Abstract
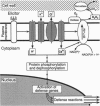
We have used suspension-cultured parsley cells (Petroselinum crispum) and an oligopeptide elicitor derived from a surface glycoprotein of the phytopathogenic fungus Phytophthora megasperma f.sp. glycinea to study the signaling pathway from elicitor recognition to defense gene activation. Immediately after specific binding of the elicitor by a receptor in the plasma membrane, large and transient increases in several inorganic ion fluxes (Ca2+, H+, K+, Cl-) and H2O2 formation are the first detectable plant cell responses. These are rapidly followed by transient changes in the phosphorylation status of various proteins and by the activation of numerous defense-related genes, concomitant with the inactivation of several other, non-defense-related genes. A great diversity of cis-acting elements and trans-acting factors appears to be involved in elicitor-mediated gene regulation, similar to the apparently complex nature of the signal transduced intracellularly. With few exceptions, all individual defense responses analyzed in fungus-infected parsley leaves have been found to be closely mimicked in elicitor-treated, cultured parsley cells, thus validating the use of the elicitor/cell culture system as a valuable model system for these types of study.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appert C., Logemann E., Hahlbrock K., Schmid J., Amrhein N. Structural and catalytic properties of the four phenylalanine ammonia-lyase isoenzymes from parsley (Petroselinum crispum Nym.). Eur J Biochem. 1994 Oct 1;225(1):491–499. doi: 10.1111/j.1432-1033.1994.00491.x. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Douglas C., Hoffmann H., Schulz W., Hahlbrock K. Structure and elicitor or u.v.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J. 1987 May;6(5):1189–1195. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldbrügge M., Sprenger M., Dinkelbach M., Yazaki K., Harter K., Weisshaar B. Functional analysis of a light-responsive plant bZIP transcriptional regulator. Plant Cell. 1994 Nov;6(11):1607–1621. doi: 10.1105/tpc.6.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
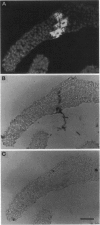
- Gross P., Julius C., Schmelzer E., Hahlbrock K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 1993 May;12(5):1735–1744. doi: 10.1002/j.1460-2075.1993.tb05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Lamb C. J., Purwin C., Ebel J., Fautz E., Schäfer E. Rapid Response of Suspension-cultured Parsley Cells to the Elicitor from Phytophthora megasperma var. sojae: INDUCTION OF THE ENZYMES OF GENERAL PHENYLPROPANOID METABOLISM. Plant Physiol. 1981 Apr;67(4):768–773. doi: 10.1104/pp.67.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H., Franke R., Krause K., Conrath U., Jeblick W., Grimmig B., Matern U. Conditioning of Parsley (Petroselinum crispum L.) Suspension Cells Increases Elicitor-Induced Incorporation of Cell Wall Phenolics. Plant Physiol. 1993 Jun;102(2):459–466. doi: 10.1104/pp.102.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
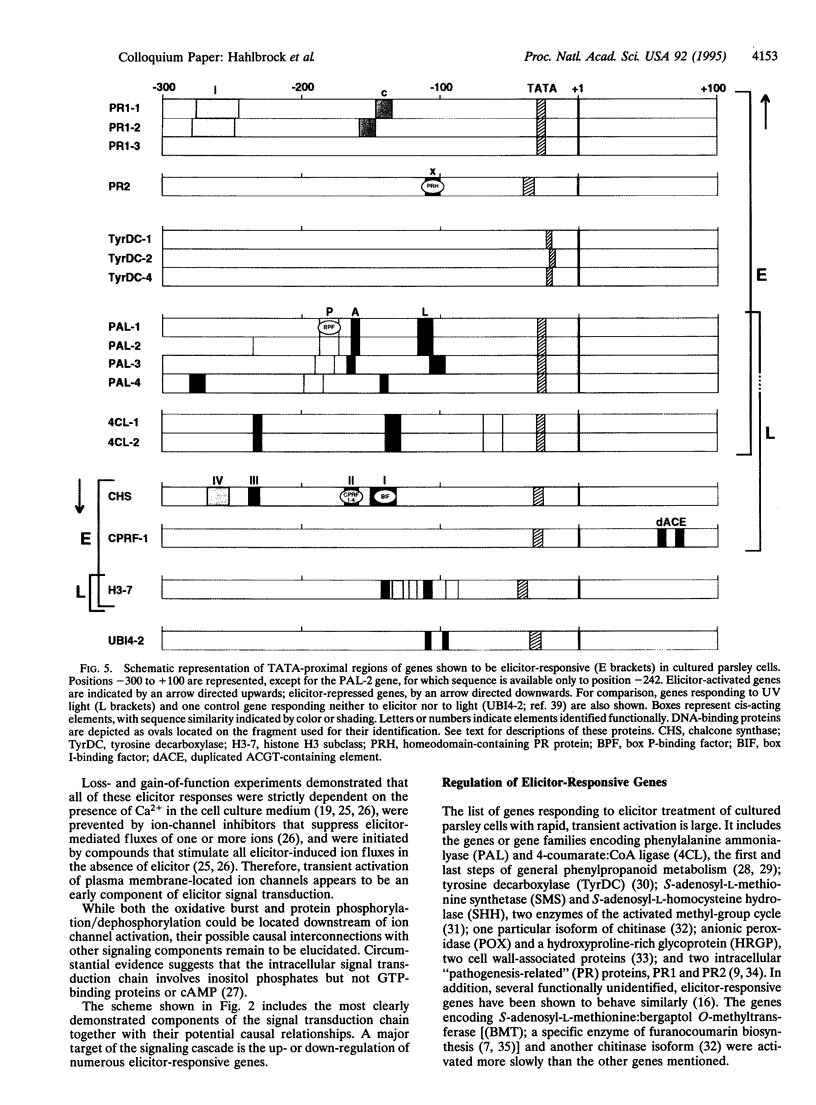
- Kawalleck P., Keller H., Hahlbrock K., Scheel D., Somssich I. E. A pathogen-responsive gene of parsley encodes tyrosine decarboxylase. J Biol Chem. 1993 Jan 25;268(3):2189–2194. [PubMed] [Google Scholar]
- Kawalleck P., Plesch G., Hahlbrock K., Somssich I. E. Induction by fungal elicitor of S-adenosyl-L-methionine synthetase and S-adenosyl-L-homocysteine hydrolase mRNAs in cultured cells and leaves of Petroselinum crispum. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4713–4717. doi: 10.1073/pnas.89.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawalleck P., Somssich I. E., Feldbrügge M., Hahlbrock K., Weisshaar B. Polyubiquitin gene expression and structural properties of the ubi4-2 gene in Petroselinum crispum. Plant Mol Biol. 1993 Feb;21(4):673–684. doi: 10.1007/BF00014550. [DOI] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Schröder M., Hahlbrock K. Several "pathogenesis-related" proteins in potato are 1,3-beta-glucanases and chitinases. Proc Natl Acad Sci U S A. 1988 Feb;85(3):782–786. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfhage U., Trezzini G. F., Meier I., Hahlbrock K., Somssich I. E. Plant homeodomain protein involved in transcriptional regulation of a pathogen defense-related gene. Plant Cell. 1994 May;6(5):695–708. doi: 10.1105/tpc.6.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Baim S. B., Shenk T., Levine A. J. Conversion of the lac repressor into an allosterically regulated transcriptional activator for mammalian cells. Mol Cell Biol. 1990 Jul;10(7):3343–3356. doi: 10.1128/mcb.10.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Hahlbrock K. Differential wound activation of members of the phenylalanine ammonia-lyase and 4-coumarate:CoA ligase gene families in various organs of parsley plants. Z Naturforsch C. 1992 Jan-Feb;47(1-2):90–94. doi: 10.1515/znc-1992-1-216. [DOI] [PubMed] [Google Scholar]
- Lozoya E., Hoffmann H., Douglas C., Schulz W., Scheel D., Hahlbrock K. Primary structures and catalytic properties of isoenzymes encoded by the two 4-coumarate: CoA ligase genes in parsley. Eur J Biochem. 1988 Oct 1;176(3):661–667. doi: 10.1111/j.1432-1033.1988.tb14328.x. [DOI] [PubMed] [Google Scholar]
- Nürnberger T., Colling C., Hahlbrock K., Jabs T., Renelt A., Sacks W. R., Scheel D. Perception and transduction of an elicitor signal in cultured parsley cells. Biochem Soc Symp. 1994;60:173–182. [PubMed] [Google Scholar]
- Nürnberger T., Nennstiel D., Jabs T., Sacks W. R., Hahlbrock K., Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994 Aug 12;78(3):449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Ryals J., Uknes S., Ward E. Systemic Acquired Resistance. Plant Physiol. 1994 Apr;104(4):1109–1112. doi: 10.1104/pp.104.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W., Nürnberger T., Hahlbrock K., Scheel D. Molecular characterization of nucleotide sequences encoding the extracellular glycoprotein elicitor from Phytophthora megasperma. Mol Gen Genet. 1995 Jan 6;246(1):45–55. doi: 10.1007/BF00290132. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Kruger-Lebus S., Hahlbrock K. Temporal and Spatial Patterns of Gene Expression around Sites of Attempted Fungal Infection in Parsley Leaves. Plant Cell. 1989 Oct;1(10):993–1001. doi: 10.1105/tpc.1.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Kawalleck P., Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Mol Gen Genet. 1988 Jul;213(1):93–98. doi: 10.1007/BF00333403. [DOI] [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Ward E. R., Uknes S. J., Williams S. C., Dincher S. S., Wiederhold D. L., Alexander D. C., Ahl-Goy P., Metraux J. P., Ryals J. A. Coordinate Gene Activity in Response to Agents That Induce Systemic Acquired Resistance. Plant Cell. 1991 Oct;3(10):1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Armstrong G. A., Block A., da Costa e Silva O., Hahlbrock K. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 1991 Jul;10(7):1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa e Silva O., Klein L., Schmelzer E., Trezzini G. F., Hahlbrock K. BPF-1, a pathogen-induced DNA-binding protein involved in the plant defense response. Plant J. 1993 Jul;4(1):125–135. doi: 10.1046/j.1365-313x.1993.04010125.x. [DOI] [PubMed] [Google Scholar]
- van de Löcht U., Meier I., Hahlbrock K., Somssich I. E. A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO J. 1990 Sep;9(9):2945–2950. doi: 10.1002/j.1460-2075.1990.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]