Abstract
Enzymes have been the centre of attention for researchers/industrialists worldwide due to their wide range of physiological, analytical, food/feed and industrial based applications. Among the enzymes explored for industrial applications, xylanases play an instrumental role in food/feed, textile/detergent, paper and biorefinery based application sectors. This study deals with the statistical optimization of xylanase production by Thielaviopsis basicola MTCC 1467 under submerged fermentation conditions using rice straw, as sole carbon source. Different fermentation parameters such as carbon source, nitrogen source, inorganic salts like KH2PO4, MgSO4 and pH of the medium were optimized at the individual and interactive level by Taguchi orthogonal array methodology (L16). All selected fermentation parameters influenced the enzyme production. Rice straw, the major carbon source mainly influenced the production of xylanase (~34 %). After media optimization, the yield of enzyme improved from 38 to ~60 IU/ml (161.5 %) indicating the commercial production of xylanase by T. basicola MTCC 1467. This study shows the potential of T. basicola MTCC 1467 for the efficient xylanase production under the optimized set of conditions.
Keywords: Xylanase, Rice straw, Thielaviopsis basicola MTCC 1467, Optimization, Submerged fermentation, L16 orthogonal array
Introduction
Rising interests in sustainable development and environmentally benign practices, microbial enzyme mediated transformation processes have shown advantages as compared to the conventional chemical conversion processes (Chandel et al. 2011a). In the last two decades, xylanases (EC.3.2.1.8) have shown a great deal of attention due to their vast biotechnological applications in food, feed and paper industries (Chandel et al. 2007). Furthermore, xylanases have shown a pivotal role for the economic production of value-added products such as D-xylitol, cellulosic ethanol, organic acids etc. (Beg et al. 2001). Hemicellulose is one of the main components of lignocellulose, the most abundant and readily available carbohydrate source on earth after cellulose (Chandel et al. 2011b). Xylan is the major backbone in the hemicellulosic fraction of the plant cell wall, linking compounds like arabinose, glucose, mannose and other sugars through an acetyl chain (Chandel et al. 2011b). Xylanases are the enzymes produced by prokaryotes, eukaryotes and mechanistically act precisely on xylan to release xylose as monomeric constituent (Beg et al. 2001).
A large number of bacteria and fungi are known to produce xylanases under varying cultivation systems using different carbon and nitrogen sources (Kulkarni et al. 1999; Subramaniyan and Prema 2002). To economize the any metabolite production from microbial systems, selection of appropriate carbon and nitrogen source is pivotal (Sreenivas Rao et al. 2008; Suresh et al. 2011; Húngaro et al. 2011). Fungi are the most potent xylanase producers, as they secrete high level of enzymes than those of yeast and bacteria (Qinnghe et al. 2004). Fungus Thielaviopsis basicola which cause black rot in roots of several plants (Baruah et al. 1980) is the selective producer of xylanases and cellulases from an industrial point of view due to the extracellular release of xylanases in fermentation broth with an appreciable yield as compared to yeast and bacteria (Haltrich et al. 1996; Steiner et al. 1987). Production of enzymes like other microbial derived metabolites is generally influenced by several physical and physico-chemical parameters such as incubation temperature, pH of medium, inorganic metals, agitation, aeration and others (Sreenivas Rao et al. 2008). In order to obtain the maximum production of enzymes from microorganism during fermentation reaction, it is essential to optimize these important parameters (Prakasham et al. 2006 and 2007a, b; Subba Rao et al. 2008a, b; Sreenivas Rao et al. 2008). Optimization of fermentation medium and other influencing parameters is also important to produce cost competitive xylanase from microbial systems. There is no defined medium for the optimum production of any metabolite due to the genetic diversity of microbial sources and their varying specific growth conditions (Subba Rao et al. 2008a, b). Therefore, it is essential to optimize all fermentation parameters, but optimizing all factors by one parameter at-a-time is a conventional methodology since it involves numerous experimental trails (Sreenivas Rao et al. 2004 and 2008). In contrast, statistically designed experiments significantly reduce the number of experiments by developing a specific design of experiments (Balakrishnan and Pandey 1996; Sreenivas Rao et al. 2008).
The main aim of this study was to provide the best production conditions for extra cellular xylanase by T. basicola MTCC 1467 under submerged fermentation conditions utilizing rice straw as main carbon source.
Materials and methods
Materials
D-glucose, Bovine serum albumin (BSA), D-xylose, Di-nitro salicylic acid (DNS), Xylan (Birchwood), Citric acid, Potato dextrose broth, Agar-Agar type I, Peptone, FeSO4.7H2O, MnSO4, CaCl2.6H2O, KH2PO4, MgSO4, Sodium potassium tartrate and Sodium hydroxide were obtained from High media, Mumbai, India. Rice straw was obtained from the local agricultural fields and was further processed in our laboratory. It was used as a sole carbon source for the xylanase production.
Microorganism and maintenance
The strain Thielaviopsis basicola MTCC 1467 was obtained from IMTECH (Institute of Microbial Technology), Chandigarh, India. It was maintained on potato dextrose agar (PDA) slants at 4 °C and was sub cultured every month.
Submerged fermentation
Experiments were performed for xylanase production optimization by T. basicola MTCC 1467 considering five factors at selected levels (Table 1). A total of 16 experimental trails were performed (Table 2). All experiments were conducted in 250 ml Erlenmeyer flasks containing 100 ml of medium with the media components such as rice straw (carbon source), peptone (nitrogen source), KH2PO, MgSO4 (Table 1) and MnSO4 0.5 mg, FeSO4.7H2O 0.15 mg and CaCl2.6H2O 0.2 mg. Prior to use, the rice straw was pretreated with NaOH (10 % w/v), autoclaved for 20 min at 121 °C. After pretreatment, the substrate was washed with running tap water to remove the traces of NaOH and dissolved lignin. Thereafter, it was dried in hot air oven at 50 °C for overnight and subsequently sieved by metal mesh (20 mesh size) to obtain uniform particle size of 4 mm. This processed rice straw was used as a carbon source in the fermentation medium. The sterilized flasks were inoculated with 5 % of inoculum and subsequently incubated in an orbital shaker (JSSI-300 C, USA) at 150 rpm at room temperature. The enzyme activities presented in this study are the average values of three individual experiments.
Table 1.
Selected fermentation factors and their assigned levels for xylanase production by Thielaviopsis basicola MTCC 1467
| Components (Factors) | L1 g/100 ml | L2 g/100 ml | L3 g/100 ml | L4 g/100 ml |
|---|---|---|---|---|
| RiceStraw | 1 | 2 | 3 | 4 |
| KH2PO4 | 0.1 | 0.2 | 0.3 | 0.4 |
| Peptone | 0.5 | 1 | 1.5 | 2 |
| MgSO4 | 0.05 | 0.1 | 0.15 | 0.2 |
| pH | 4 | 5 | 7 | 8 |
Table 2.
Experimental set up (L16 orthogonal array) and S/N ratios for xylanases by Thielaviopsis basicola MTCC 1467
| Variables trails | Rice straw | KH2PO4 | Peptone | MgSO4 | pH | Observed IU/ml | Predicted IU/ml | S/N ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 4.4 | 4.398 | 12.86905 |
| 2 | 1 | 2 | 2 | 2 | 2 | 12 | 11.998 | 21.58362 |
| 3 | 1 | 3 | 3 | 3 | 3 | 47 | 46.998 | 33.44196 |
| 4 | 1 | 4 | 4 | 4 | 4 | 55.3 | 55.298 | 34.8545 |
| 5 | 2 | 1 | 2 | 3 | 4 | 58.08 | 58.078 | 35.28053 |
| 6 | 2 | 2 | 1 | 4 | 3 | 52.2 | 52.198 | 34.35341 |
| 7 | 2 | 3 | 4 | 1 | 2 | 50.4 | 50.398 | 34.04861 |
| 8 | 2 | 4 | 3 | 2 | 1 | 55.92 | 55.918 | 34.95134 |
| 9 | 3 | 1 | 3 | 4 | 2 | 55.8 | 55.798 | 34.93268 |
| 10 | 3 | 2 | 4 | 3 | 1 | 43.08 | 43.078 | 32.68551 |
| 11 | 3 | 3 | 1 | 2 | 4 | 52.68 | 52.678 | 34.43292 |
| 12 | 3 | 4 | 2 | 1 | 3 | 41.76 | 41.758 | 32.41521 |
| 13 | 4 | 1 | 4 | 2 | 3 | 59.64 | 59.638 | 35.51075 |
| 14 | 4 | 2 | 3 | 1 | 4 | 23.04 | 23.038 | 27.24965 |
| 15 | 4 | 3 | 2 | 4 | 1 | 30.12 | 30.118 | 29.5771 |
| 16 | 4 | 4 | 1 | 3 | 2 | 34.56 | 34.558 | 30.77147 |
Xylanase optimization by L16-orthogonal array method
The orthogonal matrix L16 (44) (Fukushima and Sukahara 1990) method was used to study the relationship between the variables of medium components and to optimize their concentration for higher yields of enzyme production. This method uses a special set of arrays called orthogonal arrays. These standard arrays stipulate the way of conducting the minimal number of experiments, which could give the full information of all the factors that influence enzyme production. L16-orthogonal array method is based on modified methods described by Taguchi (Taguchi and Wu 1980 and 1986), which overcomes many problems associated with conventional methodology. The modified version consists of four distinct phases: (a) designing the experiments, (b) running and analyzing, (c) analysis and (d) validation of assumptions (Prakasham et al. 2005). Five typical fermentation factors i.e. carbon source (rice straw), nitrogen source (peptone), KH2PO4, MgSO4 and pH which have a significant influence on enzymes production were considered by performing one at-a-time experiments (data not shown) and the chosen parameters were varied at four different levels in Taguchi design of experiments (Table 1). Conditions such as temperature (30 °C), agitation (150 rpm), and media volume ratio (volume of media/volume of flask) were not changed and fixed at standard prescribed conditions for the microorganism. The selected variables were arranged into an orthogonal array (L16 orthogonal array for the representative experiments) (Table 2). With respect to medium optimization, each column would correspond to individual medium components, and each row would represent trails with different combinations of factors. Each of the above selected components were taken at four defined concentrations, covering the range over which its effect can be determined. The objective of medium optimization was to maximize the enzyme production. For this, following signal-to-noise ratio function designed by Taguchi design was used.
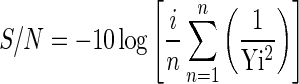 |
Where S/N is the signal-to-noise ratio, n is the number of trials with given concentration, and Yi is the yield of correspondent trials. For each component the optimal conditions are those that give the largest S/N ratio.
AEDPP, Analysis of experimental data and prediction of performance
Qualitek-4 software (Nutek Inc., MI) was used for automatic design of experiments in the present study. The data obtained was processed using Qualitek-4 software and MINITAB 15 (Trial version). The processed data was used to identify the influence of individual factors on the enzymes production and to determine the optimum fermentation conditions of T. basicola MTCC 1467 for maximum xylanase production.
Validation [V]
To validate the determined optimized methodology, fermentation experiments were carried out in triplicates and the samples collected were assayed for xylanase production profile.
Extraction of enzyme
The enzyme samples were extracted after every 24 h for about 9 days and centrifuged at 8,000 rpm at 4 °C for 30 min. Supernatant was collected and filtered through Whatman No. 1 filter paper (High media, Mumbai) to remove the suspended spores. The filtrate was used to determine the activity of xylanase.
Enzyme activity
Xylanase activity was determined by incubating 1 ml of reaction mixture which contains 0.5 ml of 2 % xylan, 0.4 ml of citrate buffer of pH 5.5 and 0.1 ml of culture filtrate at 50 °C for 10 min. The enzyme blank and substrate blank were simultaneously incubated. The reducing sugar (xylose) released was estimated by DNS method (Miller 1959). One unit of xylanase activity was defined as the number of micromoles of reducing sugar (xylose) released per ml of enzyme solution in 1 min under the assayed conditions.
Estimation of protein concentration
Protein concentration was estimated by Bradford’s method (Bradford 1976). 1 ml of Bradford’s reagent was added to suitably diluted protein samples and to the protein blank. The absorbance was read within 5 min at 595 nm. The amount of protein in the samples was determined from the standard bovine serum albumin plot.
Results and discussion
Carbon source
In order to study the influence of carbon source, xylanase fermentation studies were performed using various substrates (% w/v) such as xylose, carboxy methyl cellulose (CMC), xylan, rice straw, vegetable waste, rice brawn, wheat straw, cotton leaves and green chilly leaves (Fig. 1). The microorganism, T. basicola MTCC 1467 was able to utilize xylan and rice straw effectively as potential carbon sources for the selective production of xylanase. The carbon source is one of the most significant factors during the growth and metabolic process of any microorganism. The selection of an appropriate carbon source always plays a crucial role on the process economics of xylanase production (Beg et al. 2001; Pandya and Gupte 2012). With the aim of reducing the substrate cost, rice straw a major hemicellulose containing lignocellulosic biomass (Park et al. 2011) was selected as a principle carbon source in the present study. Several raw materials have been indicated in the literature as suitable carbon sources for the production of lignocellulolytic enzymes by microorganisms such as oat wheat, oat spelt xylan (Chivero et al. 2001; Georis et al. 2000), wheat bran, arabinoxylan (Bataillon et al. 2000), wheat bran (Fujimoto et al. 1995), sugarcane bagasse (Martınez-Trujillo et al. 2003), rice bran (Dhillon et al. 2000) and wild sugarcane (Saccharum spontaneum) leaves (Uma et al. 2008). Use of xylan and CMC as the carbon source for large scale production of enzymes is uneconomical because of their high cost. Selection of appropriate carbon source for the production of any metabolite is pivotal in any cultivation system. Production of maximum extracellular chitin deacetylase (460.4 ± 14.7 unit/g initial dry substrate) was obtained by Colletotrichum lindemuthianum ATCC 56676 under solid substrate fermentation (Suresh et al. 2011). Hence, use of cost-effective substrates such as lignocellulosic waste material is highly recommended (Wejse et al. 2003; Sá-Pereira et al. 2002).
Fig. 1.
Xylanase production by Thielaviopsis basicola MTCC 1467 using various carbon sources
Enzyme production
Xylanase production was studied at 30 °C for 10 days in production medium (100 ml) containing rice straw 2 g, MgSO4 0.1 g, KH2PO4 0.2 g, peptone 1 g, and FeSO4.7H2O 0.5 mg, MnSO4 0.15 mg and CaCl2.6H2O 0.2 mg with the medium pH of 5 (Uma et al. 2008). Xylanase production reached maximum on the 9th day (Baby Rani et al. 2008, 2012) of incubation. Xylanases are usually expressed at the end of the exponential phase and harvesting time was correlated to the medium under consideration (Wejse et al. 2003). The phenomenon of sudden increase and subsequent downfall in enzyme activities during the cultivation period has also been observed in xylanase production by Aspergillus sydowii MG-49 (Ghosh et al. 1993) and Streptomyces sp. CH-M-035 (Flores et al. 1996).
Evaluation of fermentation conditions for xylanase production
Metabolite production by microorganism depends on the physiological, nutritional, and biochemical nature of the microbe employed, improvement of production and the factors which play major role for this may vary from organism to organism (Prakasham et al. 2005; Subba Rao et al. 2008a, b). Preliminary investigations indicated that fermentation factors such as medium pH (7), carbon source (rice straw), nitrogen source (peptone), and major salts are critical for enzymes production by T. basicola 1467. Under the above optimized conditions, the enzyme yield enhanced from 38 to ~60 IU/ml indicating the significance of fermentation factors on fungal metabolism and its associated enzyme yields (Fig. 2). This data suggested that optimization for each factor of growth, nutritional requirement, and production yields are essential requirements for understanding the potential of xylanase production at the industrial scale (Sreenivas Rao et al. 2006; Prakasham et al. 2007a, b; Suvarna Lakshmi et al. 2009). Similar results were noticed for biocatalyst production with other microbial strains (Sreenivas Rao et al. 2006). However, the interactive influence of these factors on enzyme production was not possible with conventional optimization, which is known to positively regulate the microbial metabolism (Prakasham et al. 2007a, b). Therefore, optimization of fermentation factors for enzyme yields with rice straw as carbon source was investigated using five fermentation critical factors and their selected levels (Table 1).
Fig. 2.
Impact of selected variables (Rice straw, KH2PO4, MgSO4, peptone and pH) on xylanase production by Thielaviopsis basicola MTCC 1467 under submerged fermentation
Statistical optimization of xylanase production
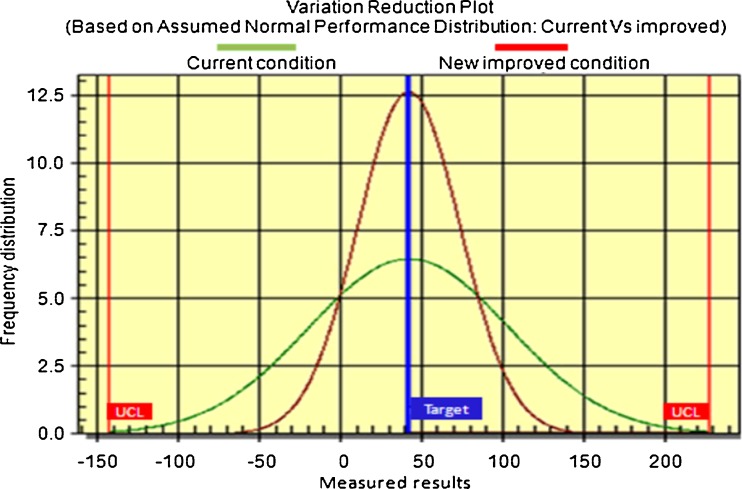
The Taguchi L16 orthogonal array revealed significant variation in the enzyme production (Table 2). The crux of the orthogonal array method lies in choosing the level combinations of the input design variables for each experiment (Fukushima and Sukahara 1990). Taguchi L16 orthogonal array method is a statistical method for design of factorial experiments and analysis of the results with accuracy eventually saving a lot of time by reducing the number of experiments (Taguchi 1986). This design has shown many advantages over conventional methodologies of media optimization and predict the accurate results by exploiting the fundamental principles of statistics, randomization, and replication (Subba Rao et al. 2008a, b). The present data suggested a significant improvement in enzyme yields compared to the conventional media optimization (Fig. 3). Similar variation of enzyme yields with optimization experiments were found for xylanase production by Aspergillus terreus (Suvarna Lakshmi et al. 2009), alkaline protease production by Bacillus circulans (Subba Rao et al. 2008a, b) and xylitol production by Candida tropicalis (Sreenivas Rao et al. 2006). Using L16 orthogonal array method the parameters were optimized for their concentrations.
Fig. 3.
Performance distribution on xylanase production by Thielaviopsis basicola MTCC 1467 under submerged cultivation conditions
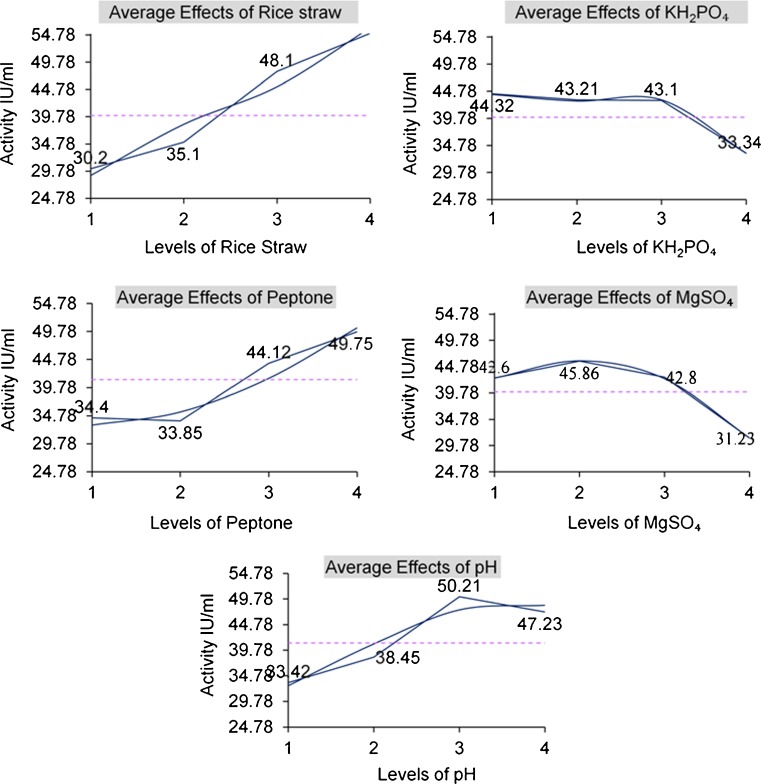
The production capacity and signal-to-noise ratio (larger is better) produced by the L16 orthogonal array is given in Table 2. The S/N ratio values help to assess which combination of factors have the greatest effect on the response characteristic of interest. A higher S/N ratio value indicates greater effect of those components in combination. The present study based on main effects of individual components (Table 3). The order of components could be ranked as rice straw>MgSO4>pH>peptone>KH2PO4, suggesting that carbon source had a major effect and KH2PO4 showed least effect on enzyme production by T. basicola MTCC 1467. The main effect plots are shown in Fig. 2. These plots show how each factor affects the response characteristic. A main effect is present when different levels of a factor affect the characteristic differently. MINITAB software creates the main effects plot by plotting the characteristic average to each factor level. A line connects the points for each factor. When the line is horizontal (parallel to the x-axis), then there is no or minimal main effect present. Different levels of the factor affect the enzyme characteristics in various manners. The greater the difference in the vertical position of the plotted points (greater the deviation from the parallel x-axis), greater is the magnitude of the main effect. In the present study, it can be seen that for each of the five variables at four levels, one level increases the activity compared to the other level. This difference is a main effect which verifies that rice straw at level 4, MgSO4 at level 2, pH at level 3, peptone at level 4, and KH2PO4 at level 1 showed main effect on xylanase production (Fig. 2). These levels also represent the optimal concentrations of the individual components in the medium. Response tables can also be used to predict the optimal levels of each component used in the study.
Table 3.
ANOVA table for xylanase production with Thielaviopsis basicola MTCC1467
| Col | Factor | DOF(f) | Sum of Sqrs (S) | Variance (V) | Pure Sum (S) | Percent (P)% |
|---|---|---|---|---|---|---|
| 1 | RS | 3 | 1463.9 | 487.966 | 1463.898 | 33.933 |
| 2 | KH2PO4 | 3 | 511.2 | 170.406 | 511.219 | 11.848 |
| 3 | Peptone | 3 | 770.2 | 256.744 | 770.234 | 17.852 |
| 4 | MgSO4 | 3 | 837.8 | 279.272 | 837.816 | 19.418 |
| 5 | pH | 3 | 731.2 | 243.761 | 731.283 | 16.949 |
| Other | Error | 0 | ||||
| Total | 15 | 4314.4 | 100 % |
Influence of selected factors interaction on xylanase production
To better understand the overall xylanase production process and activities by T. basicola MTTCC 1467, software-created interaction effects were analyzed individually since any independent factor may interact with single or all of the other factors. The severity index (SI) of each selected factor on enzyme production was estimated which can help to understand the influence of two individual factors at defined levels (Table 4). From Table 4, it can be observed that KH2PO4 and peptone interaction (level 2 and 3; column 1) had the highest impact (84.97 %). It was inquisitive to note that, in both of above interaction one of the factor such as KH2PO4 which is having a least impact percent factor of 11.848 % (Table 3) interacted with medium factor, peptone (17.85 %). Among all the selected factors, rice straw and peptone interaction showed the lowest severity index, 1.55 % (level 1 and 3; column 2). It was evident from these interaction results, that the influence of selected factors (individually) on xylanase production is different while in grouping the production yield of enzyme is independent of individual factor influence.
Table 4.
Interaction influence of selected factors on xylanase production by Thielaviopsis basicola MTCC 1467 using rice straw as sole carbon source
| Interacting factor pairs (In order of severity) | Columna | SIb (%) | Colc | Optd |
|---|---|---|---|---|
| KH2PO4 × Peptone | 2 × 3 | 84.97 | 1 | [1,4] |
| MgSO4 × pH | 4 × 5 | 81.39 | 1 | [2,3] |
| KH2PO4 × pH | 2 × 5 | 74.65 | 7 | [1,3] |
| Peptone × MgSO4 | 3 × 4 | 70.63 | 7 | [4,2] |
| KH2PO4 × MgSO4 | 2 × 4 | 59.99 | 6 | [1,2] |
| Peptone × pH | 3 × 5 | 43.7 | 6 | [4,3] |
| RS × KH2PO4 | 1 × 2 | 12.22 | 3 | [4,1] |
| RS × pH | 1 × 5 | 11.87 | 4 | [4,3] |
| RS × MgSO4 | 1 × 4 | 1.88 | 5 | [4,2] |
| RS × Peptone | 1 × 3 | 1.55 | 2 | [4,4] |
aRepresent the column locations to which the interacting factor assigned
bInteraction severity index (100 % for 90 ° angle between the lines, 0 % for parallel lines)
cShows column that should be reserved if this interaction effects were to be studied (2- L factors only)
dIndicates the factor levels desirable for the optimum condition (based strictly on the first 2 levels). If an interaction included in the study and found significant (in ANOVA) the indicated levels must replace the factor levels identified for the optimum condition without considerations of any interaction effects
ANOVA (Analysis of variance)
Understanding the impact of each individual factor on selective metabolite production is the key for a successful fermentation process. ANOVA was used to analyze the results of the orthogonal array experiments and to determine how much variation each factor has contributed towards xylanase production. The F ratio is a test static used for multiple independent variables (factors). This can be used to determine the quality of the experimental results. The main principle used in the test statistics is signal-to-noise ratio in which the systematic variance is divided by unsystematic variance and the larger ratio is better. In Table 2, the S/N ratios of the experimental results can be observed. The test static can be calculated as follows.
 |
If the variance is the measure of sum of squares (SS), it can be shown as
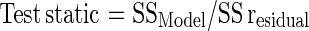 |
And the total variance is, 
Where, model and residual indicate expected (systematic) variance and random (unexpected) variance respectively.
F ratio is measured as the ratio of mean sum of squares (MS)
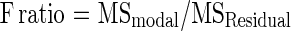 |
Where mean sum of squares (MS)=sum of squares (SS)/degrees of freedom (df)
The F ratio can also be calculated using Pearson or sample correlation coefficient (r)
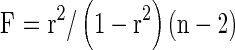 |
To obtain the optimized levels or composition of each factor, the predictive analysis based on statistical calculations is shown in Table 5. Statistical analysis of the xylanase production data in the above experimental designs revealed that among all the selected factors, Rice straw contributed the maximum impact (16.109 %) and KH2PO4 showed the least impact (9.41 %) on the overall enzyme production under the selected fermentation conditions (Table 5).
Table 5.
Xylanase production optimum conditions and performance by Thielaviopsis basicola MTCC 1467 using rice straw as carbon source
| Factors | Levels description | Levels | Contribution |
|---|---|---|---|
| RSa | 4 | 4 | 16.109 |
| KH2PO4 | 0.1 | 1 | 9.4168 |
| Peptone | 2 | 4 | 10.930 |
| MgSO4 | 0.1 | 2 | 12.147 |
| pH | 7 | 3 | 11.035 |
| Total Contribution From All Factors | 59.640 | ||
| Current Grand Average of Performance | 42.248 | ||
| Expected Result at Optimum Condition | 59.638 | ||
aRS stands for Rice Straw: Carbon source
Final medium for xylanases production by T. basicola MTCC 1467 is, rice straw 4 %, MgSO4 0.1 g, KH2PO4 0.1 g, peptone 2 g, and FeSO4.7H2O 0.5 mg, MnSO4 0.15 mg, CaCl2.6H2O 0.2 mg for 100 ml of the medium at pH 7. To confirm these results, experiments were carried out using these nutrient concentrations, and it was observed that the xylanase activity obtained was 59.64 IU/ml as compared to 59.638 IU/ml predicted using MINITAB software for the same composition. This showed that the experimental value almost matches with predicted values. The final optimized medium produced 59.64 IU/ml of xylanase activity at 9 days of incubation period as compared to 38 IU/ml before optimization [data not shown] and the activity was improved by ~161 % in the medium optimized by orthogonal matrix method. The obtained xylanase activity by T. basicola MTCC 1467 using rice straw is comparable to reported xylanase activities (Table 6). However, the conditions explored for the production of xylanase in these studies are different but the production titers of this enzyme from the present study are quite comparable with the previous reports.
Table 6.
Comparision of xylanase activity of Thielviopsis bascola MTCC 1467 with other fungal microorganisms
| Microorganism | Substrate | Type of cultivation | Xylanase activities (lU/mi) | References |
|---|---|---|---|---|
| T. harzianum T4 | Xylan, fruit stalk, extractives | Submerged | 6.69, 5.696, 0.133 | Medeiros et al. 2000 |
| Thermoascus aurantiacus | Sugarcane bagasse, Corn straw | Solid state | 11, 97 | da Silva et al. 2005 |
| A. terreus | 1 % Xylan | Submerged | 42 | Geweely et al. 2006 |
| P. oxalicum | Wheat bran | Solid state | 16 | Li et al. 2007 |
| P. clerotiorum | Theat bran | Submerged | 7.5 | Knob and Carmona (2008) |
| Fusarium solani F7 | Wheat straw | Submerged | 78.32 | Gupta et al. 2009 |
| T. harzianum | Wheat straw | Solid state | 146 | Sanghvi et al. 2011 |
| Trametes versicolor | Tomato pomace | Solid state | 50 U g−1 | Iandolo et al. 2011 |
| A. tubingensis JP-1 | Sugarcane bagasse | Solid state | 17 | Pandya and Gupte 2012 |
| Thielaviopsis basicola | Rice straw | Submerged | 59.64 | Present work |
Conclusions
Xylanases play a vital role in the production of various bio-based products of commercial significance. Of the different hydrolytic enzymes, xylanase have attracted attention because of their diverse range applications. In the present study, production of xylanase by T. basicola MTCC 1467 using rice straw, an agricultural waste as sole carbon source, was studied and the impact of critical fermentation factors at different concentrations at individual and interactive levels were studied using Taguchi L16 orthogonal array. The experimental design determined the influence of individual factors helped in establishing the relationship between variables and operational conditions as well as the optimal levels for the best performance. Significant variation in enzyme production was noticed among assigned factor levels. Evaluating the relative influence of each factor at individual level, rice straw had the major influence on the production of xylanase. Validation of the findings of the experimental design demonstrated a significant (~161 %) improvement in xylanase production by T. basicola MTCC 1467 under optimized set of conditions.
Acknowledgement
We thank AICTE and CSIR, India for the financial support.
Nomenclature
- DNS
Di-nitro salicylic acid
- ANOVA
Analysis of variance
- OA
Orthogonal array
- RS
Rice straw
Contributor Information
Anuj K. Chandel, Phone: +55-12-92205129, Email: anuj.kumar.chandel@gmail.com
A. Uma, Phone: +91-9848120819, Email: vedavathi1@jntuh.ac.in
References
- Baby Rani G, Prameela Devi Y, Narasu ML, Uma A. Production, isolation and characterization of cellulases from Thielaviopsis basicola MTCC 1467 strain by using rice straw as substrate. Bioscan J. 2008;3:537–542. [Google Scholar]
- Baby Rani G, Chiranjeevi T, Madhu C, Nagaraju N, Das A, Lakshmi Narasu M, Uma A (2012) Potential of thermo and alkali stable xylanases from Thielaviopsis basicola (MTCC-1467) in biobleaching of wood kraft pulp. Appl Biochem Biotechnol. doi:10.1007/s12010-012-9765-x [DOI] [PubMed]
- Balakrishnan K, Pandey A. Production of biologically active secondary metabolites in solid state fermentation. J Sci Ind Res. 1996;55:365–372. [Google Scholar]
- Baruah HK, Baruah P, Baruah A (1980) Textbook of plant pathology. Oxford and IBH Press
- Bataillon M, Nunes Cardinali AP, Castillon N, Duchiron F. Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzyme Microb Technol. 2000;26:187–192. doi: 10.1016/S0141-0229(99)00143-X. [DOI] [PubMed] [Google Scholar]
- Beg QA, Kapoor M, Mahajan G, Hoondal S. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Anal Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandel AK, Rudravaram R, Rao LV, Ravindra P, Narasu ML. Role of industrial enzymes in bio-industrial sector development: an Indian perspective. J Commun Biotechnol. 2007;13:283–291. doi: 10.1057/palgrave.jcb.3050065. [DOI] [Google Scholar]
- Chandel AK, Chandrasekhar G, Silva MB, Silva SS (2011a) The realm of cellulases in biorefinery development. Crit Rev Biotechnol. doi:10.3109/07388551.2011.595385 [DOI] [PubMed]
- Chandel AK, Chandrasekhar G, Radhika K, Ravinder R, Ravindra P. Bioconversion of pentose sugars into ethanol: a review and future directions. Biotechnol Mol Biol Rev. 2011;6:8–20. [Google Scholar]
- Chivero ET, Mutukumira AN, Zvauya R. Partial purification and characterization of a xylanase enzyme produced by a micro-organism isolated from selected indigenous fruits of Zimbabwe. Food Chem. 2001;72:179–185. doi: 10.1016/S0308-8146(00)00216-8. [DOI] [Google Scholar]
- da Silva R, Lago ES, Merheb CW, Macchione MM, Park YK, Gomes E. Production of xylanase and CMCase on solid state fermentation in different residues by Thermoascus aurantiacus miehe. Braz J Microbiol. 2005;36:235–241. [Google Scholar]
- Dhillon A, Gupta JK, Khanna S. Enhanced production, purification and characterization of a novel cellulase-poor thermostable, alkalitolerant xylanase from Bacillus circulans AB 16. Proc Biochem. 2000;35:849–856. doi: 10.1016/S0032-9592(99)00152-1. [DOI] [Google Scholar]
- Flores ME, Perea M, Rodriguez O, Malvaez A, Huitron C. Physiological studies on induction and catabolite repression of β-xylosidase and endoxylanases in Streptomyces sp. CH-M-1035. J Biotechnol. 1996;49:179–187. doi: 10.1016/0168-1656(96)01542-8. [DOI] [Google Scholar]
- Fujimoto H, Ooi T, Wang S-L, Takizawa T, Hidaka H, Murao S, Arai M. Purification and properties of three xylanase from Aspergillus aculeatus. Biosci Biotech Biochem. 1995;59:538–540. doi: 10.1271/bbb.59.538. [DOI] [Google Scholar]
- Fukushima M, Sukahara S. The experimental methods of biochemistry. 3. Tokyo: Society Press Centers; 1990. [Google Scholar]
- Georis J, Giannotta F, Buyl ED, Granier B, Frère JM. Purification and properties of three endo-1, 4-xylanases produced by Streptomyces spp. strain S38 which differ in their ability to enhance the bleaching of kraft pulps. Enzyme Microb Technol. 2000;26:178–186. doi: 10.1016/S0141-0229(99)00141-6. [DOI] [PubMed] [Google Scholar]
- Geweely NS, Ouf SA, Eldesoky MA, Eladly AA. Stimulation of alkalothermophilic Aspergillus terreus xylanase by low-intensity laser radiation. Arch Microbiol. 2006;186:1–9. doi: 10.1007/s00203-006-0110-z. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Das A, Mishra AK, Nanda G. Aspergillus sydowii MG 49 is a strong producer of thermostable xylanolytic enzymes. Enzym Microbial Technol. 1993;15:703–729. doi: 10.1016/0141-0229(93)90073-B. [DOI] [Google Scholar]
- Gupta VK, Gair R, Gautam N, Kumar P, Yadav IJ, Darmwal NS. Optimization of xylanase production from Fusarium solani F7. Am J Food Technol. 2009;4:20–29. doi: 10.3923/ajft.2009.20.29. [DOI] [Google Scholar]
- Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S. Production of fungal xylanases. Bioresour Technol. 1996;58:137–161. doi: 10.1016/S0960-8524(96)00094-6. [DOI] [Google Scholar]
- Húngaro HM, Calil NO, Ferreira AS, Chandel AK, Silva SS (2011) Fermentative production of ribonucleotides from whey by Kluyveromyces marxianus: effect of temperature and pH. J Food Sci Technol. doi:10.1007/s13197-011-0408-y [DOI] [PMC free article] [PubMed]
- Iandolo D, Piscitelli A, Sannia G, Faraco V. Enzyme production by solid substrate fermentation of Pleurotus ostreatus and Trametes versicolor. Appl Biochem Biotechnol. 2011;163:40–51. doi: 10.1007/s12010-010-9014-0. [DOI] [PubMed] [Google Scholar]
- Knob A, Carmona EC. Xylanase production by Penicillium sclerotiorum and its characterization. World Appl Sci J. 2008;4:277–283. [Google Scholar]
- Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Cui F, Liu Z, Xu Y, Zhao H. Improvement of xylanase production by Penicillium oxalicum ZH-30 using response surface methodology. Enzyme Microb Technol. 2007;40:1381–1388. doi: 10.1016/j.enzmictec.2006.10.015. [DOI] [Google Scholar]
- Martınez-Trujillo A, Pérez-Avalos O, Ponce-Noyola T. Enzymatic properties of a purified xylanase from mutant PN-120 of Cellulomonas flavigena. Enzyme Microb Technol. 2003;32:401–406. doi: 10.1016/S0141-0229(02)00313-7. [DOI] [Google Scholar]
- Medeiros RG, Soffner MLAP, Thome JA, Cacais AOG, Estelles RS, Salles BC, Ferreira HM, Lucena Neto SA, Silva FG, Jr, Filho EXF. The production of hemicellulases by aerobic fungi on medium containing residues of banana plant as substrate. Biotechnol Prog. 2000;16:522–524. doi: 10.1021/bp0000398. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Pandya JJ, Gupte A. Production of xylanase under solid-state fermentation by Aspergillus tubingensis JP-1 and its application. Bioprocess Biosyst Eng. 2012;35:769–779. doi: 10.1007/s00449-011-0657-1. [DOI] [PubMed] [Google Scholar]
- Park JY, Kanda E, Fukushima A, Motobayashi K, Nagata K, Kondo M, Ohshita Y, Morita S, Tokuyasu K. Contents of various sources of glucose and fructose in rice straw, a potential feedstock for ethanol production in Japan. Biomass Bioenergy. 2011;35:3733–3735. doi: 10.1016/j.biombioe.2011.05.032. [DOI] [Google Scholar]
- Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN. Alkaline protease production by an isolated Bacillus circulans under solid-state fermentation using agro industrial waste: process parameters optimization. Biotechnol Prog. 2005;21:1380–1388. doi: 10.1021/bp050095e. [DOI] [PubMed] [Google Scholar]
- Prakasham RS, Subba Rao C, Sarma PN. Green gram husk: an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour Technol. 2006;97:1449–1454. doi: 10.1016/j.biortech.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN. L-Asparaginase production by isolated Staphylococcus sp.—6A: design of experiment considering interaction effect for process parameters optimization. J Appl Microbiol. 2007;102:1382–1391. doi: 10.1111/j.1365-2672.2006.03173.x. [DOI] [PubMed] [Google Scholar]
- Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN. Enhancement of acid amylase production by an isolated Aspergillus awamori. J Appl Microbiol. 2007;102:204–211. doi: 10.1111/j.1365-2672.2006.03058.x. [DOI] [PubMed] [Google Scholar]
- Qinnghe C, Xiaoyu Y, Tiangui N, Cheng J, Qiugang M. The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Proc Biochem. 2004;39:1561–1566. doi: 10.1016/S0032-9592(03)00290-5. [DOI] [Google Scholar]
- Sanghvi GV, Koyani RD, Rajput KS. Thermostable xylanase production and partial purification by solid-state fermentation using agricultural waste wheat straw. Mycol. 2010;1:106–112. doi: 10.1080/21501203.2010.484029. [DOI] [Google Scholar]
- Sá-Pereira P, Costa-Ferreira M, Aires-Barros MR. Enzymatic properties of a neutral endo- 1,3 [4]-xylanase Xyl II from Bacillus subtilis. J Biotechnol. 2002;94:265–275. doi: 10.1016/S0168-1656(01)00436-9. [DOI] [PubMed] [Google Scholar]
- Sreenivas Rao R, Prakasham RS, Prasad KK, Rajesham S, Sarma PN, Rao LV. Xylitol production by Candida sp.: parameter optimization using Taguchi approach. Proc Biochem. 2004;39:951–956. doi: 10.1016/S0032-9592(03)00207-3. [DOI] [Google Scholar]
- Sreenivas Rao R, Jyothi PC, Prakasham RS, Sarma PN, Rao LV. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour Technol. 2006;97:1974–1978. doi: 10.1016/j.biortech.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Sreenivas Rao R, Kumar CG, Prakasham RS, Hobbs PJ. The Taguchi methodology as a statistical tool for biotechnological applications: a critical appraisal. Biotechnol J. 2008;3:510–523. doi: 10.1002/biot.200700201. [DOI] [PubMed] [Google Scholar]
- Steiner W, Laffarty RM, Gomes I, Esterbauer H. Studies on a wild type strain of Schizophyllum commune, cellulase and xylanase production and formation of the extracellular polysaccharide schizophyllan. Biotechnol Bioeng. 1987;30:169–178. doi: 10.1002/bit.260300206. [DOI] [PubMed] [Google Scholar]
- Subba Rao CH, Madhavendra SS, Sreenivas Rao R, Hobbs PJ, Prakasham RS. Studies on improving the immobilized bead reusability and alkaline protease production by isolated immobilized Bacillus circulans MTCC 6811 using overall evaluation criteria. Appl Biochem Biotechnol. 2008;15:65–83. doi: 10.1007/s12010-008-8147-x. [DOI] [PubMed] [Google Scholar]
- Subba Rao C, Sathish T, Laxmi MM, Laxmi GS, Sreenivas Rao R, Prakasham RS. Modeling and optimization of fermentation factors for enhancement of alkaline protease production by isolated Bacillus circulans using feed-forward neural network and genetic algorithm. J Appl Microbiol. 2008;104:889–898. doi: 10.1111/j.1365-2672.2007.03605.x. [DOI] [PubMed] [Google Scholar]
- Subramaniyan S, Prema P. Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol. 2002;22:33–64. doi: 10.1080/07388550290789450. [DOI] [PubMed] [Google Scholar]
- Suresh PV, Sachindra NM, Bhaskar N. Solid state fermentation production of chitin deacetylase by Colletotrichum lindemuthianum ATCC 56676 using different substrates. J Food Sci Technol. 2011;48:349–356. doi: 10.1007/s13197-011-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna Lakshmi G, Subba Rao C, Srinivas Rao R, Hobbs PJ, Prakasham RS. Enhanced production of xylanase by a newly isolated Aspergillus terreus under solid state fermentation using palm industrial waste: a statistical optimization. Biochem Eng J. 2009;48:51–57. doi: 10.1016/j.bej.2009.08.005. [DOI] [Google Scholar]
- Taguchi G. Introduction to quality engineering; Asian productivity organization. White Plains: UNIPUB; 1986. pp. 33–42. [Google Scholar]
- Taguchi G, Wu Y. Introduction to off-line quality control. Nagoya: Japan Quality Control Organization; 1980. [Google Scholar]
- Uma A, Deb JK, Baby Rani G, Prameela Devi Y, Narasu ML. Characterization and partial purification of xylanases from Thielaviopsis basicola. Technol Spect J. 2008;2:4–8. [Google Scholar]
- Wejse PL, Ingvorsen K, Mortensen KK. Xylanase production by a novel halophillic bacterium increased 20-fold by responsible surface methodology. Enzyme Microb Technol. 2003;32:721–727. doi: 10.1016/S0141-0229(03)00033-4. [DOI] [Google Scholar]