Abstract
The present study was conducted to investigate the impact of various treatments of xylanase produced by Aspergillus niger applied in bread making processes like during tempering of wheat kernels and dough mixing on the dough quality characteristics i.e. dryness, stiffness, elasticity, extensibility, coherency and bread quality parameters i.e. volume, specific volume, density, moisture retention and sensory attributes. Different doses (200, 400, 600, 800 and 1,000 IU) of purified enzyme were applied to 1 kg of wheat grains during tempering and 1 kg of flour (straight grade flour) during mixing of dough in parallel. The samples of wheat kernels were agitated at different intervals for uniformity in tempering. After milling and dough making of both types of flour (having enzyme treatment during tempering and flour mixing) showed improved dough characteristics but the improvement was more prominent in the samples receiving enzyme treatment during tempering. Moreover, xylanase decreased dryness and stiffness of the dough whereas, resulted in increased elasticity, extensibility and coherency and increase in volume & decrease in bread density. Xylanase treatments also resulted in higher moisture retention and improvement of sensory attributes of bread. From the results, it is concluded that dough characteristics and bread quality improved significantly in response to enzyme treatments during tempering as compared to application during mixing.
Keywords: Xylanase, Dough, Baking, Tempering, Dough mixing
Introduction
Xylanase is an extracellular enzyme which hydrolyses β-1, 4 D-xylosidic linkages of highly polymerized and substituted β-1, 4 linked D-xylobiose, xylotriose and glucucoronosyl residues. The enzyme holds potential for the degradation of plant cell wall materials (Kulkarni et al. 1999; Omar et al. 2008).
Microbial xylanases (β-1, 4 D-xylan xylanohydrolase, EC 3.2.1.8) are being used in various industries including food, feed, textile and paper processing industries. On account of beneficial role of enzymes different methodologies are being used for their maximum biosynthesis (Chithra and Muralikrishna 2008; Ghildyal et al. 1980; Vijaya and Joseph 1979). In food and feed, they liberate nutrients by hydrolyzing the non- degradable hemicellulose fibers thus make the nutrients available (Leisola et al. 2002).
Xylanases enhance dough quality characteristics like stability, flexibility, extensibility, coherency by modifying the elasticity of gluten network which results in better crumb structure, improvement of crumb porosity, firmness, texture profile, higher moisture retention and extend shelf life of the bread (Haros et al. 2002; Romanowska et al. 2003; Collins et al. 2005).
Xylanases due to their multidimensional role in fermentation processes have gained immense importance. Sugars like xylose, xylobiose and xylo-oligomers can be prepared by the enzymatic hydrolysis of xylan and by ethanolic extraction (Jaddou et al. 1986). The depolymerization action of xylanase results in the conversion of polymeric substances into xylo-oligosaccharides and xylose (Subramaniyan and Prema 1998; Bajpai 1999; Omar et al. 2008).
Currently, much interest has been generated in using non-starch polysaccharide hydrolyzing enzymes in juice and baking industry. Xylanases of Aspergillus niger improve the overall bread quality characteristics (Maat et al. 1992; Jiang et al. 2005; Collins et al. 2005; Rao and Narasimham 1976; Vijayanand et al. 2010).
The loaf volume is one of the most important characteristics of bread quality. The bran portion absorbs large volume of water thus gluten network is not properly developed; poorly hydrated gluten results in lower loaf volume. Starch and non-starch hydrolyzing enzymes (xylanases) release free water therefore, modify the soluble fraction of dough. These effects are apparent immediately after mixing and continue during resting that change viscoelastic properties of dough contributing to the final bread volume (Butt et al. 2008).
The improved handling properties and stability of the dough are obtained by xylanase action on both soluble and insoluble pentosans in flour, thereby improving the elasticity of the gluten network, crumb structure and bread volume. These can be used for all types of bread as an alternative to/or in combination with emulsifiers (Olse 1995). These enzymes may also contribute to eliminate the use of chemical additives such as bromate (Maat et al. 1992; Kulkarni et al. 1999). Enzyme addition significantly shortens fermentation time without affecting pH or machinability of the dough. It improves bread volume, enhances aroma and results in softer texture. (Martínez-Anaya and Jimenez 1997).
The beneficial role of xylanases is generally attributed to their property to hydrolyze the cell wall polysaccharides. The monomers and oligomers resulting from enzyme activity affect the water balance and modify the protein-starch interaction during bread storage. Xylanases are used to hydrolyze complex cell wall that ultimately improves dough properties, enhances bread quality and reduces the staling rate (Sorensen et al. 2001; Wang et al. 2004).
The present work was planned to study the potency of xylanase from Aspergillys niger in improving the dough characteristics and bread quality attributes.
Materials and methods
Materials for dough making
A Pakistani popular wheat variety, Inqulab 91 used for the production of straight grade flour was obtained from the Post-Graduate Agricultural Research Station, University of Agriculture, Faisalabad, Pakistan. The xylanase (the pH and temperature for the optimum activity of enzyme were 7.5 and 55 °C respectively. Ahmad 2009 ) produced (in another study) from an indigenous stain of Aspergillus niger in the Microbiology and Biotechnology Laboratory of NIFSAT (National Institute of Food Science and Technology) was used in dough preparation while other raw materials like sugar, shortening, sodium chloride and yeast were procured from the Local Market.
Proximate analysis
Proximate analysis of flour (straight grade flour) was carried out according to their respective methods described in AACC (2000) i.e. moisture (Method 44–15 A), ash (Method 08–01), crude fat (Method 30–10), crude protein (Method 46–10), crude fiber (Method 32–10).
Enzyme addition during tempering
Initially, different doses (200, 400, 600, 800 and 1,000 IU in T1,T2,T3,T4 & T5 respectively) of purified enzyme along with the control (T0) were applied to 1 kg of wheat grains during tempering (a preliminary step before milling in which grains were moistened up to 15.5 %) and stored for 14 h at room temperature. The samples were agitated at different intervals for uniformity in tempering. Milling of tempered samples was carried out by Quadrumate Senior Mill to obtain straight grade flour for bread preparation.
Enzyme addition during mixing
In a parallel trial, same doses (200, 400, 600, 800 and 1,000 IU) of purified xylanase as those added in tempering process were applied to 1 kg of wheat flour (straight grade flour, obtained by milling of wheat kernels at 15.5 % moisture level using Quadrumate Senior Mill) during dough mixing stage.
Dough characteristics
Effect of enzyme addition, during tempering and dough mixing, on various dough characteristics like dryness (measured by touching the dough samples), stiffness (the resistance of dough when applying manual pressure), elasticity (the capability of the dough to regain its original shape after being extended), extensibility (the deformation to fracture when extended manually with a speed of about 5 cm/s) and coherency (the sheet forming capacity) was investigated following the procedure of Hilhorst et al. (1999). The dough samples were scored by skilled professionals (n = 3) 2 min after mixing for above mentioned characteristics on 10- point scale. The parameters were scored following the scale given below:
Dryness: 1 = Extra sticky, 10 = Dry, Stiffness: 1 = Soft, 10 = Stiff, Elasticity: 1 = Non-elastic, 10 = Very elastic, Extensibility: 1 = Non-extensible, 10 = Very extensible, Coherency: 1 = Non-coherent, 10 = Coherent.
Bread production
Breads from both types of flour (straight grade flour) samples along with untreated flour (control) were prepared in the Bakery Section of NIFSAT using straight-dough method (AACC 2000). All the ingredients (flour 200 g, sugar 6, salt 2 g, shortening 10 g, yeast 4 g, water 120 ml approx) were mixed for 5 min in a Hobart mixer (Model N-50, Hobart Corp. Troy, Chio, USA) to form dough and fermented at 30 °C and 75 % relative humidity. The dough was punched after 120 and 150 min. Afterwards, the dough samples were moulded, panned (100 g capacity) and kept for final proofing for 45 min at 35 °C and 85 % RH (Relative Humidity). The breads were baked at 230 °C for 25 min.
Moisture retention
Softness and pliability of bread is related to moisture retention capacity of loaves. Bread with high water retention show better performance in relation to freshness during storage and thus lowers staling rate. Moisture content of bread samples was determined using air forced draft oven (DO-1-30/02, PCSIR (Pakistan Council for Scientific and Industrial Research) at 105 °C till constant weight according to the Method No. 44–15 A (AACC 2000).
Determination of volume
The volume of breads baked from different flour samples were determined by rapeseed displacement method as described by AACC (2000).
Determination of relative and specific volumes
Relative (volume of bread samples compared with the control sample) and specific volume (volume/mass) of the bread samples was calculated following the method of Keskin et al. (2004).
Determination of bread density
The density (mass/volume) of breads prepared from various flour samples was determined as described by Keskin et al. (2004).
Sensory evaluation
Sensory evaluation of bread samples was performed by a panel of trained judges (n = 5) to find out the influence of enzyme treatment on different external (volume, crust color, symmetry of form, evenness of bake, character of crust and break & shred) and internal characteristics (grain, crumb color, aroma, taste, texture and chew ability) of bread following the procedure of Meilgaard et al. (1999). The sensory evaluation trials were performed at room temperature under white light. The bread samples were sliced, placed in trays and labeled with random codes. The judges were provided with distilled water to rinse their oral cavities before evaluating and rating the next sample.
Statistical analysis
In case of dough characteristics and moisture analysis three while in sensory evaluation five replications were carried out. The data obtained were analyzed by Complete Randomized Design (CRD) and level of significance was determined by analysis of variance technique as described by Steel et al. (1997).
Results and discussion
Proximate analysis of wheat flour
As the dough characteristics and bread quality attributes mainly depend upon the wheat used, so its proximate analysis was performed and different components were found as moisture:11.71 ± 0.02, Total ash: 0.58 ± 0.01,Crude fat: 0.93 ± 0.02,Crude protein: 11.31 ± 0.03,Crude fiber: 0.35 ± 0.01 and Nitrogen free extract (NFE): 86.83 ± 2.34.
Dough characteristics
After the application of xylanase during two stages i.e. tempering and mixing, dough samples were studied for various quality characteristics like dryness, stiffness, elasticity, extensibility and coherency. Means shown in Fig. 1 indicate that enzyme treatments during both steps had significant effect on all bread quality parameters except density.
Fig 1.
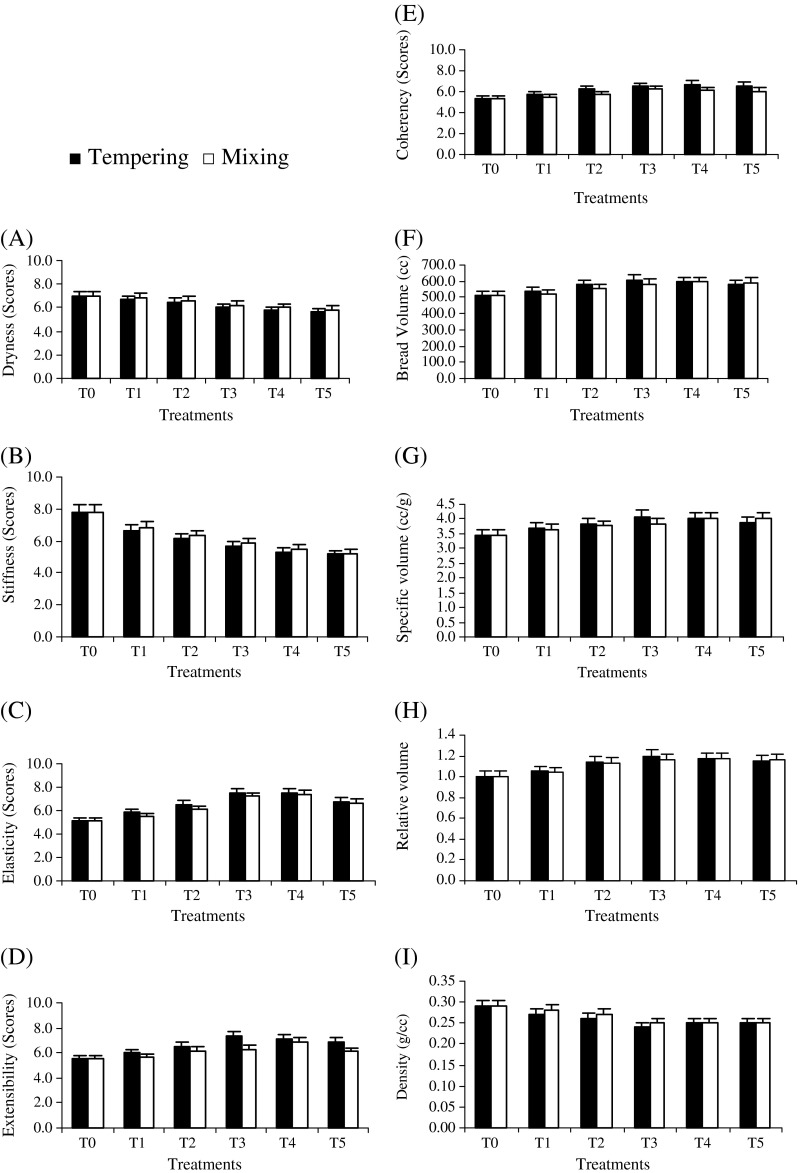
Effect of xylanase treatments during tempering of wheat kernels and mixing of wheat flour on physical quality characteristics of dough (n = 3)
Dryness
The means for dryness (Graph A) showed a negative association of dryness with enzyme concentration during tempering and dough mixing. The control sample (T0) exhibited maximum score for dryness (7.00 ± 0.502a). During tempering, when enzyme was added up to a concentration of 200 IU/kg as in treatment (T1), score for dryness i.e. 6.67 ± 0.294ab was noted, followed by T2 (400 IU/kg) with 6.50 ± 0.432abc score whereas, T5 (1,000 IU/kg) was assigned minimum score (5.67 ± 0.324d) for this trait. Likewise, in case of xylanase addition during dough mixing, 6.83 ± 0.293a score was given to T1 (200 IU/kg) and minimum 5.83 ± 0.293cd to T5 (1,000 IU/kg). The results expounded, the enzyme addition during tempering had more notable effect on dryness as compared to its application during dough mixing. The results are in agreement with Hilhorst et al. (1999) who reported similar trend for dryness of dough after xylanase treatment.
Stiffness
The highest score (7.83 ± 0.301a) for stiffness was given to untreated dough sample (Graph B). During tempering, increase in enzyme level showed inverse relation with stiffness and a score (6.67 ± 0.312bc) was noted in T1 (200 IU/kg) followed by T2 (400 IU/kg) with 6.17 ± 0.294bcde score whereas, T5 (1,000 IU/kg) was awarded minimum score (5.17 ± 0.214f). Similarly, when xylanase was used during dough mixing, a score of 6.83 ± 0.263b was given to T1 (200 IU/kg) and minimum score (5.21 ± 0.313f) was allotted to T5 (1,000 IU/kg). The results indicated that enzyme treatments exhibited capability to reduce dough stiffness as compared to control (T0) however, enzyme application during tempering showed more prominent effect to reduce stiffness as compared with its addition during dough mixing.
Elasticity
The means depicted in Graph C elucidate the impact of different xylanase treatments on the dough elasticity scores. Mean scores indicated that the elasticity of the resultant dough increased up to certain level of enzyme dosage and then started decreasing. In case of control sample (T0) the mean score for the elasticity was found to be 5.17 ± 0.324f. In case of tempering, when enzyme was added up to a concentration of 200 IU/kg (T1), a score of 5.83 ± 0.294def was noted for elasticity followed by T2 (400 IU/kg) with 6.50 ± 0.312bcd score, whereas, T3 (600 IU/kg) was assigned maximum score (7.50 ± 0.251a) for elasticity. Likewise, in case of xylanase addition during dough mixing, minimum score i.e. 5.53 ± 0.284ef was obtained by T1 (200 IU/kg) and maximum (7.33 ± 0.405ab) by T4 (800 IU/kg). The results indicated that the enzyme addition during tempering exerted momentous effect to enhance dough elasticity than that of dough mixing possibly due to more contact time of enzyme with the substrate and hence better performance.
Extensibility
Means for dough extensibility regarding variations in this attribute in response to xylanase treatments are represented in Graph D. During tempering, at a level of enzyme dosage of 200 IU/kg as in case of T1, the score increased to 6.00 ± 0.413cd then reached to its maximum (7.33 ± 0.261a) in T3 afterwards, decreased to 6.83 ± 0.29abc in T5 (1,000 IU/kg). In the same way, xylanase addition during dough mixing resulted in maximum score i.e. 6.87 ± 0.404abc for T4 (800 IU/kg) and minimum score 5.63 ± 0.373d for T1 (200 IU/kg). The results exhibited, the enzyme addition during tempering had more notable effect on dough extensibility than during dough mixing stage. Mathewson (2000) also reported increased extensibility due to enzyme supplementation in the dough making. The positive effect of xylanase on bread volume and other quality parameters is due to redistribution of water from pentosan phase to gluten phase. The increase in volume of gluten fraction increases its extensibility resulting in better oven spring and thus high loaf volume.
Coherency
It is obvious from mean scores (Graph E) that enzyme addition in either of the case i.e. wheat tempering or dough mixing resulted in increased coherency. The means demonstrated that the control treatment (T0) was assigned minimum scores i.e. 5.33 ± 0.285d. During tempering, increase in enzyme level showed positive impact on this parameter and maximum score (6.67 ± 0.284a) was granted to T4 800 IU/kg) followed by T5 (1,000 IU/kg) with 6.55 ± 0.293a score whereas, T1 (200 IU/kg) was assigned 5.67 ± 0.304bcd score. Similarly, when xylanase was added during dough mixing, 5.43 ± 0.332cd score for the parameter was observed in T1 (200 IU/kg) and maximum score i.e. 6.27 ± 0.381ab in case of T3 (600 IU/kg).
It is deduced from dough characteristics that enzyme addition is beneficial to improve the dough handling properties. Xylanase application, even at lower concentrations during tempering was more effective than at mixing stage to achieve the desired dough characteristics that make the bread making process cost effective. Hydrolysis of insoluble pentosans by xylanase promotes favorable changes in dough rheology thereby improves the gluten network; one of the important prerequisites for higher loaf volume. Overall, higher volume was observed in the enzyme treated dough showing its potential to be used in baking industry.
Effect of xylanase on bread quality
Volume and density
The means (Graph F) showed steady increase in bread volume as a function of xylanase addition during tempering up to 600 IU/kg (T3) and thereafter, a decrease in volume was observed. Minimum volume (510.0 ± 31.85g cc) was observed in control whilst, highest bread volume (610.0 ± 33.96a cc) was calculated for treatment T3 (600 IU/kg). When added during mixing stage, the parameter showed a slightly different behavior and increase in volume was noted up to a level of 800 IU/kg and then started decreasing. The results are supported by Romanowska et al. (2003) and Camacho and Aguilar (2003); they reported improvement in bread volume after xylanase treatment of bread flour. The ability of xylanase to increase the bread volume may be due the redistribution of water from pentosans to gluten phase that increases the volume of gluten fraction, results in extensible dough and thus high volume (Maat et al. 1992).
From the volume calculated through rapeseed displacement method, specific volume was calculated as presented in Graph G. Maximum value for specific volume was recorded in T3 (4.09 ± 0.231a cc/g) during tempering whereas, in the case of mixing T4 gained maximum score as 4.02 ± 0.213a cc/g. Minimum specific volume (3.47 ± 0.225b cc/g) was observed in the untreated sample (control). The findings of present work are in association with the results reported by Haros et al. (2002); they calculated an increase of 29 % in specific volume of bread in response to xylanase addition, however, xylanase application was comparatively less effective in raising bread specific volume during dough mixing. In the present study, there was an increase up to 17.86 and 15.85 % in specific volume during tempering and mixing respectively supported by the findings of Laurikainen et al. (1998), they observed 18–19 % increase in bread specific volume in response to xylanase treatment. Relative volume was calculated on the basis of volume attained through rapeseed displacement method (Graph H). Maximum relative volume (1.20 ± 0.074a) was observed in T3 (600 IU/kg) in the case of xylanase addition during tempering and when applied in dough mixing, the highest value i.e. 1.17 ± 0.062a was recorded in response to T4 (800 IU/kg).
Graph I illustrates bread density calculated on the basis of volume. The means indicated that density showed a dwindling trend in relation to increase in enzyme level up to T3 (600 IU/kg), when xylanase was applied during tempering however, as enzyme was added in dough mixing stage, decrease in density was observed up till T4 (800 IU/kg). The lowest bread density (0.24 ± 0.012a g/cc) was observed in T3 during wheat tempering and T4 (0.25 ± 0.021a g/cc) when enzyme was added during dough mixing while, maximum bread density (0.29 ± 0.022a g/cc) was calculated in the untreated bread samples. The main reason for decreased density in enzyme treated breads was their higher volume with respect to control (T0), as there is inverse relationship between volume and density; higher is the volume, lower will be the density and vice versa.
Moisture retention
In the present study, xylanase was applied at two different stages i.e. wheat tempering and dough mixing.
Means for moisture contents of untreated as well as enzyme treated breads are presented in Table 1. It is apparent from the results that treatments showed considerable variations in moisture contents; a direct correlation was observed between enzyme addition and moisture retention up to certain level and then a dwindling trend was noted for this trait.
Table 1.
Effect of treatments on moisture retention (%) of bread samples
| Treatments | Tempering | Mixing |
|---|---|---|
| T0 | 31.5 ± 1.52e | 31.5 ± 1.52b |
| T1 | 33.6 ± 1.59d | 32.4 ± 1.43b |
| T2 | 34.0 ± 1.35c | 33.2 ± 1.42b |
| T3 | 36.5 ± 1.48a | 33.6 ± 1.46b |
| T4 | 34.7 ± 1.48b | 36.3 ± 1.63a |
| T5 | 33.5 ± 1.36d | 32.8 ± 1.52b |
T0 = Control, T1 = 200, T2 = 400,T3 = 600, T4 = 800, T5 = 1,000 IU/Kg .(n = 3) (superscript ‘a’ shows the highest and ‘e’ shows the minimum moisture retained by respective samples)
In case of control (untreated sample) minimum moisture content (31.5 ± 1.52e%) was found while, maximum moisture retention 36.5 ± 1.48a was recorded in T3 (600 IU/kg) when enzyme was applied during tempering. Subsequently, a decrease in moisture was observed when enzyme dosage was raised to 800 IU/kg as in case of T4 and 1,000 IU/kg in T5 with the values 34.7 ± 1.48b and 33.5 ± 1.36d%, respectively.
Enzyme addition at mixing stage exhibited positive effect on the moisture retention capacity of breads. However, the treatments showed diverse response to varying levels of xylanase addition. Generally, the enzyme addition resulted in better moisture retention up to T4 (800 IU/kg) afterwards, a decrease was noted. Minimum value for moisture was observed in case of untreated flour (T0) whilst, maximum 36.3 ± 1.63a% moisture was observed in T4 followed by 33.6 ± 1.46b and 33.2 ± 1.42b% in T3 (600 IU/kg) and T2 (400 IU/kg), respectively.
The mean values (Table 2) depict the effect of storage intervals on moisture retention of bread samples receiving xylanase treatment during tempering and mixing. It is obvious that in case of tempering the moisture contents were maximum (39.1 ± 0.60a%) at the initiation of study followed by 36.9 ± 0.71b and 34.2 ± 0.82c% at the storage interval of 24 and 48 h, respectively, whereas minimum moisture level (29.8 ± 0.59f%) was observed at a period of 120 h. The same trend for moisture retention was observed in case of bread samples having xylanase treatment at the mixing stage; exhibiting maximum moisture contents 38.2 ± 0.26a % at the first day of storage while, the minimum 28.8 ± 0.37d% at the end of trial. The comparison of the effect of enzyme application at both stages (tempering and mixing) regarding moisture retention in bread samples expounded that moisture contents decreased gradually during storage however, xylanase addition during tempering was more effective to retain moisture over the entire study period.
Table 2.
Effect of storage (25C°) on the moisture retention (%) of bread samples
| Storage (h) | Tempering | Mixing |
|---|---|---|
| 0 | 39.1 ± 0.60a | 38.2 ± 0.26a |
| 24 | 36.9 ± 0.71b | 35.8 ± 0.42b |
| 48 | 34.2 ± 0.82c | 33.3 ± 0.56c |
| 72 | 32.8 ± 0.82d | 32.3 ± 0.70c |
| 96 | 30.9 ± 0.61e | 31.6 ± 2.03c |
| 120 | 29.8 ± 0.59f | 28.8 ± 0.37d |
(n = 3) (superscript ‘a’ shows the highest and ‘f’ shows the minimum moisture retained at different storage intervals)
Staling is generally one of the most common reasons considered for deterioration in bread quality. Among different factors contributing to staling, moisture loss is an important criterion that affects freshness of bread. Higher is the moisture loss, faster will be the bread hardening that leads to staling. Xylanase hydrolyses the hemicelluloses into smaller fractions with better water holding capacity, resulting in delayed water redistribution among different loaf components. The moisture retention capacity of these components is responsible for freshness of bread loaves as observed during the current study. However, excessive breakdown of flour components may have negative impact on dough properties; it becomes extra soft and ultimately collapse during baking leading to decreased loaf volume.
Sensory evaluation
The means (Table 3) elaborate that xylanase treatments resulted in profound effect on bread external characteristics, while in case of bread internal characteristics (Table 4) all the parameters were affected significantly.
Table 3.
Physical quality characteristics of bread prepared by different treatments
| Volume | Crust color | Symmetry of form | Evenness of bake | Character of crust | Break and shred | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | |
| To | 6.7 ± 0.08d | 6.7 ± 0.08c | 5.9 ± 0.13c | 5.9 ± 0.13c | 1.8 ± 0.04d | 1.8 ± 0.04d | 2,0 ± 0.04a | 2.0 ± 0.04a | 2.1 ± 0.04d | 2.1 ± 0.04c | 1.9 ± 0.08d | 1.9 ± 0.08d |
| T1 | 6.9 ± 0.08d | 6.7 ± 0.10c | 6.2 ± 0.10b | 6.1 ± 0.09b | 2.1 ± 0.05c | 1.9 ± 0.05c | 2.1 ± 0.05 | 2.0 ± 0.05a | 2.8 ± 0.04c | 2.2 ± 0.04b | 2.3 ± 0.09c | 2.1 ± 0.07c |
| T2 | 7.3 ± 0.05c | 7.2 ± 0.08b | 6.2 ± 0.11b | 6.1 ± 0.06b | 2.1 ± 0.05c | 2.1 ± 0.06b | 1.9 ± 0.05a | 2.0 ± 0.07a | 2.3 ± 0.05bc | 2.3 ± 0.05b | 2.4 ± 0.13a | 2.4 ± 0.09b |
| T3 | 7.9 ± 0.07a | 7.5 ± 0.11a | 6.5 ± 0.11a | 6.3 ± 0.08ab | 2.2 ± 0.05b | 2.2 ± 0.06a | 2.0 ± 0.06a | 2.1 ± 0.10a | 2.4 ± 0.08a | 2.4 ± 0.06a | 2.5 ± 0.10a | 2.4 ± 0.06a |
| T4 | 7.7 ± 0.09b | 7.7 ± 0.09a | 6.4 ± 0.12a | 6.3 ± 0.12a | 2.3 ± 0.06a | 2.2 ± 0.06a | 1.9 ± 0.06a | 2.0 ± 0.08a | 3.0 ± 0.10ab | 2.4 ± 0.06a | 2.4 ± 0.09bc | 2.4 ± 0.04a |
| T5 | 7.6 ± 0.06b | 7.6 ± 0.12a | 6.4 ± 0.09a | 6.4 ± 0.10a | 2.2 ± 0.05b | 2.2 ± 0.06a | 1.9 ± 0.05a | 1.9 ± 0.06a | 2.3 ± 0.05c | 2.2 ± 0.05b | 2.3 ± 0.05c | 2.1 ± 0.08c |
T0 = Control, T1 = 200, T2 = 400,T3 = 600, T4 = 800, T5 = 1,000 IU/Kg (n = 5 panelists, superscript ‘a’ shows the highest and ‘d’ shows the minimum score attainted by respective treatments, Temp stands for tempering and Mix for mixing)
Table 4.
Sensory quality characteristics of bread prepared by different treatments
| Grain | Color of crumb | Aroma | Taste | Texture | Chew ability | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | Temp | Mix | |
| To | 6.9 ± 0.18c | 6.9 ± 0.18b | 6.4 ± 0.17e | 6.4 ± 0.17e | 6.8 ± 0.16c | 6.8 ± 0.16d | 11.7 ± 0.15d | 11.7 ± 0.15d | 12.1 ± 0.12d | 12.1 ± 0.12cd | 7.1 ± 0.19d | 7.1 ± 0.19c |
| T1 | 7.2 ± 0.19b | 7.2 ± 0.18a | 6.8 ± 0.18d | 6.6 ± 0.18d | 7.0 ± 0.16b | 6.9 ± 0.17c | 11.9 ± 0.13cd | 11.9 ± 0.13cd | 12.3 ± 0.08bc | 12.2 ± 0.10c | 7.5 ± 0.19ab | 7.3 ± 0.19b |
| T2 | 7.4 ± 0.20ab | 7.3 ± 0.18a | 7.0 ± 0.19c | 6.9 ± 0.20c | 7.2 ± 0.17ab | 7.2 ± 0.18bc | 12.4 ± 0.16bc | 12.1 ± 0.15bc | 12.6 ± 0.14b | 12.4 ± 0.12bc | 7.5 ± 0.20ab | 7.4 ± 0.16ab |
| T3 | 7.5 ± 0.20a | 7.4 ± 0.19a | 7.6 ± 0.20a | 7.1 ± 0.20b | 7.4 ± 0.17a | 7.3 ± 0.18ab | 12.8 ± 0.16a | 12.4 ± 0.15ab | 12.9 ± 0.11a | 12.8 ± 0.13ab | 7.7 ± 0.21a | 7.4 ± 0.22ab |
| T4 | 7.3 ± 0.19ab | 7.5 ± 0.19a | 7.2 ± 0.19b | 7.3 ± 0.21a | 7.4 ± 0.17a | 7.4 ± 0.19a | 12.6 ± 0.16ab | 12.5 ± 0.15a | 12.6 ± 0.12b | 12.9 ± 0.12a | 7.4 ± 0.23bc | 7.6 ± 0.21a |
| T5 | 7.2 ± 0.19b | 6.9 ± 0.17b | 7.1 ± 0.19bc | 7.2 ± 0.20ab | 7.4 ± 0.17a | 7.4 ± 0.19ab | 12.2 ± 0.16bc | 12.3 ± 0.15ab | 12.2 ± 0.15c | 11.8 ± 0.19d | 7.2 ± 0.19cd | 7.3 ± 0.20b |
T0 = Control, T1 = 200, T2 = 400, T3 = 600, T4 = 800, T5 = 1,000 IU/Kg (n = 5 panelists, superscript ‘a’ shows the highest and ‘d’ shows the minimum score attainted by respective treatments , Temp stands for tempering and Mix for mixing)
Regarding the mean values for bread external characteristics (Table 3), the samples gained maximum scores as 7.9 ± 0.07a, 6.5 ± 0.11a and 2.4 ± 0.08a for volume, crust color and character of crust respectively when xylanase was used in a dose of 600 IU/Kg of wheat kernels (T3) during tempering. While in case of internal characteristics bread samples achieved maximum scores (Table 4) for all parameters when xylanase was applied during tempering in a dose of 600 IU/Kg of wheat kernels (T3) while in case of dough mixing, the samples achieved highest scores when the enzyme was applied at a rate of 800 IU/Kg of wheat flour (T4). So, from these results it can be concluded that application of xylanase during tempering even in smaller activities lead to better performance than enzyme addition during mixing of dough.
The present results are in conformity with the findings of Courtin and Delcour (2002); they reported that the application of enzymes in bread making improved dough softening, tolerance and oven spring. Moreover, volume and texture of bread were also modified. The present study is also in accordance with the results found by Katina et al. (2006); they reported that mixture of alpha-amylase, xylanase and lipase used in the process of bread making, exhibited significant effect on volume, texture and shelf life of wheat bread. The results are also in line with findings of Maninder and Bains (1976) who described the modification of baking quality of Indian wheats in response to amylase treatments.
The extension in shelf life of bread might be due to the anti-staling effect of enzyme. The use of xylanase leads to reduced retrogradation as a result of altered water distribution between starch-protein matrixes. Xylanase acts on hemicelluloses resulting in short chain compounds and even the monomers. These simple compounds are used by yeast during fermentation thereby facilitate the process which is essential for better bread quality; efficient process of fermentation is required for high loaf volume. Yeast utilizes monomer sugars as food resulting in higher CO2 production, along with allied products like alcohols that are required for better hedonic response. The monomers produced during fermentation process also influence the color and character of crust by affecting maillard reaction and caramelization. Moreover, the fermentation at optimum level also promotes desired crumb color.
The enzyme application makes the dough more elastic, extensible and coherent by improving the strength of gluten network that is essential to retain the produced CO2. The gas expansion during baking results in increased volume, improves the crumb structure and grain too. Enzymatic hydrolysis of complex carbohydrates improves the dough consistency that facilitates the proper flow of dough pieces and hence affects symmetry of form and break & shred. Volume and density are inversely correlated; higher volume results in lower density and ultimately better chewability. Xylanase prevents rearrangement of amylose molecules and slows down the retrogradation process thus acts as anti-staling agent; consequently, keeps the bread fresh with extended shelf life as observed during the present exploration. (Keskin et al. 2004; Haros et al. 2002; Jiang et al. 2005). The improvement in dough handling properties and final bread quality parameters advocate the fact that xylanase has better potential to be used in baking industry.
Conclusion
The results of study explicit that application of xylanase in the process of bread making improves the dough quality characteristics, retains higher moisture during storage, enhances the bread quality attributes and consumer acceptability. However, to gain maximum benefits of this enzyme it should be utilized during the tempering of wheat kernels where it finds sufficient time to play its potential role to improve the dough and bread quality in a better way as compared to its application during flour mixing.
Acknowledgment
The authors are greatly obliged to the National Institute of Food Science and Technology (NIFSAT), University of Agriculture Faisalabad, for providing the facilities to the successful completion of this project.
References
- AACC . Approved Methods of American Association of Cereal Chemists. St Paul Minnesota: The American Association of Cereal Chemists. Inc; 2000. [Google Scholar]
- Ahmad Z (2009) Production and characterization of xylanase for utilization in baking industry. PhD thesis, University of Agriculture, Faisalabad, Pakistan. (http://www.prr.hec.gov.pk/Thesis/158S.pdf, 08.04.2012)
- Bajpai P. Application of enzymes in the pulp and paper industry. Biotechnol Progr. 1999;15:147–157. doi: 10.1021/bp990013k. [DOI] [PubMed] [Google Scholar]
- Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT. Xylanases and their applications in baking industry. Food Tech Biotec. 2008;46(1):22–31. [Google Scholar]
- Camacho NA, Aguilar GO. Production, purification and characterization of low molecular mass xylanase from Aspergillus sp. and its application in baking. Appl Biochem Biotech. 2003;104:159–172. doi: 10.1385/ABAB:104:3:159. [DOI] [PubMed] [Google Scholar]
- Chithra M, Muralikrishna G. An improved method for obtaining xylanase from finger millet (Eleusine coracana var. ‘Indaf-15’) malt. J Food Sci Tech. 2008;45(2):166–169. [Google Scholar]
- Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Courtin CW, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci. 2002;35:225–243. doi: 10.1006/jcrs.2001.0433. [DOI] [Google Scholar]
- Ghildyal NP, Prema P, Srikanta S, Sreekantiah KR, Ahmed SY. Studies on the production of α-amylase in submerged culture. J Food Sci Tech. 1980;17(4):165–167. [Google Scholar]
- Haros M, Rosell CM, Benedito C. Effect of different carbohydrases on fresh bread texture and bread staling. Eur Food Res Technol. 2002;215:425–430. doi: 10.1007/s00217-002-0580-4. [DOI] [Google Scholar]
- Hilhorst R, Dunnewind B, Orsel R, Stegeman P, van Vliet T, Gruppen H, Schols HA. Baking performance, rheology, and chemical composition of wheat dough and gluten affected by xylanase and oxidative enzymes. J Food Sci. 1999;64:808–813. doi: 10.1111/j.1365-2621.1999.tb15917.x. [DOI] [Google Scholar]
- Jaddou H, Mhaisen MT, Al-Hakim M, Zeki L, Al-Ambaky MSH. Semi-pilot production of sucrose from dates and sweet sorghum using ethanolic extraction technique. J Food Sci Tech. 1986;23(4):241–243. [Google Scholar]
- Jiang Z, Li X, Yang S, Li L, Tan S. Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritime. Food Res Int. 2005;38:37–43. doi: 10.1016/j.foodres.2004.07.007. [DOI] [Google Scholar]
- Katina K, Salmenkallio-Marttila M, Partanen R, Forssell P, Autio K. Effects of sourdough and enzymes on staling of high-fibre wheat bread. Lebensm Wiss Technol. 2006;39:479–491. doi: 10.1016/j.lwt.2005.03.013. [DOI] [Google Scholar]
- Keskin SO, Sumnu G, Sahin S. Usage of enzymes in a novel baking process. Food Nahrung. 2004;48:156–160. doi: 10.1002/food.200300412. [DOI] [PubMed] [Google Scholar]
- Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Laurikainen T, Härkönen H, Autiom K, Poutanen K. Effects of enzymes in fiber-enriched baking. J Sci Food Agric. 1998;76:239–249. doi: 10.1002/(SICI)1097-0010(199802)76:2<239::AID-JSFA942>3.0.CO;2-L. [DOI] [Google Scholar]
- Leisola M, Jokela J, Pastinen O, Turunen O, Schoemaker H (2002) Industrial use of enzymes. In: Encyclopedia of Life Support Systems (EOLSS), EOLSS Publishers Co, Oxford UK
- Maat J, Roza M, Verbakel J, Stam H, MJ Santos da Silva, Bosse M, Egmond MR, Hagemans MLD, Gorcom RFM, Hessing JGM, van den CAMJJ Hondel, van Rotterdam C. Xylanases and their applications in bakery. In: MA Kusters van Someren, Beldman G, Voragen AGJ., editors. Xylan and Xylanases, progress in biotechnology No. 7. VISSER J. Amsterdam: Elsevier Science Publishers; 1992. pp. 349–360. [Google Scholar]
- Maninder K, Bains GS. Effects of amylase supplements on the rheological baking quality of Indian wheats. J Food Sci Tech. 1976;13(6):328. [Google Scholar]
- Martínez-Anaya MA, Jimenez T. Functionality of enzymes that hydrolyse starch and non-starch polysaccharide in bread making. Eur Food Res Tech. 1997;205(3):209–214. [Google Scholar]
- Mathewson PR. Enzymatic activity during bread baking. Cereal Foods World. 2000;45:98–101. [Google Scholar]
- Meilgaard D, Civille GV, Carr BT. Sensory evaluation techniques. 2. Boca Raton: CRC; 1999. [Google Scholar]
- Olse HS. Use of enzymes in food processing. In: Reed G, Nagodawithana T, editors. Enzymes, biomass, food and feed biotechnology. Weinheim: Wiley-VCH; 1995. pp. 663–736. [Google Scholar]
- Omar AW, Khataibeh MH, Abu-alruz K. The use of xylanases from different microbial origin in bread making and their effects on bread quality. J Appl Sci. 2008;8(4):672–676. doi: 10.3923/jas.2008.672.676. [DOI] [Google Scholar]
- Rao BAS, Narasimham VVL. Brewing with enzymes. J Food Sci Tech. 1976;13(3):119–123. [Google Scholar]
- Romanowska I, Polak J, Janowska K, Bieleckim S. The application of fungal endoxylanase in breadmaking. Comm Agr Appl Biol Sci. 2003;68:317–320. [PubMed] [Google Scholar]
- Sorensen JF, Sibbesen O, Poulsen CH (2001) Degree of inhibition by the endogenous wheat xylanase inhibitor controls the functionality of microbial xylanases ‘in Dough’. AACC Annual Meeting, Enzymes and Baking – 213AB, Charlotte, NC, USA
- Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics: a biometric approach. New York: McGraw Hill Book Inc; 1997. [Google Scholar]
- Subramaniyan S, Prema P. Optimization of cultural parameters for the synthesis of endoxylanase from Bacillus SSP- 34. J Sci Ind Res. 1998;57:611–616. [Google Scholar]
- Vijaya H, Joseph R. Xylanase production by ultraviolet induced variants of Streptomyces fradiae SCF-5. J Food Sci Tech. 1979;15(5):243–246. [Google Scholar]
- Vijayanand P, Kulkarni SG, Prathibha GV. Effect of pectinase treatment and concentration of litchi juice on quality characteristics of litchi juice. J Food Sci Tech. 2010;47(2):235–239. doi: 10.1007/s13197-010-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, van Vliet T, Hamer RT. Evidence that pentosans/xylanase affects the re-agglomeration of the gluten network. J Cereal Sci. 2004;39:341–349. doi: 10.1016/j.jcs.2003.12.003. [DOI] [Google Scholar]


