Abstract
During the recent decades, awareness towards the role of essential fatty acids in human health and disease prevention has been unremittingly increasing among people. Fish, fish oils and some vegetable oils are rich sources of essential fatty acids. Many studies have positively correlated essential fatty acids with reduction of cardiovascular morbidity and mortality, infant development, cancer prevention, optimal brain and vision functioning, arthritis, hypertension, diabetes mellitus and neurological/neuropsychiatric disorders. Beneficial effects may be mediated through several different mechanisms, including alteration in cell membrane composition, gene expression or eicosanoid production. However, the mechanisms whereby essential fatty acids affect gene expression are complex and involve multiple processes. Further understanding of the molecular aspects of essential fatty acids will be the key to devising novel approaches to the treatment and prevention of many diseases.
Keywords: Essential fatty acids, Omega-3 fatty acids, α-Linolenic acid, Docosapentaenoic acid, Functional foods
Introduction
There has been significant consumer interest in the health enhancing role of specific foods or physiologically-active food components. Functional foods may be defined as foods or dietary components that may provide significant health benefits in addition to basic nutrition. The term essential fatty acids (EFA) refers to those polyunsaturated fatty acids (PUFA) that must be provided by foods because these cannot be synthesized in the body yet are necessary for health. There are two families of EFA, omega-3 (ω-3) and omega-6 (ω-6). Omega-3 fatty acids have in common a final carbon–carbon double bond in the ω-3 position i.e. the third bond from the methyl end of the fatty acid whereas ω-6 fatty acids have it in the ω-6 position i.e. the sixth bond from the methyl end of the fatty acid. The importance of ω-3 and ω-6 designation is that n end is never changed during physiological transformation in the human body as it is most stable energetically. The double bonds in these ω-3 fatty acids are in the cis-configuration i.e. the two hydrogen atoms are on the same side of the double bond. α-Linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have various properties for which they can be classified as functional foods.
Sources of ALA, EPA and DHA
ALA
Alpha linolenic acid is abundant in flax seed and is present in small quantities in hemp, walnut, soybean and canola oil (Hunter 1990). It is mostly found in the chloroplast of green leafy vegetables.
EPA
Fish and fish oil are the richest sources of this fatty acid with contents ranging from 39 % to 50 % for both fresh and salt water fish (Kinsella 1990). EPA is a parent of series 3 eicosanoid hormones.
DHA
It is present in fish oil and red brown algae. It is a major brain ω-3 fatty acid and is also found in eye ball (retina). Brain is made up of about 65 % fat and out of this 50 % is DHA.
Status of ω-3 fatty acids in diet
ALA is very sensitive to destruction by light, oxygen and heat. If not protected, it becomes toxic. It is destroyed five times faster than linoleic acid (LA). Average intake of ω-3 fatty acids has decreased to less than 20 % of what was present in common diets 150 years ago. About 95–99 % of the population gets ω-3 fatty acids lesser than that required for good health, making ω-3 fatty acids an essential nutrient and therefore the most therapeutic of all the essential nutrients (20 minerals, 14 vitamins, 8–11 amino acids, 2 fatty acids).
The important ω-6 fatty acids and their sources
Linoleic acid C18:2, (Δ 9, 12)
This is abundant in safflower, sunflower and corn; present in medium quantities in soybean, sesame and almonds and in small quantities in canola, peanut and olive oils. It is very low in coconut and palm kernel. The body converts LA into other fatty acids depending upon need.
Gamma-linolenic acid, 18:3 (Δ 6, 9, 12)
It is present in evening primrose oil (7–10 %) (Hoy et al. 1983), black current (15–20 g/100 g), borage (18–26 g/100 g) (Lawson and Hughes 1988). It is also present in small amounts in organ meats and in human milk (Horrobin 1990).
Dihomo-gamma-linolenic acid, C 20:3 (Δ 8, 11, 14)
It is synthesized from GLA.
Arachidonic acid, C 20:4 (Δ 5, 8, 11, 14)
It is found in meat, eggs and dairy products.
Status of ω-6 fatty acids
LA is abundant in the diets of most people. Its intake has been doubled during the past 100 years due to increased use of corn and safflower oils. Diets too high in LA and too low in ω-3 fatty acids may lead to chronic inflammation, hypertension and blood clotting tendency that increases the risk of heart attack and stroke. Increased amount of LA slows down the metabolism of ALA to EPA and DHA by inhibiting Δ6 desaturase which may also decrease with age (Simopoulos 1996). The fatty acid composition of various oils is given in Table 1.
Table 1.
Average content of linoleic (LA 18:2 n-6) and α-linolenic (ALA 18:3 n-3) acids in oils (g/100 g fat). Presented table is based on the values reported in the literature
| Sr. No. | Oil | LA 18:2(n-6) | ALA 18:3(n-3) | Total unsaturated fatty acids |
|---|---|---|---|---|
| 1 | Soybean | 50.8 | 6.8 | 80.7 |
| 2 | Cotton seed | 50.3 | 0.4 | 69.6 |
| 3 | Corn | 57.3 | 0.8 | 82.8 |
| 4 | Safflower | 73.0 | 0.5 | 86.3 |
| 5 | Sunflower | 66.4 | 0.3 | 88.5 |
| 6 | Sesame | 40.0 | 0.5 | 80.5 |
| 7 | Olive | 8.2 | 0.7 | 81.4 |
| 8 | Peanut | 31.0 | 1.2 | 77.8 |
| 9 | Rapeseed (Zero erucic acid) | 22.2 | 11.0 | 88.0 |
| 10 | Rapeseed (High erucic acid) | 12.8 | 8.6 | 88.4 |
| 11 | Cocoa butter | 2.8 | 0.2 | 36.0 |
| 12 | Coconut | 1.8 | – | 7.9 |
| 13 | Palm kernel | 1.5 | – | 12.9 |
| 14 | Palm | 9.0 | 0.3 | 47.7 |
| 15 | Almond | 18.2 | 0.5 | 87.7 |
| 16 | Cashew | 17.0 | 0.4 | 73.8 |
| 17 | Chestnut | 35.0 | 4.0 | 75.5 |
| 18 | Walnut | 61.0 | 6.7 | 86.5 |
| 19 | Butter | 2.3 | 1.4 | 32.6 |
Metabolism of omega-6 and omega-3 fatty acids
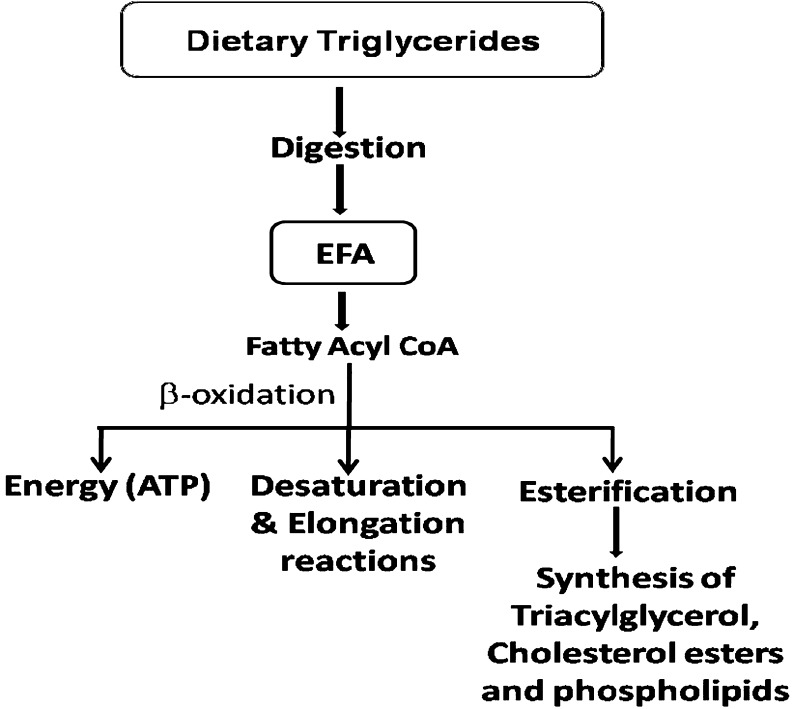
The ω-6 and ω-3 fatty acids when consumed in the diet in the form of triglycerides from various food sources undergo digestion in the small intestine which allows absorption and transport in the blood and subsequent assimilation in the body including brain, retina, heart and other tissues. These fatty acids can undergo (a) β-oxidation to provide energy in the form of ATP (b) esterification into cellular lipids including triglycerides, cholesterol esters and phospholipids and (c) converted into their important longer chain and more unsaturated products derived by a series of desaturation and elongation reactions which are particularly active in the liver and to a lesser extent in other tissues (Fig. 1).
Fig. 1.
Metabolic fate of essential fatty acids
The EFAs which are assimilated into phospholipids are particularly important in the overall structure and function of both omega-6 and omega-3 fatty acids, as phospholipids maintain both the structural integrity and the critical functioning of cellular membranes throughout the body. The above discussion indicates that some of the fatty acids present in cells and tissues of the human body are derived directly from the dietary sources and some by way of endogenous synthesis in the body itself (Table 2).
Table 2.
Sources of major physiologically-occurring fatty acids in human body
| Fatty acids | Sources (Dietary/endogenous) |
|---|---|
| 16:0 | Dietary & endogenous |
| 18:0 | Dietary & endogenous (from 16:0) |
| cis 18:1 | Dietary & endogenous (from 18:0) |
| Trans 18:1 | Dietary |
| 18:2(n-6) LA | Dietary |
| 18:3(n-3) ALA | Dietary |
| 20:4 (n-6) AA | Dietary & endogenous (from LA) |
| 20:5(n-3) EPA and | Dietary (Needs ALA to be present.) Limited conversion from ALA to EPA & DHA |
| 22:6(n-3) DHA |
ALA α-Linolenic acid; EPA eicosapentaenoic acid; DHA docosahexaenoic acid; AA arachidonic acid; LA linoleic acid
LA and ALA can only be derived from dietary sources as the enzymes needed to synthesize these two EFAs are lacking in the human body in contrast to plant cells. The pathways for desaturation and chain elongation of ω-6 and ω-3 fatty acids are given in Fig. 2.
Fig. 2.
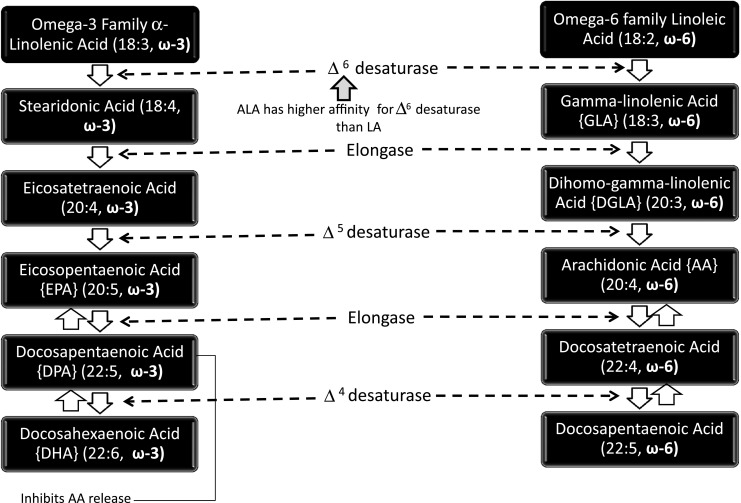
Pathways for desaturation and chain elongation of n-6 and n-3 fatty acids
The level of EPA and DHA in the tissues and cells can be increased by their direct dietary consumption. Unlike LA which is present at considerable levels in most cellular lipids (particularly membrane phospholipids), ALA does not usually accumulate to particularly high concentrations even when ingested at relatively high dietary levels. This is partly due to the fact that much of the dietary ALA undergoes β-oxidation in the mitochondria and very limited amount is available for its conversion to EPA and DHA. So, these can be consumed in the diet from fish oil or functional foods fortified with them.
Omega 3 and trans fatty acid interactions
High trans fatty acid (TFA) intake inhibit the desaturation reactions involved in the conversion of ALA to EPA and subsequently to DHA. For example, a typically Canadian diet contains about TFA: ALA ratio (ω-3) of 6:1 which is similar to the ratio found in Canadian breast milk (Chen et al. 1995). An inverse correlation has been reported by Elias and Innis (2001) between the TFA status of infants and their DHA concentrations in the circulation suggesting possible interfering effects of TFAs on fetal growth and length of gestation. Other studies have reported that TFA decrease the availability of DHA plus EPA in arterial cells, potentially enhancing the development of coronary heart disease. (Kummerow et al. 2004). Thus reducing the dietary intake of TFA is an important health goal. The potentially adverse interactive effects of TFA on omega-3 fatty acid metabolism and generation of DHA/EPA is yet another valid reason for greatly reducing intakes of TFA. Trans fatty acids increased low density lipoproteins (LDL), triglycerides and reduced beneficial high density lipoproteins (HDL) and hence have detrimental effect on health (Dhaka et al. 2011).
Essential fatty acid interactions
The actions of ω-3 and ω-6 fatty acids are characterized by their interactions and cannot be understood separately. Their action is mediated through a series of eicosanoids as discussed below.
Synthesis of eicosanoid series from fatty acids and their nomenclature
Eicosanoids are signaling molecules derived from EFAs. They constitute the major pathway by which EFAs act in the body. There are four classes of eicosanoids and two or three series within each class (Table 3). Cells outer membranes contain phospholipids. In each phospholipid, there are two types of fatty acids. Some of these fatty acids are 20 carbon PUFAs i.e. arachidonic acid (AA), eicosapentaenoic acid (EPA) or dihomo-gamma-linolenic acid (DGLA). In response to a variety of inflammatory signals, these FAs are cleaved out of phospholipids (PL) and released as free fatty acids. Then these are oxygenated (by either of two pathways) and further modified yielding the eicosanoids. Cyclooxygenase (COX) oxidation removes two C = C double bonds, leading to thromboxanes (TX), prostaglandins (PG) and prostacyclins (PGI) series (Table 3). Lipoxygenase removes no C = C double bond and leads to the formation of leukotriene (LK) (Funk 2001). The sequences of reactions for EPA and DGLA are analogous to that of AA. For example COX action on AA leads to the series 2 thromboxanes (TXA2, TXB2) each with two double bonds. COX action on EPA (with five double bonds) leads to the series 3 thromboxanes (TXA3, TXB3) each with three double bonds. The anti-inflammatory effects of eicosanoids produced by these fatty acids are shown in Table 4.
Table 3.
Three 20 carbon EFAs and the eicosanoid series derived from them
| Dietary EFA | Eicosanoid product series | ||
|---|---|---|---|
| TX | LK | Effects | |
| PG | |||
| PGI | |||
| Gamma-linolenic Acid (ω-6 18:3) via Dihomogamma linolenic Acid (ω-6 20:3) | Series-1 | Series-3 | Less inflammatory |
| Arachidonic Acid (ω-6 20:4) | Series-2 | Series-4 | More inflammatory |
| Eicosapentaenoic Acid (ω-3 20:5) | Series-3 | Series-5 | Less inflammatory |
TX thromboxanes, PG prostaglandins, LK leukotrienes, PGI prostacyclins; EFA essential fatty acid
Table 4.
Inflammatory effects of eicosanoids formed from dihomo-gamma-linolenic, arachidonic and eicosopentaenoic acids
| Fatty acid | Eicosanoids synthesized | Inflammatory effects |
|---|---|---|
| Dihomo-gamma-linolenic acid | pge1, pgf1, txa1 (inhibit AA synthesis from DGLA) | Anti-inflammatory |
| Arachidonic acid | pgd2, pge2, pgf2, txa2, lta4, ltb4, ltc4, ltd4, lte4 | Pro-inflammatory |
| Eicosopentaenoic acid | pgd3, pge3, pgf3, pgi3,txa3, Ita5, ltb5, Itc5, Itd5 | Anti-inflammatory |
tx thromboxanes, pg prostaglandins; pgi prostacyclins, lt leukotrienes; DGLA dihomo-gamma-linolenic acid, AA arachidonic acid
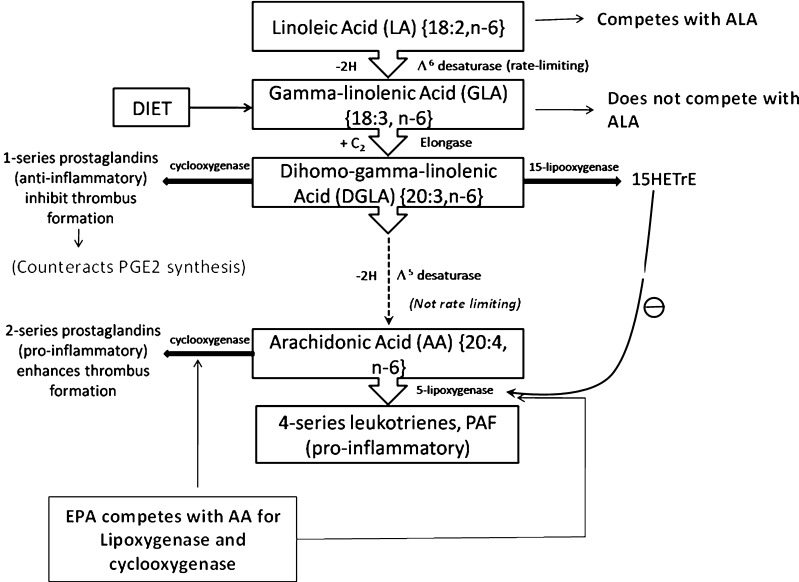
Mechanism of action of ω-3 fatty acids and GLA in countering the inflammatory effects of arachidonic acid
In the AA cascade, the dietary LA is converted to AA which is esterified into the phospholipids of cell membranes. In response to many inflammatory stimuli, eicosanoids formed from AA bind receptors on the cell. Alternately, AA can diffuse into the cell nucleus and interact with transcription factors to control DNA transcription for cytokines or other hormones. The eicosanoids from AA generally promote inflammation. Those from GLA (Via DGLA) and from EPA are generally less inflammatory, inactive or even anti-inflammatory (Kiecolt-Glaser 2010). Dietary ω-3 fatty acids and GLA counter the inflammatory effects of AA’s eicosanoids by three mechanisms namely displacement, competitive inhibition and counteraction.
Displacement
Animal studies show that increased dietary ω-3 results in decreased AA in brain and other tissues. ALA (18:3 ω-3) contributes to this by displacing LA (18:2 ω-6) from the elongase and desaturase enzymes which are involved in AA synthesis (Fig. 2). EPA inhibits phospholipase A2’s release of AA from cell membrane (Su et al. 2003). Other mechanisms involving the transport of EFAs may also play a role. High LA decreases the body’s conversion of ALA to EPA. However, this effect is not so strong, the desaturase has a higher affinity for ALA than LA (Phinney et al. 1990).
Competitive inhibition
DGLA and EPA compete with AA for access to the cyclooxygenase (COX) and lipoxygenase (LOX) enzymes. So, the presence of DGLA and EPA in tissues lowers the output of AA’s eicosanoids. For example, dietary GLA increases tissue DGLA and lowers TXB2 (Guivernau et al. 1994; Karlstad et al. 1993). Likewise EPA inhibits the production of series-2 PG and TX (Calder 2004). Although DGLA forms no LTs, a DGLA derivative blocks the transformation of AA to LTs (Fig. 3).
Fig. 3.
γ-linolenic acid (GLA) metabolic flow scheme. Platelet activating factor (PAF) (Figure modified from Fan and Chapkin 1998)
Counteraction
Some DGLA and EPA derived eicosanoids counteract their AA derived counterparts. For example, DGLA yields PGE1, which powerfully counteracts PGE2 (Fan and Chapkin 1998). EPA yields the antiaggregatory prostacyclin PGI3 and it also yields the leukotriene LTB5 which vitiates the action of the AA derived LTB4 (Prescott 1984) (Table 4).
The mechanism of action of dietary GLA as an anti-inflammatory fatty acid
Dietary LA is inflammatory. In the body, LA is desaturated to GLA, yet dietary GLA is anti-inflammatory (Table 4). Some observations partially explain this paradox. LA competes with ALA for Δ6 desaturase and thereby eventually inhibits formation of EPA (20:5 ω-3) which is an anti-inflammatory fatty acid (Fig. 2). In contrast GLA does not compete for Δ6 desaturase. GLA’s elongation product DGLA (20:3 ω-6) competes with eicosatetraenoic acid (20:4 ω-3) for Δ5 desaturase and hence it might be expected that this would indirectly make GLA inflammatory by reducing the level of EPA the products of which are anti-inflammatory but it is not so because this step is not rate limiting.
DGLA inhibits inflammation through both competitive inhibition and direct counteraction. Dietary GLA leads to sharply increased DGLA in the white blood cell’s membranes, whereas LA does not. This may reflect WBCs, lack of Δ6 desaturase. Supplementing dietary GLA increases serum DGLA without increasing serum AA (Fan and Chapkin 1998; Johnson et al. 1997). However, it is likely that some dietary GLA eventually forms AA and contributes to inflammation. However animal studies indicate that this effect was small (Karlstad et al. 1993). It has been reported that dietary GLA increases the content of elongation product DGLA within cell membranes without concomitant changes in AA. Subsequently on stimulation, DGLA can be converted to15-(S)-hydroxy-8,11,13-eicosatrienoic acid (15-HETrE) and prostaglandin E1 (Fig. 3). These compounds have both anti-inflammatory and anti proliferative properties (Fan and Chapkin 1998).
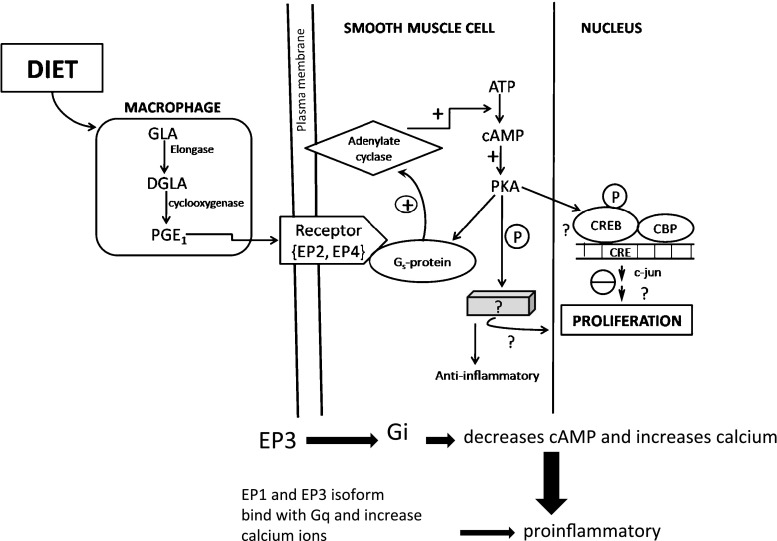
Complexity of pathways of GLA action
The eicosanoid signaling paths are complex. It is therefore difficult to characterize the action of any particular eicosanoid. For example, PGE1, formed from DGLA, binds four receptors, dubbed EP1–4. Each is coded by a separate gene and some exist in multiple isoforms. Each EP receptor in turn couples to a G-protein. The EP2, EP4 and one isoform of EP3 receptors couple to Gs. This increases intracellular cAMP which is anti-inflammatory (Fig. 4). EP1 and other EP3 isoforms couple to Gq. This leads to increased intracellular calcium and is pro-inflammatory. Finally, yet another EP3 isoform couples to Gi, which both decreases cAMP and increases calcium. Many immune system cells express multiple receptors that couple these apparently opposing pathways (Tilley et al. 2001).
Fig. 4.
Model for stimulation of DGLA-derived PGE1 biosynthesis by macrophages for further biological responses. Protein kinase A (PKA), cAMP response element binding proteins (CREB). CREB adapter binding protein (CBP), activation (+), inhibition (−), G protein stimulatory (Gs) (Figure modified from Fan and Chapkin 1998)
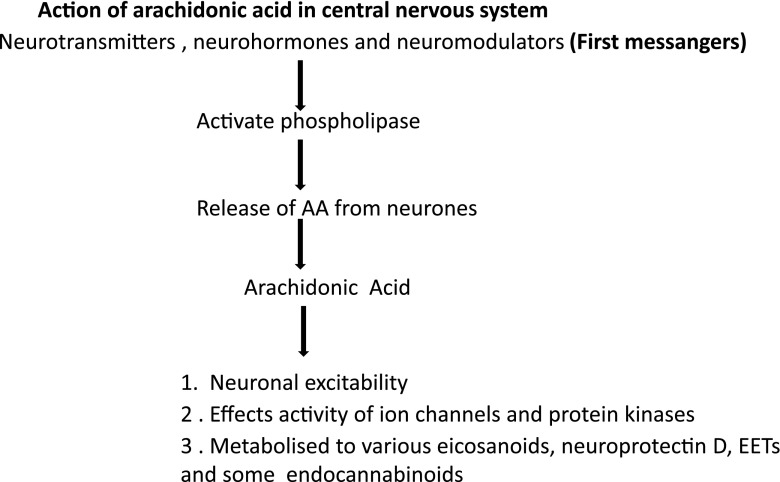
The arachidonic acid cascade in the central nervous system (CNS)
The AA cascade in the CNS is arguably the most elaborate signaling system neurobiologists have to deal with. The EPA and DGLA cascades are also present in the brain and their eicosanoid metabolites have been detected. The ways in which they differently affect mental and neural processes are not nearly as well characterized as the effects in inflammation. Neurohormones, neuromodulators or neurotransmitters act as first messengers. They activate phospolipase to release AA from neuron cell membranes as a free acid. During its short life span, free AA may affect the activity of the neurons ion channels and protein kinases or it may be metabolized to form eicosanoids, epoxyeicosatrienoic acid (EETs), neuroprotectin D or various endocannabinoids (Fig. 5).
Fig. 5.
Action of arachidonic acid in central nervous system. Epoxyeicosatrienoic acid (EET)
Significance of ω-6 to ω-3 fatty acid ratios
The concept of ω-6 to ω-3 fatty acid ratio originated in the early rodent experiments where high levels of LA (ω-6) in the diet were found to partially suppress the conversion efficiency of dietary ALA to EPA plus DHA in the body. LA and ALA are metabolized to their corresponding products via common enzyme systems (Fig. 2). Very high LA: ALA ratio resulted in smaller rise in DHA/EPA levels in tissues due to competitive inhibitory effect of LA on ALA at the level of initial desaturation reaction. Thus the lower ratios were found to provide better conversion efficiency of ALA to DHA/EPA as compared to higher ratio even when the amount of ALA was fixed at the same amount. It was indicated that lowering the ratio of LA: ALA from 27 down to 3 allowed moderately enhanced conversion of dietary ALA to EPA as revealed by moderately higher levels of EPA in blood samples taken from subjects given varying ω-6 : ω-3 ratios and amount of ALA.
Thus higher intakes of ALA and much lower ratio of LA: ALA is one strategy for moderately enhancing the conversion of ALA to EPA (Holub 2002). However, in many human studies which have lowered ratio of LA: ALA have not shown a significant rise in DHA with the lower ratio or even with higher intake of ALA despite the moderate rise in EPA (Holub 2002).
Direct consumption of preformed DHA and EPA provides for a highly efficient increase in these important long chain omega-3 fatty acids in cells and tissues such that dependency on lower LA: ALA ratios becomes questionable. So, the concept of LA: ALA ratio needs to be reconsidered in the context of dietary/health situations where DHA plus EPA are consumed directly in their preformed state. The ω-6 product formed from desaturation and elongation of LA is arachidonic acid (AA:20:4 ω-6). While small levels of AA have some important functions such as in reproduction and other processes, excessively high levels of AA are considered to be potentially problematic in the development and or progression of some chronic health conditions.
AA can be converted into neutrophils (white blood cells) to form leukotriene B4 (LTB4) which is considered to be a proinflammatory eicosanoid associated with chronic conditions such as rheumatoid arthritis, psoriasis of the skin and inflammatory in gastro intestinal disorders. In some cells and tissues AA is converted into the prostaglandin form known as prostaglandin E2 (PGE2) which has been associated with enhanced cell proliferation, mitogenesis and possibly cancer promotion. In circulating blood platelets, AA is converted to thromboxane A2 (TXA2) which is known as a prothrombolic and vasoconstrictory eicosanoid and is thought to play an important role in thrombus formation and is associated with fatal or non-fatal myocardial infarctions (heart attacks) (Wijendran and Hayes 2004).
High intake of EPA plus DHA in the diet allows for the partial replacement (reduction) of AA, thus, reducing its amount available to form the metabolites that are associated with various chronic disorders. Furthermore EPA plus DHA inhibit the conversion efficiency of AA to LTB4, PGE2 and TXA2. Finally the enzyme generated (via oxygenase activity) products of EPA plus DHA don’t appear to have the potentially harmful effects in contrast to those formed from AA by same enzymatic reactions (Wijendran and Hayes 2004).
If both ω-3 and ω-6 are present, they will compete to be transformed into their respective end products, so the ratio of ω-6: ω-3 directly affects the type of eicosanoids that are produced. This competition was recognized as important when it was found that thromboxane is a factor in the clumping of platelets, which leads to thrombosis. The leukotrienes were similarly found important in immune/inflammatory system response, and therefore relevant to arthritis, lupus and asthma. These discoveries led to greater interest in finding ways to control the synthesis of ω-6 eicosanoids. The simplest way would be by consuming more ω-3 and fewer ω-6 fatty acids. EPA forms in the body potent anti-inflammatory nanomolecules, called resolvins. Later it was found that omega-3s also turn into other anti-inflammatory molecules called omega-3-oxylipins, which partly explain the versatile health effects of fish oil (Shearer et al. 2010). Overall the positive effects of ω-3 fatty acids on health relate to inhibition or modulation of eicosanoid pathways which lead to alteration of inflammatory responses and related protein expression activity and modulation of molecules or enzymes associated with various signaling pathways involving normal and pathological cell function, incorporation of ω-3 fatty acids into membrane phospholipids and direct effects on gene expression. Because all these pathways are highly interactive, the biological potential of ω-3 fatty acids on health and disease must be due to multiple coordinated mechanisms. (Seo et al. 2005). Therefore ω-3 fatty acids are virtually functional foods.
Conversion efficiency of ALA to DHA in humans
Body converts ALA to EPA and DHA. However, the extent of this conversion appears to be minimal at current intakes and was estimated to be <1 % (Brenna 2002; Pawlosky et al. 2003). Similarly, when evaluating changes in plasma phospholipids DHA levels, supplementation of ALA, up to 5 g per day did not increase plasma (or erythrocyte) phospholipids DHA levels (Valsta et al. 1996). Depending upon several factors the range of conversion varies from <5 % to 36 % per day of the amount of ALA consumed. The major fate of supplemented ALA appears to be oxidation and not long chain ω-3 fatty acid synthesis (Brenna 2002; Valsta et al. 1996). The metabolic pathway by which dietary ALA can be converted to EPA and DHA in the mammalian liver by a series of desaturation and elongation reactions is given in Fig. 2. It has been shown that omega-3 deprivation impaired the synaptic plasticity and learning ability (Heinrichs 2010). However, extensive controlled studies in human subjects have now confirmed the very limited metabolic conversion of dietary ALA into DHA, the final end product of the sequential pathway. The common approach for assessing the apparent conversion efficiency of dietary ALA to EPA plus DHA is to determine the net increase in circulating blood levels of EPA and DHA after increasing the dietary intake of ALA from food sources. Chan et al. (1993) found moderate net rise in the level of EPA with higher levels of ALA in conjunction with ω-6: ω-3 ratios lowered to 3:1, no net rise in the level of circulating DHA was found across the various fatty acid mixtures and ratios. Feeding of 10.7 g of ALA from flaxseed oil over a 4 week period failed to provide any significant net rise in the low levels of DHA present in the breast milk of lactating women. The level of ALA supplementation in this study was approximately seven fold that recommended by Food and Nutrition Board during pregnancy (Francois et al. 2003).
With the use of radioisotopes in human studies it was reported that the conversion efficiency of ALA to DHA in young adult male subjects was 4 % (Emken et al. 1994). The overall conversion efficiency from ALA to EPA plus DHA combined was 12 %. Subsequent studies by other researchers showed estimated conversion from ALA to DHA of less than 0.1 % and a conversion of EPA plus DHA combined of less than 0.4 % efficiency (Hussein et al. 2005; Pawlosky et al. 2001). Another study compared the apparent conversion efficiency of ALA to DHA in young adult men and women. No detectable formation of DHA was found in men whereas it was about 9 % in women. Greater fractional conversion in women may be due in part to a significantly lower rate of utilization of dietary ALA for β-oxidation and/or the influence of estrogen or other hormonal factors on the conversion efficiency. In addition, the efficiency of conversion can be reduced due to controllable factors that interfere with the activity of enzymes involved in this conversion, like high intake of ω-6 fats that interfere with the conversion of ALA, significant alcohol intake, deficiency of nutritional enzyme co-factors; vitamin B3, B6 and C; minerals, zinc and magnesium and trans fatty acids from fried foods and hydrogenated oils (Burdge et al. 2002; Burdge and Calder 2005).
In conclusion, the efficiency of conversion of ALA to DHA is very limited in healthy individuals and is markedly variable between individuals within different sectors of the populations. Thus the lack of sufficient DHA in diet may have to compromise with optimal health in those with very minimal conversion capacities. These observations support the serious consideration being given to dietary DHA as an essential fatty acid and/or a conditionally essential fatty acid depending upon the conversion capacity of individuals within the population.
The question of ω-3 conversion is important, because EPA is needed to make health protecting ‘eicosanoid’ hormones and DHA is required for brain development, brain function, vision and sperm formation and has heart protective and anti inflammatory functions as well. If the body converts ALA into EPA and DHA then fish or its oil is not required in the diet. If the body cannot convert or convert at insufficient rate, we all must look to sources which can provide EPA and DHA.
Health benefits of omega-3 fatty acids
Importance of ω-3 fatty acids in human health has been established by epidemiological studies showing the relation between an ω-3 enriched diet and the prevention of some diseases like cardiovascular diseases and myocardial infarction (Kromann and Green 1980; Das 2000; VonSchacky and Harris 2007), psoriasis (Zulfakar et al. 2007), bowel disease (Diamond et al. 2008), treatment and prevention of mental illnesses (Song and Zhao 2007), prevention of several types of cancer (Chen et al. 2007; Calviello et al. 2007) or bronchial asthma (Reisman et al. 2006). Subsequently, an enormous number of epidemiological and clinical studies have dealt with the effect of ω-3 PUFA, especially EPA and DHA, in human health and the mechanism by which this effect takes place. PUFA have been reported to act as nutraceuticals (Das et al. 2011).
It is interesting to note that Greenland Eskimos consume large amount of fat from sea food, but displayed virtually no cardiovascular disease. The high level of ω-3 fatty acids consumed by the Eskimos reduced triglycerides, heart rate, blood pressure and atherosclerosis (Dyerberg et al. 1975). U.S. Food and Drug Administration have already given qualified health claim status to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) ω-3 fatty acids, stating that supportive but not conclusive research shows that consumption of EPA and DHA (ω-3) fatty acids may reduce the risk of coronary heart disease. Omega-3 fatty acids are more effective for raising energy levels, stamina and performance, improving concentration, learning, calmness, behavior and IQ, lowering cardiovascular risk factors, inhibiting cancer growth and metastasis, increasing insulin sensitivity, speeding the healing of wounds due to accidental injury, physical exertion and surgery, decreasing inflammation and joint pain, dampening the symptoms of auto immune diseases, improving bone mineral metabolism, improving weight management and increasing fat burning and decreasing fat production. The health benefits of ω-3 fatty acids are discussed below in detail.
Cardiovascular disease
Omega-3 fatty acids, especially those derived from marine sources, may be a useful tool for the primary and secondary prevention of cardiovascular disease. Omega-3s exert their cardioprotective effects through multiple mechanisms, including reducing arrhythmias and altering production of prostaglandins, which reduces inflammation and improves platelet and endothelial function. It is recommended that one serving (200–400 g) of fatty fish two times per week and a diet that includes foods rich in ALA for the primary prevention of cardiovascular disease. Also it is encouraged to have one serving (200–400 g) of fatty fish or a fish oil supplement containing 900 mg of EPA + DHA every day and a diet rich in ALA for patients with known cardiovascular disease or congestive heart failure (DeFilippis et al. 2010). The results of a major clinical study involving over eleven thousand patients with a recent myocardial infarction with 1 g per day of ω-3 fatty acids reduced the occurrence of death, cardiovascular death and sudden cardiac death by 20, 30 and 45 % respectively. Further studies indicated decreases in total mortality and cardio vascular incidents (i.e. myocardial infarction) associated with the regular consumption of fish and fish oil supplements (Wang et al. 2006; Mozaffarian and Rimm 2006).
In another study, Japanese men with unhealthy blood sugar levels were randomly given 1,800 mg daily of EPA. The thickness of the carotid arteries and blood flow were measured before and after supplementation for about 2 years. Significant decrease in the thickness of carotid artery along with improvement in blood flow was observed with EPA supplementation to patients with unhealthy blood sugar levels (Mita et al. 2007). When patients with high triglycerides >500 mg/dl and poor coronary artery health were given 4 g per day of a combination of EPA and DHA along with some monounsaturated fatty acids, it reduced their TG on an average 45 % and VLDL cholesterol (bad cholesterol) by more than >50 % (Brunton and Collins 2007).
In a study involving 18,000 patients with unhealthy cholesterol levels, patients were given 1,800 mg a day of E-EPA (ethyl esterified EPA) with a statin drug for 5 years. It was reported that EPA is a promising treatment for prevention of major coronary events (Yokoyama et al. 2007). Similarly increased HDL-cholesterol and decreased triglycerides and less heart disease was observed on ω-3 fatty acid supplementation in the diet. Eating walnuts (the ratio of ω-3 to ω-6 is about 1:4) was reported to lower total cholesterol by 4 % relative to controls when people also ate 27 % less cholesterol (Zambon et al. 2000).
Sudden death accounts for as much as 50 % of cardiovascular disease (CVD) and up to 80 % of these are due to ventricular fibrillation. A major benefit of ω-3 highly unsaturated fatty acids (HUFA) is reducing the risk of sudden death (Kang and Leaf 2000; Mozaffarian et al. 2005). It is suggested that these HUFAs reduce arrhythmic events by modifying lipid microdomains of plasma membranes, thereby affecting the conductance of ion channels (Leaf et al. 2003). PUFAs from fish oil reduce matrix metallo-proteinases (a family of collagenases involved in the degradation of extracellular matrix) expression increasing plaque stability potentially reducing sudden coronary events (Fukumoto et al. 2004; Chen et al. 2003). Proposed mechanisms of ω-3 fatty acids involve reductions in circulating triacylglycerol levels, platelet activation and the expression of vascular adhesion molecules (Kris-Etherton et al. 2001; Vanschoonbeek et al. 2003). Omega-3 triacylglycerol-enriched particles are cleared faster from blood in both humans and animals and are much less dependent on the activities of lipoprotein lipase, the LDL receptor, and apolipoprotein E-dependent pathways for blood removal and tissue targeting (Qi et al. 2002, 2003).
DHA for optimal brain and visual functioning
DHA (22:6 ω-3) is now recognized as a physiologically essential nutrient in the brain and retina of the eye where it is required in high concentrations for providing optimal mental performance (neuronal functioning) and visual activity respectively. The six cis-double bonds provide folding over the fatty acid structure (Stilwell and Wassall 2003). This unique structure along with melting point of approximately −50 °C helps the maintenance of a highly fluid microenvironment within the phospholipid components of the grey matter in mammalian brains and in other cell membranes of the nervous system (Svennerholm 1968). Its modulatory effect on the activity of ion channels underlines its role in supporting electrical signaling (Litman et al. 2001) and ultimately brain functioning such as learning ability, memory etc. The high level of DHA in the brain and nervous system are actively deposited particularly during the last trimester of pregnancy and during the first 2 months of infancy and very early years of a child’s life (Birch et al. 2000). A source of DHA to brain and nervous tissue is needed to replenish and maintain optimal DHA levels for functioning throughout life span (Youdim et al. 2000).
The highest concentration of DHA per unit tissue weight was found in the membrane phospholipid components of the photo receptor outer segments of the retina. The fluidity of retinal membrane due to DHA helps in faster response to stimulation. The optimal functioning of rhodopsin, the photopigment necessary for initiating visual sensation is considered to be supported by the presence of DHA in the retinal membrane (SanGiovanni and Chew 2005). The retina, functionally an extension of the brain, contains rods and cones with the most fluid membranes of all the body’s cell types; they are also highly enriched in DHA. The laboratory animals with experimentally induced omega-3 deficiencies showed deficits in retinal structure, visual activity development and cognitive performance (Reisbick et al. 1997; Crawford 1993).
The depletion of DHA levels to sub-optimal concentrations in the brain due to insufficient dietary intakes of ω-3 fatty acids has been found to result in cognitive deficits (impaired learning ability). A sufficient supply and accumulation of DHA appears necessary for optimal neurotransmission to support cognitive function in the brain and optimal visual transduction and functioning (Litman et al. 2001). Growing membranes must be relatively fluid and DHA is the most fluidizing element in cell membranes. Even the synapses that are the primary functional units of brain circuits are made from membranes preferentially enriched in DHA (Breckenridge et al. 1972). A benefit of ω-3 fatty acids is helping the brain to repair damage by promoting neuronal growth (Kockmann et al. 1989). In a 6 month study involving people with schizophrenia and Huntington’s disease who were treated with EPA or a placebo, the placebo group had clearly lost cerebral tissue, while the patients given the supplements had a significant incase of grey and white matter (Petrik et al. 2000).
In the prefrontal cortex (PFC) of the brain, low brain ω-3 fatty acids are thought to lower the dopaminergic neurotransmission in this brain area, possibly contributing to the negative and neurocognitive symptoms in schizophrenia. This reduction in dopamine system function in the PFC may lead to an over activity in dopaminergic function in the limbic system of brain which is suppressively controlled by the PFC dopamine system causing the positive symptoms of schizophrenia. This is called the ω-3 PUFA/dopamine hypothesis of schizophrenia (Ohara 2007). Omega-3 fatty acids have been reported to have neuroprotective action in Parkinson’s disease (Bousquet et al. 2008). High doses of ω-3 given to rats of experimental group completely prevented the neurotoxin-induced decrease of dopamine. Since Parkinson’s is a disease caused by disruption of the dopamine system, this protective effect exhibited promise for future research in the prevention of this disease.
In a recent study Perilla frutescens seed oil (PFSO) which is a rich source of omega-3 fatty acids was administered chronically to guinea pigs and neuroprotective properties were assayed ex vivo in dissociated brain cells (Eckert et al. 2010). In the brain, levels of oleic, linoleic, arachidonic and docosahexaenoic acids were significantly enhanced. Results of the study provided new insights into the potential mechanisms for the neuroprotective actions of unsaturated fatty acids and suggested PFSO as promising nutraceutical and possible alternative to fish oil supplements to provide healthful activities in the brain.
Rheumatoid arthritis
The anti-inflammatory effects of high doses of omega-3 fatty acids provide symptomatic relief and also reduce cardiovascular risk in rheumatoid arthritis. Fish oil is a convenient source of these essential fatty acids and therefore warrants consideration as a component of therapy for rheumatoid arthritis. It has been reported that in vitro anti-inflammatory activity of ω-3 fatty acids translates into clinical benefits (Wall et al. 2010; Fortin et al. 1995). Cohorts of neck pain patients and of rheumatoid-arthritis sufferers have demonstrated benefits comparable to those receiving standard non-steroidal anti-inflammatory drugs (NSAIDs). The positive effects of ω-3 fatty acids relate to inhibition or modulation of eicosanoid pathways, which lead to alteration of inflammatory responses and related protein expression and activity (Deckelbaum et al. 2006.)
C-reactive protein (CRP) is a systemic inflammatory marker and a strong predictor of stroke and cognitive impairment. Because of anti-inflammatory effects, the level of CRP was significantly lowered in subjects receiving a low dose of krill oil. The subjects were suffering from cardiovascular disease, rheumatoid arthritis or osteoarthritis and high levels of CRP (Balk et al. 2006).
Cancer
Omega-3 fatty acids reduced prostate tumor growth and increased survival (Berquin et al. 2007). High levels of DHA, the most abundant ω-3 PUFA in erythrocyte membranes, were associated with a reduced risk of breast cancer (Pala et al. 2001). Oral ω-3 fatty acid supplements benefit cancer patients, improving their appetite, weight and quality of life (Colomer et al. 2007). A supplement of EPA helped cancer patients retain muscle mass (Ryan et al. 2009). Anti-cancer effects of ω-3 fatty acids (particularly breast, colon and prostate) have been reported (Augustsson et al. 2003; De Deckere 1999; Caygill and Hill 1995; Kidd 2007). Modulation of specific genes by ω-3 fatty acid and cross talk between these genes are responsible for many effects of ω-3 fatty acids (Deckelbaum et al. 2006). In a very recent study Dimri et al. (2010) suggested that treatment of ω-3 fatty acids leads to decrease in invasion of breast cancer cells an oncogenic phenotype that is known to be associated with EZH2 (enhancer of zeste homolgue 2). It was concluded that the PcG protein (polycomb group protein) EZH2 is an important target of ω-3 PUFAs and that downregulation of EZH2 may be involved in the mediation of anti-oncogenic and chemopreventive effects of ω-3 PUFAs. The antiproliferative effects of n-3 fatty acids on cells has been shown in Fig. 4.
Omega-3 fatty acids and gene expression
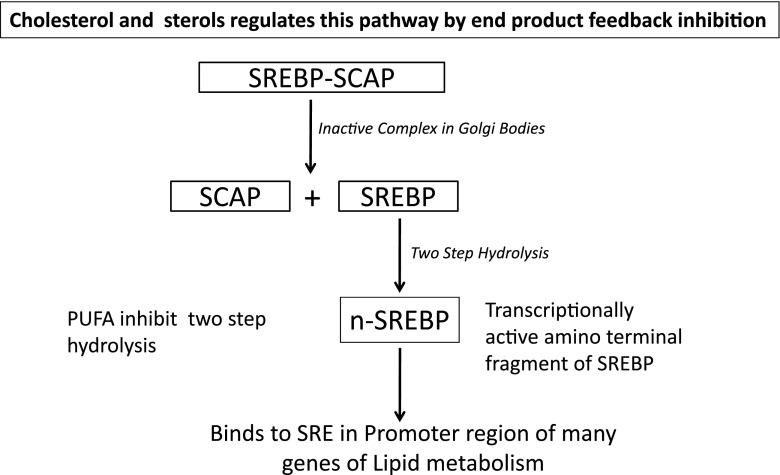
Omega-3 fatty acids are major modulators of genes and thus affecting the expression of key proteins related to inflammation, lipid metabolism and energy utilization. There could be a direct molecular interaction of ω-3 fatty acids with certain genes (Deckelbaum et al. 2006). The PUFA affect sterol regulatory element binding protein (SREBP) dependent gene expression. SREBPs are regulated post-transcriptionally and the inactive precursor form is located in the endoplasmic reticulum, where it is linked to SREBP cleavage-activating protein (SCAP). This complex is anchored by the Insig protein (Yang et al. 2002). Under conditions of sterol deprivation, Insigs dissociate from the SREBP/SCAP complex, which then translocates with the Golgi via vesicular transport. In the golgi, SREBP dissociates from SCAP and undergoes a two-step proteolysis cleavage, and the transcriptionally active amino-terminal fragment of SREBP, n-SREBP is released. SREBP binds to sterol regulatory elements in the promoter region of many genes of lipid metabolism. Cholesterol and oxysterols regulate this pathway by end product feedback inhibition (Fig. 6) (Adams et al. 2004). EPA, DHA and AA have more inhibitory capacity on SREBP processing than do the shorter chain fatty acids like 18:1, 18:2. Saturated fatty acids have no effect on SREBP processing (Worgall et al. 1998). It has been reported that addition of PUFA decrease the affinity of cholesterol for phospholipids and this in turn results in its enhanced transfer from cholesterol-rich regions (such as plasma membrane) to cholesterol-poor regions (ER), a condition that would lead to decreased SREBP transport out of the ER to the Golgi (Johnson et al. 2003).
Fig 6.
Metabolic role of SREBP-SCAP complex. sterol regulatory element binding protein (SREBP), SREBP cleavage-activating protein (SCAP) and sterol responsive element (SRE)
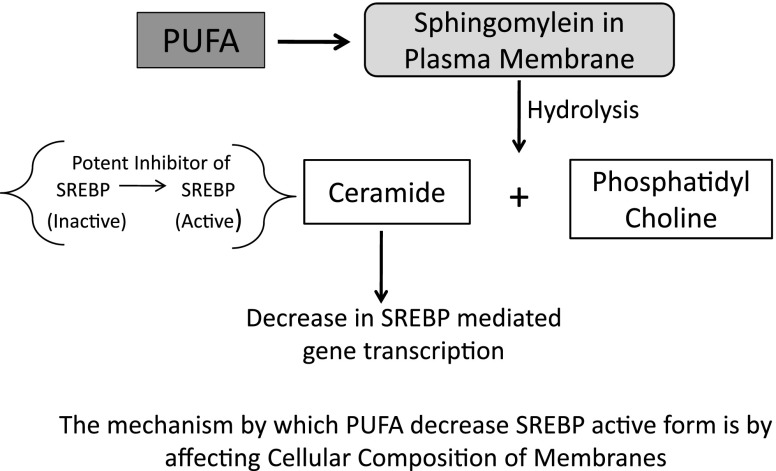
Another mechanism could be the hydrolysis of plasma membrane sphingomyelin to ceramide (Worgall et al. 2002), which affects cellular cholesterol homeostasis and gene transcription by two mechanisms. First mechanism includes lower amounts of sphingomyelin resulting in decreased ability to solubilize free cholesterol and a consequent decrease in SREBP-mediated gene transcription. In second mechanism ceramide itself acts as a potent inhibitor of SREBP (Fig. 7) processing through effects on sphingolipid synthesis (Worgall et al. 2002), a process that can regulate ER-Golgi vesicular transport (Rosenwald et al. 1992). It has been shown that ceramide synthesis is obligatory in the regulation of SREBP processing (Worgall et al. 2004). The ability of longer chain PUFA but not shorter chain or saturated fatty acids to suppress the liver X-receptors (LXRE) enhancer complex formation in the SREBP1c promoter region further exemplified the multiple mechanisms by which the fatty acids can affect gene transcription (Xu et al. 2002).
Fig 7.
Effect of polyunsaturated fatty acids (PUFA) on sterol regulatory element binding protein (SREBP)
Activation of peroxisome proliferator activated receptors (PPARs) by ω-3 fatty acids influence many critical cellular functions at multiple levels. Together with their ability to regulate SREBPs, ω-3 fatty acids can serve as master switches and there is cross talk between PPAR signaling, SREBP expression and liver-X-receptor (Yoshikawa et al. 2003; Cagen et al. 2005; Ou et al. 2001). Because of the ability of ω-3 fatty acids to inhibit inflammation and suppress genes related to lipid metabolism, these have been shown to be therapeutic agents in dyslipidemia, the metabolic syndrome, type 2-diabetes and steatohepatitis. EPA and DHA have been shown to have similar regulatory effects as glitazone, a member of the thiazolidinedione group of drugs, on a large number of genes and transcription factors (Erkkila et al. 2004; Li et al. 2004).
Health risks of high levels of EPA and DHA
Suspected risks of high levels of EPA and DHA may include the possibility of increased bleeding if overused (normally over 3 g per day) by a patient who is also taking aspirin or warfarin, hemorrhagic stroke (only in cases of very large doses), reduced glycemic control among diabetics and cardiac risk—persons with congestive heart failure, chronic recurrent angina pectoris or evidence that their heart is receiving insufficient blood flow, their doctors if advised may take these PUFA.
ω-3 fatty acids fortified foods
Omega-3 supplementation in food has been a significant recent trend in food fortification. (Whelan and Rust 2006). Flax seed oil consists of approximately 55 % ALA. Milk and cheese from grass fed cows may also be good sources of ω-3 fatty acids. Half a pint of milk provides 10 % of the recommended daily intake (RDI) of ALA while a piece of organic cheese the size of a matchbox may provide 88 % of ALA (Azona et al. 2008). Walnuts contain appreciable ω-3 fat, with approximately a 1:4 ratio of ω-3 to ω-6. Green vegetables too, contain a noteworthy amount of ω-3 fatty acids, including strawberries and broccoli.
Conclusion
Effects of EPA and DHA are mediated through direct interactions in changing the composition of cell membranes and membrane function activating or suppressing signaling molecules, interacting directly with DNA as well as with proteins that affect the processing of transcription factors and affecting enzyme activities and vesicular endoplasmic reticulum Golgi trafficking. There is a strong need for dietary supplementation of ω-3 fatty acids for reducing risk of cardiovascular morbidity and mortality in the present scenario. Omega-3 fatty acids may have beneficial effects on heart health and potentially other disease conditions such as cancer, diabetes, and neurological disorders. Anti-inflammatory effects of ω-3 fatty acids can also be used in management of inflammatory associated diseases. People at special stages in the lifecycle, such as pregnant/lactating women, infants and children may also benefit from consuming ω-3 fatty acids in adequate amounts. The current food supply offers a wide variety of sources for dietary ALA, EPA, and DHA. Sustained innovations and a growing body of scientific evidence to support dietary recommendations for ω-3 fatty acids may help the public to achieve optimal health.
References
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–67. [PubMed] [Google Scholar]
- Azona JO, Schang MJ, Garcia PT, Gallinger C, Ricardo AJ, Coates W. Omega-3 enriched broiler meat: the influence of dietary alpha-linolenic omega-3 fatty acid sources on growth, performance and meat fatty acid composition. Can J Anim Sci. 2008;88:257–269. [Google Scholar]
- Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew M, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D’Agostino R, Zhang H, Wu H, Kang JX, Yong Q. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- Bousquet M, Saint-Pierre M, Julien C, Salem N, Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acids on toxin induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008;22:1213–1225. doi: 10.1096/fj.07-9677com. [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Gombos G, Morgan IG. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta. 1972;266:695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–132. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Brunton S, Collins N. Differentiating prescription omega-3-acid ethyl esters (P-OM3) from dietary-supplement omega-3 fatty acids. Curr Med Res Opin. 2007;23:1139–1145. doi: 10.1185/030079907x188017. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Calder PC. Conversion of alpha linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principle products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB. Insulin activates the rat sterol-regulatory-element-binding protein Ic (SREBP-Ic) promoter through the combinatorial actions of SREBP, LXR, Sp-1 and NG-Y cis-acting elements. Biochem J. 2005;385:207–216. doi: 10.1042/BJ20040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. n-3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (London) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- Calviello G, Serini S, Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem. 2007;14:3059–3069. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- Caygill CP, Hill MJ. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur J Cancer Prev. 1995;4:329–332. doi: 10.1097/00008469-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Chan JK, McDonald BE, Gerrard JM, Bruce VM, Weaver BJ, Holub BJ. Effects of dietary alpha-linolenic acid and its ratio to linoleic acid on platelet and plasma fatty acids and thrombogenesis. Lipids. 1993;28:811–817. doi: 10.1007/BF02536235. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Pelletier G, Hollywood R, Ratnayake WM. Trans fatty acid isomers in Canadian human milk. Lipids. 1995;30:15–21. doi: 10.1007/BF02537037. [DOI] [PubMed] [Google Scholar]
- Chen H, Li D, Roberts GJ, Saldeen T, Mehta JL. Eicosapentaenoic acid inhibits hypoxia-reoxygenation-induced injury by attenuating upregulation of MMP-1 in adult rat myocytes. Cardiovasc Res. 2003;59:7–13. doi: 10.1016/s0008-6363(03)00349-3. [DOI] [PubMed] [Google Scholar]
- Chen YQ, Edwards IJ, Kridel SJ, Thornburg T, Berquin IM. Dietary fat–gene interaction in cancer. Cancer Metastasis Rev. 2007;26:535–551. doi: 10.1007/s10555-007-9075-x. [DOI] [PubMed] [Google Scholar]
- Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, Zarazaga A, Quecedo L, del Llano J, Usan L, Casimiro C. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97:823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- Crawford MA. The role of essential fatty acids in neural development: implications for perinatal nutrition. Am J Clin Nutr. 1993;57:703S–709S. doi: 10.1093/ajcn/57.5.703S. [DOI] [PubMed] [Google Scholar]
- Das UN. Beneficial actions of polyunsaturated fatty acids in cardiovascular diseases: but, how and why? Prostaglandins Leukot Essent Fat Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- Das L, Bhaumik E, Raychaudhuri U, Chakraborty R (2011) Role of nutraceuticals in human health. J Food Sci Technol. doi:10.1007/s13197-011-0269-4 [DOI] [PMC free article] [PubMed]
- De Deckere EA. Possible beneficial effect of fish and fish n-3 polyunsaturated fatty acids in breast and colorectal cancer. Eur J Cancer Prev. 1999;8:213–221. doi: 10.1097/00008469-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83:1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- Defilippis AP, Blaha MJ, Jacobson TA. Omega-3 fatty acids for cardiovascular disease prevention. Curr Treat Options Cardiovasc Med. 2010;12:365–380. doi: 10.1007/s11936-010-0079-4. [DOI] [PubMed] [Google Scholar]
- Dhaka V, Gulia N, Ahlawat KS, Khatkar BS. Trans-fats sources, health risks and alternative approach—a review. J Sci Food Technol. 2011;48:534–541. doi: 10.1007/s13197-010-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IR, Sterescu A, Pencharz PB, Wales PW. The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr Surg Int. 2008;24:773–778. doi: 10.1007/s00383-008-2174-0. [DOI] [PubMed] [Google Scholar]
- Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, Dimri GP. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010;31:489–495. doi: 10.1093/carcin/bgp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am J Clin Nutr. 1975;28:958–966. doi: 10.1093/ajcn/28.9.958. [DOI] [PubMed] [Google Scholar]
- Eckert GP, Franke C, Noldner M, Rau O, Wurglics M, Schubert-Zsilavecz M, Muller WE. Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Pharmacol Res. 2010;61:234–241. doi: 10.1016/j.phrs.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Elias SL, Innis SM. Infant plasma. Trans, n-6 and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation and birth weight and length. Am J Clin Nutr. 2001;73:807–814. doi: 10.1093/ajcn/73.4.807. [DOI] [PubMed] [Google Scholar]
- Emken EA, Adlof RO, Gulley RM. Dietary linolenic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- Erkkila AT, Lichtenstein AH, Mozaffarian D, Herrington DM. Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr. 2004;80:626–632. doi: 10.1093/ajcn/80.3.626. [DOI] [PubMed] [Google Scholar]
- Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411–1414. doi: 10.1093/jn/128.9.1411. [DOI] [PubMed] [Google Scholar]
- Fortin PR, Lew RA, Liang MH, Wright EA, Beckett LA, Chalmers TC, Sperling RI. Validation of a meta analysis: the effects of fish oil in rheumatoid arthritis. J Clin Epidemiol. 1995;48:1379–1390. doi: 10.1016/0895-4356(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–233. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FY, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110:1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Guivernau M, Meza N, Bajra P, Roman O. Clinical and experimental study on the long-term effect of dietary gamma-linolenic acid on plasma lipids, platelet aggregation, thromboxane formation, and prostacyclin production. Prostaglandins Leukot Essent Fat Acids. 1994;51:311–316. doi: 10.1016/0952-3278(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC. Dietary ω-3 fatty acid supplementation for optimizing neuronal structure and function. Mol Nutr Food Res. 2010;54:447–456. doi: 10.1002/mnfr.200900201. [DOI] [PubMed] [Google Scholar]
- Holub BJ. Omega-3 fatty acids in cardiovascular care. Can Med Assoc J. 2002;166:608–615. [PMC free article] [PubMed] [Google Scholar]
- Horrobin DF. Gamma-linolenic acid. Rev Contemp Pharmacother. 1990;1:1–41. [Google Scholar]
- Hoy CE, Holmer G, Kaur N, Byrjalsen I, Kirstein D. Acyl group distribution tissue lipids of rats fed evening primrose oil (γ-linolenic plus linoleic acid) or soybean oil (α-linolenic plus linoleic acid) Lipids. 1983;18:760–771. doi: 10.1007/BF02534633. [DOI] [PubMed] [Google Scholar]
- Hunter JE. n-3 fatty acids from vegetable oils. Am J Clin Nutr. 1990;51:809–814. doi: 10.1093/ajcn/51.5.809. [DOI] [PubMed] [Google Scholar]
- Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C] linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46:269–280. doi: 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Swan DD, Surettte ME, Stegner J, Chilton T, Fonteh AN, Chilton FH. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–1444. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Hamilton JA, Worgall TS, Deckelbaum RJ. Free fatty acids modulate intermembrane trafficking of cholesterol by increasing lipid mobilities: novel 13C NMR analyses of free cholesterol partitioning. Biochemistry. 2003;42:1637–1645. doi: 10.1021/bi0264465. [DOI] [PubMed] [Google Scholar]
- Kang JX, Leaf A. Prevention of fatal cardiac arrhythmias by polyunsaturated fatty acids. Am J Clin Nutr. 2000;71:202S–207S. doi: 10.1093/ajcn/71.1.202S. [DOI] [PubMed] [Google Scholar]
- Karlstad MD, DeMichele SJ, Leathem WD, Peterson MB. Effect of intravenous lipid emulsions enriched with gamma-linolenic acid on plasma n-6 fatty acids and prostaglandin biosynthesis after burn and endotoxin injury in rats. Crit Care Med. 1993;21:1740–1749. doi: 10.1097/00003246-199311000-00025. [DOI] [PubMed] [Google Scholar]
- Kidd PM. Omega-3 DHA and EPA for cognition, behaviour and moods: clinical findings and structural functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12:207–227. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella JE. Sources of omega-3 fatty acids in human diets. In: Lees RS, Karel M, editors. Omega-3 fatty acids in health and disease. New York: Marcel Dekker; 1990. pp. 157–200. [Google Scholar]
- Kockmann V, Spielmann D, Traitler H, Lagarde M. Inhibitory effect of Stearidonic acid (18: 4 n-3) on platelet aggregation and arachidonate oxygenation. Lipids. 1989;24:1004–1007. doi: 10.1007/BF02544069. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton P, Eckel RH, Howard BV, Jeor SS, Bazzarre TL. Lyon diet heart study. Benefits of a mediterranean—style national cholesterol education program/American heart association step 1 dietary pattern on cardio vascular disease. Circulation. 2001;103:1823–1825. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- Kromann N, Green A. Epidemiological studies in the Upenavik district, Greenland. Acta Med Scand. 1980;208:401–406. [PubMed] [Google Scholar]
- Kummerow FA, Zhou Q, Mahfouz MM, Smiricky MR, Grieshop CM, Schaeffer DJ. Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells. Life Sci. 2004;74:2707–2723. doi: 10.1016/j.lfs.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Lawson LD, Hughes BG. Triacylglycerol structure of plant and fungal oils containing γ-linolenic acid. Lipids. 1988;23:313–317. doi: 10.1007/BF02537340. [DOI] [PubMed] [Google Scholar]
- Leaf A, Xiao YF, Kang JX, Billman GE. Prevention of sudden cardiac deaths by n-polyunsaturated fatty acids. Pharmacol Ther. 2003;98:355–377. doi: 10.1016/s0163-7258(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16:237–242. doi: 10.1385/JMN:16:2-3:237. [DOI] [PubMed] [Google Scholar]
- Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2-diabetes. Atherosclerosis. 2007;191:162–167. doi: 10.1016/j.atherosclerosis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB. Fish intake, contaminants and human health: evaluating the risks and the benefits. J Am Med Assoc. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Longstreth WT, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, Siscovick DS. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–206. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara K. The n-3 polyunsaturated fatty acid/dopamine hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:469–474. doi: 10.1016/j.pnpbp.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ou J, Tu H, Shan B, Luk A, Debose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci USA. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala V, Krogh V, Muti P, Chajes V, Riboli E, Micheli A, Saadatian M, Sieri S, Berrino F. Erythrocyte membrane fatty acids and subsequent breast cancer: a prospective Italian Study. J Natl Cancer Inst. 2001;93:1088–1095. doi: 10.1093/jnci/93.14.1088. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Lin Y, Goodson S, Riggs P, Sebring N, Brown GL, Salem N. Effect of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am J Clin Nutr. 2003;77:565–572. doi: 10.1093/ajcn/77.3.565. [DOI] [PubMed] [Google Scholar]
- Petrik MB, McEntee MF, Johnson BT, Obukowicz MG, Whelan J. Highly unsaturated (n-3) fatty acids, but not alpha—linolenic, conjugated linoleic or gamma linolenic acids, reduce tumorigenesis in Apc (Min/+) mice. J Nutr. 2000;130:2434–2443. doi: 10.1093/jn/130.10.2434. [DOI] [PubMed] [Google Scholar]
- Phinney SD, Odin RS, Johnson SB, Holman RT. Reduced arachidonate in serum phospholipids and cholesteryl esters associated with vegetarian diets in humans. Am J Clin Nutr. 1990;51:385–392. doi: 10.1093/ajcn/51.3.385. [DOI] [PubMed] [Google Scholar]
- Prescott S. The effect of eicosapentaenoic acid on leukotriene B production by human neutrophils. J Biol Chem. 1984;259:7615–7621. [PubMed] [Google Scholar]
- Qi K, Seo T, Al-Haideri M, Worgall TS, Vogel T, Carpentier YA, Deckelbaum RJ. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry. 2002;41:3119–3127. doi: 10.1021/bi015770h. [DOI] [PubMed] [Google Scholar]
- Qi K, Al-Haideri M, Seo T, Carpentier YA, Deckelbaum RJ. Effects of particle size on blood clearance and tissue uptake of lipid emulsions with different triglyceride compositions. J Parenter Enteral Nutr. 2003;27:58–64. doi: 10.1177/014860710302700158. [DOI] [PubMed] [Google Scholar]
- Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ. Visual attention in infant monkeys: effects of dietary fatty acids and age. Dev Psychol. 1997;33:387–395. doi: 10.1037//0012-1649.33.3.387. [DOI] [PubMed] [Google Scholar]
- Reisman J, Schachter HM, Dales RE, Tran K, Kourad K, Barnes D, Sampson M, Morrison A, Gaboury I, Blackman J. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6:26–34. doi: 10.1186/1472-6882-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald AG, Machamer CE, Pagano RE. Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry. 1992;31:3581–3590. doi: 10.1021/bi00129a005. [DOI] [PubMed] [Google Scholar]
- Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:353–363. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Seo T, Blaner WS, Deckelbaum RJ. N-3 fatty acids: molecular approaches to optimal biological outcomes. Curr Opin Lipidol. 2005;16:11–18. doi: 10.1097/00041433-200502000-00004. [DOI] [PubMed] [Google Scholar]
- Shearer GC, Harris WS, Pedersen TL, Newman JH. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 and ethyl esters. J Lipid Res. 2010;51:2074–2081. doi: 10.1194/jlr.M900193-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Part I: metabolic effects of omega-3 fatty acids and essentiality. In: Spiller GA, editor. Handbook of lipids in human nutrition. Boca Raton: CRC; 1996. pp. 51–73. [Google Scholar]
- Song C, Zhao S. Omega-3 fatty acid eicosapentaenoic acid. A new treatment for psychiatric and neurodegenerative diseases: a review of clinical investigations. Expert Opin Investig Drugs. 2007;16:1627–1638. doi: 10.1517/13543784.16.10.1627. [DOI] [PubMed] [Google Scholar]
- Stilwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trials. Eur Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- Tilley L, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsta LM, Salminen I, Aro A, Mutanen M. Alpha-linolenic acid in rapeseed oil partly compensates for the effect of fish restriction on plasma long chain n-3 fatty acids. Eur J Clin Nutr. 1996;50:229–235. [PubMed] [Google Scholar]
- Vanschoonbeek K, de Maat MP, Heemskerk JW. Fish oil consumption and reduction of arterial disease. J Nutr. 2003;133:657–660. doi: 10.1093/jn/133.3.657. [DOI] [PubMed] [Google Scholar]
- vonSchacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res. 2007;73:310–315. doi: 10.1016/j.cardiores.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 fatty acids from fish oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies, a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- Whelan J, Rust C. Innovative dietary sources of N-3 fatty acids. Annu Rev Nutr. 2006;26:75–100. doi: 10.1146/annurev.nutr.25.050304.092605. [DOI] [PubMed] [Google Scholar]
- Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J Biol Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- Worgall TS, Johnson RA, Seo T, Gierens H, Deckelbaum RJ. Unsaturated fatty acid-mediated decreases in sterol regulatory element-mediated gene transcription are linked to cellular sphingolipid metabolism. J Biol Chem. 2002;277:3878–3885. doi: 10.1074/jbc.M102393200. [DOI] [PubMed] [Google Scholar]
- Worgall TS, Juliano RA, Seo T, Deckelbaum RJ. Ceramide synthesis correlates with the posttranscriptional regulation of the sterol-regulatory element-binding protein. Arterioscler Thromb Vasc Biol. 2004;24:943–948. doi: 10.1161/01.atv.0000125703.20434.4d. [DOI] [PubMed] [Google Scholar]
- Xu J, Cho H, O’Malley S, Park JH, Clarke SD. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-I and -2 in three distinct stages and by different mechanisms. J Nutr. 2002;132:3333–3339. doi: 10.1093/jn/132.11.3333. [DOI] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida S, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolemic patients (JELIS): a randomised open-label, blinded end-point analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Ide T, Shimano H, Yahagi N, Amemiya-Kudo M, Matsuzaka T, Yatoh S, Kitamine T, Okazaki H, Tamura Y, Sekiya M, Takahashi A, Hasty AH, Sato R, Sone H, Osuga J, Ishibashi S, Yamada N. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and Liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. I. PPARs suppress sterol regulatory element binding protein-Ic promoter through inhibition of LXR signaling. Mol Endocrinol. 2003;17:1240–1254. doi: 10.1210/me.2002-0190. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health Implications. Int J Dev Neurosci. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Zambon D, Sabate J, Munoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting Walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–546. doi: 10.7326/0003-4819-132-7-200004040-00005. [DOI] [PubMed] [Google Scholar]
- Zulfakar MH, Edwards M, Heard CM. Is there a role for topically delivered eicosapentaenoic acid in the treatment of psoriasis? Eur J Dermatol. 2007;17:284–291. doi: 10.1684/ejd.2007.0201. [DOI] [PubMed] [Google Scholar]