Abstract
Caralluma adscendens (Roxb.) Haw var. fimbriata (wall.) Grav. & Mayur. is a traditional food consumed as vegetable or pickle in arid regions of India and eaten during famines. In Indian traditional medicine, the plant is used to treat diabetes, inflammation and etc. The aim of this study was to evaluate the antioxidant properties (DPPH, TEAC, TAA, FRAP, OH˙ and NO˙ radical scavenging activities) of the different extracts from aerial parts. The levels of total phenolics and flavonoids of the extracts were also determined. The extracts were found to have different levels of antioxidant properties in the test models used. Methanol and water extracts had good total phenolic and flavonoid contents showed potent antioxidant and free radical scavenging activities. The antioxidant activity was correlated well with the amount of total phenolics present in the extracts. The extracts and its components may be used as an additive in food preparations and nutraceuticals.
Keywords: Amino acid analysis, Caralluma adscendens var. fimbriata, FRAP, Phosphomolybdenum method, Ascorbic acid equivalent antioxidant capacity (AEAC)
Introduction
Reactive oxygen species are highly reactive molecules formed during aerobic life in living organisms include free radicals such as superoxide anion radical (O2˙−), DPPH, hydroxyl radical (HO˙) and non free radical such as singlet oxygen (1O2) or hydrogen peroxide (H2O2) (Ahn et al. 2007). Generally, the production of appropriate Reactive oxygen species (ROS) that is controlled by the antioxidant system in living organism may be essential for many cellular functions such as killing phagocytes, bacterial ingestion and redox regulation of signal transduction. However, the overproduction of ROS in living organism can lead to attract DNA, cell membrane, proteins and other cellular components and consequently induce degeneration, destruction and toxicity of various molecules that play an important role in metabolism of life. Also, ROS are known as the molecules that cause qualitative decay of food (Heo et al. 2005). Therefore, antioxidants with radical scavenging activities could have great relevance as prophylactic and therapeutic agents in diseases in which oxidants or free radicals are implicated (Valento et al. 2002). Dietary antioxidants can stimulate cellular defenses and help to prevent cellular components against oxidative damage. In addition some synthetic antioxidants like butylhydroxyanisole (BHA) and butylhydroxytoluene (BHT) were needed to be replaced with natural antioxidants because of their potential health risks and toxicity (Dudonne et al. 2009). Fruits and vegetables contain significant levels of natural antioxidant phytonutrients with physiological and biochemical functions which benefit human health. For this reason, fruit and vegetables represent a major source of dietary antioxidants (Tavarini et al. 2008).
Caralluma is a genus of the family, Asclepiadaceae of about hundred species was distributed in Africa, Spain, Saudi Arabia, Middle East, Pakistan and India. Caralluma species have been used for centuries in semi-arid areas of Pakistan as emergency foods (Atal et al. 1980). Caralluma adscendens (Roxb.) Haw var. fimbriata (Wall.) Grav. & Mayur. is a succulent perennial herb, growing wild in the states of Andhra Pradesh, Karnataka and Tamil Nadu of India (Kunert et al. 2008). C. adscendens is commonly or locally known as “Makadshenguli/Shengulmakad”. In Indian traditional medicine, the plant is used to treat diabetes, pain, fever, inflammation and has also been used as an appetite suppressant. C. adscendens is a traditional food consumed in the form of pickle and vegetable and also eaten during famines (The Wealth of India 1992). Antioxidant activity, hypolipidemic and hypoglycemic effect of various extracts (Tatiya et al. 2010; Mali et al. 2009) of C. adscendens whole plant has been investigated in alloxan induced diabetic rats. The phytochemical screening of C. adscendens revealed the presence of pregnane glycosides (caratubersides A and B and various boucerocides), flavonoids, saponins and triterpenoids (Tatiya et al. 2010). Pregnane and flavone glycosides were isolated from the whole plant of C. adscendens var. fimbriata (Kunert et al. 2008).
C. adscendens is traditionally consumed in the form of pickle and vegetable. So our interest was to analyse the proximate and amino acid composition. This is the first study exploring nutritional composition. In the present study, the less explored C. adscendens var. fimbriata was analyzed for in vitro antioxidant activity through several biochemical assays: 2, 2-diphenyl-1-picrylhydrazyl (DPPH˙) radical scavenging assay, trolox equivalent antioxidant capacity (TEAC) assay, ferric reducing antioxidant power (FRAP) assay, total antioxidant activity (TAA) by phosphomolybdenum assay, hydroxyl radical scavenging and nitric oxide radical scavenging activities.
Materials and methods
Chemicals
Potassium ferricyanide, ferric chloride, 2,2-diphenyl-1-picryl-hydrazyl (DPPH), butylated hydroxyanisole (BHA), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), potassium persulfate, 2,2′-azinobis(3-ethylbenzothiozoline-6-sulfonic acid) diammonium salt (ABTS˙+), 2,4,6-tripyridyl-S-triazine (TPTZ), ferrous ammonium sulphate, ethylenediamine tetraacetic acid (EDTA) disodium salt, trichloroacetic acid (TCA), folin ciocalteu’s reagent, aluminium chloride, ascorbic acid, ammonium acetate, glacial acetic acid, acetyl acetone, sodium nitroprusside, sulfanilic acid, naphthyl ethylene diamine dihydrochloride, sodium phosphate and ammonium molybdate were obtained from Himedia, Merck (Damstadt, Germany) or Sigma-Aldrich (St. Louis, MO, USA). All other reagents used were of analytical grade.
Plant material and extract preparation
The aerial parts of C. adscendens var. fimbriata were collected from Coimbatore district (Mettupalayam) of Tamil Nadu, India during the month of October- 2008. The plant was identified and authenticated by Dr. R. Gopalan, Taxonomist, Karpagam University, Coimbatore and Dr. V.S. Raju, taxonomist, Kakatiya University, Andhra Pradesh. The freshly collected plant materials were washed thoroughly in tap water, shade dried at room temperature and powdered. Extracts were prepared using solvents of increasing polarity. A portion (50 g) of powdered plant material was extracted with 250 ml (1:5 v/v) of chloroform followed by ethyl acetate, methanol and water in a Soxhlet apparatus (12 h for each solvent). Consequently, the solvent extracts were evaporated to dryness at 40 °C in a rotary evaporator (Buchi type, Flawil, Switzerland) and the water extract was cooled and lyophilised (Alpha 1-2 LD model, CHRIST, German). The yield of each extract constituents were calculated and stored in the dark at 4 °C prior to use. The extracts obtained were used for the assessment of antioxidant properties using various chemical assays.
Proximate composition
Chemical analysis to determine proximate composition of sample was carried out using standard procedures. The Kjeldahl method was used for total nitrogen determination using a Kjeltec System. Protein was calculated from total nitrogen using a factor of 6.25. The Soxhlet method (AOAC 1990) was used for total fat determination. Total fat was obtained from 6 h hexane extraction. Crude fibre was obtained after samples digestion with boiling diluted acid and alkali (AOAC 1990). Moisture was determined from sample weight loss after oven drying at 110 °C for 4 h (AOAC 1990). Ash content was calculated after heating the sample at 550 °C for 2 h. Carbohydrates was determined by difference. All samples were analyzed in triplicate.
Amino acid composition analysis
The powdered sample of C. adscendens var. fimbriata was hydrolyzed with 6N HCI at 110 °C for 24 h. Amino acid analysis was performed on reverse phase-high pressure liquid chromatography (HPLC) (Shimadzu LC-10 AD, Shimadzu Corporation, Kyoto, Japan). Samples were analyzed on Shimpack amino-Na type column (10 cm × 6.0 mm) obtained from Shimadzu Corporation. The post column samples were derivatized with o-phthaldialdehyde (OPA) (Ishida et al. 1981) and data were integrated using an integrator model C-R7A (Shimadzu chromatopac data processor). The amount of each amino acid present in the sample was calculated in mg/100 g dry weight.
Determination of total phenol content
The content of total phenolics was determined by using Folin-Ciocalteu method Singleton et al. (1999). An aliquot of the sample extract (0.1 ml) was mixed with distilled water (3 ml). To this 0.5 ml of Folin-ciocalteu reagent was added. After 3 min 2 ml of 20 % sodium carbonate was added and mixed thoroughly. The tubes were incubated in a water bath (100 °C) for exactly 1 min. It was then cooled and the absorbance was measured at 650 nm using spectrophotometer (Shimadzu, UV 2450) against the reagent blank. The results were expressed as milligram gallic acid equivalent per gram sample dry weight.
Determination of total flavonoid content
The content of total flavonoid in the extract was determined based on the method described by Ordon Ez et al. (2006). A volume of 0.5 ml of 2 % AlCl3 ethanol solution was added to 0.5 ml of sample solution. After 1 h at room temperature, the absorbance was measured at 420 nm with UV-Visible spectrophotometer (Shimadzu, UV 2450). A yellow color indicated the presence of flavonoids. Extract samples were evaluated at a final concentration of 0.1 mg/ml. Total flavonoid content were calculated as milligram quercetin equivalent per gram sample dry weight.
2, 2-Diphenyl-1-picrylhydrazyl (DPPH˙) radical scavenging assay
The DPPH radical scavenging assay was determined according to the method designed by Leong and Shui (2002). Briefly, 2 ml of 0.15 mM DPPH (in methanol) was added to the different concentrations of the extract (amounting to 1 ml). The reaction mixture was incubated for 30 min after which its absorbance was measured at 517 nm, where methanol was used as both a blank and negative control. The results were expressed as ascorbic acid equivalent antioxidant capacity (AEAC) and defined as mg ascorbic acid equivalents (AA)/100 g of fresh weight.
Trolox equivalent antioxidant capacity (TEAC) assay
This method was based on the reduction of the ABTS radical cations (ABTS˙+) by antioxidants present in extracts according to the procedure prescribed by Re et al. (1999). ABTS˙+ radical cation was produced by reacting 7 mM aqueous ABTS with 2.45 mM potassium persulfate and kept in the dark at room temperature for 16 h. The blue green solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. The stock solution of sample extracts was diluted. After the addition of 1 ml of diluted ABTS˙+ solution to 10 μl of sample extracts or trolox standards in ethanol, it was incubated at 30 °C exactly for 30 min. Appropriate solvent blanks were also run in each assay. Results were expressed as trolox equivalent antioxidant capacity (TEAC). The unit of antioxidant activity was defined as the concentration of trolox having the equivalent antioxidant activity expressed as mM Trolox equivalent per gram sample dry weight.
Hydroxyl radical scavenging activity (HRSA)
The scavenging activity of hydroxyl radical (OH˙) was measured according to the method of Klein et al. (1991). Various concentrations of extracts and standards (25–150 μg/ml) were added to 1 ml of iron-EDTA solution (0.13 % ferrous ammonium sulphate and 0.26 % EDTA), 0.5 ml of EDTA solution (0.018 %), and 1 ml of DMSO (0.85 % v/v in 0.1 M phosphate buffer, pH 7.4). The reaction was initiated by adding 0.5 ml of ascorbic acid (0.22 %) and incubated at 80–90 °C for 15 min in a water bath. After incubation the reaction was terminated by the addition of 1 ml of ice-cold TCA (17.5 % w/v). Three ml of Nash reagent (75 g of ammonium acetate, 3 ml of glacial acetic acid, and 2 ml of acetyl acetone were mixed and raised to 1 L with distilled water) was added and left at room temperature for 15 min. The reaction mixture without sample was used as control. The intensity of the colour formed was measured at 412 nm using a spectrophotometer. The % hydroxyl radical scavenging activity was calculated by the following formula:
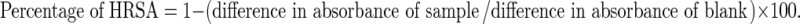 |
Ferric reducing antioxidant power (FRAP) assay
The ability to reduce ferric ions was measured using a modified version of the method described by Benzie and Strain (1996). An aliquot (500 μl) of an extract (with appropriate dilution, if necessary) was added to 3 ml of FRAP reagent (10 parts of 300 mM sodium acetate buffer at pH 3.6, 1 part of 10 mM TPTZ solution and 1 part of 20 mM FeCl3 . 6H2O solution) and the reaction mixture was incubated in a water bath at 37 °C. The increase in absorbance at 593 nm was measured at 30 min. The antioxidant capacity based on the ability to reduce ferric ions of the extract was expressed as μmol Trolox equivalents per gram of plant material on dry weight basis.
Nitric oxide radical (NO˙) scavenging activity
Sodium nitroprusside in aqueous solution at physiological pH, spontaneously generates NO, which interacts with oxygen to produce nitrite ions, which can be estimated by the use of Griess Illosvoy reaction (Garrat 1964). The reaction mixture (3 ml) contains sodium nitroprusside (10 mM, 2 ml), phosphate buffer saline (0.5 ml) and extract or standard solution (25–150 μg/ml) was incubated at 25 °C for 150 min. After incubation, 0.5 ml of the reaction mixture containing nitrite was pipetted and mixed with 1 ml of sulfanilic acid reagent (0.33 % in 20 % glacial acetic acid) and allowed to stand for 5 min for completing diazotization. Then, 1 ml of naphthyl ethylene diamine dihydrochloride was added, mixed and allowed to stand for 30 min at 25 °C. A pink coloured chromophore is formed in diffused light. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions.
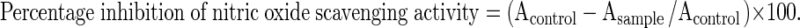 |
Where, A control is the absorbance of the control and A sample is the absorbance of the test.
Total antioxidant activity (TAA) by phosphomolybdenum method
The total antioxidant activity of the extracts was evaluated by the phosphomolybdenum method (Prieto et al. (1999). Briefly, 0.5 ml of each sample solution or ascorbic acid (200–1000 mM) was combined with 3 ml of reagent (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). A typical blank solution contained 3 ml of reagent solution and the appropriate volume of the same solvent used for the sample. All tubes were capped and incubated in a boiling water bath at 95 °C for 90 min. After the samples has been cooled to room temperature, the absorbance of the solution of each sample was measured at 695 nm against the blank using a UV–Vis spectrophotometer (Shimadzu, UV 2450). Total antioxidant activity (TAA) based on the ability to reduce Mo (VI) to Mo (V) by the extracts were expressed as μmol ascorbic acid (AA) equivalents per gram of plant material on dry weight basis.
Statistical analysis
The data obtained from antioxidant assays were presented as means of three replicate determinations ± standard deviation (SD). Statistical analysis of data involved one-way analysis of variance (ANOVA). Differences were considered statistically significant when P < 0.05. The INSTAT software program was used in the statistical analysis (INSTAT™, version 3, Graphpad software, San Diego, USA).
Results and discussion
Proximate analysis
The proximate analysis of C. adscendens var. fimbriata aerial parts is presented in Table 1. From the results, it can be inferred that the protein and available carbohydrate contents were 3.5 % and 55.4 %, respectively. The aerial parts contained 82 % moisture, 5.6 % lipid and 27.5 % total free amino acids. However, the results also showed that the crude fiber and ash content of the aerial parts was high (15.3 % and 2.1 %, respectively). The crude lipid content of the stems of Caralluma adscendens var. attenuata and C. pauciflora are comparable with our results. The presence of fiber in the diet is necessary for digestion and for elimination of wastes. The contraction of muscular walls of the digestive tract is stimulated by fiber, thus counteracting constipation (Narasinga Rao et al. 1989). The World Health Organization (WHO) has recommended an intake of 22–23 kg of fiber for every 1000 K.cal. diet. The crude fibre content in the presently investigated C. adscendens var. fimbriata is found to be more than that of Caralluma adscendens var. attenuata and C. pauciflora.
Table 1.
Proximate and amino acid composition of Caralluma adscendens var. fimbriata aerial parts
| Parameters | Concentration (%) |
|---|---|
| Proximate composition a | |
| Moisture | 82 ± 0.51 |
| Lipid | 5.6 ± 0.1 |
| Carbohydrates | 55.4 ± 0.4 |
| Protein | 3.5 ± 0.7 |
| Total free amino acid | 27.5 ± 0.5 |
| Crude fibre | 15.3 ± 0.2 |
| Ash | 2.1 ± 0.8 |
| Amino acid composition (mg/100 g dry weight) | |
| Aspartic acid | 21.6 |
| Glutamic acid | negligible |
| Alanine | 120.72 |
| Methionine | 22.56 |
| Tyrosine | 130.08 |
| Lysine | 316.56 |
| Theronine | negligible |
| Proline | 483.8 |
| Isoleucine | 1578.24 |
| Phenylalanine | 141.58 |
| Tryptophane | 157.36 |
| Serine | negligible |
| Glycine | 108.29 |
| Valine | 342.95 |
| Leucine | 22.77 |
| Histidine | 84.48 |
| Arginine | 51.58 |
1Values are mean ± standard deviation of three replicates
aAOAC 1990
Amino acid composition of C. adscendens var. fimbriata aerial parts
The data on amino acid composition of C. adscendens var. fimbriata aerial parts are presented in Table 1. The highest amounts of the essential amino acids were of isoleucine (1578.24 mg/100 g sample dry weight), valine (342.95 mg/100 g sample dry weight) and lysine (316.56 mg/100 g sample dry weight). However, the aerial parts contained lower concentrations of essential amino acids, viz. methionine and leucine (22.56 and 22.77 mg/100 g sample dry weight, respectively). Off all non essential amino acids, the highest amount was of proline (483.8 mg/100 g sample dry weight) and tryptophane (157.36 mg/100 g sample dry weight). The negligible amount was observed in threonine, glutamic acid and serine. Olaofe and Akintayo (2000) and Hassan and Umar (2006) reported comparatively similar results for legumes and leaves of other vegetables, respectively.
Extract yield
Single solvent could not extract all the antioxidants with different polarities and solubilities from the plant materials. In this aspect, we used different polarity of solvents for extraction of antioxidant compounds from C. adscendens var. fimbriata aerial parts (Table 2). The yield percentage of different extracts ranged from 3.6 to 11.5 % (w/w) and was 3.6 ± 1.20, 5.9 ± 0.78, 11.5 ± 1.26 and 8.4 ± 1.13 % for chloroform, ethyl acetate, methanol and water extracts, respectively. Extractions with methanol and water resulted in the highest amount of extractable compounds. As the results presented in Table 3, the extraction ability of water and ethanol were very similar to one another, whereas the extraction yield with ethyl acetate was only small in comparison with that of the other solvents. This indicated that most of the soluble compounds in the vegetables were high in polarity.
Table 2.
Extract yield, total phenol and flavonoid contents of different solvent extracts of C. adscendens var. fimbriata aerial parts
| Sample1 | Extract Yield (%) | Total Phenol (mg GAE/g DW) | Total Flavonoid (mg QE/g DW) |
|---|---|---|---|
| CAFC | 3.6 ± 1.2c2 | 8.7 ± 0.6b | 1.1 ± 0.9a |
| CAFE | 5.9 ± 0.8e | 14.9 ± 0.4d | 1.3 ± 0.7c |
| CAFM | 11.5 ± 1.3a | 21.0 ± 0.6b | 3.7 ± 1.2d |
| CAFW | 8.4 ± 1.1b | 18.8 ± 0.9a | 3.1 ± 0.5b |
1 CAFC C. adscendens var. fimbriata chloroform extract; CAFÉ C. adscendens var. fimbriata ethyl acetate extract; CAFM C. adscendens var. fimbriata methanol extract; CAFW C. adscendens var. fimbriata water extract. GAE Gallic acid equivalent; QE Quercetin equivalent
2Values are mean ± standard deviation of three replicates
Means followed by different letters in the same column are significantly different (P < 0.05)
Table 3.
Antioxidant capacity of DPPH, FRAP, TEAC and TAA of different solvent extracts of C. adscendens var. fimbriata aerial parts
| Samples1 | DPPH (mg AA/100 g FW) | FRAP (μmol FeSO4/g DW) | TEAC (μmol TE/g DW) | TAA (μmol AA/g DW) |
|---|---|---|---|---|
| CAFC | 5093.3 ± 0.8b2 | 2912 ± 0.4c | 6550 ± 2.5e | 654 ± 0.7a |
| CAFE | 5457.1 ± 1.9a | 4240 ± 0.6d | 7500 ± 1.9c | 1072 ± 0.4d |
| CAFM | 6063.5 ± 1.4d | 4700 ± 0.5b | 8150 ± 0.9b | 1303 ± 0.8b |
| CAFW | 5701.5 ± 1.6c | 5860 ± 0.7a | 8350 ± 1.7a | 1375 ± 0.9c |
1 CAFC C. adscendens var. fimbriata chloroform extract; CAFÉ C. adscendens var. fimbriata ethyl acetate extract; CAFM C. adscendens var. fimbriata methanol extract; CAFW C. adscendens var. fimbriata water extract
2Values are mean ± standard deviation of three replicates
Means followed by different letters in the same column are significantly different (P < 0.05)
Total phenol and flavonoid contents of the extracts
The phenolic compounds are powerful antioxidants and act in a structure-dependent manner; they can scavenge reactive oxygen species (ROS), and chelate transition metals which play vital roles in the initiation of deleterious free radical reactions. Obviously, total phenolic contents could be regarded as an important indication of antioxidant properties of plant extracts (Wang et al. 2010). Table 2 illustrates the total phenolic content in different solvent extracts of C. adscendens var. fimbriata aerial parts. It can be seen, the phenolic content of methanol extract (21.0 ± 0.59 mg GAE/g sample dry weight) is significantly higher than that of water extract (18.8 ± 0.98 mg GAE/g sample dry weight), which in turn significantly higher than that of ethyl acetate extract (14.9 ± 0.40 mg GAE/g sample dry weight) and chloroform extract (8.7 ± 0.63 mg GAE/g sample dry weight). Marwah et al. (2007) observed the total phenol content of C. flava (23.6 ± 0.5 mg GAE/g of ethanol extract) and C. quadrangula (18.7 ± 0.4 mg GAE/g of ethanol extract) aqueous ethanol extract. These values are similar and comparable with that of C. adscendens var. fimbriata extracts.
Flavonoids are one of the most diverse and widespread group of natural compounds are probably the most important natural phenolics. The flavonoids containing multiple hydroxyl groups have higher antioxidant activities against peroxyl radicals than do phenolic acids and possess antioxidant capacity towards a variety of easily oxidizable compounds. The antioxidant activities of flavonoids increased with the number of hydroxyl groups substituted on the B-ring (Pyo et al. 2004). The flavonoid contents of water and methanol extracts (3.1 ± 0.54 and 3.7 ± 1.16 mg QE/g sample dry weight, respectively) were relatively higher compared to the levels of ethyl acetate (1.3 ± 0.75 mg QE/g sample dry weight) and chloroform (1.1 ± 0.92 mg QE/g sample dry weight) extracts (Table 2). The lowest amount of total phenol and flavonoid contents were shown by the chloroform extract. These results showed that, polyphenol content was strongly dependent on the solvents. Polar extracts had more phenolics in them than non-polar extracts (Hayouni et al. 2007). Our results clearly showed that a higher content of polyphenols was obtained with an increase in the polarity of the solvent used.
2, 2-Diphenyl-1-picrylhydrazyl (DPPH˙) radical scavenging assay
The model of scavenging DPPH˙ radical is especially used to evaluate chain-breaking activity in the propagation phase of lipid (and protein) oxidation. The effect of antioxidants on DPPH˙ radical scavenging was thought to be due to their hydrogen donating ability (Mao et al. 2006). In the present study, the scavenging activity of C. adscendens var. fimbriata extracts against DPPH˙ radicals was expressed in terms of AEAC. The AEAC values of the chloroform, ethyl acetate, methanol and water extracts were found to be 5093.3 ± 0.83, 5457.1 ± 1.92, 6063.5 ± 1.38 and 5701.5 ± 1.60 mg AA/100 g FW, respectively. Methanol extract that contained the highest amount of total phenolics, was found to be the most active radical scavenger followed by water and ethyl acetate extracts. However, the chloroform extract was not as effective as the other three extracts (Table 3). These results showed that, DPPH˙ radical scavenging ability of all solvent extracts was related to various amount of antioxidants. Tatiya et al. (2010) observed that the butanol extract showed high DPPH scavenging activity than other extracts (methanol, aqueous and petroleum ether) of C. adscendens and the IC50 values were found in the range between 157.62 and 458.71 μg/ml. The aqueous methanol extract of C. edulis (charungli) showed a strong DPPH scavenging activity between 80 and 100 % (Ansari et al. 2005). The DPPH˙ radical scavenging activity of C. flava aqueous ethanol extracts exhibited the percentage activity of 31.5 ± 1.0 at 50 μg/ml. Whereas, the aqueous ethanol extracts of C. quadrangula, lacks antioxidant activity in the DPPH assay. The preliminary phytochemical investigation of the aqueous ethanol extracts of C. quadrangula, the most abundant constituents are not phenolics but pregnane glycosides (Marwah et al. 2007). In general, the polyphenol concentrations are positively correlated with antioxidant activity due to their hydrogen donating abilities. These reports indicated that the radical scavenging capacity of extracts might be mostly affected by the presence and position of phenolic hydroxyl group. The radical scavenging activity of phenolic compounds depends on their molecular structure, that is, on the availability of phenolic hydrogens and on the possibility for stabilization of the resulting phenoxyl radicals formed by hydrogen donation (Jung et al. 2006).
Trolox equivalent antioxidant capacity (TEAC)
TEAC is a quantification of the effective antioxidant activity of the extract; a higher TEAC would imply greater antioxidant activity of the samples. Generation of ABTS˙+ radical cation (Re et al. 1999) forms the basis of one of the spectrophotometric methods that have been applied to the measurement of the total antioxidant activity of solutions of pure substances, aqueous mixtures and beverages widely used for the assessment of antioxidant activity of various substances. This method measures the relative antioxidant ability to scavenge the radical ABTS˙+ as compared with a standard amount of Trolox, and is an excellent tool for determining the antioxidant activity of hydrogen donating antioxidants and of chain breaking antioxidants (Egea et al. 2010). In this study all the tested C. adscendens var. fimbriata extracts exhibited strong ABTS˙+ scavenging capacities, with a TEAC value range of 6550–8350 μmol TE/g sample dry weight (Table 3). The water extract showed the greatest TEAC value of 8350 ± 1.71 μmol TE/g sample dry weight, followed by that of methanol (8150 ± 0.89 μmol TE/g sample dry weight), ethyl acetate (7500 ± 1.92 μmol TE/g sample dry weight) and chloroform (6550 ± 2.46 μmol TE/g sample dry weight) extracts. The reason for the difference in the TEAC values can lie in the different extraction procedures of the plant. The higher TEAC value reported for the water extract of the plant can be due to the higher content of phenolic substances. Hagerman et al. (1998) have reported that the high molecular weight phenolics have more ability to quench free radicals (ABTS˙+) and their effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl group’s substitution than the specific functional groups.
Hydroxyl radical (OH˙) scavenging activity
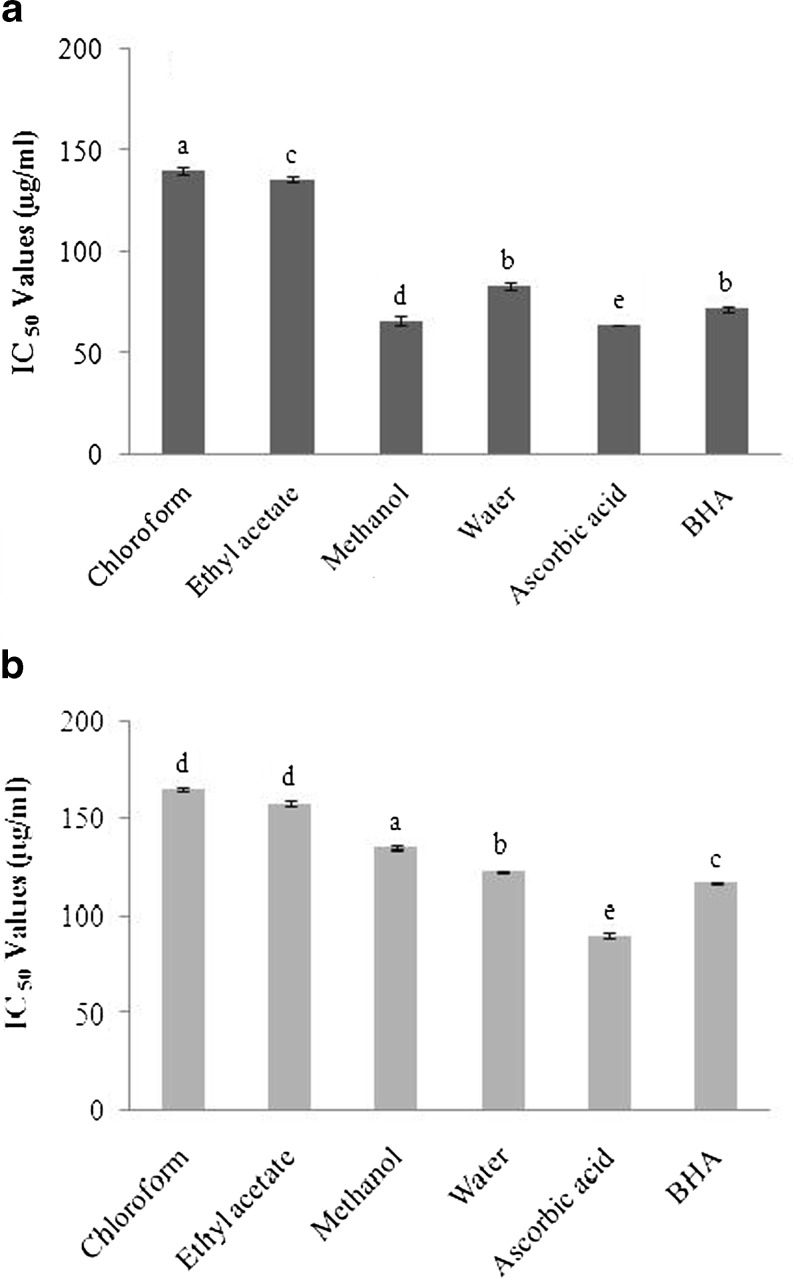
The OH˙ radical has the capacity to join nucleotides in DNA and cause strand breakage, which contributes to carcinogenesis, mutagenesis, and cytotoxicity. In addition, this species is considered to be one of the quick initiators of the lipid peroxidation process, abstracting hydrogen atoms from unsaturated fatty acids (Singh et al. 2007). The OH˙ scavenging activity of the extracts of C. adscendens var. fimbriata by generating the hydroxyl radicals using ascorbic acid–iron EDTA was shown in Fig. 1a. Of all extracts studied, the methanol extract had the strongest OH˙ scavenging activity with an IC50 value of 66 ± 2.19 μg/ml. The ability of water extract to scavenge OH˙ was observed to be 83 ± 1.98 μg/ml. On the other hand, extracts prepared with chloroform and ethyl acetate exhibited moderate radical scavenging activity when compared with those of others given above (140 ± 1.73 and 136 ± 1.26 μg/ml, respectively). The concentration of the positive controls, ascorbic acid and BHA required to scavenge 50 % of the free radical (IC50) was 64 ± 0.35 and 72 ± 0.41 μg/ml, respectively. The ability of the extracts to quench OH˙ radicals was observed to be directly related to the prevention of propagation of the process of lipid peroxidation by scavenging the active oxygen species and thus reducing the rate of chain reaction (Shukla et al. 2009). In this respect, it can be concluded that the difference of antioxidant activity from each extract of C. adscendens var. fimbriata aerial parts might be related to their phenolic content.
Fig. 1.
Hydroxyl radical scavenging activity (a) and Nitric oxide radical scavenging activity (b) of different solvent extracts of C. adscendens var. fimbriata aerial parts. Values are mean ± standard deviation of three replicates. Means followed by different letters in the same column are significantly different (P < 0.05)
Ferric reducing antioxidant power (FRAP) assay
FRAP assay measures the reducing potential of an antioxidant reacting with a ferric tripyridyltriazine (Fe3+–TPTZ) complex and producing a coloured ferrous tripyridyltriazine (Fe2+–TPTZ) (Benzie and Strain 1996). Generally, the reducing properties are associated with the presence of compounds which exert their action by breaking the free radical chain by donating a hydrogen atom (Kubola and Siriamornpun 2008). In our study, FRAP values of the different extracts of C. adscendens var. fimbriata aerial parts were shown in Table 3. Water extract had the highest FRAP value of 5860 ± 0.71 μmol FeSO4/g dry weight followed by methanol (4700 ± 0.46 μmol FeSO4/g dry weight), ethyl acetate (4240 ± 0.62 μmol FeSO4/g dry weight), and chloroform (2912 ± 0.41 μmol FeSO4/g dry weight) extracts. These results indicated that methanol extract had significantly stronger FRAP than other extracts. Due to different antioxidant potentials of different compounds, the antioxidant activity of Caralluma extracts is strongly dependent on the extraction solvent. Tatiya et al. (2010) found the reducing capacity of C. adscendens was increased in a dose dependent manner, with butanol extract showed the maximum absorbance (0.65–1.7 OD value at sample concentration between 250 and 2000 mg/ml) than other extracts such as methanol, aqueous and petroleum ether. The FRAP assay treats the antioxidants contained in the samples as reductants in a redox linked colorimetric reaction and the value reflects the reducing power of the antioxidants.
Nitric oxide (NO˙) radical scavenging activity
The nitric oxide radical (NO˙), which is produced in vivo by variety of cell types, is an important bioregulatory molecule with a number of physiological functions. However, under oxidative stress this RNS reacts with other reactive species to produce more toxic RNS and ROS (Dastmalchi et al. 2008). In the present study, different solvent extracts of the C. adscendens var. fimbriata aerial parts were checked for its inhibitory effect on nitric oxide production. Nitric oxide radical generated from sodium nitroprusside at physiological pH was found to be inhibited by the extracts. The NO˙ radical scavenging activities of the water, methanol and ethyl acetate extracts, showed to be equally potent in scavenging NO˙, by 48.6 ± 0.34–55.6 ± 0.39 %, respectively at a concentration of 150 μg/ml, while chloroform extract was considerably less effective NO˙ scavenger (43.1 ± 0.47 %) at the same concentration. As compared with the IC50 values (Fig. 1b), the nitric oxide scavenging activities of water (123 ± 0.5 μg/ml), methanol (135 ± 0.28 μg/ml) and ethyl acetate (158 ± 0.46 μg/ml) extracts were more effective than chloroform (165 ± 0.17 μg/ml) extract. The activities were lower than those obtained for ascorbic acid (90 ± 0.33 μg/ml) but the IC50 values of the methanol and water extracts were comparable with that of BHA (117 ± 0.18). The NO˙ generated from sodium nitroprusside reacts with oxygen to form nitrite. The extract inhibits nitrite formation by competing with oxygen to react with nitric oxide directly and also to inhibit its synthesis. Scavengers of NO compete with oxygen leading to reduced production of nitric oxide (Sfahlan et al. 2009). The plant extracts may have the property to counteract the effect of NO formation and preventing the ill effects of excessive NO generation in the human body. Further, the scavenging activity may also help to arrest the chain of reactions initiated by excess generation of NO that are detrimental to human health (Kumaran and Karunakaran 2006).
Total antioxidant activity (TAA) by phosphomolybdenum method
Total antioxidant capacity of C. adscendens var. fimbriata was evaluated in this study by the phosphomolybdenum method based on the reduction of Mo (VI) to Mo (V) by the antioxidant compounds and the formation of a green Mo (V) complex at a low pH with a maximal absorbance at 695 nm. A high absorbance value indicates that the sample possesses high antioxidant activity (Prieto et al. 1999). In the study, extracts were found to have different levels of antioxidant activity in the systems tested (Table 3). The methanol extract of aerial parts has the higher antioxidant capacity (1375 ± 0.89 μM AA/g sample DW) than the other three extracts which showed antioxidant capacity in the order: water extract (1303 ± 0.81 μM AA/g sample DW) > ethyl acetate extract (1072 ± 0.43 μM AA/g sample DW) > chloroform extract (654 ± 0.74 μM AA/g sample DW). In this assay, methanol and water extracts were found to be rather similar in their action, chloroform extract being the lowest in activity. The extracts demonstrated electron-donating capacity and thus they may act as radical chain terminators, transforming reactive free radical species into more stable non-reactive products (Dorman et al. 2003). The total antioxidant capacity of plant extracts may be attributed to their chemical composition and phenolic content. However, TAA values were in good agreement with C. quadrangula (899 ± 29.2 mg GAE/g of ethanol extract) and C. flava (335 ± 0.5 mg GAE/g of ethanol extract) (Marwah et al. 2007).
Linear correlation coefficients between polyphenol contents and the different antioxidant activities
Correlations were tested to link the antioxidative capacities measured by the different assays used with each other, as well as the polyphenolic contents of the extracts. Table 4 summarizes the linear correlation coefficients between all analyses carried out on the extracts. In the present study, total phenol content was shown to provide the highest association with TAA assay (r2 = 0.990). Similar results were also found for DPPH (r2 = 0.952) and TEAC (r2 = 0.927), FRAP (r2 = 0.708), hydroxyl (r2 = 0.814) and nitric oxide (r2 = 0.730) scavenging activities confirm that phenols are mainly responsible for the antioxidant activity of extracts. These results indicated that the radical scavenging capacity of each extract might be mostly related to their concentration of phenolic hydroxyl group. Phenolic compounds are attracting considerable interest in the field of food chemistry and medicine due to their promising antioxidant potential (Sultana et al. 2007). The good correlations between total phenol content and total flavonoid content (r2 = 0.836) was reported in our study. This indicates that the flavonoids are the major phenolic compounds present in the C. adscendens var. fimbriata aerial parts. Correlation coefficient between total flavonoid content with antioxidant assays (r2 = 0.756–0.999) studied in this study were found to be very strong. Whereas moderate correlation was found between total flavonoid content and reducing capacity, measured by the FRAP method (r2 = 0.560, Table 4) was might be attributed to the fact that most of the flavonoids are in their glycoside form, which are less effective as compared to their respective aglycone forms (Shahidi 1997). Several studies have reported a good correlation between the phenolic compounds of plant extracts and antioxidant activity (Santas et al. 2008; Kubola and Siriamornpun 2008).
Table 4.
Linear correlation coefficients of phenolic compounds with antioxidant activities
| TPC | TFC | DPPH | TEAC | TAA | FRAP | OH˙ | NO˙ | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1.000 | |||||||
| TFC | 0.836 | 1.000 | ||||||
| DPPH | 0.952 | 0.875 | 1.000 | |||||
| TEAC | 0.927 | 0.756 | 0.785 | 1.000 | ||||
| TAA | 0.990 | 0.781 | 0.902 | 0.960 | 1.000 | |||
| FRAP | 0.708 | 0.560 | 0.509 | 0.913 | 0.776 | 1.000 | ||
| OH˙ | 0.814 | 0.999 | 0.859 | 0.734 | 0.756 | 0.542 | 1.000 | |
| NO˙ | 0.730 | 0.810 | 0.615 | 0.864 | 0.746 | 0.873 | 0.805 | 1.000 |
Data from this study indicated that DPPH scavenging activity had a good correlation with TAA (r2 = 0.902), TEAC (r2 = 0.785), hydroxyl (r2 = 0.859) and nitric oxide (r2 = 0.615) radical scavenging activities. However, moderate correlation was found with FRAP method (r2 = 0.509). Amongst the methods used for quantifying antioxidant activities, the correlation between TEACs and other assays such as TAA, FRAP, hydroxyl and nitric oxide activities was r2 = 0.960, r2 = 0.913, r2 = 0.734 and r2 = 0.864, respectively. Upon comparing the antioxidant capacity results obtained with the other methods we find good correlations values. Although, a moderate correlation (r2 = 0.542) was observed between FRAP and HRSA.
In conclusion, the antioxidant activities of extracts from C. adscendens var. fimbriata aerial parts were evaluated in this study with different in vitro testing systems. The extracts of aerial parts are capable of scavenging a wide range of synthetic and naturally occurring free radicals. Methanol and water proved to be the most efficient solvent for extraction of antioxidants from aerial parts as the related extract contained the highest amount of phenolic compounds exhibited the strongest antioxidant capacity in all the assays tested. The antioxidant activity was correlated well with the amount of total phenolics present in the extracts in each assay. The extracts and its components may be used as an alternative to synthetic antioxidants as additive in food preparations and nutraceuticals.
Acknowledgement
The authors are grateful to the Management, Karpagam Educational Institutions for generous support and encouragement to carry out the study.
References
- Ahn GN, Kim KN, Cha SH, Song CB, Lee J, Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, Heu MS, Jeon YJ. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol. 2007;226:71–79. doi: 10.1007/s00217-006-0510-y. [DOI] [Google Scholar]
- Ansari NM, Houlihan L, Hussain B, Pieroni A. Antioxidant activity of five vegetables traditionally consumed by South-Asian Migrants in Bradford, Yorkshire, UK. Phytother Res. 2005;19:907–911. doi: 10.1002/ptr.1756. [DOI] [PubMed] [Google Scholar]
- AOAC (1990) Official methods of analysis, 14th edn. Washington DC, USA
- Atal CK, Sharma BM, Bhatia AK. Search of emergency foods through wild flora of Jammu and Kashmir state: Sunderbani area -1. Indian Forester. 1980;106:211–219. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Dastmalchi K, Dorman HJD, Oinonen PP, Darwis Y, Laakso I, Hiltunen R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT- Food Sci Technol. 2008;41:391–400. doi: 10.1016/j.lwt.2007.03.007. [DOI] [Google Scholar]
- Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Egea I, Sánchez-Bel P, Romojaro F, Pretel MT. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Foods Hum Nutr. 2010;65:121–129. doi: 10.1007/s11130-010-0159-3. [DOI] [PubMed] [Google Scholar]
- Garrat DC. The quantitative analysis of drugs, 3rd vol. Japan: Chapman and Hall Ltd; 1964. pp. 456–458. [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hassan LG, Umar KJ. Nutritional value of Balsam apple (Momordica balsamina L.) leaves. Pak J Nutr. 2006;5:522–529. doi: 10.3923/pjn.2006.522.529. [DOI] [Google Scholar]
- Hayouni EA, Abedrabba M, Bouix M, Hamdi M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007;105:1126–1134. doi: 10.1016/j.foodchem.2007.02.010. [DOI] [Google Scholar]
- Heo SJ, Park EJ, Lee KW, Jeon YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol. 2005;96:1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Fujita T, Asai K. New detection and separation method for amino acids by high performance liquid chromatography. J Chromat. 1981;204:143–148. doi: 10.1016/S0021-9673(00)81650-7. [DOI] [PubMed] [Google Scholar]
- Jung CH, Seog HM, Choi IW, Park MW, Cho HY. Antioxidant properties of various solvent extracts from wild ginseng leaves. LWT- Food Sci Technol. 2006;39:266–274. doi: 10.1016/j.lwt.2005.01.004. [DOI] [Google Scholar]
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system. Biochemistry. 1991;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Kubola J, Siriamornpun S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008;110:881–890. doi: 10.1016/j.foodchem.2008.02.076. [DOI] [PubMed] [Google Scholar]
- Kumaran A, Karunakaran RJ. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006;97:109–114. doi: 10.1016/j.foodchem.2005.03.032. [DOI] [Google Scholar]
- Kunert O, Rao VG, Babu GS, Sujatha P, Sivagamy M, Anuradha S, Rao BVA, Kumar BR, Alex RM, Schuehly W, Kuehnelt D, Rao GV, Rao AVNA. Pregnane glycosides from Caralluma adscendens var. fimbriata. Chem Biodiv. 2008;5:239–250. doi: 10.1002/cbdv.200890021. [DOI] [PubMed] [Google Scholar]
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- Mali KK, Dias RJ, Hawaldar VD, Mahajan NS. Hypoglycemic activity of Caralluma adscendens in alloxan induced diabetic rats. Int J Chem Sci. 2009;7:517–522. [Google Scholar]
- Mao LC, Pan X, Que F, Fang XH. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur Food Res Technol. 2006;222:236–241. doi: 10.1007/s00217-005-0007-0. [DOI] [Google Scholar]
- Marwah RG, Fatope MO, Mahrooqi RA, Varma GB, Abadi HA, Al-Burtamani SKS. Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 2007;101:465–470. doi: 10.1016/j.foodchem.2006.02.001. [DOI] [Google Scholar]
- Narasinga Rao BS, Deosthale YG, Pant KC (1989) Nutritive value of Indian Foods. National institute of nutrition, Indian Council of Medical Research, Hyderabad, India
- Olaofe O, Akintayo ET. Production of isoelectric points of legume and oil seed proteins from their amino acid composition. J Technol Sci. 2000;4:49–53. [Google Scholar]
- Ordon Ez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex, Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Pyo YH, Lee TC, Logendra L, Rosen RT. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004;85:19–26. doi: 10.1016/S0308-8146(03)00294-2. [DOI] [Google Scholar]
- Re R, Pellegirini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Santas J, Carbo R, Gordon MH, Almajano MP. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–1216. doi: 10.1016/j.foodchem.2007.09.056. [DOI] [Google Scholar]
- Sfahlan AJ, Mahmoodzadeh A, Hasanzadeh A, Heidari R, Jamei R. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 2009;115:529–533. doi: 10.1016/j.foodchem.2008.12.049. [DOI] [Google Scholar]
- Shahidi F. Natural antioxidants, chemistry, health effects and applications. Champaign: AOCS Press; 1997. [Google Scholar]
- Shukla S, Mehta A, Bajpai VK, Shukla S. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chemic Toxicol. 2009;47:2338–2343. doi: 10.1016/j.fct.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Singh R, Singh S, Kumar S, Arora S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem Toxicol. 2007;45:1216–1223. doi: 10.1016/j.fct.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Singleton V, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chem. 2007;104:1106–1114. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- Tatiya AU, Kulkarni AS, Surana SJ, Bari ND. Antioxidant and hypolipidemic effect of Caralluma adscendens Roxb. in alloxanized diabetic rats. Int J Pharmacol. 2010;6:362–368. [Google Scholar]
- Tavarini S, DeglInnocenti E, Remorini D, Massai R, Guidi L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008;107:282–288. doi: 10.1016/j.foodchem.2007.08.015. [DOI] [Google Scholar]
- The Wealth of India . A dictionary of Indian raw materials and industrial products. New Delhi: Vedams; 1992. pp. 266–267. [Google Scholar]
- Valento P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J Agric Food Chem. 2002;50:4989–4993. doi: 10.1021/jf020225o. [DOI] [PubMed] [Google Scholar]
- Wang H, Gan D, Zhang X, Pan Y (2010) Antioxidant capacity of the extracts form pulp of Osmathus fragrans and its components. LWT–Food Sci Technol 43:319–325