Abstract
Sunflower meal protein isolate (SMPI) is a promising food additive in different matrices. However, the uses of SMPI are limited because of the presence of antinutritional compounds like polyphenolic substances. Chlorogenic and caffeic acids are the dominants polyphenolics in the SMPI. These substances cause significant changes of the colour of the meal, proteins and food matrices during their extraction and use as food additives. Moreover, these substances lower the nutritional value of the end product due to their interaction with some amino acids such as lysine and methionine. Thus, the removal of these substances is important to enable the use of the SMPI and meal in general in a greater extent in food applications and replacing more expensive protein sources such as soy proteins. The aim of this work was to study the production of functional bread by supplementing wheat flour with sunflower meal protein isolate (SMPI). SMPI with low content of chlorogenic and caffeic acid was usefully produced following alkaline extraction and purification with succinic acid. Purified SMPI showed well balanced amino acid profile and was characterized by high water and fat absorption capacities. It was incorporated to dough formula at 8–12 % of the total wheat flour. The results showed that production of bread supplemented with SMPI was technologically feasible. The supplemented bread had high mass volume and nutritional quality compared to the control bread. The optimal SMPI to incorporate into dough formula without significant alteration of the final bread colour was established at 10 %. This study will be helpful to find economic ways to enhance the nutritional quality of wheat bread and to improve the profitability of sunflower meal residue.
Keywords: Chlorogenic acid, Caffeic acid, Bread, Sunflower meal protein, Wheat flour, Nutritional value
Introduction
Bread has always been an important component of the human nutrition and in many countries it constitutes the most important source of different nutrients such as proteins, fibres, minerals and vitamins (Thompkinson et al. 2012; Ahmed et al. 2011). During the last two decades, real trends toward healthy eating and developments of functional foods were noticed worldwide (Annapure et al. 1998). The use of plant ingredients in different food matrices was chosen as a potential mean to increase the use of plant ingredients in human diet and to develop novel functional foods. Bread is a potential vehicle of many essential nutrients (Khoshgozaran-Abras et al. 2012). Traditionally, bread is made from wheat flour of different type such as whole, bleached and refined. However, it was well established that bread and bakery products made from refined wheat flower are not sufficient to provide the human organism with all the essential nutrients (Dodok et al. 1993; Gómez et al. 2008). This is mainly due to the deficiency of the wheat proteins from some essential amino acids and the loss of minerals and vitamins during the refining and bleaching processing. Thus, the task of increasing nutritional and biological value of bread can be solved with the introduction of additional types of protein-containing materials rich in proteins, essential amino acids, fibres, and vitamins, especially vitamins of the B-group (Martinez and Duvnjak 2006). A promising source of proteins is the protein-containing vegetable raw materials, including secondary materials such as oil seed meals; a residual material of the oil extraction industry. Among the potential meals, sunflower meal is observed as a highly promising one (Rodrigues et al. 2012). It is a protein-rich ingredient and can be used to extract highly valuable proteins and other compounds for the development of functional bread and bakery products that meet the requirements of the modern functional diets. By this way, bread will be really an important source of functional proteins, carbohydrates, vitamins of B-group and minerals (Chavan et al. 1987).
According to the Food and Agriculture Organization of the United Nations (FAO 2012), apart soybean and rapeseed, sunflower seeds are among the world’s most important oilseeds with a world production of ≅ 472 million tonnes in 2011/2012 (FAO 2012). From nutritional point of view, sunflower seeds contain approximately 20 % crude proteins, whereas total protein content of the sunflower press cake is 30–50 % (Dorrell and Vick 1997). The techno-functional properties of sunflower proteins are almost similar or comparable with those of leguminous proteins (Gonzalez-Perez et al. 2005). One of the most important particularities of the sunflower proteins is the fact that although they are lower in sulphur-containing (−S) amino acids than other protein-rich foods, they provide an array of other amino acids, notably glutamic and aspartic acids. These proteins are considered as a valuable alternative as food ingredients (Rajasekaran and Kalaivani 2012), since they are low in antinutritional compounds and devoid of toxic substances (González-Pérez and Vereijken 2007). Direct use of sunflower meal in food is not suitable because of its comparatively high content of phenolic compounds (1–4 %) in defatted flour from dehulled seeds, mainly chlorogenic and caffeic acids (Sripad et al. 1982; Saeed and Cheryan 1988; Saeed and Cheryan 1989). This is the main restriction of sunflower use in food formulation even if sunflower meal protein isolate (SMPI) is a promising food additive in different matrices. In fact, the uses of SMPI are limited because of the presence of antinutritional compounds like polyphenolic substances (Kumar et al. 2010). Chlorogenic and caffeic acids are the dominants polyphenolics in the SMPI. These substances cause significant changes of the colour of the meal, proteins and food matrices during their extraction and use as food additives. Moreover, these substances lower the nutritional value of the end product due to their interaction with some amino acids such as lysine and methionine. Thus, the removal of these substances is important to enable the use of the SMPI and meal in general in a greater extent in food applications and replacing more expensive protein sources such as soy proteins (Martinez and Duvnjak 2006). To overcome this inconvenient, proteins are extracted from sunflower meal and purified from the antinutritional compounds (Sosulski 1979; González-Pérez et al. 2002; Naczk and Shahidi 2004). After purification, sunflower protein isolates can thus be used to overcome the deficiency of wheat flour, a factor which will contribute to reduce world protein malnutrition (Livingstone et al. 1993).
Classical technology of SMPI extraction consists of dispersing the meal in an extracting medium, which may be water, salt solution, alkalis, acids or any organic solvent with subsequent precipitation in its isoelectric point with hydrochloric acid or sodium hydroxide. However, the most inconvenient of these extraction methods is the high content of phenolic compounds in the extracted protein, a fact which reduces the bioavailability of protein and give them a dark color, thus limiting there use in bread making. The known methods of purifying the protein products from phenolic compounds, in particular chlorogenic and caffeic acids, mostly limited to washing with solvents or using membrane technology. However, in most cases, their application is inefficient for an adequate removal of the phenolic compounds (Dominguez et al. 1995).
The main objective of this study was to extract protein isolate from sunflower meal and to investigate the production of fortified white wheat bread by the addition of SMPI to the bread formula.
Materials and methods
Sunflower meal
Sunflower meal produced by Oil factory “Krasnodar”, Krasnodar, Russia was used. The main characteristics of the meal as the follows: Dry matter 91 ± 1.15 %, fat 1.20 ± 0.25, total crude protein 39.2 ± 0.51 %, total soluble protein 68.65 ± 0.63 %, ash 0.65 ± 0.11 %, total fibres 20.63 ± 0.11 %. The mean size of the meal particles was <2 mm.
Wheat flour
Standardized wheat flour was used for dough (bread) preparation (Russian standard Р 52189–2003). The main characteristics of the flour are as follows: total humidity 13.32 ± 0.15 %, acidity 3.3 ± 0.05°, ash 0.73 ± 0.05 %, whiteness 44.75 ± 2.5 units, gluten 15 ± 1.35 %.
Raw SMPI extraction
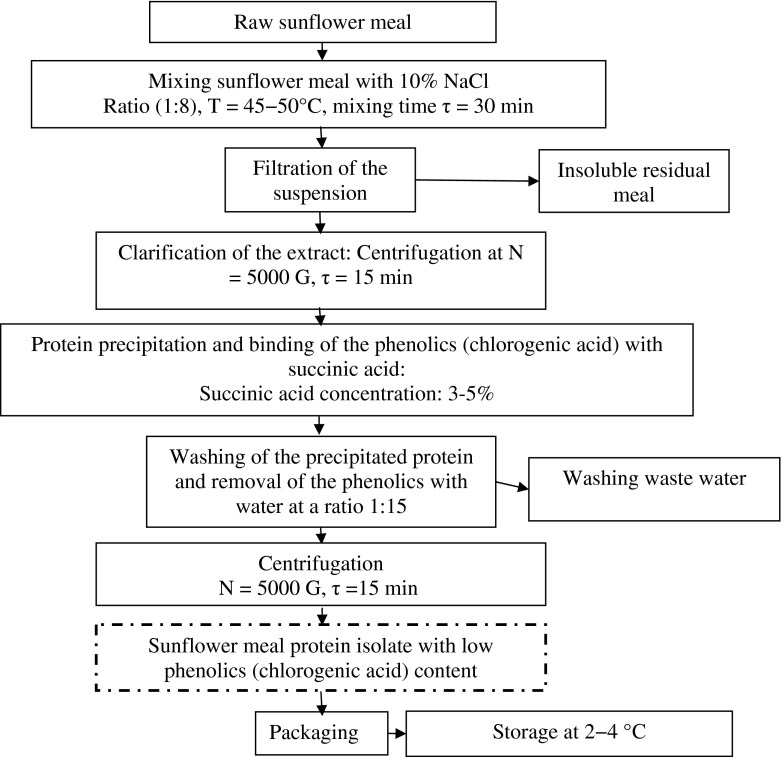
Figure 1 shows the general technological scheme with optimal conditions used to produce sunflower meal protein isolates. The extraction of protein from sunflower meal was performed with 10 % aqueous sodium chloride solution at a ratio 1:8 by weight between sunflower meal and NaCl solution. The extraction time was varied between 10 and 120 min at a temperature of 25, 35–45 and 50 °C. Upon completion of extraction, the insoluble meal residue was separated from the meal extract by filtration. The remained suspension was then clarified by centrifugation at 5000 g for 15 min. Extraction pH was set at 3, 5, 7, 8 and 10.
Fig. 1.
Flow diagram of the general technological scheme with optimal conditions used to produce sunflower meal protein isolate (SMPI)
SMPI purification from chlorogenic acid
In the present study, sunflower meal protein isolates with minimal content of phenolic compounds (chlorogenic and caffeic acids) was obtained by using 3–5 % succinic acid aqueous solution as purifying agent. A series of experiments aimed to obtain the optimal conditions (parameters) of protein precipitation and removal of phenolic compounds with succinic acid were conducted. As a result of mathematical processing of the experimental data by using a multiple regression equation, a surface response was obtained to predict the protein content in the final product with minimal amount of phenolic compounds. The following equation was obtained:
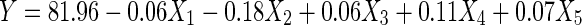 |
1 |
Where:
- Y
The protein content in the final extracted product,%
- X1
The concentration of sodium chloride,%
- X2
The succinic acid concentration,%
- X3
The ratio “protein/succinic acid”
- X4
Treatment duration, min
- X5
Temperature, °C.
Proximate analysis
Moisture content was determined gravimetrically according to AACC method 44–15A (American Association of Cereal Chemists, 1983). For this, the moisture-containing materials were dried at 100 °C in a forced-air oven overnight. The moisture content was calculated as (100 % - total dry matter). Oil content was determined by defatting the sample with hexane in a Soxhlet extractor. The oil was recovered and dried at 105 °C in the forced-air oven for 3 h before being weighed. Total protein content was determined by the Kjeldahl method according to AACC Method 46–12 (American Association of Cereal Chemists, 1983) and the conversion coefficient (6.25) was used to calculate protein content from the nitrogen number.
Chlorogenic and caffeic acid analysis
Chlorogenic and caffeic acid content was determined by the method described in (Fujioka and Shibamoto 2008) with slight modification. First, polymeric components from sunflower and SMPI samples were eliminated by using Carrez reagents I and II (Rincón et al. 2003). A sample of 3 ml of each material was mixed with 0.1 ml each of Carrez reagents I and II. A volume of 0.8 ml methanol was added and the mixture was well mixed by vortexing. It was then allowed to equilibrate during 20 min. The precipitated insoluble material was eliminated by centrifugation at 3500 g for 30 min. The obtained clarified solution was filtered with a with 0.2 μm filter. Chlorogenic and caffeic acid were analysed with a Zorbax Eclipse XDB C-18 5 μ column (150 × 4.6 mm i.d.) in an Agilent-1100 HPLC system equipped and a multiple wavelength detector. Two mobile phases were used: the phase-A was a 10 mM citric acid and the phase-B was HPLC-grade methanol. The analysis was carried out by using variable gradient mode. The initial was set at A/B ratio of 85/15 from 0 to 5 min, and then it was linearly increased up to 60/40 until 85 min. The flow rate was 0.7 ml/min. The injection volume was 10 μl. All analyses were carried out in triplicate.
Functional properties of the SMPI
Fat absorption capacity (FAC)
FAC value of the SMPI was evaluated as follows: 5 g of the SMPI were placed in a pre- weighed graduated centrifuge tube to which 30 ml of refined and deodorized sunflower oil was added. The mixture was stirred for 1 mine at 1000 g. The mixture oil/protein was left to stand for 30 min, and then centrifuged for 15 min at 4000 g. The non adsorbed oil was discharged and the tube/protein/oil complex was weighed. Fat absorption capacity was calculated by the following formula:
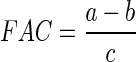 |
2 |
Where:
- a
the mass of the tube with the protein isolate and absorbed oil, g.
- b
the mass of the tube and protein isolate, g.
- c
Mass of the protein isolate, g.
Water-holding capacity (WHC)
The WHC of the SMPI was determined similarly to the fat absorption capacity, instead of oil using water.
Fat emulsifying capacity (FEC)
FEC of the SMPI was determined by the maximum amount of the oil introduced into a colloidal system of proteins to achieve coacervation under controlled conditions. To determine the fat emulsifying capacity of protein isolate, a 7 g sample was placed in a mixer to which 100 ml of distilled water was added. The mixture was suspended for 1 min in a mixer at a stirring speed of 4000 g. Then, refined and deodorized sunflower oil was added to the water/protein suspension and emulsified in a blender for 5 min. The resulting emulsion was placed in a graduated centrifuge tube and centrifuged for 5 min at 2000 g. Fat emulsifying capacity (%) of the SMPI was calculated using the following formula:
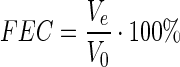 |
3 |
Where: Ve in the volume of the emulsified layer, (ml) and V0 is the initial volume of the mixture, (ml).
Foaming properties
To determine the foaming properties of the sunflower meal protein isolate, a sample of 6 g (by dry matter) was used. A weighed sample was placed in a beaker to which a volume of 25 ml of distilled water was added and carefully stirred until a homogeneous suspension was formed. The resulting slurry was quantitatively transferred into a graduated cylinder, and the total amount of the fluid was adjusted with distilled water until a final volume of 300 ml. A control experiment was prepared in which the SMPI was replaced by egg protein. The control and experimental samples were simultaneously shaken for 1 min. The formed foams were measured. The foaming ability (capacity) and foam stability of the SMPI were measured as follows:
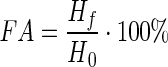 |
4 |
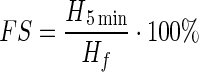 |
5 |
Where:
- FA
Foaming ability, %.
- Hf
Height of the foam, ml.
- H0
Height of the solution before the foam was formed, ml.
- FS
Foam stability, %.
- H5min
Height of the foam after 5 min, ml.
Dough preparation
All calculations were made as % to the initial wheat flour. Dough for bread making was prepared according to the Russian standard ТУ 9114-269-02067862-2009 (Puchkov 2004) as follows: A sample of 100 g of wheat flour was considered as the basic reference and all other ingredients were added as % of flour. Farinograph data were used to determine the amount of water to add the dough formula. Sourdough was used as fermentation tool and was prepared by adding instant baking yeast and salt in the amounts of 1.5 % and 1.3 %, respectively to wheat flour. The fortified bread with SMPI was prepared by adding 8-10-12 and 14 % (w/w) protein isolate to dough formula. Dependently of the amount of the SMPI used, water was added in the range of 30–37 %. The ingredients were then mixed during 15 min and the mixture was proofed for 210 min. The proofing procedure was carried in a proofer chamber at 35–40 °C and a relative humidity of 85 %. After fermentation, the dough was divided into equal pieces, put in special moulds and proofed 30 min at 35 °C and a relative humidity of 85 %.
Analysis of Vol. of CO2
The measurement of the produced carbone dioxide in the dough was measured through the expended volume of the dough after fermentation. This was based on the fact that among the gazes, during the fermentation process, the baking yeasts produce mainly CO2.
Determining the dough strength and bread baking
The dough strength was determined on a 40 g sample by using a penetrometer AP-4/2. After the dough was prepared, it was kneaded at a temperature of 35 °C for 30 s. Then it was divided into three equal parts. Each part was fixed on special nozzles and then placed in an incubator at fixed temperature of 35 °C for 60 min. The dough sample was covered with moistened glasses. After that, each dough sample was placed on a table to determine the depth of a 50 g K-60 probe introduced into the dough for 5 s. The penetrometer is scaled so as to give indication of the dough strength dependently of the value reached by the penetrometer as follows: 100 = very strong, 101–150 = strong, 151–200 = average, 201–250 = weak, 250 = very weak. Dough was baked duration 25 min at 230 °C in a rotational oven.
Amino acid composition
The nutritional value was measured through the product amino acid profile analysis. Amino acid composition of the end product (bread) supplemented with the SMPI was analyzed according to the method described in (Das et al. 2009). Before amino acid analysis, each bread sample was hydrolyzed in 6.0 N HCl. The solution was sealed in tube under nitrogen and incubated at a temperature of 110 °C for 24 h and dried in a desiccator. A derivatization procedure was followed according to this procedure: to a dried hydrolysed sample of the standard amino acid mixture, 1 mL of 1 mole/L sodium borate buffer at pH 6.6 containing 0.02 % of sodium azide and 0.8 μl of diethyl ethoxymethylenemalonate was added. The reaction was carried out at 50 °C for 60 min with vigorous shaking. The resulting mixture was cooled to room temperature and 20 μL was injected in to a Waters HPLC system equipped with a 4.6 × 150 mm C18 reversed phase column using a binary gradient system (Nova-Pack C 18, Waters, On, Canada). The solvents used were: solvent (A) 25 mM sodium acetate at pH 6 and acetonitrile as solvent (B). The solvent (A) was mixed with 0.02 % sodium azide. The flow rate was set at 1 mL/ min as follows: time 0–2 min, elution with A/B (95:5); from 2 to 40 min linear gradient A/B (95:5) to A/B (70:30); from 40 to 45 min A/B (70:30) to A/B (60:40); from 45 to 50 min A/B (60:40) to A/B (90:10). 10 μl of a standard mix (equivalent to 10 pmol of each amino acid) were used as reference material.
Sensory analysis
Sensory analysis of the bread supplemented with sunflower meal protein isolate (SMPI) was performed by a trained panel (ten persons) and by an untrained panel (15 persons). A descriptive sensory analysis was first performed. Bread samples were cut in slices of 2 cm thick on glass dishes equipped with code random numbers. They were served in a totally randomized manner. Bread samples were served at ambient temperature of approximately 22 ± 1 °C and the analyses were carried out under normal lightening conditions. After tasting a sample, the bread attributes were described: soft texture, sticky texture, mouthfeel, crust colour, pore size distribution, regularity and uniformity of the pores, visual bread mass volume, aroma, presence of specific smells like mould odour, sunflower odour, taste, bitter taste, yeast taste, salty taste, and moist odour. The attribute intensities were rated on scales of 0–10, where the value 0 is the lowest intensity and the value 10 corresponded to the highest intensity of the evaluated sensory attribute (Schoenlechner et al. 2010).
Statistical analysis
All experiments were carried out in triplicate. Results are shown as mean values. Data were statistically analysed by Statgraphic Plus for Windows v. 5.1 (StatPoint Inc., Virginia, USA). One-way ANOVA was used to compare the sample mean values at P < 0.05.
Results and discussion
Protein extractability
The extraction yield of the SMPI ranged between 46.4 ± 1.26 and 50.35 ± 1.19 %. The end product extracted from the sunflower meal contained 70–72 % humidity (28–30 %) dry matter and its purity in terms of total protein content was 94.42 ± 1.09 %. The highest yield was obtained at an extraction temperature of 45–50 °C. A ratio 1:8 of the initial sunflower meal to 10 % NaCl solution was also found to be an optimal extraction parameter. Effect of the duration of the extraction procedure reached a plateau after 30 min, suggesting that the extractable proteins reached the maximal level. This value was taken as an optimal extraction time in the optimized technological scheme. Removal of the suspended non soluble residual meal particles were removed by simple centrifugation at rotational speed from 5000 to 30000 g. It was found that the protein solution obtained by clarification at 5000 g did not significantly (p > 0.084) differ from the one clarified at the highest centrifuge speed. The extraction pH had significant effect (P < 0.001) and the highest yield was obtained under alkaline conditions at pH 10. The obtained SMPI was collected and dried under mild conditions and the final product had white color whereas classically the same product is generally characterized by dark color. The obtained results were in good agreement with the previously reported information in scientific literature. Indeed, in (Pickardt et al. 2009), it was reported that NaCl was highly effective to extract protein from sunflower meal. Because of the impaired solubility of sunflower proteins at low pH, the potential of sodium chloride (NaCl) to improve protein extractability was evaluated in the pH range 2–11. The authors found that increasing NaCl concentration up to 2.8 mole/L significantly enhanced protein extractability. In our study, 10 % NaCl solution corresponded to molar concentration of 1.77 mole/L. Similarly, in (Weisz et al. 2010) it was reported that sunflower proteins was successfully extracted using 1.3 mole/L NaCl and pH 6.
Chlorogenic and caffeic acid content
The results on chlorogenic and caffeic acids contents in the purified SMPI by treating in succinic acid are 412.8 ± 20.31 mg/kg and 2.2 ± 0.19 mg/kg respectively. The same treatment in hydrochloric acid yielded a meal protein isolate with chlorogenic and caffeic acids contents of 590.8 ± 31.28 mg/kg and 8.3 ± 1.18 mg/kg, respectively. According to the obtained results, it was found that the purified SMPI using succinic acid had chlorogenic acid content of 30 % lower than the protein purified in hydrochloric acid. At the same time, the caffeic acid content in the targeted meal protein isolate was 4 times lower than in the protein isolate obtained using hydrochloric acid for phenolics removal. Thus, the SMPI purified with the use of succinic acid contained minimal amount of phenolic compounds (chlogenic and caffeic acids), a factor making this protein as a suitable ingredient to be used in bread making technology to improve the nutritional quality (total protein content and specific amino acid composition) of wheat bread flour without any risk of deterioration of the color of the crumb.
Analysis of the regression equation (Eq. 1) showed that the total protein content in the final product was significantly influenced by the “protein/succinic acid” ratio, duration and temperature of the purification process in the succinic acid solution. The optimal parameters were the followings protein/succinic acid ratio of 1:11; process duration of 25 min and treatment temperature of 50 °C. The obtained SMPI was characterized by low chlorogenic and caffeic acids contents. In the present study, the targeted SMPI was first extracted under alkaline conditions with NaOH and then simultaneously precipitated/purified by both HCl and succinic acid at pH 4.5, corresponding to the isoelectric point of the major part of the proteins contained in the isolate. The pH 4.5 was favourable because the interaction between chlorogenic acid with sunflower protein isolate is the lowest at this condition. Indeed, Saeed and Cheryan (1989) investigated the interaction between these components with continuous diafiltration at 3 ligands to protein molar ratios and pH values 3, 5, 7 and 9. They reported that pH influenced the extent of binding. The binding ratio was lowest at pH 5, irrespective of ligand to protein molar ratio. Binding was greater at pH 7 and pH 3. At pH 9, binding was lower than at pH 3 up to a certain free chlorogenic acid concentration. Above this concentration, binding was the higher at pH 9. Binding increased as the ligand to protein molar ratio increased irrespective of pH. There are two groups of binding sites in sunflower proteins at pH 3, 5, and 7 and three groups at pH 9. The observed difference between the effects of HCl and succinic acid on total chlorogenic and caffeic acids in the final protein isolate could be attributed to the affinity of these molecules to succinic acid.
Colour of the sunflower meal protein isolate
The obtained sunflower meal protein isolate (SMPI) following HCl and succinic acid purification was evaluated in terms of visual appearance and colour characteristics. The end products looked differently from the control bread made without adding SMPI. The HCl purified SMPI was dark-green while the succinic acid purified SMPI was characterized by a white to light creamy colour. According to the Hunter Lab scale colour measurement system, significant differences were observed. The HCl and succinic acid purified SMPI were characterized by L* values of 53.15 ± 1.26 and 94.12 ± 2.19, respectively. The problematic of the dark colour of sunflower protein isolate was previously reported by (Taha et al. 1981). They indicated that washing with water and drying with acetone improved the purity of the protein, but only little improvement in colour was attained. However, addition of sodium sulphite to the sodium hydroxide solution used for the extraction of the protein results in a significant improvement in the colour of the isolated protein. Nevertheless, this approach is not suitable since the extraction procedure is complicated by the need to use different chemicals. The use of succinic acid as simultaneous precipitation/purification agent is more suitable and economically more interesting. The colour improvement of the end product will enhance the potential use of sunflower meal derived proteins in food applications because the food use of sunflower protein isolates is limited by their dark colour which is due to a high content of chlorogenic acid. The later is readily oxidized, the oxidation products combine with the protein forming coloured compounds (Kratch et al. 1986) and (Lapteva et al. 1985).
Functional properties of sunflower meal protein isolate
Functional properties of the investigated sunflower meal protein isolate (SMPI) were compared with the functional properties of other protein components from plant and animal origin, and the results are presented in Table 1. The obtained results showed that the fat absorption capacity (FAC) of the SMPI exceed the FAC of wheat gluten and egg powder by 37.5 and 6.25 %, respectively. However, it was lower than the FAC of soybean lour and milk powder. It was found that water-holding capacity (WHC) and fat emulsifying capacity (FEC) of SMPI exceed the WHC and FEC of the other proteins, except the WHC of the whole soy flour. It was noted that the foaming capacity (FC) of the SMPI was higher than the FC of milk and egg powder by 3.3 and 4.9 times, respectively. The FC of the SMPI was higher than that of the whole soy flour by 44 %. However, the FC of the SMPI was lower than that of the other proteins. Regarding the foam stability (FS), the obtained results showed that foam formed by SMPI was lower only from the FS of the wheat gluten and whole soy flour. Based on these results, it was concluded that the traditionally used proteins in the bread making industry have significantly lower functional properties than those of SMPI, a fact which allows this ingredient to be used for the production of new a bakery products.
Table 1.
Functional properties of sunflower meal protein isolate (SMPI), wheat gluten, whole soy flower, soy protein isolate, skim milk powder, and egg powder
| Ingredient | Total protein content, % | g/g | % | |||
|---|---|---|---|---|---|---|
| FAC | WHC | FEC | FC | FS | ||
| Sunflower meal protein isolate (SMPI) | 94.9 ± 1.1a | 1.9 ± 0.24b | 2.0 ± 0.23b | 74.6 ± 1.7a | 49.0 ± 1.0c | 35.7 ± 1.2b |
| Wheat gluten | 81.3 ± 1.3b | 1.2 ± 0.12d | 1.9 ± 0.22b | 68.0 ± .01ab | 67.0 ± 2.1b | 47.0 ± 2.1a |
| Whole soy flower | 38.6 ± 1.7d | 4.4 ± 0.32a | 2.1 ± 0.14b | 46.0 ± 1.2c | 27.0 ± 0.95d | 36.0 ± 1.8b |
| Soy protein isolate | 95.7 ± 2.1a | 2.5 ± 0.21b | 3.5 ± 0.61a | 74.0 ± 2.9a | 113.0 ± 9.1a | 7.0 ± 0.8c |
| Skim milk powder | 46.0 ± 1.8c | 2.4 ± 0.27b | 0.4 ± 0.09c | 12.0 ± 1.8e | 15.0 ± 0.27e | 5.0 ± 0.22cd |
| Egg powder | 26.0 ± 0.96e | 1.8 ± 0.11bc | 1.9 ± 0.08b | 32.0 ± 2.1d | 10.0 ± 0.94f | 0 |
*(FAC) Fat absorption capacity, (WHC) Water-holding capacity, (FEC) Fat emulsifying capacity, (FC) Foaming capacity and (FS) Foam stability
Each observation is a mean ± SD of 3 replicate experiments
Influence of sunflower meal protein isolate on dough rheological properties
Effect of SMPI on the rheological properties of the wheat dough was investigated by determining the texture strength, elasticity and plasticity of the dough (Fig. 2a). According to the obtained results, it was established that the introduction of SMPI to dough formula has a strengthening effect on the dough. By adding 8, 10 and 12 % of SMPI, the K60 index was reduced by 9.6 %, 14.8 % and 17.3 %, respectively, compared with the control dough. The strengthening effect of the SMPI was confirmed by the changes of the plasticity determined by texturometric analysis (Fig. 2b). By adding 8, 10 and 12 % of SMPI to the wheat flour, the plastic properties of the obtained dough were reduced by 8.1 %, 17.2 % and 20.5 %, respectively. At the same time, data analysis showed that elastic properties of the supplemented dough decreased by 9.1 % 15.1 % and 22.7 % respectively. Taking into account that the gliadin has an isoelectric point at pH 9.8, and the isoelectric point of the used SMPI is at pH 4.7, one can assume that due to their opposite electric charges, protein-protein electrostatic interaction may occur between protein components of the wheat flour and SMPI. Thus, the introduction of the SMPI to dough formula reinforced its structure due to electric interactions.
Fig. 2.
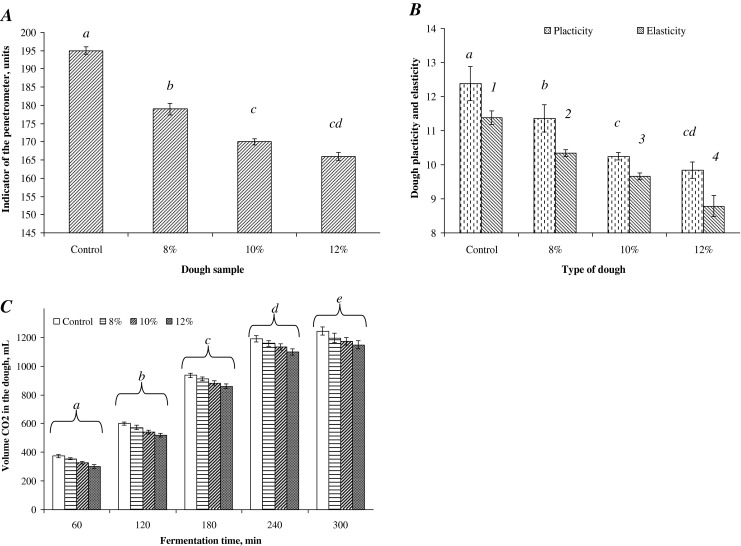
Effect of the amount of sunflower meal protein isolate SMPI added to the wheat flour on: a dough strength, b elastic and plasticity of the dough, and c volume of CO2 formed during fermentation. Each observation is a mean ± SD 3 replicate experiments
Effect of the SMPI on wheat flour baking properties
It was found that the addition up to 10 % of SMPI to the wheat flour did not significantly affect the techno-functional properties of the final product, whereas when more than 12 % of the SMPI was added a significant deterioration of the color of bread was observed. Effect of SMPI on the content and quality of gluten in the final bread formula is shown in Table 2. From these data, it appeared that the addition of SMPI to the wheat flour did not significantly decrease the amount of wet gluten, while it caused significant increase of the amount of the dry gluten compared to the control wheat flour. The increase of the dry gluten varied between 2.5 and 11 %, depending of the amount of the protein isolate added. This phenomenon was due to partial involvement of the SMPI in the protein complex involving gluten. The used sunflower protein showed a strengthening effect on wheat gluten, as confirmed by the indicator of the depth of gluten penetrometer K20, a measure of the compressive strain of gluten and its stretch. When 8, 10, 12 and 14 % of the SMPI was added to the wheat flour, the deformation index decreased by 13 %, 20.6 %, 26 % and 30.5 %, respectively, in comparison with the control sample. At the same time, the index K20 –was also reduced by 8.5 %, 12.8 %, 16.2 % and 20.1 %, respectively. The gluten extensibility of the supplemented samples also decreased by 3.9 %, 8.5 %, 13 % and 18 %, as the protein isolate was added. The obtained results allowed us to select a supplementation (dosage) level in the range of 8–12 % of the sunflower meal protein isolate. Over 12 % of the protein isolate added, the gluten became stiff, and acquired a non elastic structure and gray color. The strengthening of the gluten structure may be due to the fact that the SMPI is characterized by a high content of mono amine-dicarboxylic acids (glutamic and aspartic acid) and basic amino acids histidine and arginine. All these amino acids, by their reactive functional groups, they can interact with reactive functional groups of wheat gluten. Taking into account this fact, it is possible to assume the existence of ionic and electrostatic interactions between the reactive groups of gluten and those of the used sunflower protein isolate, causing the appearance of an additional type of intermolecular interactions, resulting in strengthening the structure of wheat gluten. Moreover, due to the presence of hydroxyl amino acids (serine and threonine) in the sunflower meal protein isolate, interactions between reactive groups of gluten molecules and the used protein isolate are really possible through hydrogen bonds. Effect of the used SMPI was studied on the gas formation (dough expansion) as functions of the amount of the protein isolate used (Fig. 2c). Analysis of the obtained results showed that for 5 h of dough fermentation, the volume of carbon dioxide emitted by the introduction of 8, 10 and 12 % of the SMPI was reduced by 6.8 %, 11.1 % and 14.2 %, respectively, compared with the control sample. This may be due to the inhibitory effect of the acidic protein isolate on yeast cells.
Table 2.
Effect of sunflower meal protein isolate (SMPI) on quality and quantity of gluten in the supplemented wheat flour
| SMPI dosage,% | Wet gluten content,% | Dry gluten content,% |
|---|---|---|
| 0 | 28.3 ± 1.0a | 9.4 ± 0.64a |
| 8 | 28.1 ± 0.95a | 9.64 ± 0.31a |
| 10 | 27.8 ± 1.6a | 10.1 ± 0.11a |
| 12 | 27.5 ± 0.81a | 10.3 ± 1.0a |
| 14 | 27.1 ± 1.08a | 10.4 ± 0.84a |
*Each observation is a mean ± SD of 3 replicate experiments
Influence of SMPI on the bread quality
To investigate the possibility of using SMPI in the formulation of bread to increase its biological value without visible deterioration of the quality indicators, a series of laboratory test baking were conducted. On the basis of the published data on the technological incompatibility between sunflower proteins and wheat flour as well as on the results we obtained on the effects of SMPI on the properties of the gluten, it was concluded that the optimal way to overcome this incompatibility reducing is a two-stage method of dough preparation with the introduction of the SMPI directly to the dough. Effect of SMPI on the total acidity of wheat dough during fermentation was studied. After 60 min fermentation, comparative analysis of the obtained data showed that at supplementation levels of 8, 10 and 12 % protein isolate, dough made with SMPI had total acidity higher than that of the control dough of 2.8 %, 5.7 % and 8.6 %, respectively. It was noticed that with the use of sunflower protein isolate, the optimal value of the dough acidity 20–25 min earlier than that of the control dough. Thus, the change in the acidity of the dough during fermentation indicates an intensification of the process of acid formation, due to the introduction of supplementary nitrogen compounds, particularly amino acids contained in the sunflower meal protein isolate, and some extend because of the presence of succinic acid. This can be considered as a positive factor in the technological scheme of bread making, because it reduces the duration of the fermentation test. Comparative analysis of quality control of the experimental samples of bread baked by using regular and thick sourdough starters as fermentation tool with different dosages of the sunflower protein isolate showed a significant difference in the organoleptic and physico-chemical properties of the different bread samples. By using 8 and10% sunflower meal protein isolate, crumb color of the bread prepared by the regular and thick sourdough starter was not significantly different from the control bread, while by adding 12 % and more of the sunflower protein isolate, the bread was gray (Table 3). Analysis of physico-chemical parameters of the obtained bread showed that the best results were obtained in the preparations where SMPI was added directly to fermentation thick sourdough starter. Specific volume of the bread was significantly higher by 5–7 % compared with similar bread samples prepared by conventional fermentation sourdough starter, bread dimensional stability by 5–7.5 %, porosity by 4.2–5.5 %, and total compressibility of the crumb by to 11.3–14.4 %, respectively. It was also noticed that the addition of 8 %, 10 % and 12 % SMPI to the dough made with regular and thick sourdough starter, the acidity of the final products similarly increased compared with the control by 3.5 %, 7.4 %, and 14.8 %, respectively. This observation was probably due to the presence of some residual succinic acid in then used protein isolate. A further increase of added SMPI to the bread formula yielded and excessive increase of the dough acidity, a factor which negatively affected the quality of the final product. It was established that the average values of the physico-chemical properties of the bread made with various amounts of the SMPI and thick fermentation sourdough starter were at least 10 % higher than the physico-characteristics properties of the bread prepared with the regular sourdough starter. Thus, the most significant influence on the physico-chemical quality of the final bread made with the addition of SMPI was the type of the fermentation sourdough starter. The best results were obtained with the use of thick fermentative sourdough starter. This was due to restricted contact between meal protein isolate and the other compounds of the wheat flour.
Table 3.
Effect of sunflower meal protein isolate (SMPI) on the quality of wheat bread according to different preparation modes of the dough
| Quality index | Methods of dough preparation | |||||||
|---|---|---|---|---|---|---|---|---|
| With regular sourdough starter | With thick sourdough starter | |||||||
| Control | Amount of SMPI, % flour mass | Control | Amount of SMPI, % flour mass | |||||
| 8 | 10 | 12 | 8 | 10 | 12 | |||
| Bread mass volume, mL/100 g | 320 ± 1.1b | 328 ± 3.1b | 336 ± 2.2a | 330 ± 3.1b | 324 ± 1.2b | 340 ± 2.1a | 348 ± 2.1a | 346 ± 1.7a |
| Dimensional stability, | 0.40 ± 0.11a | 0.41 ± 0.11a | 0.42 ± 0.11a | 0.43 ± 0.17a | 0.40 ± 0.11a | 0.42 ± 0.09a | 0.45 ± 0.04a | 0.46 ± 0.11a |
| Acidity, degrees | 2.7 ± 1.1a | 2.8 ± 0.1a | 2.9 ± 0.11a | 3.1 ± 0.27a | 2.7 ± 0.17a | 2.8 ± 0.27a | 2.9 ± 1.31a | 3.1 ± 2.19a |
| Porosity, % | 70 ± 1.2b | 71 ± 2.1b | 73 ± 3.1b | 72 ± 1.1b | 72 ± 2.1b | 75 ± 1.9a | 77 ± 2.2a | 76 ± 2.9a |
| Structural and mechanical properties of the crumb | ||||||||
| ∆Нtotal | 92 ± 1.1c | 96 ± 1.3b | 99 ± 3.1b | 97 ± 2.1b | 97 ± 2.1b | 108 ± 2.1a | 115 ± 2.1a | 111 ± 2.1a |
| ∆Н(plasticity) | 68 ± 0.91b | 70 ± 2.1b | 72 ± 2.1b | 70 ± 1.9b | 72 ± 1.1b | 81 ± 1.1ab | 85 ± 1.9a | 83 ± 1.2a |
| ∆Н(elasticity) | 24 ± 0.21b | 26 ± 0.94ab | 27 ± 0.19ab | 27 ± 0.27ab | 25 ± 0.61b | 27 ± 0.09ab | 30 ± 0.94a | 28 ± 0.64ab |
| Organoleptic evaluation: Color of the crumb | Typical for this type of product | Light | Light | With a touch of gray color | Typical for this type of product | Light | Light | With a touch of gray color |
*Each observation is a mean ± SD of 3 replicate experiments
To determine the optimal dosage of the sunflower meal protein isolate, mathematical methods were used. A full factorial experimental design was used. Statistical analysis of the experimental data allowed obtaining regression equation (polynomial equation of first degree), adequately describing the effect of the SMPI concentration (amount added to the bread formula), (D1) and gluten quality (D2) on final bread mass volume (P1) and bread porosity (P2):
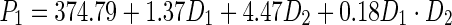 |
6 |
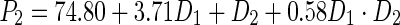 |
7 |
From Eq. (6), it seems that the mass volume of final product was affected by both the amount of the added SMPI and quality of gluten. At the same time, Eq. (7) shows that the porosity of the bread was mostly affected only by the amount of the added SMPI to the bread formula. Determination of the optimal values of the amount of the added SMPI and quality of gluten was performed by the method of penalty functions. This approach permitted obtaining of the maximum values of D1 and D2 as follows: D1 = 10.02 % and D2 = 92 units. These values provided the optimal conditions for the best values of the bread mass volume and porosity. Thus, based on the research work conducted in the present project, it was possible to conclude that the calculated optimal amount of the SMPI to be added to the bread formula was in good agreement with the experimental results. Thus, a dosage of 10 % meal protein isolate by weight of flour was chosen for further experiments. The best way to add the SMPI to the dough was studied using its particular functional properties. Given the high water-holding capacity and fat absorption ability of the protein isolates, it was added in two forms to the dough: (1) in the form of protein-aqueous suspension or (2) as an emulsion. The protein suspension was prepared from the SMPI and water in a ratio of 1:3. Protein emulsion was prepared by intensive mechanical mixing of the sunflower meal protein isolate, unrefined sunflower oil and water in a ratio of 3:1:2. The control sample of the dough was prepared refined sunflower oil without the addition of sunflower meal protein isolate. The dough was baked with the use of a fermentative thick sourdough starter with the introduction of the SMPI at the mixing stage. Analysis of the obtained results showed that the introduction of the SMPI in the form of a protein aqueous suspension and protein-fat emulsion affected the organoleptic and physico-chemical properties of the final product. They were all characterized by a well-developed porosity compared with the control bread. By introducing the investigated SMPI as protein-water slurry (suspension), the targeted bread specific volume was higher by 2.4 % compared to the control. It had also porosity, total compressibility, and crumb dimensional stability higher by 8.2 %, 5 %, 2.7 %, respectively, compared with the control bread. The addition of the SMPI in the form of a protein emulsion increased the bread parameters in comparison with the control as follows: bread specific volume by 7.9 %, porosity by 6.8 % and total compressibility of the bread crumb by 19.5 %, and dimensional stability by 10 %. Thus, based on the obtained results, the addition of SMPI as a protein suspension and protein emulsion had a positive impact on the overall quality of the prepared wheat bread. However, it is important to mention that the introduction of the SMPI in the form of a protein emulsion had the highest significant impact on the bread quality. Indeed, the bead specific volume was higher by 5.3 % compared with the bread made by introducing the protein isolate as a protein-water suspension, a higher porosity by 4 %, and total compressibility of the bread crumb by 10.5 %, and product dimensional stability higher by 4.7 %.
In the work of (Gatta and Piergiovanni 1996), a study has been reported in which baking and nutritional properties were investigated on mixtures of wheat flour and defatted sunflower meal. Loaves prepared with different mixtures from 5 to 20 % sunflower meal were evaluated for volume, weight and external score. According to the results they reported, it was found that the addition of sunflower meal enhanced protein content but had a detrimental effect on bread quality. They reported that incorporation of 10 % sunflower meal was found to be acceptable. To improve bread appearance they suggested the addition of maltose to dough. From nutritional value point of view, they stated that the incorporation of sunflower meal produced bread with a remarkable content of trypsin inhibitors compared with the control bread made with regular wheat flour. Consequently, proteins are less susceptible to proteolysis. This lower availability of proteins partially nullifies the improvement of the nutritional quality related to the incorporation of sunflower meal. In (Taha et al. 1982), a study was reported in which sunflower seed protein concentrate was used to enrich wheat bread. Bread was prepared by adding 5 and 10 % proteins to the basic formula. According to the reported results, chemical analysis of the enriched bread revealed significant increase in the protein content by values ranging from 21.55 to 56.88 %. Sensory analysis of the supplemented bread included aroma, crumb colour, crust colour, texture, flavour, and overall acceptability. The mean scores for these characteristics show that the addition of sunflower meal protein to wheat bread formula was favourably accepted, especially at 5 % level.
Bread nutritional value
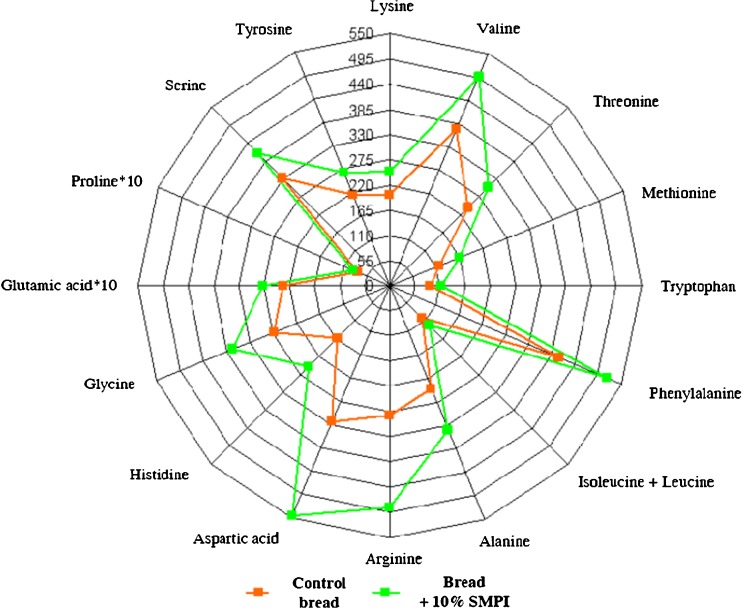
In the present study, SMPI was added to bread formula to improve its nutritional value. Indeed, systematical analysis of the information reported in many research papers on the biological or nutritional value of bread showed that white bread has a deficit of three essential amino acids (Onishchenko 2007). It is therefore interesting to evaluate the effect of the SMPI on the nutritional value of wheat bread. According to the results obtained in our study (Fig. 3), it has been established that the incorporation of SMPI in the wheat bread formula increased its content of the both essential and nonessential amino acids. At a supplementation level of 10 % with SMPI, bread content of most essential amino acids (lysine, threonine, tryptophan, leucine, isoleucine and phenylalanine) increased by 12–13 %. Moreover, valine and methionine content in the supplemented bread increased by 16 % and 20 %, respectively. Histidine and arginine by 29 % and 33 %, respectively, compared with the control read. The overall content of the other amino acids increased by an average value of 20 %. Thus, the addition of SMPI to wheat bread recipe significantly (P < 0.001) improved its amino acid composition, especially the essential amino acids.
Fig. 3.
Nutritional value of the sunflower meal protein isolate (SMPI) supplemented bread expressed as its amino acid profile
Moreover, the degree of satisfaction of the daily requirement of protein and essential amino acids for adults and children by the use of 10 % SMPI supplemented bread was calculated. According to the obtained data, (Tables 4 and 5), it has been found that the child’s daily need for protein and essential amino acids by consuming 150 g of white bread supplemented with 10 % SMPI is satisfied by 23.2 % and 38.9 %, respectively (Onishchenko 2008). At the same time, the daily need of primary school age child is covered for lysine by 11 %, threonine and methionine by 20 %, phenylalanine and valine by 29 % and 32 %, respectively. The daily needed amount of leucine and isoleucine, arginine is covered by 38 %, for tryptophan by 46 %, and histidine by 64 %. The adult daily requirement for total protein and essential amino acids by the consumption of 350 g of white bread supplemented with 10 % SMPI is satisfied by 34 % and 55.6 %, respectively. The degree of daily requirement for adult is covered for lysine and methionine by 25.5 % and 29 %, respectively. For tryptophan - 35 %, valine - 43 %. The total daily amount of leucine, isoleucine, and threonine is satisfied by 48 %. Phenylalanine by 81 %. Thus, the use of bakery products enriched with SMPI, plays an essential role to satisfy the protein requirements and essential amino acids, a condition which allows us to position SMPI supplemented products as foods of high biological/nutritional value.
Table 4.
Satisfaction of the daily requirement for total protein and essential amino acids for primary school children (7 to 11 years old, average weight 35 kg) (Onishchenko 2007; Onishchenko 2008)
| Component | Daily recommended value | Content in 150**g bread with 10 % SMPI | Daily satisfaction, % of the recommended value |
|---|---|---|---|
| Total protein, g | 63 | 14.6 | 23.2 |
| Essential amino acids, mg | |||
| Lysine | 3043 | 337 | 11.1 |
| Valine | 1758 | 558 | 31.7 |
| Leucine + Isoleucine | 4366 | 1657 | 37.9 |
| Threonine | 2192 | 411 | 18.7 |
| Methionine | 1096 | 219 | 20 |
| Tryptophane | 321 | 152 | 47.3 |
| Phenylalanine | 2362 | 694 | 29.3 |
| Arginine | 1686 | 637 | 37.8 |
| Histidine | 529 | 342 | 64.6 |
| Total amino acids | 12855 | 5008 | 38.9 |
*SMPI Sunflower meal protein isolate
Table 5.
Satisfaction of the daily requirement for total protein and essential amino acids for adult (Onishchenko 2007; Onishchenko 2008)
| Component | Daily recommended value | Content in 350**g bread with 10 % SMPI | Daily satisfaction, % of the recommended value |
|---|---|---|---|
| Total protein, g | 77 | 34 | 44.1 |
| Essential amino acids, mg | |||
| Lysine | 3000 | 787 | 26.2 |
| Valine | 3000 | 1302 | 43.4 |
| Leucine + Isoleucine | 8000 | 3867 | 48.3 |
| Threonine | 2000 | 959 | 47.9 |
| Methionine | 2000 | 511 | 25.5 |
| Tryptophane | 1000 | 353 | 35.3 |
| Phenylalanine | 2000 | 1620 | 81 |
| Total amino acids | 21000 | 11686 | 55.6 |
*SMPI Sunflower meal protein isolate
Conclusions
The present work showed that some technologically important functional properties of SMPI such as fat absorption and water holding capacity are higher than those of wheat gluten. The introduction of SMPI to dough (bread) formula strengthens the impact of gluten complex of wheat flour, improves the consistency, plasticity and elasticity of the dough made from flour containing low quality gluten. The optimum dosage of the SMPI to be added to wheat dough formula was fixed to 10 % flour basis. The best way to introduce the SMPI to the dough formula was found to be in a form of protein-lipid emulsion and dough preparation on thick sourdough starter. The use of SMPI as ingredient in wheat bread making increased the biological and nutritional value of the bread by improved the qualitative and quantitative scores of the amino acid composition of the final product. The use of SMPI can be extended to other product such as traditional Indian bread (Naan), popular Kabylian bread (Tamtunt) and more. It can be also used to fortify what or cereal based food matrices such as spaghetti and macaronis.
Acknowledgments
The authors express deep gratitude to Professor Bochkova L. K. for scientific consultation.
References
- AACC . Official methods of analysis. Method 44–15A. American association of cereal chemistry. 8. St Paul: American Association of Cereal Chemistry; 1983. [Google Scholar]
- Ahmed A, Anjum F, Randhawa M, Farooq U, Akhtar S, Sultan M (2011) Effect of multiple fortification on the bioavailability of minerals in wheat meal bread. J Food Sci Technol (Online First). doi:10.1007/s13197-010-0224-9 [DOI] [PMC free article] [PubMed]
- Annapure US, Singhal RS, Kulkarni PR. Studies on deep-fat fried snacks from some cereals and legumes. J Sci Food Agr. 1998;76(3):377–382. doi: 10.1002/(SICI)1097-0010(199803)76:3<377::AID-JSFA957>3.0.CO;2-R. [DOI] [Google Scholar]
- Chavan JK, Kadam SS, Salunkhe DK, Beuchat LR. Biochemistry and technology of chickpea (Cicer arietinum L.) seeds. Crit Rev Food Sci Nutr. 1987;25(2):107–158. doi: 10.1080/10408398709527449. [DOI] [PubMed] [Google Scholar]
- Das R, Bhattacherjee C, Ghosh S. Preparation of mustard (brassica juncea L.) protein isolate and recovery of phenolic compounds by ultrafiltration. Ind Eng Chem Res. 2009;48:4939–4947. doi: 10.1021/ie801474q. [DOI] [Google Scholar]
- Dodok L, Ali MA, Hozová B, Halasová G, Polacek I. Importance and utilization of chickpea in cereal technology. Acta alimentaria. 1993;22:119–129. [Google Scholar]
- Dominguez H, Nunez MJ, Lema JM. Aqueous processing of sunflower kernels with enzymatic technology. Food Chem. 1995;53(4):427–434. doi: 10.1016/0308-8146(95)99838-Q. [DOI] [Google Scholar]
- Dorrell DG, Vick BA. Properties and processing of oilseed sunflower. In: Schneiter AA, editor. Sunflower technology and production Madison. Wisconsin: American Society of Agronomy; 1997. pp. 709–744. [Google Scholar]
- FAO (2012) FAOSTAT-Agriculture. http://faostat.fao.org/. Accessible date 2012-07-10
- Fujioka K, Shibamoto T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008;106(1):217–221. doi: 10.1016/j.foodchem.2007.05.091. [DOI] [Google Scholar]
- Gatta CD, Piergiovanni AR. Technological and nutritional aspects in hyperproteic bread prepared with the addition of sunflower meal. Food Chem. 1996;57(4):493–496. doi: 10.1016/0308-8146(95)00200-6. [DOI] [Google Scholar]
- Gómez M, Oliete B, Rosell CM, Pando V, Fernández E. Studies on cake quality made of wheat–chickpea flour blends. LWT - Food Sci Technol. 2008;41(9):1701–1709. doi: 10.1016/j.lwt.2007.11.024. [DOI] [Google Scholar]
- González-Pérez S, Merck KB, Vereijken JM, van Koningsveld GA, Gruppen H, Voragen AGJ. Isolation and characterization of undenatured chlorogenic acid free sunflower (helianthus annuus) proteins. J Agr Food Chem. 2002;50(6):1713–1719. doi: 10.1021/jf011245d. [DOI] [PubMed] [Google Scholar]
- González-Pérez S, Vereijken JM. Sunflower proteins: overview of their physicochemical, structural and functional properties. J Sci Food Agr. 2007;87(12):2173–2191. doi: 10.1002/jsfa.2971. [DOI] [Google Scholar]
- Gonzalez-Perez S, Vereijken JM, Merck KB, Gruppen H, Voragen AGJ. Emulsion properties of sunflower (helianthus annuus) proteins. J Agricultural Food Chem. 2005;53(6):2261–2267. doi: 10.1021/jf0486388. [DOI] [PubMed] [Google Scholar]
- Khoshgozaran-Abras S, Azizi M, Bagheripoor-Fallah N, Khodamoradi A (2012) Effect of brown rice flour fortification on the quality of wheat-based dough and flat bread. Journal of Food Science and Technology (Online First). doi:10.1007/s13197-012-0716-x [DOI] [PMC free article] [PubMed]
- Kratch VV, Lapteva NA, Alexeyeva MV, Vaintraub IA. Sunflower protein isolates chromaticity Part 2. Effect of chlorogenic acid binding on the chromaticity and available lysine content. Food /Nahrung. 1986;30(3–4):459–462. doi: 10.1002/food.19860300387. [DOI] [Google Scholar]
- Kumar S, Chauhan J, Kumar A. Screening for erucic acid and glucosinolate content in rapeseed-mustard seeds using near infrared reflectance spectroscopy. Journal of Food Science and Technology. 2010;47:690–692. doi: 10.1007/s13197-010-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapteva NA, Brega VO, Kratch VV, Alexeyeva MV, Vaintraub IA. Sunflower protein isolates chromaticity Part 1. Comparison of isolates obtained by various methods and a simplified method of estimation (Short communication) Food/Nahrung. 1985;29(8):803–806. doi: 10.1002/food.19850290821. [DOI] [Google Scholar]
- Livingstone AS, Feng JJ, Malleshi NG. Development and nutritional quality evaluation of weaning foods based on malted, popped and roller dried wheat and chickpea. Int J Food Sci Technol. 1993;28(1):35–43. doi: 10.1111/j.1365-2621.1993.tb01249.x. [DOI] [Google Scholar]
- Martinez E, Duvnjak Z. Enzymatic degradation of chlorogenic acid using a polyphenol oxidase preparation from the white-rot fungus Trametes versicolor ATCC 42530. Process Biochem. 2006;41:1835–1841. doi: 10.1016/j.procbio.2006.03.036. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054(1–2):95–111. doi: 10.1016/j.chroma.2004.08.059. [DOI] [PubMed] [Google Scholar]
- Onishchenko GG (2007) Recommended average daily sets of products for babies 7–11 and 11–18 years. Guidelines № 01100/8604-07-34: MM: Moscow, Russia
- Onishchenko GG (2008) Norms of physiological requirements for energy and nutrients for different groups of the Russian Federation: Guidelines № 2.3.1.2432-08 M. Moscow, Russia
- Pickardt C, Neidhart S, Griesbach C, Dube M, Knauf U, Kammerer DR, Carle R. Optimisation of mild-acidic protein extraction from defatted sunflower (Helianthus annuus L.) meal. Food Hydrocolloids. 2009;23(7):1966–1973. doi: 10.1016/j.foodhyd.2009.02.001. [DOI] [Google Scholar]
- Puchkov LI (2004) Laboratory handbook on baking technology. 4th ed (in Russian). ISBN 5-901065-65-4.
- Rajasekaran A, Kalaivani M (2012) Designer foods and their benefits: A review. Journal of Food Science and Technology (Online First). doi:10.1007/s13197-012-0726-8 [DOI] [PMC free article] [PubMed]
- Rincón F, Martínez B, Delgado JM. Detection of factors influencing nitrite determination in meat. Meat Sci. 2003;65(4):1421–1427. doi: 10.1016/S0309-1740(03)00065-2. [DOI] [PubMed] [Google Scholar]
- Rodrigues IM, Coelho JFJ, Carvalho MGVS. Isolation and valorisation of vegetable proteins from oilseed plants: Methods, limitations and potential. J Food Eng. 2012;109:337–346. doi: 10.1016/j.jfoodeng.2011.10.027. [DOI] [Google Scholar]
- Saeed M, Cheryan M. Sunflower protein concentrates and isolates low in polyphenols and phytate. J Food Sci. 1988;53:1127–1131. doi: 10.1111/j.1365-2621.1988.tb13545.x. [DOI] [Google Scholar]
- Saeed M, Cheryan M. Chlorogenic acid interactions with sunflower proteins. J Agr Food Chem. 1989;37(5):1270–1274. doi: 10.1021/jf00089a015. [DOI] [Google Scholar]
- Schoenlechner R, Mandala I, Kiskini A, et al. Effect of water, albumen and fat on the quality of gluten-free bread containing amaranth. Int J Food Sci Technol. 2010;45:661–669. doi: 10.1111/j.1365-2621.2009.02154.x. [DOI] [Google Scholar]
- Sosulski F. Organoleptic and nutritional effects of phenolic compounds on oilseed protein products: A review. J Am Oil Chem Soc. 1979;56(8):711–715. doi: 10.1007/BF02663047. [DOI] [Google Scholar]
- Sripad G, Prakash V, Narasinga Rao MS. Extractability of polyphenols of sunflower seed in various solvents. J Biosc. 1982;4:145–152. doi: 10.1007/BF02702723. [DOI] [Google Scholar]
- Taha FS, Abbasy M, El-Nockrashy AS, Shoeb ZE. Countercurrent extraction-isoelectric precipitation of sunflower seed protein isolates. J Sci Food Agr. 1981;32(2):166–174. doi: 10.1002/jsfa.2740320212. [DOI] [Google Scholar]
- Taha FS, Attia M, Shehata NA. Protein enrichment of bread: I. Chemical and sensoric evaluation. Z Ernährungswiss. 1982;21(1):77–82. doi: 10.1007/BF02023043. [DOI] [PubMed] [Google Scholar]
- Thompkinson D, Bhavana V, Kanika P (2012) Dietary approaches for management of cardio-vascular health- a review. Journal of Food Science and Technology (Online First) [DOI] [PMC free article] [PubMed]
- Weisz GM, Schneider L, Schweiggert U, Kammerer DR, Carle R. Sustainable sunflower processing-I. Development of a process for the adsorptive decolorization of sunflower (Helianthus annuus L.) protein extracts. Innovative Food Sci Emerg Technol. 2010;11(4):733–741. doi: 10.1016/j.ifset.2010.05.005. [DOI] [Google Scholar]