Abstract
This work evaluated the effect of carnauba and mineral oil coatings on the bioactive compounds and antioxidant capacity of tomato fruits (cv. “Grandela”). Carnauba and mineral oil coatings were applied on fresh tomatoes at two maturity stages (breaker and pink) over 28 day of storage at 10 °C was evaluated. Bioactive compound and antioxidant activity assays included total phenols, total flavonoids, ascorbic acid (ASA), lycopene, DPPH radical scavenging activity (%RSA), trolox equivalent antioxidant capacity (TEAC) and oxygen radical absorbance capacity assay (ORAC). The total phenolic, flavonoid and lycopene contents were significantly lower for coated fruit than control fruits. However, ascorbic acid content was highest in fruits treated with carnauba, followed by mineral oil coating and control fruits. The ORAC values were highest in breaker tomatoes coated with carnauba wax, followed by mineral oil-coated fruits and controls. No significant differences in ORAC values were observed in pink tomatoes. % RSA and TEAC values were higher for controls than for coated fruit. Edible coatings preserve the overall quality of tomatoes during storage without affecting the nutritional quality of fruit. We found that the physiological response to the coatings is in function of the maturity stage of tomatoes. The information obtained in this study support to use of edible coating as a safe and good alternative to preserve tomato quality, and that the changes of bioactive compounds and antioxidant activity of tomato fruits, was not negatively affected. This approach can be used by producers to preserve tomato quality.
Keywords: Tomato, Edible coatings, Bioactive compounds, Antioxidant capacity
Introduction
The used of edible coating has received more attention in recent years, due to the growing interest for reducing environmental pollution caused by plastics, the need to extend the shelf life of foods, and the increasing demand for healthier and ecological foods (Espino-Díaz et al. 2010). Edible coatings are composed of hydrocolloids (polysaccharides or proteins), hydrophobic compounds (lipids or waxes) or a combination of both (composite coatings) that may enhance the coating properties for optimal handling (Espino-Díaz et al. 2010).
Edible coatings preserve fruit quality by surrounding the product with a modified atmosphere that serves as a partial barrier to gases (such as O2 and CO2), water vapor and aroma compounds, decreasing the respiration and water loss rates of the fruit and preserving texture and flavor (Espino-Díaz et al. 2010; Gonzalez-Aguilar et al. 2010a).
The perishability of tomatoes requires the development of technologies that reduce their postharvest deterioration and extend their shelf life (Gonzalez-Aguilar et al. 2009). Several studies have reported the use of edible coatings for the preservation of fruits and vegetables during storage, and many edible coatings on the market today are used mainly to preserve the quality of tomatoes (Olivas et al. 2008). Some edible coatings, such as chitosan have antibrowning characteristics and can maintain tissue firmness and reduce microbial decay in harvested tomato fruits for extended periods (Liu et al. 2007). Dávila-Aviña et al. (2011) evaluated the effect of carnauba and mineral oil coatings on the postharvest quality of tomato fruits and their results show that respiration rate, color, weight loss and enzyme activity were positively affected by mineral oil coating in two maturity stages, concluding that mineral oil coating could be a good alternative to preserve the quality and extend the postharvest life of tomato fruit.
Tomato fruit (Lycopersicon esculentum Mill.) is one of the most widely consumed produce items throughout the world. Some of its popularity may arise from the growing public awareness of tomato products’ health benefits. The beneficial effects on human health are believed to be due at least partially to the action of antioxidant compounds that reduce oxidative damage in the human body. Tomatoes are rich in health-related compounds, as they are good sources of phenolic and flavonoid compounds, lycopene and ascorbic acid (Frusciante et al. 2007).
The use of edible coatings to extend the shelf-life of fresh fruits is widely recognized; however more research is necessary to address the potential of this technology for improving nutritional qualities in fruit, especially with regard to antioxidant contents (Gonzalez-Aguilar et al. 2010b).
The objective of this study was to evaluate the effect of edible coatings (carnauba and mineral oil) on the bioactive compounds (total phenols, flavonoids, ascorbic acid, and lycopene) and antioxidant capacity (DPPH•, TEAC, and ORAC) of tomato fruits (cv. Grandela) at two maturity stages over 28 day of storage at 10 °C.
Materials and methods
Plant material
Fresh tomato fruits (cv. “Grandela”) were greenhouse-produced in Obregón, Sonora, Northwest México. Upon arrival, the fruits were selected based on their size, weight, color and external appearance. Samples were washed using chlorinated water (200 ppm) for 2 min and then left to dry at room temperature for 1 h. Fruit samples were classified according to their size, uniformity and maturity stage (2 and 4). At stage 2 (i.e., “breaker”), fruits showed less than 10 % green color, while at stage 4 (i.e., “pink”), fruits showed a color other than green on less than 30–60 % of the surface of the whole fruit (Dávila-Aviña et al. 2011).
Edible coatings
Commercial carnauba Stafresh 2505™ (SF 2505) and mineral oil Stafresh 151™ (SF 151) coatings were provided by FMC Foodtech (Riverside, CA).
Treatments
Fruits were divided into two batches based on subjective evaluations. In both batches, fruits classified as being stage 2 and 4 mature were subdivided into three groups (control, mineral oil and carnauba coatings). For each maturity stage, carnauba and mineral oil edible coatings at 1 L/ton were manually applied using ArtexMR brushes S-1110ª (México).
One hundred eighty fruits per maturity stage (60 fruits per treatment) were evaluated and stored at 10 °C. Total phenols and flavonoids, ascorbic acid, lycopene, % RSA, TEAC and ORAC were recorded on Days 0, 5, 10, 15, 21 and 28 of storage.
Bioactive compounds
Total phenols and flavonoids
Hydrophilic extracts were obtained according to the method of Shivashankara et al. (2004) with some modifications. Tomato sample (30 g) was homogenized in 10 mL of 80 % methanol, using an Ultra Turrax®T25 basic homogenizer (IKA Works, Wilmington, NC, USA). The homogenate was sonicated for 30 min in a Branson 2510 ultrasonic cleaner and centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant was collected and the precipitate was extracted again with 5 m L o f 80 % methanol, under the conditions previously described. The two supernatants were mixed and filtered using Whatman filter paper No. 1 and stored at −30 °C until use in determination of the bioactive compound and antioxidant capacity assays. The extraction process was performed in triplicate for each maturity state and sampling day.
Total phenols were measured spectrophotometrically using Folin–Ciocalteu reagent with gallic acid as a standard (Gao et al. 2011). Briefly, 50 μL of tomato extract (0.804 and 0.755 g L−1, for breaker and pink respectively) were added to 3 mL of deionized water plus 250 μL of Folin–Ciocalteu reagent (1 N). After a 5 min reaction time, 750 μL of 20 % Na2CO3 solution was added. The mixture was increased to 5 mL by adding deionized water. The phenols were measured at 760 nm after a 30 min reaction time. The results were reported in mg of gallic acid equivalent (GAE) per 100 g of fresh weight.
Flavonoid content was determined based on the methods described by Zhishen et al. (1999). The extract (1 mL) was mixed with 4 mL of deionized H2O and 300 μL of NaNO2 (5 %) and equilibrated for 5 min. After equilibrium, 300 μL of AlCl3 (10 %) were added. The extracts were rested for 1 min, and then, 2 mL of NaOH (1 M) were added. The volume was increased to 10 mL by adding deionized water while stirring. The absorbance was determined at 415 nm using a UV–vis Varian Cary 50 BIO spectrophotometer. Total flavonoids were expressed on a fresh-weight basis as mg of rutin equivalents/100 g of fresh weight.
Lycopene
Sample preparation for determining carotene content was conducted according to Mejia et al. (1988) with some modifications. Tomato tissue (3 g) was homogenized with 25 mL of tetrahydrofuran (THF) containing 0.01 % butylated hydroxytoluene (BHT). The mixture was centrifuged for 15 min at 10 000 g, and filtered through membrane filter (0.45-μm). The analysis was performed by HPLC (Varian 9012 solvent delivery system, CA, USA) using a Microsorb RP-C18 analytical column (4.6 mm × 100 mm, 3 μm) with a 10-μL loop injector. The mobile phase was acetonitrile: methanol: THF (58:35:7, v/v/v), with isocratic flow at a rate of 1 mL/min−1. Lycopene content was detected by UV–vis at 460 nm. The lycopene concentration was calculated using lycopene as the external standard and expressed as mg lycopene per 100 g of fresh weight.
Ascorbic acid
Three grams of sample was added to 20 mL of a mixture of metaphosphoric acid: glacial acetic: water (30:80:890 w/v/v). The sample was homogenized, and an aliquot of the supernatant was transferred to Eppendorf tubes. The extracts were centrifuged (14,000 rpm, 15 min, at 4 °C) and the supernatant was filtered through a 0.22-μm-pore membrane. Samples were analyzed with an HPLC system (Varian 9012 solvent delivery system, CA, USA) equipped with a variable wavelength UV–vis detector (Varian 9050, CA, USA). Ascorbic acid was analyzed using a water microbondapack-NH2 analytical column (3.9 × 300 mm, 10 μm) with a 10-μL loop injector. The mobile phase was acetronitrile: 0.05 M KH2PO4 (75:25 v/v) at a flow rate of 1.0 mL/min, and the detector wavelength was set at 268 nm (Doner and Hicks 1981). The concentration of ascorbic acid was expressed as mg/100 g fresh tissue.
Antioxidant activity
Oxygen radical absorbance capacity assay (ORACFL)
ORAC values was determined according to Robles-Sánchez et al. (2009). AAPH was used as peroxyl radical generator, fluorescein as a fluorescent probe and Trolox as standard. The reaction mixture contained 100 μL of extract, 1.65 mL of 75-mM phosphate buffer (pH 7), 150 μL of 0.8-M AAPH, and 100 μL of 0.106-μM fluorescein. Phosphate buffer was used as a blank. The samples, phosphate buffer and fluorescein were pre-incubated at 37 °C for 15 min. AAPH was added to initiate the reaction. Fluorescence was measured and recorded every 5 min until it declined to less than 5 % of the initial value. The excitation and emission wavelengths were set at 484 and 515 nm, respectively, and each extract was measured in triplicate. The values were calculated using a regression equation between the trolox concentration and the net area under the fluorescein decay curve, and results were expressed as trolox equivalents (μmol TE)/g of fresh weight.
Trolox equivalent antioxidant capacity (TEAC)
TEAC value was determined according to Miller et al. (1996) and Re et al. (1999). ABTS•+ cation was generated through the interaction of 19.2 mg of ABTS (2′2-azino-bis(3-ethylbenzotriazoline-6-sulfonic acid)) dissolved in 5 mL of HPLC-grade water and 88 μL of potassium persulfate (K2S2O8) (0.0378 g mL−1). It was incubated in the dark at room temperature for 16 h; then 1 mL of ABTS-activated radical was taken, and 88 mL of ethanol was added. The radical was adjusted at an absorbance of 0.7 ± 0.02 at 734 nm. The reaction was initiated by adding 2,970 μL of ABTS•+ and 30 μL of the extract or trolox standard solution in methanol, and absorbance was monitored at 734 nm at 1 and 6 min. The percentage of inhibition was calculated, and results were expressed as μmol of TE/g of fresh weight.
Radical scavenging activity using DPPH method
DPPH was determined according to the Kedare and Singh (2011) technique, with some modifications. The stock solution was prepared by mixing 2.5 mg of DPPH radical with 100 mL of pure methanol. The solution was adjusted at an absorbance of 0.7 ± 0.02 at 515 nm. Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic) was used as a standard and 80 % methanol was used as a blank. DPPH radical (3.9 mL) was placed in a test tube, and 100 μL of the extract (2:8 dilution) were added. The mixture was shaken in a vortex and kept in the dark for 30 min. The absorbance was then read with an UV–vis Varian Cary 50 Bio spectrophotometer at a wavelength of 515 nm. Results were expressed in EC50 (concentration of antioxidant required to reduce the absorbance of the radical by 50 %) in g/mL. Analyses were performed in triplicate for each treatment/day.
Statistical analysis
The data were analyzed as a randomized complete block design using the GLM procedure of the Number Cruncher Statistical System version 6.0 software (NCSS, LLC). Storage time was chosen as the blocked factor to observe the effect of the edible coatings on tomato antioxidants. Differences between treatments were determined using Tukey’s comparisons test. P ≤ 0.05 was considered significant. Each experiment was performed in triplicate.
Results and discussion
Bioactive compounds
Total phenols and flavonoids
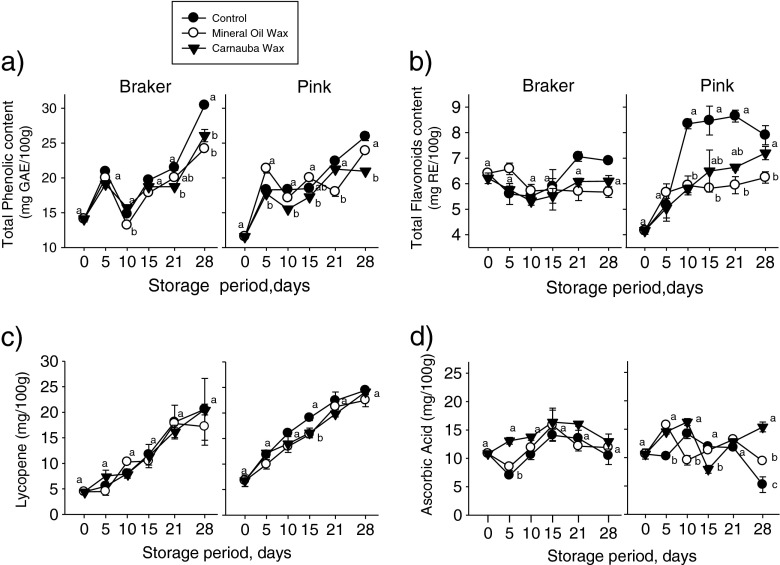
The results describe the overall effect of the use of edible covered in tomato at 28 days of storage. Figure 1a shows the changes in total phenolic content of tomato fruit at two maturity stages (breaker and pink) treated with mineral oil and carnauba coatings over 28 days of storage at 10 °C. Edible coatings had a significant effect (P < 0.05) on the total phenolic content of tomato fruit at both maturity stages. Breaker tomatoes showed significant differences (P < 0.05) among edible coating treatments and control fruits; however, no significant differences (P > 0.05) were observed between waxes. Phenolic contents were 20.22, 18.22, 18.74 (mg GAE/100 g) for control, mineral oil and carnauba, respectively. Pink tomatoes showed significant differences between carnauba and mineral oil treatments and between carnauba and control, with phenolic contents of 19.15, 18.65, 17.37 (mg GAE/100 g) for control, mineral oil and carnauba, respectively. No significant differences were observed between controls and mineral oil treated pink tomatoes (P > 0.05).
Fig. 1.
Changes in bioactive compounds of tomato fruits at two maturity stages treated with mineral wax and carnauba during storage at 10 °C. (n = 4). Different letters represents significant differences (p < 0.05) between treatments and between days. Each observation is a mean ± SD of three replicates (n = 9)
The edible coatings’ effects on total flavonoids were different depending on the maturity stages of treated tomatoes (Fig. 1b). The edible coatings’ effects on the flavonoid content of breaker tomatoes was not significant (P = 0.16); total flavonoid content values were 6.22, 5.97 and 5.83 (mg RE/100 g) for control, mineral oil and carnauba, respectively. However, for pink tomatoes, significant differences (P < 0.05) were observed among controls and treatments, with flavonoid content values of 7.16, 5.62 and 5.88 (mg RE/100 g) for control, mineral oil and carnauba, respectively. No significant differences (P > 0.05) were observed between coating treatment groups; differences were only evident when compared to control.
Edible coatings can produce abiotic stress on produce, modifying its metabolism and affecting the production of such secondary metabolites as phenolic and flavonoid compounds (Gonzalez-Aguilar et al. 2010b). The application of edible coatings to fresh fruit has been associated with an accumulation of phenolic compounds and ascorbic acid, causing an increase in the antioxidant capacity of the fruit (Frusciante et al. 2007). The accumulation of phenolic compounds may be promoted by PAL activity, which is activated under stress conditions. Previous studies showed that low O2 (2.5 KPa) and high CO2 (7 kPa) concentrations increased the production of phenolic compounds during the storage of fresh cut melons, which was related to oxidative stress (Frusciante et al. 2007). In grapes treated with edible chitosan coatings, an increase in the PAL enzyme responsible for synthesizing phenolic compounds was observed (Romanazzi et al. 2002). Phenolic and flavonoid compounds are secondary metabolites in plants with the ability to protect human body tissue against oxidative attacks (Romanazzi et al. 2002). However, even when the use of mineral oil and carnauba edible coatings modifies the metabolism of tomato with positive effects on the organoleptic quality of the fruit (Dávila-Aviña et al. 2011), there is a stress that alters the production of secondary metabolites, as found in this study.
Lycopene
Figure 1c shows the changes in the lycopene content of tomato fruits at two maturity stages treated with mineral oil and carnauba over 28 days of storage at 10 °C. For breaker tomatoes, no significant effect of the treatments on lycopene content was observed (P = 0.61); mean values for control, mineral oil and carnauba were 11.38, 10.77 and 11.35 mg/100, respectively. For pink tomatoes, the edible coatings had a significant effect on lycopene content (P < 0.05); mean values of lycopene were 16.73, 14.92, and 15.47 mg/100 for control, mineral oil and carnauba, respectively. No significant differences were observed between mineral oil and carnauba treatments.
Lycopene is the major carotenoid compound in tomatoes; it gives the fruit its characteristic red color (Frusciante et al. 2007). Different studies have shown that applying edible wax reduces tomato metabolism, thus increasing shelf life. The lycopene content of tomatoes has been previously reported to be in the range of 1.86–14.62 mg per 100 g of fw (Frusciante et al. 2007). Therefore, differences in lycopene content could be attributed to the retardation of the fruit maturity process caused by the combination of temperature of storage and use edible coatings.
Ascorbic acid
Figure 1d shows the changes in the ascorbic acid content of tomato fruit at two maturity stages, treated with mineral oil and carnauba, over 28 days of storage at 10 °C. Significant differences among treatments (P < 0.05) for both maturity stages were observed. For breaker tomatoes, the mean through the storage of ascorbic acid content was 10.87, 11.84 and 13.85 mg/100 fw for control, mineral oil and carnauba, respectively. However, there were no significant differences in ascorbic acid content between control tomatoes and those treated with mineral oil wax. Pink tomatoes showed ascorbic acid values of 10.65, 11.61, and 12.92 mg/100 g fw for control, mineral oil, and carnauba treatments. Significant differences (P < 0.05) were observed among all groups.
The ascorbic acid content of tomatoes has been previously reported in the range of 2.2–21 mg per 100 g of fw (Frusciante et al. 2007). These results are consistent with those found in this study. Ascorbic acid content increases with ripening and storage time; however, once the fruit is fully ripe, the ascorbic acid content starts to decline (Kalt 2005). In this study, however, ascorbic acid content increased more slowly in breaker tomatoes during the storage period. Ali et al. (2010) reported a similar slowing-down of ascorbic acid increase during ripening. They concluded that this slower increase in ascorbic acid in coated fruit suggests that the coating slowed down, but did not prevent the synthesis of ascorbic acid during ripening. On the other hand, the ascorbic acid content of pink tomatoes decreased during the storage period. Similar results were reported by Tigist et al. (2011) who found that a general trend of increase in ascorbic acid content, followed by a fall during the full ripening stage.
Antioxidant capacity
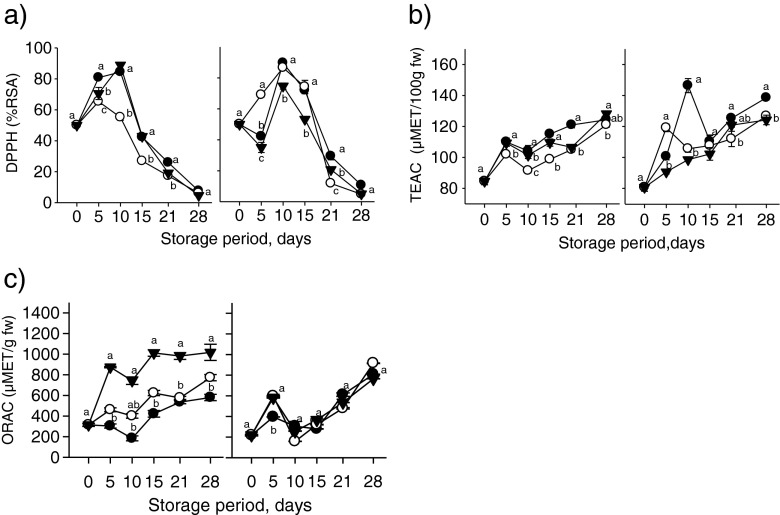
Figure 2a shows the DPPH radical-scavenging activity of tomato fruits at two maturity stages treated with mineral oil and carnauba over 28 days of storage at 10 °C. For breaker-stage tomatoes, the analysis of variance for % RSA showed significant differences (P < 0.05) among treatments. A global effect showed mean values of 48.48, 36.79 and 46.02 % for control, mineral oil, and carnauba, respectively. The DPPH radical-scavenging activity showed significant differences (P < 0.05) for pink tomatoes; the values observed were 48.93, 49.38 and 39.77 % for control, mineral oil, and carnauba, respectively. No significant differences were observed between control tomatoes and those treated with mineral oil coating.
Fig. 2.
Changes in antioxidant activity of tomato fruits at two maturity stages treated with mineral wax and carnauba during storage at 10 °C.(n = 4). Different letters represents significant differences (p < 0.05) between treatments
Figure 2b shows the antioxidant capacity, expressed as trolox equivalents (TEAC), of tomato fruit at two maturity stages treated with mineral oil and carnauba over 28 days of storage at 10 °C. Breaker tomatoes showed significant differences (P < 0.05) among treatments. TEAC values were 109.83, 100.44 and 106.60 μmol TE/100 g for control, mineral oil, and carnauba, respectively. Statistical differences were also observed for pink tomatoes, which had mean values of 116.94, 108.59 and 102.84 μmol TE/100 g for the control, mineral oil and carnauba groups, respectively. Although untreated pink tomatoes showed higher TEAC values than treated ones, on day 5 the fruit treated with mineral oil wax had much higher TEAC values than the controls and the carnauba -treated fruits, showing significant differences (P < 0.05).
Figure 2c shows the changes in antioxidant capacity, expressed as the ORAC, of tomato fruit at two maturity stages treated with mineral oil and carnauba over 28 days of storage at 10 °C. Breaker tomatoes showed significant differences among treatments (P < 0.05); the values observed were 395.51, 530.34, 853.29 μmol TE/g fw for control, mineral oil, and carnauba, respectively. The initial antioxidant activity of tomato fruits was 350.85 μmol TE/g fw. This value tended to increase during storage, reaching a peak of 1017.098 μmol TE/g fw in fruits treated with carnauba and 771.72 μmol TE/g fw in fruits treated with mineral oil. These values were 75.54 % and 33.07 % higher than in controls at 28 days of storage. For pink-stage tomatoes, however, significant differences were not observed (P = 0.1870), and the mean values were 434.80, 446.78 and 452.33 μmol TE/g for control, mineral oil, and carnauba, respectively. The regression analysis (data not shown) to correlate the antioxidant activity of tomato samples with bioactive compounds analyzed in this study show a high correlation of ORAC assay for ascorbic acid, total phenolics and lycopene for breaker maturity state and total phenolics, flavonoids and lycopene for pink maturity state. Of the three methods assayed to analyze antioxidant capacity, ORAC assay shows the highest correlation with the major bioactive compounds analyzed in this study regardless of maturity state.
The production of volatile compounds, such as ethanol and acetaldehyde, appears to play an important role in the induction of antioxidant activity. Ethanol has been associated with the induction of ROS-scavenging enzymes, such as SOD, POD and CAT (Chanjirakul et al. 2006) while acetaldehyde induces antifungal compounds, such as limonene and phytoalexins (Fisher and Phillips 2008). Therefore, these volatile compounds may also induce the accumulation of antioxidant compounds, inactivating ROS with scavenging enzymes and improving the antioxidant status of produce and increasing the health benefits to the consumer. We found that the use of edible coating did not affect the ethanol and acetaldehyde content of tomatoes compared with controls (data not shown). It appears that internal atmosphere created by coatings was barely affected and did not induce significantly the anaerobic metabolism of tomatoes.
Most post-harvest treatments involve altering the natural conditions of the fruit to prolong post-harvest life. It has been shown that as a secondary response, some post-harvest treatments could induce mechanisms that affect the metabolic activity of the treated produce, such as triggering the fruit antioxidant mechanism (Gonzalez-Aguilar et al. 2010b). The activation of the antioxidant system is a response to post-harvest stress; this can be considered a helpful response that improves the antioxidant status of tropical fruits.
Conclusions
Based on the obtained results, edible coatings of carnauba and mineral oil had a significant effect on the bioactive compounds and antioxidant capacity of fresh tomatoes. Although phenolic compounds and lycopene values were, in general, higher in control fruits, the ORAC assays showed that treated fruit presented higher antioxidant capacity values. Probably other bioactive compounds such as ascorbic acid and other flavonoids could be more related to the high antioxidant capacity. Further studies are needed in order to understand the possible mode of action of edible coatings on antioxidant capacity and how to modulate the stress induced response in order to increase the content of health related compounds. Therefore, complementary studies will need to be conducted in order find the right combination of edible coatings with antioxidant and antimicrobial natural products, to preserve tomatoes quality without affecting the sensorial attributes.
Acknowledgments
The authors are thankful to Consejo Nacional de Ciencia y Tecnología and Centro de Investigación en Alimentación y Desarrollo for funding support and to C. Mariana Rodríguez-Armenta for technical assistance.
References
- Ali A, Maqbool M, Ramachandran S, Alderson PG. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Tech. 2010;58:42–47. doi: 10.1016/j.postharvbio.2010.05.005. [DOI] [Google Scholar]
- Chanjirakul K, Wang SY, Wang CY, Siriphanich J. Effect of natural volatile compounds on antioxidant capacity and antioxidant enzymes in raspberries. Postharvest Biol Tech. 2006;40:106–115. doi: 10.1016/j.postharvbio.2006.01.004. [DOI] [Google Scholar]
- Dávila-Aviña JE, Villa-Rodríguez JA, Cruz-Valenzuela R, Rodríguez-Armenta CM, Olivas GI, González-Aguilar GA. Effect of edible coatings, storage time and maturity stage on quality of tomatoes fruit. Am J Agric Biol Sci. 2011;6:162–171. doi: 10.3844/ajabssp.2011.162.171. [DOI] [Google Scholar]
- Doner LW, Hicks KB. High-performance liquid chromatographic separation of ascorbic acid, erythorbic acid, dehydroascorbic acid, dehydroerythorbic acid, diketogulonic acid, and diketogluconic acid. Anal Biochem. 1981;115:225–230. doi: 10.1016/0003-2697(81)90550-9. [DOI] [PubMed] [Google Scholar]
- Espino-Díaz M, De Jesús Ornelas-Paz J, Martínez-Téllez MA, Santillán C, Barbosa-Cánovas GV, Zamudio-Flores PB, Olivas GI. Development and characterization of edible films based on mucilage of opuntia ficus-indica (L.) J Food Sci. 2010;75:E347–E352. doi: 10.1111/j.1750-3841.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- Frusciante L, Carli P, Ercolano MR, Pernice R, Di Matteo A, Fogliano V, Pellegrini N. Antioxidant nutritional quality of tomato. Mol Nutr Food Res. 2007;51:609–617. doi: 10.1002/mnfr.200600158. [DOI] [PubMed] [Google Scholar]
- Gao H, Cheng N, Zhou J, Wang B, Deng J, Cao W (2011) Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J Food Sci Technol. doi:10.1007/s13197-011-0591-x [DOI] [PMC free article] [PubMed]
- Gonzalez-Aguilar GA, Valenzuela-Soto E, Lizardi-Mendoza J, Goycoolea F, Martínez-Téllez MA, Villegas-Ochoa MA, Monroy-García IN, Ayala-Zavala JF. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J Sci Food Agric. 2009;89:15–23. doi: 10.1002/jsfa.3405. [DOI] [Google Scholar]
- Gonzalez-Aguilar G, Ayala-Zavala J, Olivas G, de la Rosa L, Alvarez-Parrilla E. Preserving quality of fresh-cut products using safe technologies. J Verbr Lebensm. 2010;5:65–72. doi: 10.1007/s00003-009-0315-6. [DOI] [Google Scholar]
- Gonzalez-Aguilar GA, Villa-Rodriguez JA, Ayala-Zavala JF, Yahia EM. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci Technol. 2010;21:475–482. doi: 10.1016/j.tifs.2010.07.004. [DOI] [Google Scholar]
- Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:R11–R19. doi: 10.1111/j.1365-2621.2005.tb09053.x. [DOI] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tian S, Meng X, Xu Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol Tech. 2007;44:300–306. doi: 10.1016/j.postharvbio.2006.12.019. [DOI] [Google Scholar]
- Mejia LA, Hudson E, De Mejia EG, Vazquez F. Carotenoid content and vitamin A activity of some common cultivars of Mexican peppers (Caps&m annuum) as determined by HPLC. J Food Sci. 1988;53:1440–1443. doi: 10.1111/j.1365-2621.1988.tb09295.x. [DOI] [Google Scholar]
- Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996;384:240–242. doi: 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- Olivas GI, Davila-Aviña JE, Salas-Salazar NA, Molina FJ. Use of edible coatings to preserve the quality of fruits and vegetables during storage. Stewart Postharvest Rev. 2008;4:1–10. doi: 10.2212/spr.2008.3.6. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Robles-Sánchez RM, Islas-Osuna MA, Astiazarán-García H, Vázquez-Ortiz FA, Martín-Belloso O, Gorinstein S, González-Aguilar GA. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh-cut “ataulfo” mangoes (Mangifera Indica L.) as affected by low-temperature storage. J Food Sci. 2009;74:S126–S134. doi: 10.1111/j.1750-3841.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- Romanazzi G, Nigro F, Ippolito A, DiVenere D, Salerno M. Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J Food Sci. 2002;67:1862–1867. doi: 10.1111/j.1365-2621.2002.tb08737.x. [DOI] [Google Scholar]
- Shivashankara KS, Isobe S, Al-Haq MI, Takenaka M, Shiina T. Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of irwin mango fruits stored at low temperature after high electric field pretreatment. J Agric Food Chem. 2004;52:1281–1286. doi: 10.1021/jf030243l. [DOI] [PubMed] [Google Scholar]
- Tigist M, Workneh T, Woldetsadik K (2011) Effects of variety on the quality of tomato stored under ambient conditions. J Food Sci Technol. doi:10.1007/s13197-011-0378-0 [DOI] [PMC free article] [PubMed]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]