Abstract
This work aims to investigate the hygroscopic behavior of grugru palm powder through adsorption isotherms and its degree of caking. The powders of grugru palm (T1 - without maltodextrin, T2 – with 8 % of maltodextrin) were obtained by oven drying at 65 °C for 25 h. The experimental data was obtained through static gravimetric method at temperatures of 25, 30, 35 and 40 °C with different saturated salt solutions. The models of GAB, BET, Henderson, and Oswin were fitted to experimental data. The values of hygroscopicity were 6.39 and 5.17 % and degrees of caking were 3.11 and 0.03 % for T1 and T2, respectively. The adsorption isotherms from mathematical models can be classified as Type III. The GAB and Oswin models were the best representing the behavior of the powder isotherms, T1 and T2, respectively. The grugru palm powder proved to be non-hygroscopic and non-agglomerating. The T2 with 8 % of maltodextrin presented the lowest hygroscopicity.
Keywords: Acrocomia aculeata, Sorption isotherms, Food drying, Degree of caking, Isotherms, Mathematical modeling, Powder technology
Introduction
Brazil has a wide variety of oil plants, natives or introduced; among these species, the grugru palm (Acrocomia aculeata) stands out for being highly productive and native to arid and semiarid regions (Kopper et al. 2009). The pulp is eaten fresh or used in the production of powders that can be used in drinks, candies, ice creams, custards, cakes, jellies and juices (Brasil 2002).
The grugru palm presents a pulp which is consumed fresh or utilized in the production of flour that can be employed in different foods; this fruit is sweet, mucilaginous and can be cooked for soft drinks and ice cream (Brasil 2002).
According to Ramos et al. (2008), the bioavailability of β-carotene, a precursor of vitamin A, in the pulp of grugru palm is greater than the bioavailability of pure β-carotene.
According to Fernandes et al. (2011), it is very important and critical in the economic process to select a suitable drying process for preservation of fruit characteristics. The research on drying of common fruits has been extensively carried out; however, studies on exotic, tropical and native fruits are scarce.
During storage, foods exposed to several relative humidities and temperatures; and tends to reach equilibrium with the environment and accordingly adjust the moisture (Toneli et al. 2008). Mathlouthi and Rogé (2003) stated that to explain the hygroscopic behavior of different food products, mathematical models of adsorption isotherms have been proposed.
The understanding of adsorption isotherms of food moisture is very important in science and technology of food for many purposes, such as design and optimization of industrial processes, e.g., drying, packaging and storing, so it is crucial to model moisture changes taking place during dehydration and in order to predict the stability of shelf life (Jamali et al. 2006).
According to Jaya and Das (2004) the hygroscopicity is the ability of food powder to absorb moisture from high relative humidity environment. In the case of fruit powders, glucose and fructose are responsible for strong interaction with the water molecule due to the polar terminals present in these molecules.
Studies for obtaining grugru palm powder is very important for food technology, because it is necessary to discover the best techniques to attain the best storage conditions. Hence, the objective is to investigate the hygroscopic behavior, the degree of caking and to apply mathematical models to predict the adsorption isotherms of grugru palm powders obtained by oven drying with forced air ventilation.
Material and methods
Raw material
The grugru palm fruits were harvested in the Araripe Plateau, Cariri region, Ceará State, Brazil, and taken to the Laboratory of Food Quality Control and Drying. The fruits were selected according to the level of maturity and sanity and later were hygienized for the peeled and then the pulp was separated through a stainless steel knife. The pulp was stored in a vertical freezer (Esmaltec, 340) at −20 °C until the the drying process initiated.
Two grugru palm powders were utilized:
Control treatment (T1) – oven drying of pulp without addition of drying adjunvant.
Treatment 2 (T2) – oven drying of pulp with addition of drying adjuvant (8 % of maltodextrin ‘Maltogill 20®’, dextrose equivalent - DE 20). An aqueous maltodextrin solution was prepared and homogenized with triturated pulp before drying.
Oven drying
The pulp was unfrozen and crushed, the pulp for T1 was distributed in stainless steel trays with a diameter of 25 cm; the pulp for T2 was prepared with an aqueous maltodextrin solution and was homogenized and distributed into stainless steel trays with a diameter of 25 cm; and the trays were placed in an oven (Tecnal, model TE-394/l) with forced air ventilation at 65 °C for a period 25 h.
Right after drying, the product was taken to a rotatory blade mill (MA 048, Marconi) and then sieved (mesh of 1,200 μm) to obtain a homogeneous powder.
Modeling of adsorption isotherms
In determining the moisture adsorption isotherms, a static gravimetric method described by Wolf et al. (1985) was applied using saturated solutions of salts as described by Greenspan (1977). The salt solutions were prepared and put in tempered glass containers sealed with silicone and stored at room temperature (21 ± 2 °C).
Measurements of adsorption isotherms were performed in triplicate. For each cell about 0.20 g of each treatment were weighed in pre-librated aluminum crucibles. Afterward, the crucibles were taken to cells, which contained the saturated solutions of salts.
The process was monitored through sample weighing on analytical balance every 24 h during 10 days until equilibrium moisture was reached. After balanced moisture, the water activity of the samples was determined at temperatures of 25, 30, 35 and 40 °C using aw meter (AQUALab 4TEV). Then were determinate of moisture content in an oven (Tecnal, model TE-394/l) with forced air ventilation at 75 °C to constant weight.
The equilibrium moisture (Xeq, Equation 1) was calculated by the difference between the mass of balanced sample and the dried mass:
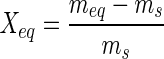 |
1 |
Where: Xeq = equilibrium moisture (d.b.); meq = mass of balanced sample (g); ms = mass of dried sample (g).
For adjusting of the experimental data from adsorption isotherms of grugru palm powder, mathematical models of GAB, BET, Henderson and Oswin were used, represented respectively by the equations in Table 1. The parameter calculations for each model were performed by the software Statistic, version 7.0 (Statsoft 2007).
Table 1.
Mathematical models used to adjust the experimental data of adsorption isotherms
| Models | Equationsa | |
|---|---|---|
| GAB (Guggenheim–Andersen–de Boer) (Van Den Berg and Bruin 1981) |
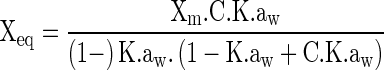
|
(2) |
| BET (Brunauer et al. 1938) |
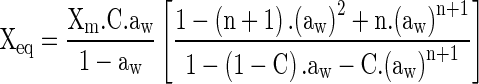
|
(3) |
| Henderson (Henderson 1952) |
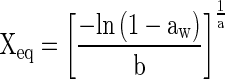
|
(4) |
| Oswin (Oswin 1946) |
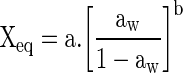
|
(5) |
aaw = water activity; Xm = moisture content in the molecular monolayer (g H2O g−1 dry basis); X eq = equilibrium moisture content (g H2O g−1 dry basis); C = constant related to heat of sorption of the molecular layer; a, b, K = adjusting parameters.
The quality of adjusting different models was evaluated through the best values of the determination coefficient (R2) and the relative mean deviation (E%, Equation 6), defined by Iglesias and Chirife (1976):
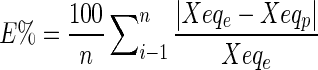 |
6 |
Where: E% = relative mean error; Xeqe = experimental values; Xeqp = values predicted by the model; n = number of experimental data.
Hygroscopicity
The analysis was performed using the methodology A14a, described by GEA Niro Research Laboratory (2003), consisting of powder exposure to air relative humidity (RH) of 79.5 % and checking of weight increase every 10 min until the maximum weight is reached. Approximately 0.5 g of the sample was weighed and evenly spread on a plate, then the plate was placed in the apparatus and the analysis was started. The calculation of hygroscopicity is given by Equation 7. The parameters used to characterize the hygroscopicity of grugru palm powder were obtained as described by GEA Niro Research Laboratory (2003).
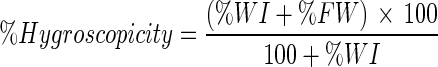 |
7 |
Where: %FW = % free water;
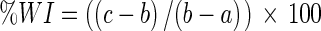 ; a = weight of plate (g); b = weight of plate + powder (g); c = weight of plate + powder in equilibrium (g).
; a = weight of plate (g); b = weight of plate + powder (g); c = weight of plate + powder in equilibrium (g).
Degree of caking
The analysis was performed through the methodology A15a, described by GEA Niro Research Laboratory (2003), which consists of exposing the powder to absorb air moisture (79.5 % relative humidity) until reaching of equilibrium as described in hygroscopicity. Afterward, the powder was oven dried at 105 °C and then sieved under standard conditions (sieve mesh modified to 1,200 μm). What was left in the sieve was expressed as degree of caking. The calculation of the degree of caking is given by Equation 8. The parameters to characterize the degree of caking of grugru palm powder were obtained as described by GEA Niro Research Laboratory (2003).
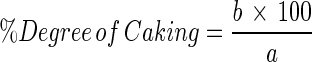 |
8 |
Where: a = grams of powder used; b = grams of powder retained in the sieve.
Statistical analysis
All analysis was determined in triplicate. Data were analyzed statistically through analysis of variance (ANOVA), and differences between the averages for hygroscopicity and degree of caking were determined by Tukey test at 5 % probability using the software Statistic, version 7.0 (Statsoft 2007).
Results and discussion
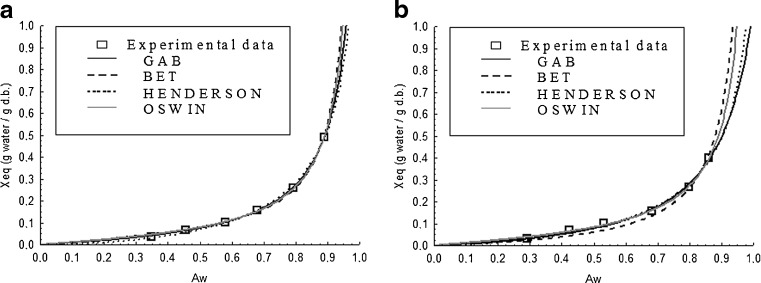
The adsorption isotherms of grugru palm powders showed a behavior characteristic to Type III, according to Brunauer classification (Rizvi 1995), as can be seen in Figs. 1a and b. Note that the values of the experimental data of equilibrium moisture (Xeq) increased due to the increased water activity under condition of constant temperature, as e.g. at 25 °C.
Fig. 1.
Adsorption isotherms of grugru palm powder at 25 °C without maltodextrin - T1 (a) and with maltodextrin 8 % - T2 (b). (Abbreviations: aw = water activity; Xeq = equilibrium moisture content g H2O g−1 dry basis)
From the analysis of Figs. 1a and b, the grugru palm powder tend to gain moisture under environment with aw above 0.1, indicating increased Xeq independently of the conditions of relative humidity. Noting at these figures, one can say that for T1 (Fig. 1a) GAB, Oswin and BET models adjusted best in the range of aw from 0.1 to 0.6, but all models had excellent fits from 0 6 to 1.0. In Fig. 1b, all models adjusted to the experimental data of aw from 0.1 to 0.3 and from 0.8 to 1.0, and only GAB, Henderson and Oswin models presented the best adjustments for aw from 0.3 to 0.7.
Table 2 contains the parameters obtained by GAB, BET, Henderson and Oswin models for grugru palm powders (T1 and T2) measured at temperatures of 25, 30, 35 and 40 °C.
Table 2.
Adjustment results of experimental data of adsorption isotherms at 25, 30, 35 and 40 °C for powders of grugru palm
| Models | Parametersc | T1a | T2b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 40 °C | 25 °C | 30 °C | 35 °C | 40 °C | ||
| GAB | Xm | 0.0790 | 0.0749 | 0.0903 | 0.0887 | 0.1351 | 0.9838 | 0.1101 | 0.1129 |
| C | 1.0632 | 1.1767 | 0.8827 | 0.9021 | 0.5786 | 0.0912 | 0.7175 | 0.7018 | |
| K | 0.9674 | 0.9901 | 0.9807 | 0.9995 | 0.8969 | 0.7516 | 0.9453 | 0.9451 | |
| R2 | 0.9996 | 0.999 | 0.9991 | 0.9983 | 0.9928 | 0.9851 | 0.9825 | 0.9956 | |
| E% | 0.26 | 0.43 | 0.40 | 0.47 | 0.98 | 1.34 | 1.32 | 0.79 | |
| BET | Xm | 0.0772 | 0.1079 | 0.1390 | 0.1619 | 0.2083 | 0.2341 | 0.111 | 0.1129 |
| C | 1.4445 | 0.7924 | 0.6164 | 0.4364 | 0.3615 | 0.2541 | 0.8590 | 0.8719 | |
| n | 1.3535 | 1.2986 | 1.2656 | 1.1246 | -0.7521 | -0.5822 | 1.3158 | 1.2681 | |
| R2 | 0.9979 | 0.999 | 0.9989 | 0.9983 | 0.9741 | 0.9708 | 0.9882 | 0.9987 | |
| E% | 0.62 | 0.41 | 0.41 | 0.46 | 1.82 | 2.31 | 1.00 | 0.41 | |
| Henderson | a | 0.5935 | 0.5691 | 0.5655 | 0.5473 | 0.661 | 0.6737 | 0.6324 | 0.6248 |
| b | 3.3831 | 3.1328 | 3.0034 | 2.8181 | 3.6838 | 3.6527 | 3.3485 | 3.1871 | |
| R2 | 0.9969 | 0.9946 | 0.9964 | 0.9951 | 0.9905 | 0.9863 | 0.9758 | 0.9897 | |
| E% | 0.70 | 0.88 | 0.77 | 0.89 | 1.18 | 1.31 | 1.56 | 1.23 | |
| Oswin | a | 0.0794 | 0.0799 | 0.0831 | 0.0843 | 0.0842 | 0.0874 | 0.0849 | 0.0902 |
| b | 0.8758 | 0.9382 | 0.9626 | 1.0187 | 0.8525 | 0.8571 | 0.9311 | 0.9398 | |
| R2 | 0.999 | 0.999 | 0.9989 | 0.9983 | 0.9981 | 0.996 | 0.9875 | 0.9984 | |
| E% | 0.38 | 0.41 | 0.38 | 0.47 | 0.52 | 0.68 | 1.05 | 0.45 | |
aT1: powder without addition of maltodextrin; bT2: powder with addition of 8 % maltodextrin. cAbbreviations: R 2 determination coefficient; E% relative average error; X m moisture content in the monolayer (g H2O g−1 dry basis); C constant related to the heat of sorption in the molecular layer. K GAB constant related to multi-layers; n BET constant related to multi-layers; a & b adjusting parameters of the Henderson and Oswin models.
The parameters of the models applied to the experimental data of adsorption isotherms of grugru palm powders (determination coefficients - R2 and the relative average deviations - E%) were utilized as evaluation criteria for the representation of isotherms are shown in Table 2.
According to the results shown in Table 2, all models studied (GAB, BET, Henderson and Oswin) can represent the behavior of grugru palm powders, as they exhibited excellent values of R2 and E%, respectively from 0.97411 to 0.99961 and from 0.26 % to 1.82 %; these values are below the criteria recommended by Aguerre et al. (1989), where E% less than 10 % indicates a reasonable representation of the models, and by Labuza et al. (1985), in which the representation of isotherms is considered extremely good for E% less than 5 %. Thus, the GAB and Oswin models were the best representing the experimental data of T1 and T2, respectively.
The GAB model is reported by several researchers in forecasting adsorption isotherms of various dehydrated foods, particularly Ferreira and Pena (2003) in obtaining adjustment of this model with R2 of 0.9996 and 0.9991 for peach-palm powder, Silva et al. (2005) which found R2 values higher than 0.99 for yellow mombin powder, and Alam and Singh (2011) who obtained the maximum R2 for aonla flakes.
The moisture values in the monolayer (Xm) of GAB model for T1, between temperatures 25 and 40 °C, showed random fluctuations (Table 2) ranging from 0.079002 g H2O.g−1 to 0.090334 g H2O.g−1 on dry basis, with the highest value in the temperature of 35 °C. The same was observed for constants C and K. The values of C and K correspond to classification Type III, according to Brunauer 0 < K < 1 and 0 ≤ C ≤ 2 (Blahovec, 2004).
Gabas et al. (2007) reported that strong adsorbate-adsorbent interactions are favored by lower temperatures, causing an increase in C, which explain the temperature variation and may be the result of a mathematical compensation between C and K.
The values of Xm are in accordance to the values reported for fruits comprising 4–15 % on dry basis (Moreira et al. 2008); however, Koua et al. (2012) obtained values for cassava ranging from 6.16g H2O.100 g−1 to 3.66 g H2O.100 g−1 on dry basis. The (GAB) Xm parameter is important because it can be related to the beginning of a series of chemical reactions of food decaying (Ferreira and Pena 2003).
The Xm parameter also characterizes the moisture content under which food is stable, because at lower values of Xm biochemical changes can be observed - lipid oxidation (Ordóñez 2005). Thus, the values of Xm (Table 2) for grugru palm show low moisture content in the monolayer, hence lipid oxidation can occur during storage in the temperatures here studied. So to prevent and minimize such oxidative processes, packaging should be impervious to air and light.
The Oswin model also showed the best fitting for dehydrated garlic with R2 ranging from 0.9148 to 0.9488 (Arora et al. 2011); Basu et al. (2011) studying pectin found values of R2 between 0.82 and 0.97, while E% was less than 0.012. Lomauro et al. (1985), quoted by Al-Muhtaseb et al. (2002), reported the Oswin model just fitted 57 % of the isotherms described for foods. For grugru palm powder in this study, the Oswin model presented R2 value of 0.99818 and E% of 0.52.
Jain et al. (2010) stated that the Oswin model can be used to plan and evaluate drying, storage conditions and moisture amount to be removed during dehydration. Sopade et al. (1996) reported that this model can also be used to predict the extension of hydration or dehydration required.
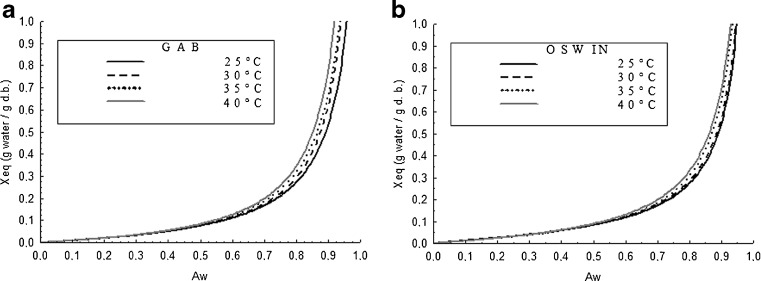
Figures 2a and b show the graphical presentations of adsorption isotherms of moisture for grugru palm powder, in all temperatures, adjusted by GAB and Oswin models for T1 and T2, respectively, which represented the best adjustment.
Fig. 2.
Adsorption isotherms at different temperatures of the best model (GAB) for grugru palm powder without maltodextrin - T1 (a), and best model (Oswin) of for grugru palm powder with maltodextrin 8 % - T2 (b). (Abbreviations: aw = water activity; Xeq = equilibrium moisture content g H2O g−1 dry basis)
Figures 2a and b show that with increasing temperature there is an increase in equilibrium moisture values of aw higher than 0.6 for T1 and T2. Also there is little influence of temperature on Xeq of grugru palm powder with aw below 0.6. Studies carried out with surinam-cherry powder indicated influence of temperature on equilibrium moisture from aw above 0.3 (Vieira et al. 2007); this shows the grugru palm powder has a hygroscopicity lower than that of surinam-cherry powder.
Table 3 shows the values of hygroscopicity and degree of caking for T1 and T2. Treatment T1 rendered higher hygroscopicity and caking degree in relation to T2. This can be related to the addition of drying adjuvant (maltodextrin), which results in decreased powder hygroscopicity, and this fact was also found in the adsorption isotherms of this work.
Table 3.
Hygroscopicity and degree of caking of powders of grugru palm
| Treatmenta | Hygroscopicity (%) | Degree of caking (%) |
|---|---|---|
| T1 | 6.39 a* | 3.11 a |
| T2 | 5.17 b | 0.03 b |
aT1 = powder without maltodextrin; T2 = powder with 8 % maltodextrin.
*Equal low case letters in the same column do not differ by Tukey test at 5 % probability.
The powder caking is an undesirable reaction, consisting initially in the powder transformation into an agglomerated and sticky material and resulting in decreased functionality, smoothness and quality loss; the main cause of agglomeration is the presence of plasticizing water onto the surface of particles (Aguilera et al. 1995).
According to GEA Niro Research Laboratory (2003), which characterizes the hygroscopic behavior and the degree of caking, the grugru palm powder is classified as a non- hygroscopic and non-caking product.
Studies about sucrose by Mathlouthi and Rogé (2003) reported that caking is linked to solid bridges created during the agglomeration process, which turned sucrose more hygroscopic. The same can be seen in T1 in which the powder had the highest caking and thereafter making grugru palm powder with higher hygroscopicity, because this is a sweet product with about 17.5 % of sucrose.
Costa et al. (2003) reported that the variations in hygroscopic behavior of food powders are also assigned to the powder granulation, as products of finer particles have a greater surface of contact and therefore a greater number of active sites. This report can explain the non-hygroscopicity of grugru palm powder when compared to other high hygroscopic fruits described in the literature, as the particle size of powder of grugru palm is 1,200 μm and most of other fruit powders present a particle size ranging from 500 μm to 600 μm.
Conclusion
The adsorption isotherms of grugru palm powders adjusted by the mathematical models can be classified as Type III.
All models adjusted to the powder of grugru palm. However, it is recommend the GAB model in describing the equilibrium of adsorption isotherms for T1 and Oswin for T2.
The grugru palm powder showed a relative increase in moisture in the monolayer (Xm) along with increasing temperature. The values of C and K are in conformity with the classification Type III, and according to Brunauer 0 < K < 1 and 0 ≤ C ≤ 2.
The grugru palm powder is characterized as non-hygroscopic and non-caking. The T2 treatment, with maltodextrin, showed lesser hygroscopicity.
Acknowledgments
To Laboratory of Food Quality Control and Drying (UFC) for the research supporting; to CNPq through INCT, and to Araucaria Foundation for the scholarship granting.
Contributor Information
Dalany Menezes Oliveira, Email: dalany5@yahoo.com.br.
Edmar Clemente, Phone: +55-44-30113659, FAX: +55-44-30118940, Email: eclemente@uem.br.
José Maria Correia da Costa, Email: correiacostaufc@gmail.com.
References
- Aguerre RJ, Suarez C, Viollaz PE. New BET type multilayer sorption isotherms Part II: Modelling water sorption in foods. LWT - Food Sci Technol. 1989;22:192–195. [Google Scholar]
- Aguilera JM, Del Valle JM, Karel M. Caking phenomena in amorphous food powder. Trends in Food Sci Technol. 1995;6:149–155. doi: 10.1016/S0924-2244(00)89023-8. [DOI] [Google Scholar]
- Alam MS, Singh A. Sorption isotherm characteristics of aonla flakes. J Food Sci Technol. 2011;48(3):335–343. doi: 10.1007/s13197-011-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhtaseb AH, McMinn WAM, Magee TRA. Moisture sorption isotherm characteristics of food products: a review. Trans I Chem E C. 2002;80:118–128. [Google Scholar]
- Arora S, Sharma SR, Kumar S. Thermodynamic models for water sorption by garlic. J Food Sci Technol. 2011;48:604–609. doi: 10.1007/s13197-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Shivhare US, Muley S (2011) Moisture adsorption isotherms and glass transition temperature of pectin. J Food Sci Technol. doi:10.1007/s13197-011-0327-y [DOI] [PMC free article] [PubMed]
- Blahovec J. Sorption isotherms in materials of biological origin mathematical and physical approach. J Food Eng. 2004;65:489–495. doi: 10.1016/j.jfoodeng.2004.02.012. [DOI] [Google Scholar]
- Brasil (2002) Ministério da Saúde. Alimentos regionais brasileiros/ Ministério da Saúde, Secretaria de Políticas de Saúde, Coordenação-Geral da Política de Alimentação e Nutrição. Brasília: Ministério da Saúde.
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layer. J Am Chem Soc. 1938;60:309–319. doi: 10.1021/ja01269a023. [DOI] [Google Scholar]
- Costa JMC, Medeiros MFD, Mata ALML. Isotermas de adsorção de pós de beterraba (Beta vulgaris L.), abóbora (Cucurbita moschata) e cenoura (Daucuscarota) obtidos pelo processo de secagem em leito dejorro: estudo comparativo. Rev Ciência Agronômica. 2003;34:5–9. [Google Scholar]
- Fernandes FAN, Rodrigues S, Law CL, Mujumdar AS. Drying of exotic tropical fruits: a comprehensive review. Food Bioprocess Technol. 2011;4:163–185. doi: 10.1007/s11947-010-0323-7. [DOI] [Google Scholar]
- Ferreira CD, Pena RS. Comportamento higroscópico da farinha de pupunha (Bactris gasipaes) Ciência Tecnol Alimentos. 2003;23:251–255. doi: 10.1590/S0101-20612003000200025. [DOI] [Google Scholar]
- Gabas AL, Telis VRN, Sobral PJA, Telis-Romero J. Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. J Food Eng. 2007;82:246–252. doi: 10.1016/j.jfoodeng.2007.02.029. [DOI] [Google Scholar]
- GEA Niro Research Laboratory (2003) Analytical methods of dry milk products. GEA Niro analytical methods 15 a. Soeborg, Denmark.
- Greenspan L. Humidity fixed points of binary satured aqueuos solutions. J Res Natl Stand A. J Phys Chem. 1977;81:89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- Henderson SM. A basic concept of equilibrium moisture. Agric Eng. 1952;33:29–32. [Google Scholar]
- Iglesias HA, Chirife J. A model for describing the water sorption behaviour of foods. J Food Sci. 1976;41:984–992. doi: 10.1111/j.1365-2621.1976.tb14373.x. [DOI] [Google Scholar]
- Jain SK, Verma RC, Sharma GP, Jain HK. Studies on moisture sorption isotherms for osmotically dehydrated papaya cubes and verification of selected models. J Food Sci Technol. 2010;47:343–346. doi: 10.1007/s13197-010-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali A, Kouhila M, Mohamed LA, Jaouhari JT, Abdenouri N. Sorption isotherms of Chenopodium ambrosioides, leaves at three temperatures. J Food Eng. 2006;72:77–84. doi: 10.1016/j.jfoodeng.2004.11.021. [DOI] [Google Scholar]
- Jaya S, Das H. Effect of maltodextrin, glycerol monostearate and tricalcium phosphate on vaccum dried mango powders properties. J Food Eng. 2004;63:125–134. doi: 10.1016/S0260-8774(03)00135-3. [DOI] [Google Scholar]
- Kopper AC, Saravia APK, Ribani RH, Lorenzi GMAC. Utilização tecnológica da farinha de bocaiuva na elaboração de biscoitos tipo cookie. Alim Nutr. 2009;20(3):463–469. [Google Scholar]
- Koua BK, Koffi PME, Gbaha P, Toure S (2012) Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta). J Food Sci Technol. doi:10.1007/s13197-012-0687-y [DOI] [PMC free article] [PubMed]
- Labuza TP, Kaanane A, Chen JY. Effects of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci. 1985;50:385–392. doi: 10.1111/j.1365-2621.1985.tb13409.x. [DOI] [Google Scholar]
- Lomauro CJ, Bakshi AS, Labuza TP (1985) Evaluation of food moisture sorption isotherm equations. Part I. Fruit, vegetable and meat products. LWT 18:111–117
- Mathlouthi M, Rogé B. Water vapour sorption isotherms and the caking of food powders. Food Chem. 2003;82:61–71. doi: 10.1016/S0308-8146(02)00534-4. [DOI] [Google Scholar]
- Moreira R, Chenlo F, Torres MD, Vallejo N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J Food Eng. 2008;88:514–521. doi: 10.1016/j.jfoodeng.2008.03.011. [DOI] [Google Scholar]
- Ordóñez JA. Tecnologia de alimentos: componentes dos alimentos e processos. Sao Paulo: Artmed editora; 2005. [Google Scholar]
- Oswin CR. The kinetics of packing life. III. The isotherm. J Chem Ind. 1946;65:419–423. doi: 10.1002/jctb.5000651216. [DOI] [Google Scholar]
- Ramos MIL, Ramos Filho MM, Hiane PA, Braga Neto JA, Siqueira EMA. Qualidade nutricional da polpa de bocaiúva Acrocomia aculeata (Jacq.) Lodd. Ciência Tecnol Alimentos. 2008;28:90–94. doi: 10.1590/S0101-20612008000500015. [DOI] [Google Scholar]
- Rizvi SSH. Thermodynamic properties of foods in dehydration. In: Rao MA, Rizvi SSH, editors. Eng Prop Foods. New York: Marcel Dekker; 1995. pp. 223–310. [Google Scholar]
- Silva YC, Mata MERMC, Duarte MEM (2005) Atividade de água em pó microencapsulado com amido modificado: estudo de dois modelos matemáticos. In: Simpósio brasileiro de pós colheita de frutos tropicais, João Pessoa, BR
- Sopade PA, Ajisegiri ES, Abass AB. Moisture sorption isotherms of dawadawa, a fermented African locust bean (Parkia biglobosa Jacq. Benth) Food Control. 1996;1:153–156. doi: 10.1016/0956-7135(96)00022-9. [DOI] [Google Scholar]
- Statsoft . Statistica for window – computer programa manual. Versão 7.0. Tulsa: Statsoft Inc; 2007. [Google Scholar]
- Toneli JTCL, Park KJ, Murr FEX, Negreiros AA. Efeito da umidade sobre a microestrutura da inulina em pó. Ciência Tecnol Alimentos. 2008;28:122–131. doi: 10.1590/S0101-20612008000100018. [DOI] [Google Scholar]
- Van Den Berg C, Bruin S. Water activity and its estimation in food systems: theoretical aspects. In: Rockland LB, Stewart GE, editors. Water activity; influence on food quality. New York: Academic Press; 1981. pp. 45–58. [Google Scholar]
- Vieira AH, Figueirêdo RMF, Queiroz AJM. Isotermas de adsorção de umidade da pitanga em pó. Rev Biologia Ciências da Terra. 2007;7:11–20. [Google Scholar]
- Wolf W, Spiess WEL, Jung G. Standarization of isotherm measurements. In: Simatos D, Multon JL, editors. Properties of water in foods. The Netherlands: Martinus Nijhoff; 1985. pp. 661–679. [Google Scholar]