Abstract
The recovery of phenolic compounds of Eugenia pyriformis using different solvents was investigated in this study. The compounds were identified and quantified by reverse-phase high-performance liquid chromatography coupled with ultraviolet–visible diode-array detector (RP-HPLC-DAD/UV–vis). Absolute methanol was the most effective extraction agent of phenolic acids and flavonols (588.31 mg/Kg) from Eugenia pyriformis, although similar results (p ≤ 0.05) were observed using methanol/water (1:1 ratio). Our results clearly showed that higher contents of phenolic compounds were not obtained either with the most or the least polar solvents used. Several phenolic compounds were identified in the samples whereas gallic acid and quercetin were the major compounds recovered.
Keywords: Phenolic acids, Flavonols, Extraction, Solvents, HPLC, Eugenia pyriformis
Introduction
Phenolic acids and flavonoids are ubiquitous bioactive compounds which belong to a diverse group of secondary metabolites and are universally present in higher plants. These phytochemicals have shown to possess significant antioxidant capacities that can protect the human body from health problems (Robards et al. 1999). “These compounds posses an aromatic ring bearing one or more hydroxyl groups and their structures may range from that of a simple phenolic molecule to that of a complex high-molecular mass polymer” (Balasundram et al. 2006).
Extraction and isolation are the first important steps for separation, characterization, and quantification of polyphenols from plant material (Kim and Lee 2001). Polyphenols are often most soluble in organic solvents less polar than water. The solubility is dependent on the polar properties of the polyphenols. The appropriate selection of the extracting solvent is not as simple as it may seem (Kim and Lee 2001). Solvent extraction, extraction with supercritical fluid and ultrasound-assisted extraction are the most common used techniques for the isolation of phenolic compounds (Ignat et al. 2011). Several solvents and conditions have been used to achieve optimal extraction. The application of acidified methanol, ethanol, acetone, ethyl acetate and mixtures of solvents with water has been widely reported in literature (Annegowda et al. 2011; Durling et al. 2007; Haminiuk et al. 2011; Luthria 2008; Luthria and Pastor-Corrales 2006; Prasad et al. 2011; Pushp et al. 2011; Ramful et al. 2011; Russell et al. 2009; Wang et al. 2008; Wijekoon et al. 2011; Sasidharan and Menon 2011). Therefore, the aim of this work was to evaluate the use of different solvents in the extraction of the phenolic acids and flavonols of a typical fruit from the Brazilian Atlantic Forest, Eugenia pyriformis.
Material and methods
Sample preparation
The purée of Uvaia (Eugenia pyriformis) was utilized to evaluate the extraction of phenolic compounds and it was provided by Sitio do Belo (Paraibuna, SP, Brazil). Absolute methanol (AM), absolute ethanol (AE), distilled water (DW) and a combination of methanol/water (MA) (1:1, v/v) and ethanol/water (EA) (1:1, v/v) were used in order to evaluate the phenolic compounds extraction. The purée was thawed and macerated (10 g) with the different solvents (40 mL). The mixture was agitated in a test tube rotary mixer for 1 h (Haminiuk et al. 2011) and the samples were then centrifuged at 6,000 rpm for 10 min. Before analysis, the supernatant was filtered with Spritzen syringe filter of 0.22 μm (Trasadingen, Switzerland). The filtrate (extract) was used to quantify the phenolic acids and flavonols of Eugenia pyriformis by HPLC analysis.
Quantification of individual phenolic compounds –RP- HPLC-DAD/UV
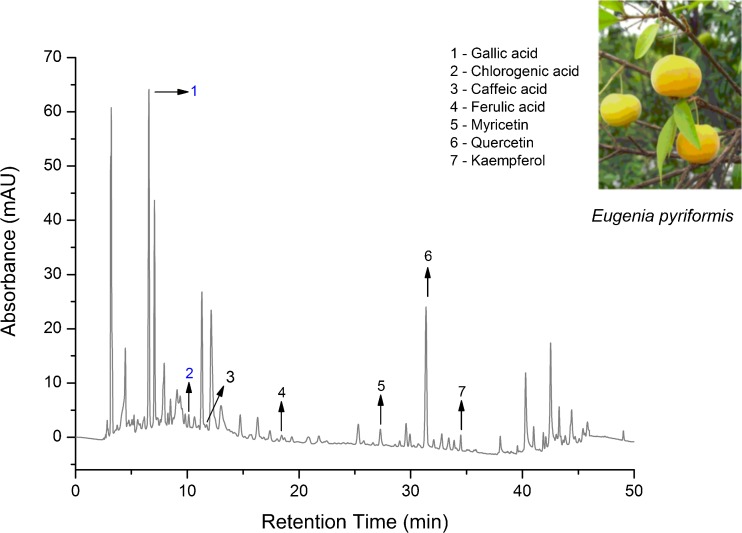
For high-performance liquid chromatography coupled with a ultraviolet–visible diode-array detection (HPLC-DAD/UV–vis), a Dionex UltiMate 3000 HPLC system (Dionex, Idstein, Germany) equipped with UltiMate 3000 Pump, UltiMate 3000 Autosampler Column Compartment, UltiMate 3000 Photodiode Array Detector and Chromeleon software was used. A reversed phase Acclaim® 120 column, C18 5 μm 120 Å (4.6 mm × 250 mm), was used in the experiments. The column was maintained at 40 °C throughout the analysis and the detection was recorded at 280, 300 and 320 nm. Phenolic acids and flavonols are usually detected at wavelengths between 210 and 320 nm. The injection volume was 10 μL (Maisuthisakul and Gordon 2012). The mobile phases consisted of acidified water with phosphoric acid 1 % and methanol. The solvent gradient was as follows: 0–15 % B in 2 min, 15–25 % B in 5 min, 25–30 % B in 10 min, 30–35 % B in 15 min, 35–50 % B in 25 min, 50–60 % B in 30 min, 60–80 % B in 35 min, 80–100 % B in 45 min and 100–5 % B in 60 min. The flow rate was 1 mL/min (Kelebek and Selli 2012). The following standards were used in this study: phenolic acids (gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid) and flavonols (rutin, myricetin, quercetin and kaempferol). Stock solutions of all standards were prepared in methanol and the calibration curves were obtained from duplicate injections of at least five concentrations. For the HPLC analysis, phenolic compounds were identified by comparing their retention times with those of pure standards (Granato et al. 2011). Figure 1 presents the chromatogram of phenolic acids and flavonols standards used in this work at 280 nm.
Fig. 1.
Chromatogram of phenolic compounds standards at 280 nm
Statistical analysis
Assays were performed in triplicate for each sample (Yogesh and Ali 2012). Results were expressed as mean values ± standard deviation (SD). Student’s t-test was used for comparison between two means, and the one-way ANOVA was used for comparison of more than two means. A difference was considered statistically significant when p ≤ 0.05. The statistical analysis was carried out using Statistica 7.1 software (StatSoft, Tulsa, OK, USA).
Results and discussion
The recovery of polyphenols from plant materials is influenced by the solubility of the phenolic compounds in the solvent used for the extraction process (Rodríguez-Carpena et al. 2011). Furthermore, solvent polarity will play a key role in increasing phenolic solubility (Naczk and Shahidi 2006). Table 1 presents the effect of different solvents on the extraction of phenolic compounds from Eugenia pyriformis. It is evident that the recovery of phenolic compounds was dependent on the solvent used and its polarity (Alothman et al. 2009). Polyphenols are often most soluble in organic solvents less polar than water. An effective extraction of plant material depends on a proper solvent selection, elevated temperatures, and mechanical agitation to maximize polyphenols recovery (Kim and Lee 2001). The chromatografic profile of Eugenia pyriformis at 280 nm using absolute methanol as extracting agent is shown in Fig. 2. The recovery of phenolic compounds content varied from 255.91 to 588.31 mg/Kg of fresh weight. Absolute methanol (AM) was the most effective extraction agent on the recovery of phenolic acids and flavonols from Eugenia pyriformis. However, similar results (p ≤ 0.05) were obtained by the use of methanol/water (MA) (1:1 ratio). The lowest amount of the bioactive compounds was obtained ethanol/water (EA) (1:1 ratio). In terms of polarity, the solvents used in this work can be classified according to their dielectric constant as follows: ethanol < methanol < water. Our results clearly showed that higher contents of phenolic acids and flavonols were not obtained either with the most or the least polar solvents used.
Table 1.
Effect of different solvents on phenolic compounds extraction
| Compounds | λ (nm) | RT (min) | AMa | AEa | DWa | MAa | EAa |
|---|---|---|---|---|---|---|---|
| Gallic acid | 320 | 6.60 | 346.1 ± 7.43e | 75.1 ± 4.63a | 255.8 ± 6.91c | 299.2 ± 7.73d | 215.1 ± 3.56b |
| Chlorogenic acid | 280 | 10.14 | 38.4 ± 1.51c | 27.2 ± 2.04a | 33.4 ± 1.54b | 42.3 ± 2.04d | 31.6 ± 1.34b |
| Caffeic acid | 280 | 11.73 | 5.2 ± 0.64a | Nd | Nd | Nd | Nd |
| p-coumaric acid | 320 | 13.75 | 1.5 ± 0.12b | 0.92 ± 0.02a | 2.9 ± 0.12d | 1.8 ± 0.09c | 1.2 ± 0.15a |
| Ferulic acid | 320 | 18.45 | 3.4 ± 0.19c | 2.3 ± 0.09a | 2.7 ± 0.16b | 3.3 ± 0.12c | 2.3 ± 0.17a |
| Rutin | 320 | 19.92 | 1.1 ± 0.07c | 0.85 ± 0.03a | 0.83 ± 0.01a,b | 1.1 ± 0.14c | 0.85 ± 0.07b |
| Myricetin | 280 | 27.28 | 29.5 ± 1.67c | 22.9 ± 1.31a | 23.0 ± 1.25a | Nd | 25.4 ± 1.26b |
| Quercetin | 280 | 31.37 | 149.7 ± 5.35d | 124.0 ± 4.81b | 114.2 ± 3.52a | 180.9 ± 4.22e | 133.9 ± 2.38c |
| Kaempferol | 300 | 34.48 | 13.4 ± 1.16c | 2.7 ± 0.13b | Nd | 2.8 ± 0.11b | 2.6 ± 0.18a |
| Total | 588.3 ± 13.18e | 413.1 ± 11.42b | 432.7 ± 12.16c | 531.4 ± 12.71d | 255.9 ± 9.14a | ||
amg/Kg of fresh weight, Means followed by the same letters are not statistically different in the same line (p ≤ 0.05). Nd Not determined. λ Wavelength, RT Retention time. AM Absolute methanol, AE Absolute ethanol, DW Distilled water, MA Methanol/water (1:1, v/v), EA Ethanol/water (1:1, v/v). Data presented are mean values ± SD; n = 3
Fig. 2.
Chromatografic profile of Eugenia pyriformis at 280 nm with absolute methanol as extraction agent
The results obtained in this research are in agreement to Liazid et al. (2007), Navarro et al. (2006), Espinosa-Alonso et al. (2006) and Wijngaard et al. (2009) that used absolute methanol to obtain phenolic compounds from vegetable matrix. The extraction of phenolic compounds has been widely studied (Kosanić et al. 2011) and several methods have been developed to recovery these substances. Alothman et al. (2009) studied the antioxidant capacity and the phenolic content of selected tropical fruits from Malaysia. According to their results, the recovery of phenolic compounds was dependent on the fruit type and the solvent system, and acetone and ethanol were the most efficient solvents for extracting phenols. In our study, all the nine standards were identified in Eugenia pyriformis.
The phenolic acids and flavonols showed a wide range of variation according to Table 1: gallic acid (75.08–346.12 mg/Kg), chlorogenic acid (27.16–42.31 mg/Kg), caffeic acid (0.00–5.20 mg/Kg), p-coumaric acid (0.92–2.89 mg/Kg), ferulic acid (2.31–3.42 mg/Kg), rutin (0.77–1.14 mg/Kg), myricetin (0.00–29.52 mg/Kg), quercetin (114.16–180.88 mg/Kg) and kaempferol (0.00–13.36 mg/Kg). Gallic acid and quercetin were the major compounds recovered among all the other phenolic compounds. Gallic acid (3,4,5-trihydroxybenzoic acid), an endogenous plant phenol, is abundantly present in tea, grapes, different berries, fruits, as well as in wine (Prince et al. 2011) and has received much attention because of its potent free radical scavenging and antioxidant action. On the other hand, quercetin (3,3’,4’,5,7-pentahydroxyflavone), is a typical flavonoid ubiquitously present in vegetables and fruits (Murota and Terao 2003). The beneficial effects of quercetin are related to its anti-oxidation properties which may result from its ability in scavenging free radical and chelating metal ions. These properties allow it to inhibit lipid peroxidation. Both substances have been widely found in plant materials (Hollman and Katan 1997). Fu et al. (2011) studied the antioxidant capacity and total phenolic content of 62 fruits. Interestingly, among other phenolic compounds, gallic acid and quercetin were found in most of the fruits evaluated.
As previously stated, the highest results were obtained with absolute methanol and a mixture of methanol and water. In literature, it was not found a common sense of which solvent or mixture of solvents are more suitable to recovery the bioactive compounds among the different plant materials. However, it is clear that alcoholic extraction still remains as a good alternative to obtain phenolic compounds from plant materials.
Conclusion
The results demonstrate the importance of selecting the most suitable solvent for the extraction of phenolic compounds from plant material. Among the solvents tested, absolute methanol was the most effective extraction agent on the recovery of phenolic compounds. The content of phenolic acids and flavonols identified by HPLC analysis in Eugenia pyriformis ranged from 255.91 to 588.31 mg/Kg. The compounds with highest concentrations were gallic acid and quercetin.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq; Process Number 501535/2009-8) for financial support.
References
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Annegowda H, Bhat R, Min-Tze L, Karim A, Mansor S (2011) Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. J Food Sci Technol. doi:10.1007/s13197-011-0435-8 [DOI] [PMC free article] [PubMed]
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, Perry NB. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101:1417–1424. doi: 10.1016/j.foodchem.2006.03.050. [DOI] [Google Scholar]
- Espinosa-Alonso LG, Lygin A, Widholm JM, Valverde ME, Paredes-Lopez O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.) J Agric Food Chem. 2006;54:4436–4444. doi: 10.1021/jf060185e. [DOI] [PubMed] [Google Scholar]
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ, Li HB. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Granato D, Katayama FCU, de Castro IA. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem. 2011;129:366–373. doi: 10.1016/j.foodchem.2011.04.085. [DOI] [PubMed] [Google Scholar]
- Haminiuk CWI, Plata-Oviedo MSV, Guedes AR, Stafussa AP, Bona E, Carpes ST. Chemical, antioxidant and antibacterial study of Brazilian fruits. Int J Food Sci Technol. 2011;46:1529–1537. doi: 10.1111/j.1365-2621.2011.02653.x. [DOI] [Google Scholar]
- Hollman PCH, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51:305–310. doi: 10.1016/S0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Kelebek H, Selli S (2012) Identification of phenolic compositions and the antioxidant capacity of mandarin juices and wines. J Food Sci Technol. doi:10.1007/s13197-011-0606-7 [DOI] [PMC free article] [PubMed]
- Kim D-O, Lee CY (2001) Extraction and isolation of polyphenolics. Current Protocols in Food Analytical Chemistry: John Wiley & Sons, Inc
- Kosanić M, Ranković B, Vukojević J. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48:584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liazid A, Palma M, Brigui J, Barroso CG. Investigation on phenolic compounds stability during microwave-assisted extraction. J Chromatogr A. 2007;1140:29–34. doi: 10.1016/j.chroma.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Luthria DL. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008;107:745–752. doi: 10.1016/j.foodchem.2007.08.074. [DOI] [Google Scholar]
- Luthria DL, Pastor-Corrales MA. Phenolic acids content of 15 dry edible bean (Phaseolus vulgaris L.) varieties. J Food Compos Anal. 2006;19:205–211. doi: 10.1016/j.jfca.2005.09.003. [DOI] [Google Scholar]
- Maisuthisakul P, Gordon M (2012) Characterization and storage stability of the extract of Thai mango (Mangifera indica Linn. Cultivar Chok-Anan) seed kernels. J Food Sci Technol. doi:10.1007/s13197-011-0604-9 [DOI] [PMC free article] [PubMed]
- Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/S0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Navarro JM, Flores P, Garrido C, Martinez V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006;96:66–73. doi: 10.1016/j.foodchem.2005.01.057. [DOI] [Google Scholar]
- Prasad KN, Hassan FA, Yang B, Kong KW, Ramanan RN, Azlan A, Ismail A. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. Peels. Food Chem. 2011;128:1121–1127. doi: 10.1016/j.foodchem.2011.03.105. [DOI] [Google Scholar]
- Prince PSM, Kumar MR, Selvakumari CJ. Effects of gallic acid on brain lipid peroxide and lipid metabolism in streptozotocin-induced diabetic wistar rats. J Biochem Mol Toxicol. 2011;25:101–107. doi: 10.1002/jbt.20365. [DOI] [PubMed] [Google Scholar]
- Pushp P, Sharma N, Joseph G, Singh R (2011) Antioxidant activity and detection of (−)epicatechin in the methanolic extract of stem of in the methanolic extract of stem of Tinospora cordifolia. J Food Sci Technol. doi:10.1007/s13197-011-0354-8 [DOI] [PMC free article] [PubMed]
- Ramful D, Aumjaud B, Neergheen VS, Soobrattee MA, Googoolye K, Aruoma OI, Bahorun T. Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res Int. 2011;44:1190–1196. doi: 10.1016/j.foodres.2010.09.024. [DOI] [Google Scholar]
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. doi: 10.1016/S0308-8146(99)00093-X. [DOI] [Google Scholar]
- Rodríguez-Carpena JG, Morcuende D, Andrade MJ, Kylli P, Estévez M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem. 2011;59:5625–5635. doi: 10.1021/jf1048832. [DOI] [PubMed] [Google Scholar]
- Russell WR, Labat A, Scobbie L, Duncan GJ, Duthie GG. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 2009;115:100–104. doi: 10.1016/j.foodchem.2008.11.086. [DOI] [Google Scholar]
- Sasidharan I, Menon A. Effects of temperature and solvent on antioxidant properties of curry leaf (Murraya koenigii L.) J Food Sci Technol. 2011;48:366–370. doi: 10.1007/s13197-010-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun BG, Cao YP, Tian YA, Li XH. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- Wijekoon M, Bhat R, Karim AA. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J Food Compos Anal. 2011;24:615–619. doi: 10.1016/j.jfca.2010.09.018. [DOI] [Google Scholar]
- Wijngaard HH, Rossle C, Brunton N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009;116:202–207. doi: 10.1016/j.foodchem.2009.02.033. [DOI] [Google Scholar]
- Yogesh K, Ali J (2012) Antioxidant potential of thuja (Thuja occidentalis) cones and peach (Prunus persia) seeds in raw chicken ground meat during refrigerated (4 ± 1 °C) storage. J Food Sci Technol. doi:10.1007/s13197-012-0672-5 [DOI] [PMC free article] [PubMed]