Abstract
Investigations were carried out to study the influence of vacuum packaging and long term storage on quality in red chilli. Chilli fruits were stored in vacuum packed and jute bags at two moisture levels (10 % and 12 %) in room and cold environments under both light and dark conditions for a period of 24 months. During storage period, average room and cool chamber temperatures were 25 ± 2 °C and 4 ± 1 °C, respectively. Changes of moisture (Halogen moisture analyzer), capsaicin (HPLC-UV), oleoresin and total extractable colour (spectrophotometer) were analyzed at 3 months interval up to 12 months and 6 months interval from 12 to 24 months. Statistical analysis (ANOVA) and Duncan’s test were applied to the analytical data to evaluate the effect of treatments applied. It was observed that the vacuum packed chillies under cold storage were found to have the least per cent decline in various quality parameters. Chillies with 12 % moisture and stored in vacuum packaged bags recorded better quality parameters over 10 % moisture.
Keywords: Chilli, Vacuum packaging, Moisture, Capsaicin, Oleresin, Total extractable colour
Introduction
Quality of any produce drives its market and is a crucial factor that determines its demand. Chilli [Capsicum annuum L.] is an important spice crop in India and is grown in the tropical, subtropical and temperate regions of the world. The superior intrinsic quality (attractive red colour and pungency) of Indian chillies accounts for its global demand (Rithesh et al. 2000). The world production of red chilli is estimated to be around 21 lakh tons, 45 % of which is produced in India. The FAO world spice production statistics records a bulk of 86 % by volume, making the country the largest producer of spices, in addition to it being the largest consumer and exporter of spices in the global context (Peter et al. 2006). The consumption of chilli apart from its taste and colour has immense medicinal value due to the presence of capsaicin (Lee et al. 2007), oleoresin, carotenoids, total extractable colours and minerals (Kang et al. 1995; Rithesh et al. 2000; Deepa et al. 2011).
The main quality contributing factors of chillies viz., colour and pungency are sensitive to the vagaries of climate and are affected by factors like high temperature and humidity, moisture and oxygen, respiration, insects, pests and microorganisms, which work together in causing deterioration. The red pepper owes its intense red colour to around 40 carotenoid pigments, noteworthy among which are capsanthin, capsorubin and capsanthin 5, 6 epoxide (Davies et al. 1970). The rising resentment against artificial food colouring substances has created a promising deal for red chillies that naturally have a high colour value. Carotenoids have received increased attention in recent years as a potential neutraceutical component due to their powerful antioxidant function (Matsufuji et al. 1998).
Chillies are also valued for their pungency, the active principle of which is capsaicin, an amide derivative of vanillylamine and 8-methyl-non- trans -6 enoic acid (Bernal et al. 1993). Carotenoid loss or destruction has been recognized as one of the major causes for colour change in dried red pepper products (Ramakrishnan and Francis 1973). Oxidation of food ingredients such as vitamins, pigments and aroma compounds is one of the most important causes of quality loss and is the major deteriorative reaction in microbiologically safe foods as dry and frozen products (Anderson and Lingnert 1997).
Dry chillies though microbiologically safe when compared to the fresh produce, are not safe from oxidation of carotenoid pigments. Studies on vacuum packaging are therefore expected to address some of these problems and thus maintain quality for a relatively longer period. Vacuum packaging is the procedure that results in a reduced oxygen level in sealed package. An evacuated pack collapse around the product so that the pressure inside is seldom much less than atmosphere. The anaerobic environment of vacuum packaging prevents the growth of spoilage microorganisms especially aerobic ones which are responsible for off odor, slime and texture changes (Nunez et al. 1986).
The aim of the work is to find out the effect of vacuum packaging on quality parameters like moisture content, capsaicin, oleoresin, and total extractable colour during long term storage.
Material and methods
Sample collection, vacuum treatment and storage
Chilli samples (var. Kundagol Deluxe), a low pungent high colour variety of red chilli was collected at uniform crop maturity, dried to constant moisture contents of 10 and 12 %, and subjected to two packaging and three storage conditions. Chillies used for vacuum packaging were destemmed to remove stalk to avoid disruption of vacuum. Dried chillies were vacuum packed (OLPACK 510, Interprise breussel S.A., Belgium) in multi layer polythene bags of 350 × 180 × 150 mm dimension. For comparison, they were also stored in jute bags (farmer’s practice of storage). Both vacuum packed bags and jute bags were stored at room temperature (25 ± 2 °C) and cold storage (4 ± 1 °C) under normal light and dark. For the light treatments, the bags were stored in the laboratory with normal diffused light as existed without any additional lighting. For dark treatments, the racks were covered with thick black cloth to protect the infiltration of even diffused light and the light intensity was zero. The observations on moisture content, oleoresin extractable color, total extractable color and capsaicin content were recorded at 3 months interval up to 12 months and 6 months interval from 12 to 24 months.
Biochemical analysis
Moisture content
The moisture content was determined by Halogen Moisture Analyzer (MB 45) from Ohaus, USA, which works on thermo gravimetric principle. i.e. the moisture is determined from the weight loss of a sample by heating. It has a built in precision balance and a halogen dryer unit that ensures fast heating of the sample and thus guarantees the rapid availability of the measurement results.
Capsaicin content
Capsaicin content was analyzed as per the method 21.3 of ASTA (Anonymous 2004a). For the extraction of capsaicinoids, 25 g of ground chilli sample was weighed into a 500 ml soxhlet flask together with 200 ml of rectified spirit and several glass beads were added to avoid bumping. This was refluxed gently for 5 h by heating in Soxhlet apparatus. The extract was cooled and filtered into a stoppered test tube through Whatman No.1 filter paper pre-wetted with rectified spirit. This sample was then analyzed by HPLC [Waters 2487, dual wavelength detector, 515 HPLC pump, IN-LINE degasser, pump control module 2.], the symmetry C18 column [250 mm length, 0.25 mm i.d, 250 μ particle size] was used, with the combination of acetonitrile and 1 % acetic acid in water (60:40 v/v) as a mobile phase, flow rate of the mobile phase was kept at 0.8 ml/min. The HPLC was monitored at 280 nm and the injection volume was 20 μl. For peak identification, solution of reference standards were analyzed under similar conditions and their retention time were compared to those of samples. 0.1 g of standard N-vanillyl-n-nonamide (Sigma 97 %), was made up to 100 ml with rectified spirit and was kept as a stock solution (1,000 ppm) in amber colored standard flask. The working standard was prepared by adding 10 ml of stock and was made up to 100 ml with rectified spirit and was stored in refrigerator. The contribution of each identified compound was expressed as the percentage of its peak area. The samples were analyzed by Empower-2 software. The capsaicin in the whole chilli sample was calculated in Scoville Heat Units (SHU) (Scoville 1912) as follows,
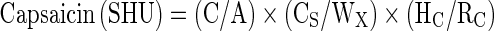 |
where,
- C
Average peak area of capsaicin
- A
Average peak area of standard
- CS
Concentration of standard (mg/ml)
- WX
Weight of sample (mg/ml)
- HC
Heat factor for capsaicin
- RC
Response factor for capsaicin relative to standard.
Capsaicin content in per cent was calculated as follows,
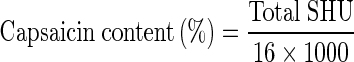 |
Total extractable colour
The total extractable colour or capsicum extractable colour in chillies was measured as per the method 20.1 stipulated in the ASTA analytical methods (Anonymous 2004b). For which, 100 mg of ground chilli sample was weighed accurately and then transferred to a 100 ml volumetric flask. This was diluted to volume with acetone and tightly capped with stopper. The flask was shaken vigorously and incubated at room temperature in dark for 16 h. After the incubation period, the flask was shaken again and left for sufficient time for particles to settle. The absorbance of the sample was recorded at 460 nm in UV–VIS dual beam spectrophotometer [Labomed, Inc., USA]. The capsicum extractable colour was computed using the following formula and expressed as ASTA units.
Total extractable colour
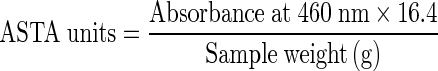 |
Oleoresin extractable colour
The oleoresin extractable colour in chilli was measured as per the procedure laid down in ASTA 20.1 analytical method (Anonymous 2004b). For this, 100 mg of ground chilli was transferred quantitatively to a 100 ml volumetric flask. This was diluted to volume with acetone, shaken and then left to stand for 2 min. From this, 10 ml of the extract was pipetted into another 100 ml volumetric flask, diluted to volume with acetone and was then vigorously mixed. The absorbance of the sample was recorded at 460 nm in UV–VIS dual beam spectrophotometer [Labomed, Inc., USA]. The oleoresin extractable colour was then determined as per the following formula and expressed as ASTA units.
Oleoresin extractable colour
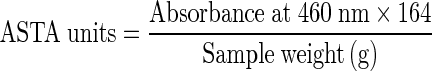 |
Statistical analysis
The interpretation of analytical data was performed by the application of analysis of variance (ANOVA). Two factors of variation (time and storage atmosphere) were considered. Duncan’s test was applied to those parameters which showed statistical significant variation in the samples due to any of these factors.
Results and discussion
Physical parameters
The samples stored in vacuum packed bags had maroon red colour fruits, characteristic flavor of capsaicin with no extraneous matter thus indicating no deterioration. On the contrary, the samples stored in jute bags were infested with mold and characteristic white patches on the entire surface coupled with odd odor. This is mainly attributed to the variations in the moisture content of the fruits which was constant for vacuum packed bags. While, it fluctuated in jute bags, depending on the relative humidity of the atmosphere. Changes in the moisture content of dried and frozen foods is known to affect nutritional quality. Increase in moisture content of dried food will promote microbial deterioration and accelerate rancidity and oxidation of vitamins. While, the loss of moisture will cause fat oxidation and browning reactions to occur resulting in off-flavor and off-colour. Reduction in rehydrating capacity of the samples increased with increase in storage period (Satish Naik et al. 2005). However, reduction was less for those packed in vacuum package as compared to conventional package. This might be attributed to the reduction of water binding sites due to chemical and structural changes in cells of the seed samples. Similar results were observed in mushroom by Kumar and Sreenarayanam (2000) and Deshpande and Tamhane (1981). Temperature fluctuation can increase the rate of deterioration of foods, contribute to colour fading of highly coloured products. Prolonged exposure to heat, light and oxygen resulted in 20–53 % loss of the initial carotenoids (De Guevara et al. 2002; Minguez-Mosquera and Hornero-Mendez 1993). Kader et al. (1989), Wright and Kader (1997) reported that low levels of O2 and high CO2 influence both the reduction of carotenoid loss by oxidation, and the inhibition of its biosynthetic pathway.
Table 1 shows that, in general, there is not much change in the moisture content of vacuum packed bags during storage for 24 months though there was a slight decline in the moisture content with advancement in the storage period, which was almost negligible. Lot of fluctuations were noticed in the moisture content of whole chilli stored in jute bags, irrespective of storage at room temperature or cold storage; either at 10 or 12 % moisture level. During the sixth month of storage, jute bags of 12 % moisture under cold storage had the maximum moisture content of 16.08 % which was significantly higher compared to all other treatments, followed by jute bag 10 % moisture under cold storage at third and sixth month of storage. The lowest moisture content (8.86 %) was recorded at the twelfth month in jute bags of 10 % moisture stored under light at room temperature followed by same treatment at twelfth month and ninth month of storage. These increases and decreases equilibrate well with the ambient relative humidity prevailing during that period. This has also been confirmed from earlier studies, where paprika samples stored under different moisture conditions were found to equilibrate with the ambient relative humidity (Osuna-Garcia and Wall 1998). The moisture content of cold stored jute bag samples were found to be quite high, which is mainly due to high humidity values maintained in cold storages. However, the moisture content of vacuum packaged chillies were not influenced by the storage condition i.e., it did not vary with room temperature, light or dark or cold storage. This was mainly attributed to the barrier properties of the packaging film.
Table 1.
Influence of vacuum packaging on biochemical parameters in whole chilli at different storage conditions
| Parameters | Storage months | Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
T1 (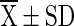 ) ) |
T2 (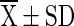 ) ) |
T3 (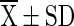 ) ) |
T4 (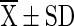 ) ) |
T5 (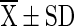 ) ) |
T6 (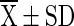 ) ) |
T7 (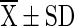 ) ) |
T8 (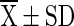 ) ) |
T9 (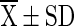 ) ) |
T10 (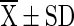 ) ) |
||
| Moisture content (%) | 0 | 10.0 ± 0.01 | 10.0 ± 0.03 | 10.0 ± 0.02 | 11.9 ± 0.03 | 12.0 ± 0.03 | 12.0 ± 0.02 | 10.0 ± 0.04 | 10.0 ± 0.02 | 12.0 ± 0.03 | 11.9 ± 0.05 |
| 3 | 10.1 ± 0.03 | 10.0 ± 0.01 | 10.1 ± 0.02 | 11.9 ± 0.02 | 11.9 ± 0.02 | 12.1 ± 0.02 | 14.0 ± 0.01 | 15.6 ± 0.01 | 14.6 ± 0.01 | 15.3 ± 0.02 | |
| 6 | 10.1 ± 0.01 | 10.1 ± 0.01 | 10.2 ± 0.01 | 11.8 ± 0.01 | 11.9 ± 0.00 | 12.0 ± 0.02 | 13.8 ± 0.01 | 15.6 ± 0.01 | 13.6 ± 0.02 | 16.0 ± 0.00 | |
| 9 | 9.9 ± 0.02 | 10.1 ± 0.00 | 10.1 ± 0.00 | 11.7 ± 0.01 | 11.9 ± 0.01 | 11.9 ± 0.01 | 9.1 ± 0.01 | 15.3 ± 0.02 | 9.3 ± 0.00 | 14.8 ± 0.00 | |
| 12 | 9.9 ± 0.01 | 9.6 ± 0.01 | 9.8 ± 0.00 | 11.8 ± 0.02 | 11.7 ± 0.01 | 11.8 ± 0.02 | 8.8 ± 0.00 | 14.1 ± 0.00 | 9.8 ± 0.01 | 13.9 ± 0.02 | |
| 18 | 9.9 ± 0.00 | 9.5 ± 0.00 | 9.7 ± 0.02 | 11.6 ± 0.01 | 11.7 ± 0.01 | 11.9 ± 0.01 | 13.3 ± 0.01 | 14.1 ± 0.00 | 13.8 ± 0.01 | 14.0 ± 0.01 | |
| 24 | 9.6 ± 0.00 | 9.4 ± 0.02 | 9.6 ± 0.01 | 11.4 ± 0.01 | 11.3 ± 0.01 | 11.5 ± 0.00 | 9.0 ± 0.02 | 14.0 ± 0.01 | 9.3 ± 0.01 | 14.0 ± 0.02 | |
| Average | 9.9G | 9.8H | 9.9G | 11.6E | 11.8D | 11.9C | 11.2F | 14.1B | 11.8D | 14.3A | |
| Capsaicin content (%) | 0 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 |
| 3 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | |
| 6 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | |
| 9 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | |
| 12 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | |
| 18 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| 24 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| Average | 0.07B | 0.07B | 0.08A | 0.07B | 0.07B | 0.08A | 0.05C | 0.05C | 0.05C | 0.05C | |
| Total extractable color (%) | 0 | 327.3 ± 0.02 | 331.5 ± 0.01 | 328.6 ± 0.01 | 329.5 ± 0.02 | 330.3 ± 0.02 | 328.5 ± 0.04 | 325.2 ± 0.02 | 328.4 ± 0.00 | 330.5 ± 0.02 | 327.7 ± 0.01 |
| 3 | 323.0 ± 0.02 | 327.8 ± 0.02 | 327.6 ± 0.01 | 320.4 ± 0.02 | 329.6 ± 0.01 | 327.1 ± 0.01 | 312.9 ± 0.02 | 322.1 ± 0.02 | 311.6 ± 0.02 | 323.8 ± 0.04 | |
| 6 | 319.9 ± 0.02 | 325.5 ± 0.02 | 324.0 ± 0.00 | 320.4 ± 0.01 | 327.1 ± 0.03 | 325.8 ± 0.00 | 223.0 ± 0.02 | 256.8 ± 0.01 | 221.8 ± 0.00 | 252.2 ± 0.02 | |
| 9 | 285.6 ± 0.02 | 291.0 ± 0.01 | 301.4 ± 0.02 | 289.3 ± 0.02 | 296.2 ± 0.03 | 306.4 ± 0.01 | 194.4 ± 0.02 | 216.7 ± 0.01 | 147.4 ± 0.01 | 222.8 ± 0.02 | |
| 12 | 232.6 ± 0.01 | 236.6 ± 0.00 | 264.9 ± 0.02 | 238.4 ± 0.01 | 248.8 ± 0.01 | 283.7 ± 0.01 | 130.9 ± 0.01 | 180.4 ± 0.02 | 118.8 ± 0.02 | 200.2 ± 0.02 | |
| 18 | 225.4 ± 0.02 | 227.1 ± 0.02 | 234.4 ± 0.02 | 230.3 ± 0.02 | 239.4 ± 0.01 | 261.7 ± 0.02 | 121.2 ± 0.02 | 172.4 ± 0.02 | 102.5 ± 0.00 | 193.7 ± 0.01 | |
| 24 | 221.3 ± 0.02 | 222.1 ± 0.02 | 230.5 ± 0.03 | 229.4 ± 0.00 | 236.6 ± 0.02 | 256.7 ± 0.02 | 116.3 ± 0.01 | 168.4 ± 0.02 | 95.4 ± 0.02 | 184.2 ± 0.0 | |
| Average | 276.5F | 280.3D | 287.4B | 279.7E | 286.9C | 298.6A | 203.5I | 235.0H | 189.7J | 243.6G | |
| Oleoresins extractable colour (%) | 0 | 220.6 ± 0.10 | 222.1 ± 0.10 | 230.3 ± 0.07 | 218.3 ± 0.03 | 219.2 ± 0.04 | 219.8 ± 0.02 | 218.3 ± 0.04 | 219.5 ± 0.03 | 220.5 ± 0.02 | 220.6 ± 0.02 |
| 3 | 217.2 ± 0.03 | 219.5 ± 0.02 | 220.1 ± 0.06 | 214.4 ± 0.03 | 215.2 ± 0.04 | 215.9 ± 0.02 | 211.6 ± 0.02 | 213.1 ± 0.04 | 216.7 ± 0.03 | 217.2 ± 0.02 | |
| 6 | 215.5 ± 0.02 | 215.3 ± 0.02 | 216.0 ± 0.06 | 211.5 ± 0.04 | 212.1 ± 0.01 | 212.9 ± 0.01 | 141.8 ± 0.01 | 142.4 ± 0.02 | 145.2 ± 0.02 | 145.9 ± 0.07 | |
| 9 | 204.5 ± 0.02 | 205.1 ± 0.04 | 205.8 ± 0.02 | 206.9 ± 0.00 | 206.9 ± 0.02 | 207.3 ± 0.03 | 134.0 ± 0.05 | 134.7 ± 0.02 | 133.4 ± 0.01 | 134.0 ± 0.03 | |
| 12 | 195.7 ± 0.01 | 196.2 ± 0.02 | 196.1 ± 0.02 | 196.2 ± 0.02 | 196.8 ± 0.02 | 196.9 ± 0.03 | 114.8 ± 0.02 | 115.1 ± 0.02 | 110.6 ± 0.03 | 110.9 ± 0.02 | |
| 18 | 191.0 ± 0.09 | 191.8 ± 0.03 | 192.7 ± 0.02 | 189.0 ± 0.05 | 189.1 ± 0.13 | 189.9 ± 0.03 | 54.1 ± 0.04 | 54.4 ± 0.13 | 47.5 ± 0.01 | 47.9 ± 0.01 | |
| 24 | 186.0 ± 0.02 | 186.6 ± 0.04 | 186.9 ± 0.02 | 184.0 ± 0.05 | 184.3 ± 0.01 | 184.5 ± 0.02 | 25.0 ± 0.05 | 25.2 ± 0.05 | 20.0 ± 0.02 | 20.2 ± 0.02 | |
| Average | 204.5C | 205.3B | 20.6.9A | 202.9F | 203.4E | 203.9D | 128.5H | 129.2G | 127.7J | 128.2I | |
T1 = Vacuum packed chilli with 10 % moisture at room temperature (light), T2 = Vacuum packed chilli with 10 % moisture at room temperature (Dark), T3 = Vacuum packed chilli with 10 % moisture at cold storage, T4 = Vacuum packed chilli with 12 % moisture at room temperature (light), T5 = Vacuum packed chilli with 12 % moisture at room temperature (Dark), T6 = Vacuum packed chilli with 12 % moisture at cold storage, T7 = Jute bag packed chilli with 10 % moisture at room temperature, T8 = Jute bag packed chilli with 10 % moisture at cold storage, T9 = Jute bag packed chilli with 12 % moisture at room temperature, T10 = Jute bag packed chilli with 12 % moisture at cold storage
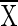 =mean value (n = 4), SD = Standard Deviation, Superscript indicate the differences based on Duncan’s Multiple Range Test (DMRT) value
=mean value (n = 4), SD = Standard Deviation, Superscript indicate the differences based on Duncan’s Multiple Range Test (DMRT) value
Pungency as influenced by packaging and storage
The data on capsaicin content (Table 1) was found to be stable up to 3 months of storage without any significant differences between the treatments, irrespective of whether it was vacuum packed or stored in gunny bags, 10 or 12 % moisture, cold stored or ambient stored under dark or light. Earlier work also support the relatively stable property of capsaicin where it was found that despite the varietal character for capsaicin content, it was stable throughout storage, regardless of storage conditions (Aczel 1985). However, from the sixth month, capsaicin content started declining with up to 25 % loss in jute bag stored under room temperature. There is a progressive degradation in capsaicin of chillies exposed to the ambient condition at 12 % moisture increasing up to 87.5 % by the twenty-fourth month. Cold stored samples however had higher capsaicin content. No decline in the capsaicin content up to 6 months was observed in all the vacuum packed bags regardless of their storage conditions, i.e. light, dark or cold storage and the moisture content before packaging. There was 12.5 % reduction in the capsaicin content starting from the 9 month which continues until 12 months of storage and no significant difference were observed between vacuum packed treatments. Similar results were observed in chilli seeds by Deepa et al. (2011). A similar influence of storage conditions has been reported by Ayub Hossain and Gottschalk (2009) and Zanoni et al. (2000) in dried tomato halves wherein, a significant loss of colour and other quality parameters were found at room temperature compared to cool chamber. The deterioration of capsaicin content in jute bags was probably because of higher oxidation and absorption of moisture from ambient atmosphere. The samples held under vacuum packaging are unlikely to suffer from oxidation and maintain the original moisture content because of the property of the films used for packaging. Storage of bunched onions under modified atmospheric packaging and moderate vacuum packaging also indicated superiority in maintaining various quality parameters over conventional storage (Hong and Kim 2004). To substantiate this, Naik et al. (2001), study can be quoted which reveal that chillies analyzed after 135 days of storage period, had almost no colour value but the capsaicin content was comparable with control sample. Van Blaricom and Martin (1951) stated that the factors responsible for pungency are not correlated with colour retention, since the pungent component, capsaicin is retained for long periods and is still present in samples which have lost their original colour. These results, thus establish vacuum packaging as a better technology to slow down the deterioration of capsaicin in chillies.
Colour as influenced by packaging and storage
Table 1, shows that there was a general decline in the oleoresin extractable colour for all the treatments. However, the decline in vacuum packed bags was controlled while that in jute packed chilli, the deterioration was rapid resulting in very low colour values. Among all the treatments, vacuum packed bags with 12 % moisture and kept under cold storage had better colour values. The degradation was much faster in jute bags stored at room temperature, since there was a free exchange of O2 and moisture content between the atmosphere and the containers as jute bags do not possess barrier properties unlike polyfilms used in vacuum packaging. In addition, the oxidative properties are enhanced at a relatively higher temperature and hence the faster degradation.
The conjugated system of double bonds in carotenoids that is responsible for their rich, intense colours is also the source of their susceptibility to oxidation (Bunnell and Bauernfeind 1962). Relatively better retention of colour stored under cold storage can be justified, since lower temperature retard the degradation process. This is in agreement with Lease and Lease (1956) who reported that the loss of colour is markedly slower at low temperature storage. The carotenoids are sensitive to light and this sensitivity is dependent on the presence of oxygen, the light thus acting as a catalyst to induce oxidation. However, in the complete absence of air, light has little effect (Bunnell and Bauernfeind 1962). This property of light acting as a catalyst can perhaps serve as an answer to the relatively higher degradation of colour among light exposed vacuum packed bags. Moisture may protect carotenoids from oxidation through a direct effect on the free radicals produced during pigment oxidation. Increased moisture content decreases the number of free radicals and thereby slows the oxidation rate (Labuza et al. 1970). A better retention of colour in the vacuum packed treatments involving 12 % moisture may be attributed to the protective function of moisture.
Conclusion
Vacuum packaging has been found to be a superior technology in preserving the quality of whole chillies for up to 24 months when compared to jute bags where chilli can be stored for only a short period. Various quality parameters viz., total extractable colour, oleoresin extractable colour and capsaicin content were very high in vacuum packaging treatments compared to jute bags which are again due to the impermeability of packaging film to air and moisture. Among various treatments, vacuum packed chillies under cold storage were found to have the least per cent decline in various quality parameters. Chillies with 12 % moisture and stored in vacuum packaged bags recorded better quality parameters over 10 % moisture reportedly because of moisture’s protective property against oxidation.
Acknowledgments
This work was funded by Interprise Brussels, Bruxtainer Division, Belgium and University of Agricultural Sciences, Dharwad. The authors greatly acknowledge the support of Jean-Marie GILLE, Paul Graindorge, Mohan Bajikar and the Vice-Chancellor, UAS, Dharwad.
References
- Aczel A (1985) Application of over pressured layer chromatography in food stuff examinations. Variation in capsaicin content of red pepper millings as a function of storage. In: Euro Food Pack. International Conference on Food Packaging: 325–330
- Anderson K, Lingnert H. Infleunce of oxygen concentration on the storage stability of cream powder. Lebensm Wiss Technol. 1997;30:147–154. [Google Scholar]
- Anonymous (2004a) Pungency of capsicums and their oleoresins (HPLC method). Method 21.3, 4th edn (revised). Official Analytical Methods of the American Spice Trade Association, New Jersey, pp. 111–115
- Anonymous (2004b) Extractable colour in capsicums and their oleoresins, 4th edn (revised). Method 20.1 Official Analytical Methods of the American Spice Trade Association, New Jersey, pp. 89–91
- Bernal MA, Antonio Calderon A, Pedreno MA, Romualdo M, Barcelo AR, Merino de Caceres F. Capsaicin oxidation by peroxidase from Capsicum annuum (var. annuum) fruits. J Agric Food Chem. 1993;41:1041–1044. [Google Scholar]
- Bunnell RH, Bauernfeind JC. Chemistry, uses and properties of carotenoids in foods. Food Technol. 1962;16(7):36–43. [Google Scholar]
- Davies BH, Matthews S, Kirk JTO. The nature and biosynthesis of the carotenoids of different colour varieties of Capsicum annuum. Phytochem. 1970;9:797–805. [Google Scholar]
- De Guevara RGL, Gonzalez M, Gracia-Meseguer MJ, Nieto JM, Amo M, Varon R. Effect of adding natural antioxidants on colour stability of paprika. J Sci Food Agric. 2002;82(9):1061–1069. [Google Scholar]
- Deepa GT, Chetti MB, Khetagoudar MC, Adavirao GM (2011) Influence of vacuum packaging on seed quality and mineral contents in chilli (Capsicum annuum L.). J Food Sci Technol, online journal,doi:10.1007/s13197-011-0241-3 [DOI] [PMC free article] [PubMed]
- Deshpande AG, Tamhane DV. Studies on dehydration of mushrooms (Volvariella Volvancea) J Food Sci Technol. 1981;18(3):96–101. [Google Scholar]
- Hong S-I, Kim D. The effect of packaging treatment on the storage quality of minimally processed bunched onions. Int J Food Sci Technol. 2004;39:1033–1041. [Google Scholar]
- Hossain MA, Gottschalk K. Effect of moisture content, storage temperature and storage period on colour, ascorbic acid, lycopene and total flavanoids of dried tomato halves. Int J Food Sci Technol. 2009;44:1245–1253. [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. Crit Rev Food Sci Nutr. 1989;28(1):1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Kang JY, Teng CH, Wee A, Chen FC. Effect of capsaicin and chilli on ethanol induced gastric mucosal injury in rat. Int J Gastroenterol Hepatol. 1995;36(5):664–669. doi: 10.1136/gut.36.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sreenarayanam VV. Studies on storage of dehydrated onion flakes. Indian Food Packag. 2000;54(2):72–75. [Google Scholar]
- Labuza TP, Tannenbaum SR, Karel M. Water content and stability of low and intermediate moisture foods. Food Technol. 1970;24:546–549. [Google Scholar]
- Lease JG, Lease EJ. Factors affecting the retention of red colour in peppers. Food Technol. 1956;10:368–375. [Google Scholar]
- Lee IO, Lee KH, Pyo JH, Choi YJ, Lee YC. Anti- inflammatory effect of capsaicin in Helicobacter Pylori infected gastric epithelial cells. Helicobacter. 2007;12(5):510–517. doi: 10.1111/j.1523-5378.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- Matsufuji H, Nakamusa H, Chino M, Takeda M. Antioxidant activity of capsanthin and the free fatty acid esters in paprika (Capsicum annuum L.) J Agric Food Chem. 1998;46(9):3468–3472. [Google Scholar]
- Minguez-Mosquera MI, Hornero-Mendez D. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reverse-phase HPLC. J Agric Food Chem. 1993;41(10):1616–1620. [Google Scholar]
- Naik PJ, Nagalakshmi S, Balasubrahmanyam N, Dhanaraj S, Shankaracharya NB. Packaging and storage studies on commercial varieties of Indian chillies (Capsicum annuum) J Food Sci Technol. 2001;38(3):227–230. [Google Scholar]
- Naik S, Ramachandra M, Tulasidas T, Rajashekharappa KS, Murali K, Mallesha BC. Post harvest (storage) studies for dried oyster mushroom (Pleurotus Florida) J Dairying Foods Home Sci. 2005;24(2):146–149. [Google Scholar]
- Nunez MP, Gaya M, Madena M, Rodriguezmarian MA, Garcia-Acer C. Changes in microbiological, chemical, rheological and sensory characteristics during ripening of vacuum packaged Manchego cheese. J Food Sci. 1986;21(2):112–115. [Google Scholar]
- Osuna-Garcia JA, Wall MM. Pre-storage moisture content affects colour loss of ground paprika (Capsicum annuum L.) under storage. J Food Qual. 1998;21:251–259. [Google Scholar]
- Peter KV, Nybe EV, Mini Raj N. Available technologies to raise yield. Surv. Ind. Agric. Chennai: The Hindu Year Book; 2006. pp. 82–86. [Google Scholar]
- Ramakrishnan TV, Francis FJ. Colour and carotenoid changes in heated paprika. J Food Sci. 1973;38:25–28. [Google Scholar]
- Rithesh M, Dangi RS, Dass SC, Malhothra RC. The hottest chilli variety in India. Curr Sci. 2000;79(3):287–288. [Google Scholar]
- Scoville WL. Note on capsicum. J Am Pharm Assoc. 1912;1:453. [Google Scholar]
- Van Blaricom LO, Martin JA. Retarding the loss of red colour in cayenne pepper with oil antioxidants. Food Technol. 1951;5:337–339. [Google Scholar]
- Wright KP, Kader AA. Effect of controlled atmosphere storage on the quality and carotenoid content of sliced persimmons and peaches. Postharvest Biol Technol. 1997;10:89–97. [Google Scholar]
- Zanoni B, Pagliarini E, Foschino R. Study of the stability of dried tomato halves during shelf-life to minimize oxidative damage. J Sci Food Agric. 2000;80:2203–2208. [Google Scholar]


