-STAR211>3.0.CO;2-M. [DOI] [Google Scholar]
- Kuakpetoon D, Wang YJ. Structural characteristics and physicochemical properties of oxidized corn starches varying in amylose content. Carbohydr Res. 2006;341:1896–1915. doi: 10.1016/j.carres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Lawal OS, Adebowale KO, Ogunsanwo BM, Barba LL, Ilo NS. Oxidized and acid thinned starch derivatives of hybrid maize: functional characteristics, wide-angle X-ray diffractometry and thermal properties. Int J Biol Macromol. 2005;35:71–79. doi: 10.1016/j.ijbiomac.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Martinez-bustos F, Amaya-llano SL, Carbajal-arteaga JA, Chang YK, Zazueta-morales JJ. Physicochemical properties of cassava, potato and jicama starches oxidised with organic acids. J Sci Food Agric. 2007;87:1207–1214. doi: 10.1002/jsfa.2805. [DOI] [Google Scholar]
- Muhrbeck P, Eliasson AC, Salomonsson AC. Physical characterization of bromine oxidized potato starch. Starch-Starke. 1990;42:418–420. doi: 10.1002/star.19900421103. [DOI] [Google Scholar]
- Pongsawatmanit R, Srijunthongsiri S. Influence of xanthan gum on rheological properties and freeze–thaw stability of tapioca starch. J Food Eng. 2008;88:137–143. doi: 10.1016/j.jfoodeng.2008.02.009. [DOI] [Google Scholar]
- Rocha TS, Demiate IM, Franco CML. Structural and physicochemical characteristics of Peruvian carrot (Arracacia xanthorrhiza) starch. Cienc Tecnol Alim. 2008;28:620–628. doi: 10.1590/S0101-20612008000300018. [DOI] [Google Scholar]
- Sánchez-Rivera MM, García-Suárez FJL, Velázquez Del Valle M, Guttierrez-Meraz F, Bello-Pérez LA. Partial characterization of banana starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2005;62:50–56. doi: 10.1016/j.carbpol.2005.07.005. [DOI] [Google Scholar]
- Sangseethong K, Lertpanit S, Sriroth K (2006) Hypochlorite oxidation of cassava starch. Starch-Starke Lectures of the 57th Starch Convention. http://www.agfdt.de/ie/downlst.htm. Accessed 22 nov 2008
- Steffe JF, Castell-Perez ME, Rose KJ, Zabik ME. Rapid testing method for characterizing the rheological behavior of gelatinizing corn starch slurries. Cereal Chem. 1989;66:65–68. [Google Scholar]
- Suh DS, Jane J. Comparison of starch pasting properties at various cooking conditions using the micro visco-amylo-graph and the rapid visco analyser. Cereal Chem. 2003;80:745–749. doi: 10.1094/CCHEM.2003.80.6.745. [DOI] [Google Scholar]
- Silva RM, Ferreira GF, Shirai MA, Haas A, Scherer ML, Franco CML, Demiate IM. Características físico-químicas de amidos modificados com permanganato de potássio/ácido lático e hipoclorito de sódio/ácido lático. Cienc Tecnol Alim. 2008;28:66–77. doi: 10.1590/S0101-20612008000100011. [DOI] [Google Scholar]
- Sumerly R, Alvarex H, Cereda MP, Vilpoux O. Hidrólise do amido. In: Cereda MP, Vilpoux OF, editors. Tecnologia, usos e potencialidades de tuberosas amiláceas latino americanas. São Paulo: Fundação Cargill; 2003. pp. 377–395. [Google Scholar]
- Taggart P. Starch as an ingredient: manufacture and applications. In: Eliasson AC, editor. Starch in food: structure, function and applications. Boca Raton: CRC Press; 2004. p. 605. [Google Scholar]
- Takizawa FF, Silva GO, Konkel FE, Demiate IM. Characterization of tropical starches modified with potassium permanganate and lactic acid. Braz Arch Biol Technol. 2004;47:921–931. doi: 10.1590/S1516-89132004000600012. [DOI] [Google Scholar]
- Van Soest JJG, Tournois H, De Wit D, Vliegenthart JFG. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr Res. 1995;279:201–214. doi: 10.1016/0008-6215(95)00270-7. [DOI] [Google Scholar]
- Wang YJ, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2003;52:207–217. doi: 10.1016/S0144-8617(02)003041. [DOI] [Google Scholar]
- Wurzburg OB. Modified starches: properties and uses. Boca Raton: CRC Press; 1989. p. 288. [Google Scholar]
- Wurzburg OB. Modified starches. In: Stephen AM, Phillips GO, Williams PA, editors. Food polysaccharides and their applications. Boca Raton: CRC Press; 2006. pp. 87–118. [Google Scholar]
- Zaidul ISM, Yamauchi H, Kim S-J, Hashimoto N, Noda T. RVA study of mixtures of wheat flour and potato starches with different phosphorus contents. Food Chem. 2007;102:1105–1111. doi: 10.1016/j.foodchem.2006.06.056. [DOI] [Google Scholar]
- Zortéa MEB, Demiate IM, Praxedes MA, Wosiacki G. Avaliação da viscosidade aparente de pastas de amidos nos viscosímetros Brookfield RVDV-II+PRO e rápido visco-analisador RVA-4. Rev Bras Tecnol Agroind. 2011;5:326–335. [Google Scholar]