Abstract
The effect of vacuum packaging and pomegranate peel extract on ground goat meat and cooked nuggets during refrigerated storage (4 ± 1 °C) was evaluated. Three different treatments evaluated were: I). Aerobic packaging (AP); II) Vacuum packaging (VP) and III). Vacuum packaging along with 1 % pomegranate peel extract (VP + PPE). Results of quality evaluation showed that VP and VP + PPE maintained a more stable colour than AP. In all treatments, a significant (P < 0.05) increase in hardness and gumminess of nuggets was observed during the storage. But, VP nuggets showed minimum changes in texture parameters. TBARS values were significantly (P < 0.05) lower in VP and VP + PPE than AP. In ground meat, VP reduced the TBARS by 27 % and PPE reduced the TBARS by 41 %. In nuggets, TBARS was decreased by 17 % and 40 % in VP and VP + PPE respectively. Total plate counts were significantly higher (>log 7) in AP than VP meat and nuggets. Thus VP and PPE have a synergistic antioxidant effect and VP extended the refrigerated shelf life of goat meat and nuggets.
Keywords: Vacuum packaging, Natural extract, Lipid oxidation, Texture profile, Goat meat
Introduction
Meat and meat products are susceptible for rapid quality and shelf-life deteriorations because of their rich nutritional composition. Major quality deterioration is due to oxidation and microbial spoilage. These changes are responsible for meat products to become unacceptable from a sensory, nutritional, microbiological or safety perspective. Food additives that are classified as GRAS (generally recognized as safe) are used to extend the shelf life and overall quality through inhibition of oxidative rancidity and spoilage bacteria. Further vacuum packaging can be combined to maintain microbial and sensory quality of the meat products. (Tang et al. 2001). Use of synthetic chemicals is limited by food legislation and therefore, use of natural ingredients has been encouraged as consumers and processors are concerned about the safety of synthetic food additives. Rosemary, sage, thyme, chitosan, fenugreek, pomegranate, grape seed etc have been studies as source of natural antioxidants in different meat products (Nassu et al. 2003; Rojas and Brewer 2008; Devatkal et al. 2011). However, very few studies have been carried out on combined effect of vacuum packaging and natural antioxidant in meat and meat products. Therefore, the objective of this study was to evaluate the combined effect of vacuum packaging and pomegranate peel extract on quality characteristics of ground goat meat and cooked goat meat nuggets stored at 4 ± 1 °C.
Material and methods
Meat and raw materials
Fresh goat meat (thigh muscles of goats slaughtered at an age about 2 year and weighing 18–20 kg) was obtained from local retail meat processing units. Meat was stored at 4 °C for approximately 4 h before use. Fresh meat samples were obtained separately for each of the replications. Fresh pomegranate (Punica granatum) was also obtained from retail fruit market. Pomegranate peel extract (PPE) was prepared by extracting in sterile distilled water for 1 h at 60 °C. Nylon-6 packaging film (100 μm) obtained from local manufacturer was used for packaging. Microbiological media (Himedia) and other chemicals used were of analytical grade.
Preparation goat meat samples
About 3 kg goat meat was minced twice 10 mm plate followed by 8 mm plates using a meat mincer (Sirman, Italy). After mincing the meat samples (500 g each) were assigned to one of the following three treatments : I. AP/control (meat with 2 % salt and packed aerobically); II. VP (meat with 2 % salt and vacuum packed) and III. VP + PPE (meat with 2 % salt and 1 w/v % pomegranate peel extract and vacuum packed) and all samples were stored at 4 ± 1 °C for 9 days. During this storage period color, TBARS values and total plate counts were evaluated at an interval of 3 days.
Preparation of goat meat nuggets
About 5 kg of goat meat was deboned, chilled overnight (4 ± 1 °C), ground twice through 8 mm plate using a meat mincer (Sirman, Italy) and used for meat nuggets preparation. Freshly obtained meat was used for each replication. Sodium chloride, sodium tripolyphosphate, sodium nitrite, spice mix, condiments (onion and garlic paste) and refined sunflower oil were other ingredients used for the preparation of goat meat nuggets. The formulation was similar to all treatments except that 1 % w/v PPE was added in VP + PPE treatment. This emulsion was manually filled in stainless steel boxes (about 450 g) and lids of the boxes were tightly closed. The moulds were steam cooked to an internal temperature of 80–82 °C. Internal temperature of blocks was measured with a digital thermometer having a probe. After, internal temperature reached the 80–82 °C, blocks were held at this temperature for 15 min. After this process meat blocks were cooled to room temperature. Nuggets from these cooked blocks were used for packaging and analysis. These nuggets (500 g each) were assigned to one of the following three treatments : I. AP/control (aerobically packed nuggets ); II. VP (vacuum packed nuggets) and III. VP + PPE (nuggets with1% w/v pomegranate peel extract and vacuum packed) and all samples were stored at 4 ± 1 °C for 25 days. During this storage period color, texture, TBARS values and total plate counts were evaluated at an interval of 5 days.
Instrumental colour evaluation
Colour properties of goat meat and nuggets was evaluated by Hunter Colorimeter (Hunter and Harold 1987). Colorimetric analysis on ground meat samples and cooked nuggets was performed using a Hunter Lab Miniscan XE Plus colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA) with 25 mm aperture set for illumination D65, 10° standard observer angle. Hunter L (lightness), a (red ± green) and b (yellow ± blue) values were measured on the outer and inner surfaces of horizontally cut nuggets. The hue and chroma values were calculated by using formulae, tan−1b/a and (a2 + b2)1/2 respectively. Total color difference (ΔE) was calculated using the following equation: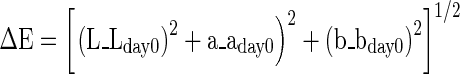 (Seydim et al. 2006).
(Seydim et al. 2006).
Texture profile analysis (TPA)
The textural properties of nuggets were evaluated using a texture analyzer (Stable Micro System, Model No TA. HDi UK). Texture profile analysis (Bourne 1978) was performed using five pieces of each sample (2 cm × 2 cm), which were compressed twice to 50 % of the original height by a compression probe (P 75). A crosshead speed of 2 mm/s was used. The following parameters were determined: hardness (N) = maximum force required to compress the sample; springiness (cm) = ability of sample to recover its original form after a deforming force was removed; cohesiveness = extent to which sample could be deformed prior to rupture (A2/ A1, A1 being the total energy required for first compression and A2 the total energy required for the second compression); chewiness (N-cm) = work to masticate the sample for swallowing (S × gumminess) (Bourne 1978).
Thiobarbituric acid reactive substances (TBARS) value
Lipid oxidization was monitored by measuring thiobarbituric acid reactive substances at different intervals of storage. TBARS were determined using extraction method described by Witte et al (1970). TBARS were extracted in chilled 20 % trichloroacetic acid. Thiobarbituric acid extracts of each sample were used for measuring the absorbance at 520 nm using a uv-visible spectrophotometer (UV-1800 Shimadzu Corp. Japan). 1, 1, 3,3, tetraethoxypropane (Sigma Aldrich, New Delhi, India) was used as standard for TBARS assay. TBARS numbers were calculated as mg of malonaldehyde per kg of meat sample.
Determination of total plate counts
A 10 g of meat sample was homogenized in 90 ml of sterile 0.1 % peptone water using sterile pestle and mortar for 2 min. Appropriate decimal dilutions were prepared in 0.1 % sterile peptone water and used for assessing the microbial quality using pour plate method as described in ICMSF (1978). Total plate counts were assessed using poured plates of plate count agar, incubated at 37 °C for 48 h. Microbial colonies from the plates containing 30–300 colonies were counted and expressed as log10 cfu/g.
Statistical analysis
Three replications of the study were performed and measurements of all parameters were made in duplicate. Mean values for various parameters were calculated and compared by analysis of variance using the SPSS software for windows (version 13.0). Storage data of TBARS values were analyzed using two-way ANOVA with treatment and storage time as main effects. Statistical significance was identified at the 95 % confidence level (P < 0.05). The average values were reported along with mean standard error (± standard error).
Results and discussion
Changes in coulor, lipid oxidation and total plate counts of ground meat
Results of instrumental color evaluation of stored ground meat are presented in Table 1. Lightness significantly (P < 0.05) increased during storage in all treatments. Although vacuum packaged treatment had higher lightness, no significant difference was seen between the treatments on any given days of storage. Redness and chroma values increased significantly in all treatments during storage. However, started decreasing when storage period advanced. However, Berruga et al. (2005) found that redness decreased and b value increased significantly in aerobic packaged lamb meat and colour was more stable in vacuum packaging. Kerry et al. (2000) found that ‘L’and ‘b’ values showed no overall trends during MAP storage of lamb patties. They further observed that lamb patties packaged under vacuum had higher Hunter ‘a’ values compared to all other packaging systems. Similarly Smith et al. (1983) studied the evolution of colour in fresh lamb meat packed under MAP and vacuum, and found a more desirable appearance of lamb meat packed in vacuum than in MAP.
Table 1.
Effect of vacuum packaging and pomegranate peel extract on colour , TBARS values and total plate counts of ground goat meat stored at 4 ± 1 °C
| Treatments | Storage period (days) | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | Treatment means | MSE (±) | |
| L value | ||||||
| AP | 39.1a | 45.4b | 45.6b | 46.7b | 44.2 | 0.30 |
| VP | 39.1a | 43.5b | 48.0b | 44.4b | 43.7 | 0.37 |
| VP + PPE | 43.4a | 47.2c | 43.4b | 46.0c | 45.0 | 0.74 |
| a value | ||||||
| AP | 11.8a | 13.2b | 14.4c | 13.3b | 11.9 | 0.30 |
| VP | 11.7a | 14.3c | 13.2b | 13.8b | 12.1 | 0.26 |
| VP + PPE | 11.8a | 13.1b | 13.7b | 11.8a | 12.6 | 0.53 |
| b value | ||||||
| AP | 12.1 | 12.1 | 12.4 | 12.1 | 12.2 | 0.20 |
| VP | 12.1 | 11.6 | 11.1 | 11.1 | 11.4 | 0.17 |
| VP + PPE | 11.9 | 12.4 | 10.3 | 11.3 | 11.2 | 0.35 |
| Chroma | ||||||
| AP | 13.8a 1 | 17.9b | 19.0b | 18.0b | 17.2b | 0.32 |
| VP | 13.8a 1 | 18.5b | 17.2b | 17.7b | 16.8b | 0.28 |
| VP + PPE | 16.1a 2 | 17.9b | 17.2b | 16.4b | 16.9b | 0.56 |
| Hue | ||||||
| AP | 41.1 | 42.3 | 40.4 | 42.3 | 46.5 | 0.61 |
| VP | 41.1 | 39.2 | 39.6 | 38.3 | 44.5 | 0.53 |
| VP + PPE | 42.4 | 43.6 | 37.0 | 43.5 | 41.6 | 1.06 |
| TBARS values (mg malonaldehyde/kg meat) | ||||||
| AP | 0.15a | 0.21a | 0.28a2 | 0.24a2 | 0.222 | 0.07 |
| VP | 0.13a | 0.16a12 | 0.19a1 | 0.17a1 | 0.161 | 0.06 |
| VP + PPE | 0.09a | 0.12a1 | 0.17a1 | 0.14a1 | 0.131 | 0.03 |
| Total plate counts (log10 cfu/g) | ||||||
| AP | 4.8a | 5.6b | 7.6c2 | 7.7c2 | 6.42 | 0.29 |
| VP | 4.6a | 5.5b | 6.2c2 | 6.6c1 | 5.81 | 0.25 |
| VP + PPE | 4.5a | 5.4b | 6.1c1 | 6.5c1 | 5.61 | 0.51 |
N = 15( color) , 6( TBARS and total plate counts) Mean values with different superscript within a row and numerical ( within a column) are significantly different. AP Aerobic Packaging, VP Vacuum packaging, VP + PPE Vacuum packaging +1 % pomegranate peel extract
TBARS values of raw goat meat during storage significantly increased. However the pattern of this increase was different in three treatments. In control, TBARS increased during all storage intervals, but in vacuum packaged meat, TBARS decreased after 6 days of storage period. Further, on any given day of storage TBARS was significantly lower in VP and VP + PPE than AP. Observation on percent increase in TBARS indicated an increase by 46 % in AP, 26 % in VP and 30 % in VP + PPE treatments. Thus a significant difference between AP and VP was observed for percent increase in lipid oxidation. Among three treatments the overall TBARS was significantly lower in VP + PPE followed by VP and highest in AP indicating a significant reduction in TBARS by VP and PPE. Observation on percent reduction indicated that VP reduced the TBARS of control by 27 % and addition of PPE reduced the TBARS of control by 41 %. Formanek et al (1998) reported that vacuum packing was most effective in controlling the development of lipid oxidation in ground beef. Kerry et al. (2000) observed the lower TBARS values in lamb patties packaged under vacuum compared to other packaging systems and suggested that vacuum is very effective in controlling lipid oxidation. Similarly Berruga et al. (2005) observed a lower lipid oxidation in lamb meat packed in vacuum in comparison to those packaged in modified atmospheres. The higher malondialdehyde levels in AP than in vacuum may consequently be due to greater levels of residual oxygen (Smiddy et al. 2002). The concentration of O2 in the package atmosphere is the determining factor for the rate of lipid oxidation. Exclusion or limiting of the oxygen content VP packaging atmospheres limits oxidation and thus resulted in lower TBA values for these meat samples (Seydim et al. 2006).
The observation on correlation between TBARS values and colour properties suggested that a negative correlation between redness and TBARS values but a positive correlation of TBARS with b values. Similar observations were also reported by Berruga et al. (2005) in vacuum packaged lamb. Oxidation might also be responsible for the formation of metmyoglobin and gradual changes in colour attributes during storage (Jeremiah 2001). Several researchers have suggested that lipid oxidation promotes oxymyoglobin oxidation (Yin and Fautsman 1993; O’Grady et al. 2000).
Total plate counts significantly increased in all treatments during storage of fresh goat meat (Table 1). Although a linear increase in APC was seen in all treatments, the threshold of log7 was observed on 6th day in AP. But, in vacuum packaged treatments, the APC did not reach the threshold limit of log7 even after 9 day. Observation on percent change in total plate counts indicated an increase by 60 %, 45 % and 44 % in AP, VP and VP + PPE treatments. Babji et al (2000) have also reported a lower total plate counts in vacuum packaged goat meat as compared to aerobic packaged. It has been reported that microbial spoilage of meat occurs with APC at levels of 7–8 log cfu/cm2 or g (Jeremiah 2001). In general, vacuum-packaged meats are generally quite stable at low temperatures (Labadie 1999). While the shelf life of meat that is packaged with films that are highly permeable to oxygen is approximately 1 week, the shelf life of vacuum- packaged meat is around 3–12 weeks, when stored at 0 °C (Gill and Newton 1978). Further, in vacuum-packaged meat, spoilage is determined by temperature, relative humidity, partial oxygen and carbon dioxide pressure and storage conditions usually favour the growth of lactic acid bacteria in this type of product (Brightwell et al. 2009).
Changes in coulor, texture, lipid oxidation and total plate counts of nuggets
The effect of packaging and storage period on colour attributes of cooked nuggets is given in Table 2. Lightness value did not show any significant change during storage in all treatments. Redness value was not significantly affected by packaging method but storage period had a significant effect on redness in all treatment. Further, storage period and packaging method did not affect significantly the yellowness, chroma and hue values of cooked nuggets. Colour difference was also not affected by the storage time, but vacuum packaging and PPE significantly (P < 0.05) reduced the colour difference. During later days of storage, colour difference was significantly lower in VP + PPE. This indicates the useful effect of VP and PPE in minimizing the colour loss during the storage. The evolution of L, a and b values suggests that vacuum, followed by VP + PPE, maintained a more stable colour than AP.
Table 2.
Effect of vacuum packaging and pomegranate peel extract on colour properties of goat meat nuggets stored at 4 ± 1 °C
| Treatments | Days | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | Treatment means | MSE (±) | |
| L (Lightness)value | ||||||||
| AP | 58.9 | 57.3 | 57.8 | 57.2 | 58.2 | 58.2 | 57.9 | 0.16 |
| VP | 58.9 | 57.8 | 58.7 | 58.1 | 59.3 | 58.2 | 58.2 | 0.11 |
| VP + PPE | 58.4 | 57.6 | 57.3 | 57.4 | 57.8 | 57.3 | 57.6 | 0.28 |
| a (redness) value | ||||||||
| AP | 11.8b | 10.4a | 9.5a | 9.8a | 10.6a | 10.6a | 10.4 | 0.88 |
| VP | 11.8b | 10.4a | 10.5a | 10.9a | 11.4a | 11.3a | 11.0 | 0.62 |
| VP + PPE | 11.1b | 10.4a | 10.1a | 10.6a | 10.5a | 11.0a | 10.6 | 0.15 |
| b (yellowness) value | ||||||||
| AP | 20.9 | 22.4 | 22.5 | 22.6 | 21.3 | 21.3 | 21.8 | 0.13 |
| VP | 21.1 | 21.7 | 21.1 | 21.2 | 21.1 | 20.4 | 21.1 | 0.94 |
| VP + PPE | 20.8 | 20.7 | 19.8 | 20.0 | 20.2 | 19.6 | 20.2 | 0.23 |
| Chroma (colour intensity) | ||||||||
| AP | 24.0 | 24.8 | 24.5 | 24.6 | 23.9 | 23.8 | 24.3 | 0.11 |
| VP | 24.2 | 24.1 | 23.6 | 23.9 | 24.1 | 23.4 | 23.9 | 0.81 |
| VP + PPE | 23.6 | 23.2 | 22.2 | 22.7 | 22.8 | 22.5 | 22.8 | 0.20 |
| Colour difference | ||||||||
| AP | 19.6 | 20.3 | 20.7 | 17.52 | 19.62 | 23.42 | 20.22 | 0.48 |
| VP | 20.1 | 20.3 | 20.2 | 17.42 | 21.52 | 21.51 | 20.12 | 0.34 |
| VP + PPE | 19.6 | 19.8 | 18.1 | 15.81 | 18.71b | 21.01 | 18.81 | 0.83 |
| Hue | ||||||||
| AP | 60.5 | 65.0 | 66.9 | 66.4 | 63.4 | 63.3 | 64.2 | 0.27 |
| VP | 60.7 | 64.3 | 63.4 | 62.7 | 61.4 | 61.1 | 62.2 | 0.19 |
| VP + PPE | 61.9 | 63.2 | 62.8 | 61.8 | 62.4 | 60.7 | 62.1 | 0.46 |
N = 15. Mean values with different superscript within a row (alphabets) and within a column (numerical) are significantly (P < 0.05) different. AP Aerobic packaging, VP Vacuum packaging, VP + PPE Vacuum packaging +1 % pomegranate peel extract
Textural properties of nuggets during storage period are depicted in Table 3. Hardness significantly increased during the storage period irrespective of packaging method. Maximum change of hardness was observed in AP and VP + PPE as compared to VP. The increase in hardness might be due to loss of moisture during storage. Use of vacuum packaging assisted in maintaining the hardness values to the original values. Springiness and cohesiveness did not show any significant trend and storage and vacuum packaging did not have any significant effect on cohesiveness and springiness of cooked nuggets. Chewiness significantly increased after 5th day of storage as compared to initial storage period. After 5th day, chewiness remained constant throughout the storage. This trend was similar in all treatments. Springiness was significantly higher in VP + PPE and AP than VP nuggets. In earlier studies also it has been reported that loss of moisture during storage would increase the textural parameters in meat products (Martinez et al. 2004). Results of lipid oxidation in nuggets are presented in Table 2. TBARS significantly increased during the storage period. However this increase was significantly higher in AP and VP but extract containing sample showed no significant change in TBARS values. At any given interval of storage, significantly higher TBARS value was observed in AP and lowest in VP + PPE. The nuggets containing PPE and packaged in VP showed a significant lower TBARS than AP and VP. The overall treatment means of TBARS was significantly higher in AP, followed by VP and lowest in VP + PPE. There was a significant reduction in TBARS values by VP and further reduction of TBARS by use of PPE. Observation on percent changes in TBARS showed that an increase by 66 % in AP, 64 % in VP but a negative change (−9.6 %) in VP + PPE. Similarly percent reduction of TBARS values indicated that VP reduced TBARS by 17 % and 40 % in VP + PPE. Thus vacuum packaged nuggets showed a significant lower increase in TBARS during storage period. Further use of PPE also significantly reduced TBARS in vacuum packaged meat. Devatkal and Naveena (2010) have also demonstrated the antioxidant effect of pomegranate peel extract in goat and chicken meat. They have reported that PPE is rich in total phenolics having strong antioxidant effect. The results of vacuum packaging are in agreement with the findings of earlier studies on vacuum packaged meat products (Kerry et al 2000; O’Grady et al. 2000; Jeremiah 2001).
Table 3.
Effect of vacuum packaging and pomegranate peel extract on textural properties of goat meat nuggets stored at 14 ± 1 °C
| Treatments | Days | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | Treatment | MSE (±) | |
| Hardness | ||||||||
| AP | 17.6a | 22.5b | 25.0c | 21.1b | 20.5b | 21.6b | 21.41 | 0.83 |
| VP | 17.8a | 21.9b | 26.9c | 25.8c | 21.7b | 19.8b | 22.32 | 0.59 |
| VP + PPE | 21.7a | 22.1a | 23.8b | 23.4b | 21.1a | 26.9b | 23.22 | 1.45 |
| Cohesiveness | ||||||||
| AP | 0.61 | 0.60 | 0.54 | 0.58 | 0.60 | 0.58 | 0.58 | 0.03 |
| VP | 0.62 | 0.59 | 0.53 | 0.55 | 0.59 | 0.61 | 0.58 | 0.08 |
| VP + PPE | 0.61 | 0.59 | 0.56 | 0.56 | 0.58 | 0.58 | 0.58 | 0.03 |
| Springiness | ||||||||
| AP | 0.98 | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | – |
| VP | 0.98 | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | – |
| VP + PPE | 0.98 | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | – |
| Chewiness | ||||||||
| AP | 10.7a | 13.3b | 13.1b | 12.1b | 11.9a | 12.3b | 12.2 | 0.47 |
| VP | 11.1a | 12.7b | 13.8b | 13.7b | 12.5b | 12.02b | 12.0 | 0.33 |
| VP + PPE | 13.1b | 12.7a | 13.2b | 12. 2a | 12.8a | 15.2c | 13.3 | 0.81 |
| Gumminess | ||||||||
| AP | 10.8a | 13.4b | 13.2b | 12.2b | 12.1b | 12.5b | 12.4 | 0.46 |
| VP | 11.2a | 12.8b | 13.9c | 13.8c | 12.6b | 12.1b | 12.7 | 0.32 |
| VP + PPE | 13.3a | 12.8a | 13.4a | 12.9a | 12.3a | 15.4b | 13.3 | 0.79 |
N = 6. Mean values with different superscript within a row (alphabets) and within a column (numerical) are significantly (P < 0.05) different. AP Aerobic packaging, VP Vacuum packaging, VP + PPE Vacuum packaging +1 % pomegranate peel extract
Total aerobic plate counts of nuggets during storage period are presented in Table 4. Initial aerobic counts were not significantly different in three treatments. APC significantly increase during all intervals in AP but in VP and VP + PPE this significant increased was only observed from 10th day of storage. Thus a slow growth of APC was observed in VP and VP + PPE. In AP, maximum prescribed limit of log7 cfu /g was observed on 15th day of storage and it reached an unacceptable level on 20th days of storage. In contrast, in VP and VP + PPE the maximum prescribed limit was observed on 25th day of storage and even after 25 days of storage total plate counts remained with in maximum permissible limits of log7 cfu /g. The observation on percent increase in APC during storage showed a 300 % increase in AP and 145 % in vacuum packaged nuggets. The overall treatments means of APC was sign ificantly higher in AP (log 6.3) as compared to vacuum packaged nuggets (log 5.4)
Table 4.
Effect of vacuum packaging and pomegranate peel extract on TBARS values and microbial quality of goat meat nuggets stored at 4 ± 1 °C
| Treatments | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | Treatment means | MSE (±) | |
| TBARS numbers (mg malonaldehyde/kg meat) | ||||||||
| AP | 0.33a1 | 0.44b3 | 0.47b3 | 0.50c3 | 0.56c3 | 0.552c | 0.483 | 0.02 |
| VP | 0.31a1 | 0.31a2 | 0.40b2 | 0.43b2 | 0.45b2 | 0.51c2 | 0.402 | 0.05 |
| VP + PPE | 0.31b1 | 0.26a1 | 0.28a1 | 0.31a1 | 0.31a1 | 0.28a1 | 0.291 | 0.08 |
| Total plate counts (log10 cfu/g) | ||||||||
| AP | 2.2a | 4.9a | 6.4a2 | 7.0a2 | 7.6a2 | 8.8a2 | 6.3a2 | 0.19 |
| VP | 2.2a | 4.6a | 5.2a1 | 6.2a1 | 6.6a1 | 7.4a1 | 5.5a1 | 0.14 |
| VP + PPE | 2.1a | 4.1a | 5.1a1 | 6.6a1 | 6.2a1 | 7.3a1 | 5.4a1 | 0.34 |
N = 6. Mean values with different superscript within a row (alphabets) and within a column (numerical) are significantly (P < 0.05) different. AP Aerobic packaging, VP Vacuum zpackaging, VP + PPE Vacuum packaging +1 % pomegranate peel extract
Conclusion
The results of present study showed the synergistic antioxidant effect of vacuum packaging and pomegranate peel extract. The overall shelf life of ground goat meat was increased by 3 days and that of nuggets by more than 5 days by using vacuum packaging.
References
- Babji YT, Murthy TRK, Anjaneyulu ASR. Microbial and sensory quality changes in refrigerated minced goat meat stored under vacuum and in air. Small Ruminant Res. 2000;36:75–84. doi: 10.1016/S0921-4488(99)00106-6. [DOI] [Google Scholar]
- Berruga MI, Vergara H, Gallego L. Influence of packaging conditions on microbial and lipid oxidation in lamb meat. Small Ruminant Res. 2005;57:257–264. doi: 10.1016/j.smallrumres.2004.08.004. [DOI] [Google Scholar]
- Bourne MC. Texture profile analysis. J Food Sci. 1978;32:62–67. [Google Scholar]
- Brightwell G, Clemens R, Adam K, Urlich S, Boerema J. Comparison of culture-dependent and independent techniques for characterization of the microflora of peroxyacetic acid treated, vacuum packaged beef. Food Microbiol. 2009;26:283–288. doi: 10.1016/j.fm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Naveena BM. Effect of salt, kinnow and pomegranate fruit by-product powders on color and oxidative stability of raw ground goat meat during refrigerated storage. Meat Sci. 2010;85:306–311. doi: 10.1016/j.meatsci.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Jaiswal P, Jha SN, Bharadwaj R, Viswas KN (2011) Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. Food Sci Technol. doi:10.1007/s13197-011-0351-y [DOI] [PMC free article] [PubMed]
- Formanek Z, Kerry JP, Buckley DJ, Morrissey PA, Farkas Effects of dietary vitamin E supplementation and packaging on the quality of minced beef. Meat Sci. 1998;50:203–210. doi: 10.1016/S0309-1740(98)00031-X. [DOI] [PubMed] [Google Scholar]
- Gill CO, Newton KG. The ecology of bacterial spoilage of fresh meat at chill temperatures. Meat Sci. 1978;2:207–217. doi: 10.1016/0309-1740(78)90006-2. [DOI] [PubMed] [Google Scholar]
- Hunter RS, Harold RW. The measurement of appearance. New York: John Wiley and Sons; 1987. [Google Scholar]
- ICMSF . International commission on microbiological specifications for foods. Microorganisms in foods. 1. Their significance and methods of enumeration. Canada: University of Toronto Press; 1978. [Google Scholar]
- Jeremiah LE. Packaging alternatives to deliver fresh meats using short or long-term distribution. Food Res Int. 2001;34:749–772. doi: 10.1016/S0963-9969(01)00096-5. [DOI] [Google Scholar]
- Kerry JP, O’Sullivan MG, Buckley DJ, Lynch PB, Morrissey PA. The effects of dietary a-tocopheryl acetate supplementation and modified atmosphere packaging (MAP) on the quality of lamb patties. Meat Sci. 2000;56:61–66. doi: 10.1016/S0309-1740(00)00021-8. [DOI] [PubMed] [Google Scholar]
- Labadie J. Consequences of packaging on bacterial growth. Meat is an ecological niche. Meat Sci. 1999;52:299–305. doi: 10.1016/S0309-1740(99)00006-6. [DOI] [PubMed] [Google Scholar]
- Martinez O, Salmerón J, Guillén MD, Casas C (2004) Texture profile analysis of meat products treated with commercial liquid smoke flavourings. Food Control 15:457–461
- Nassu RT, Goncalves LAG, da Silva MA, Beserra FJ. Oxidative stability of fermented goat meat sausage with different levels of natural antioxidant. Meat Sci. 2003;63:43–49. doi: 10.1016/S0309-1740(02)00051-7. [DOI] [PubMed] [Google Scholar]
- O’Grady MN, Monahan FJ, Burke RM, Allen P. The effect of oxygen level and exogenous a-tocopherol on the oxidative stability of minced beef in modified atmosphere packs. Meat Sci. 2000;55:39–45. doi: 10.1016/S0309-1740(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Rojas MC, Brewer MS. Effect of natural antioxidants on oxidative stability of frozen vacuum packaged beef and pork. J Food Quality. 2008;31:173–185. doi: 10.1111/j.1745-4557.2008.00196.x. [DOI] [PubMed] [Google Scholar]
- Seydim AC, Acton JC, Hall MA, Dawson PL. Effects of packaging atmospheres on shelf-life quality of ground ostrich meat. Meat Sci. 2006;73:503–510. doi: 10.1016/j.meatsci.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Smiddy M, Papkovskaia N, Papkovsky DB, Kerry JP. Use of oxygen sensors for the non-destructive measurement of the oxygen content in modified atmosphere and vacuum packs of cooked chicken patties: impact of oxygen content on lipid oxidation. Food Res Int. 2002;35:577–584. doi: 10.1016/S0963-9969(01)00160-0. [DOI] [Google Scholar]
- Smith GC, Seideman SC, Savell JW, Dill CW, Vanderzant Vacuum packaging versus modified atmosphere packaging of lamb loins. J Food Protec. 1983;46:47–51. doi: 10.4315/0362-028X-46.1.47. [DOI] [PubMed] [Google Scholar]
- Tang S, Kerry JP, Sheehan D, Buckley DJ, Morrissey PA. Antioxidative effect of added tea catechins on susceptibility of cooked red meat, poultry and fish patties to lipid oxidation. Food Res Int. 2001;34:651–657. doi: 10.1016/S0963-9969(00)00190-3. [DOI] [Google Scholar]
- Witte VC, Krauze GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]
- Yin MC, Fautsman C. Influence of temperature, pH, and phospholipids composition upon the stability of myoglobin and phospholipids: a liposome model. J Agric Food Chem. 1993;41:853–885. doi: 10.1021/jf00030a002. [DOI] [Google Scholar]


