Abstract
The changes in the main nutrient and medicinal components during the storage of the Chinese yam (Dioscorea opposita) tubers were studied. The harvested tubers were stored under ambient conditions (10 °C to 18 °C, 60 % to 80 % Relative Humidity) and cold temperature and packaged conditions (4 °C, 60 % to 65 % Relative Humidity) for 45 day. The allantoin, starch, total alcohol-soluble sugar, reducing sugar, protein, and moisture contents of the samples were evaluated. Their amylase activities were also investigated. Results of ambient conditions indicated that, during storage, moisture decreased by 67.96 % to 56.51 %, and total sugars, reducing sugars, and protein increased by 6.49 % to 9.81 %, 1.7 % to 2.27 %, and 13.02 % to 14.55 %, respectively. Starch and enzyme activities increased during the early days of storage and progressively decreased, and the content of allantoin changed in volatility. The changes were more significant at cold temperatures and packaged conditions than at ambient conditions. This result suggests that after-ripening occurred in the early stages of Chinese yam tubers, which positively affected the nutritional potential of the tubers by a marked increase in nutrients. Low-temperature sweetening greatly affects the nutritional potential of tubers by a series of complicated interactions between starch and sugars at 4 °C.
Keywords: Chinese yam, Nutrients, Storage, After-ripening, Low temperature sweetening
Introduction
The Chinese yam (Dioscorea opposita) is one of the endemic crops of China (Flora Republicae Popularis Sinicae 2004) that has nutritional and economic significance in China. It is widely cultivated in the northeastern, central, and southeastern regions of China, and it is also distributed in Korea and Japan. Henan province, the main producing area, cultivates the species with the highest quality. The Chinese yam is rich in nutrients including proteins (3.59 % to 8.93 %), amino acids (2.31 % to 7.26 %), starches (43.7 %), sugars (3.39 %), vitamins, and amylases, among others. (Nie et al. 1993; Omonigho and Ikenebomeh 2000; Yuan 2008; Nie et al. 1993; Zhou et al. 2004; Omonigho and Ikenebomeh 2000). In addition, numerous active constituents are present in Chinese yam tubers, such as allantoin (Fu et al. 2006), which promotes wound healing, speeds up cell regeneration, and exhibits a keratolytic effect, among others. Steroidal saponins, especially diosgenin and its glycoside, are the typical bioactive components for most plants of the family Dioscoreaceae (Yang and Lin 2008). These components are rarely detected in the Chinese yam (Zhang et al. 2011). The Chinese yam is very a popular edible and medicinal plant that promotes health and longevity (Yang and Lin 2008). It is not only used as a vegetable; it is also processed into various kinds of products. Therefore, yams are in great demand. However, the supply of yams is unable to meet the demand because of serious losses during transportation and distribution. Most yams are susceptible to mechanical injury, weight loss, and fungal decay during retail and distribution. Although some scientific methods of yam storage have been improved, such as the use of γ-radiation, edible coatings (Dhall et al. 2011; Mishra et al. 2010; Dhall et al. 2011; Rekha and Vijayalakshmi 2011), fungicides (Hussain et al. 2012; Sudha et al. 2011; Wijewardane and Guleria 2011; Bajwa and Sandhu 2011)biocides, Osmo-air drying (Pisalkar et al. 2011; Jain et al. 2011), plastic packaging (Azene et al. 2011; Kaur et al. 2011; Shakerardekani and Karim 2012) and modified atmosphere packaging (Alam and Goyal 2011; Majidi et al. 2012; Kudachikar et al. 2011; Stasiewicz et al. 2012; Alam and Goyal 2011; Kudachikar et al. 2011), these techniques are restricted in commercial applications. These post-harvest storage methods are technically complex and quite expensive for adoption by small-scale farmers who produce the bulk of yam tubers. Compared with γ-radiation and biocide techniques, the low-temperature storage method is more popular because it is relatively inexpensive and not technically complex (Ahuja and Goyal 2012; Mahalingaiah et al. 2012; Oliveira et al. 2012). Low-temperature (4 °C) storage is more effective and popular for the storage, transportation, and retail of yams in China.
Differences in the storage conditions or treatment of yams may produce some changes in nutrients that could affect the sensory qualities. Recent studies have reported these changes during storage in some cultivars grown in the tropics, such as Dioscorea batatas (Fu et al. 2006), Dioscorea alata (Chou et al. 2006; Sahore et al. 2007; Chou et al. 2006; Shittu 2011), Dioscorea. dumetorum (Afoakwa and Sefa-Dedeh 2002; Afoakwa and Sefa-Dedeh 2001; Medoua et al. 2005; Sefa-Dedeh and Emmanuel 2002), Dioscorea rotundata (Omonigho and Ikenebomeh 2000; Morse et al. 2000; Efiuvwere and Nwachukwu 1998; Oyebanji et al. 2003), Dioscorea. pseudojaponica (Fu et al. 2006; Yang and Lin 2008), and other Dioscorea species (Lee et al. 2007). However, the quality of Chinese yams grown in the North Temperate Zone is different from those grown in the tropics because of climate and environment. In addition, very little information is available on the changes of nutrients in the Chinese yam (Dioscorea opposita) occurring during storage.
Accordingly, the present study aims to determine the changes in nutrients and medicinal composition during storage of the Chinese yam (Dioscorea opposita) under different temperature conditions. Starches, proteins, sugars, and amylases are the main nutrients in the Chinese yam. As such, starch, protein, total alcohol-soluble sugars, reducing sugars, and amylase activities were selected as the indexes. Allantoin plays an important role in the nitrogen economy of plants (Fu et al. 2006). Recently, allantoin has been found in some plants, such as soybean (Matsumoto et al. 1997) and alfalfa (Cheng et al. 1999). However, studies on the allantoin content of Chinese yams and changes during storage of Chinese yam tubers are rare. Therefore, allantoin was also included as an index.
Materials and methods
Materials
Mature Chinese yam (Dioscorea opposita) tubers were harvested in October from a farm in Henan, a province in central China. They were immediately transported to the laboratory in Tianjin for subsequent chemical analyses. All tubers, except the wounded and mildewed ones, were thoroughly washed with distilled water, wiped with sterilized dry cloth, and then randomly divided into two groups. One group of tubers was stored under ambient conditions (10 °C to 18 °C, 60 % to 80 % Relative Humidity), and another group was packaged with polyethylene film and stored under conditions reflecting cold room temperature (4 °C, 60 % to 65 % Relative Humidity).
Sample preparation
Tuber samples from the two groups were collected at fixed time intervals (days 0, 3, 6, 9, 12, 18, 24, 30, 36, and 45). Three portions in each condition were taken out randomly for analyses. The yams were peeled prior to testing.
The samples were tested for moisture, allantoin, protein, starch, total alcohol-soluble sugars, reducing sugars, and amylase (α + β) activities. The moisture, allantoin, starch, total alcohol-soluble sugars, and reducing sugars of dried yams were determined. The samples were cut into 0.5 cm thick slices and deactivated enzymes at 100 °C to 105 °C for 20 min using a drying oven and then dried to constant weight at 80 °C. The dried samples were reduced to coarse particles via milling, and the particles were packaged into polypropylene bags and kept at ambient temperature conditions (10 °C to 18 °C) for analysis. Protein and amylase (α + β) activities of fresh yams were determined. Fresh yams were ground and centrifuged at 4 °C (12,000 × g). The supernatant was then stored at −20 °C for analysis of protein and amylase (α + β) activities. To remove the effect of dehydration, protein and amylase (α + β) activities contents expressed by fresh weight were converted to ones expressed by dry weight in accordance with Formulas (1) and (2), respectively.
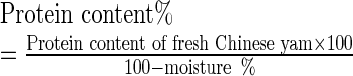 |
1 |
 |
2 |
Analytical methods
The samples were analyzed in triplicate for moisture content using Association of Official Analytical Chemist Approved methods 925.10 AOAC (1980). Moisture content was determined as weight loss after drying at 105 °C until reaching constant weight.
Starch was determined using the iodine colorimetry method described by Xu et al. (1998) Briefly, 1 mL of sample, 20 mL of distilled water, and 0.5 mL of I2-KI reagent were put in a 25 mL brown volumetric flask, shaken, and let stand. After 5 min, the mixture was added to make 25 mL with distilled water. The absorbance of mixture was measured at 620 nm using a Ultraviolet-wisible(UV–VIS) spectrophotometer (Tuopu Instrument Co., Ltd, China). Soluble starch was used to determine the standard curve: y = 0.3117× + 0.011 (R2 = 0.9999).
Total sugars were determined according to the method used by Lane and Eynon (1923), with some modifications. Briefly, a solution containing 0.05 % anthrone, 33.95 % distilled water, and 66 % by volume concentrated sulfuric acid was used. This acid solution (5 mL) was added to the yam extract with water (1 mL). The resulting mixture was warmed to 90 °C for 5 min, occasionally shaking the flask to mix the contents, and then set and cooled to room temperature. The absorbance was measured at 620 nm using a UV–VIS spectrophotometer. Glucose was used to determine the standard curve: y = 6.52× + 0.1152 (R2 = 0.9994).
Reducing sugars and amylase activities were determined according to the method proposed by Bernfeld (1955) using 3.5 dinitrosalycilic acids. Briefly, 1 mL sample and 2 mL 3,5-dinitrosalicylic acid reagent were pipetted into a 10 mL volumetric flask. The mixture was boiled for 2 min, and the absorbance of the mixture was measured at 540 nm using a spectrophotometer. Glucose was used to determine the standard curve: y = 0.2005× – 0.0474 (R2 = 0.9996).
The presence of protein was determined using the Coomassie Brilliant Blue G250 method described by Sedmak and Grossberg (2005). Immediately after the dye solution was mixed with a protein sample, the absorbance of the mixture was measured at 595 nm. The absorbance of the solution was stable for 60 min to 90 min at room temperature. Bovine serum albumin was used to determine the standard curve: y = 6.455× + 0.1371 (R2 = 0.9949).
Analyses of allantoin were performed on an Agilent 1100 High Performance Liquid Chromatography (HPLC) instrument (Agilent, Waldbronn, USA) consisting of a G1311A Quaternary Pump, a G1322A degasser, a G1314A Ultraviolet(UV) Detector, a G1316A column, and the HPLC ChemStation software. The conditions were as follows: HPLC column: Kromasil C18 (4.6 × 250 mm, 5 μm); mobile phase: EtOHCH3OH/H2O (10/90); flow rate: 0.4 mL/min; UV detective wavelength: 224 nm; and column temperature: 30 °C. Before injection, the samples were filtered through a 0.22 μm millipore filter. The standards for allantoin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products, China. The standard curve was: y = 12.593× + 60.32 (R2 = 0.9996).
Statistical analysis
All experimental groups were each composed of three samples and all experiments were replicated three times. The statistical significance of difference was assessed via analysis of variance followed by the Dunnett t-test; P < 0.05 was considered to indicate statistical significance.
Results and discussion
Changes in the moisture content of Chinese yam tubers during storage
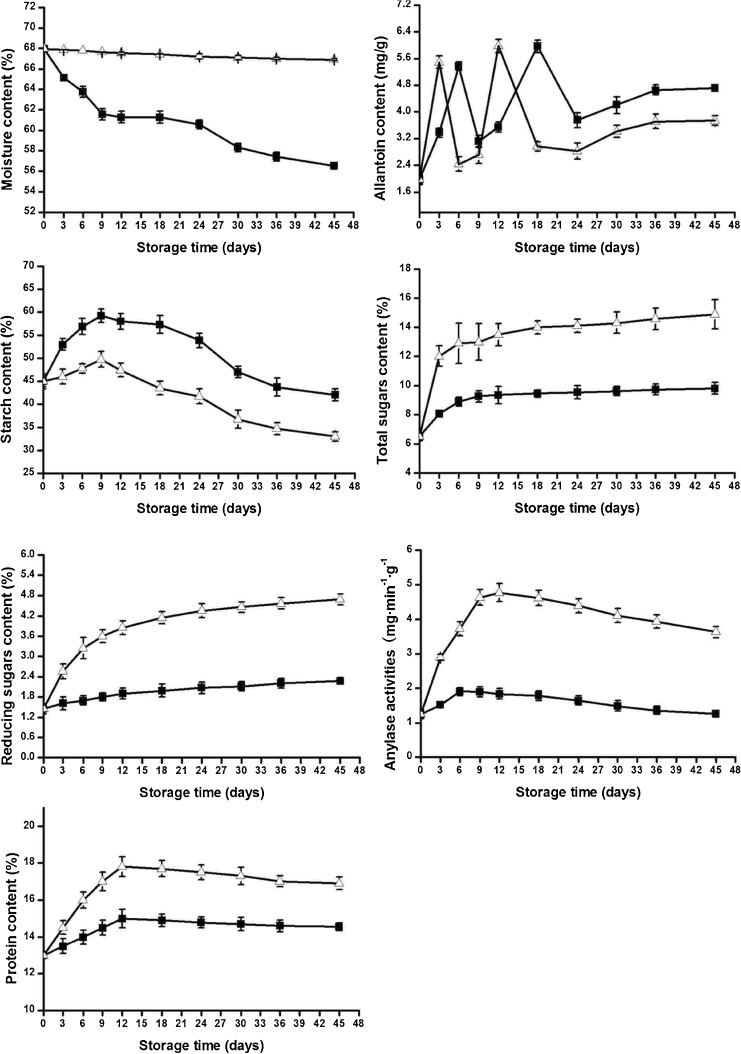
The results of the changes of main nutrients and medicinal composition from Chinese yam tubers during storage are shown in Table 1 and the trend of changes is shown in Fig. 1. A rapid drop in moisture was observed for all the samples stored under ambient temperature conditions after harvest. During the first 9 day of storage, the moisture of yam tubers decreased from 67.96 % to 61.55 %. However, during the following 15 day, the samples showed lower rates of moisture loss, ranging from 61.55 % to 60.58 %. After the 25th d, the moisture loss rates decreased progressively to 57.43 %. The trend of rapid-slow-rapid dehydration was similar to that obtained by Medoua et al. (2005) in Dioscorea. dumetorum. However, these rates of moisture losses were higher than the 5.8 % observed in Dioscorea. dumetorum after 56 day (Medoua et al. 2005). The samples stored at cold temperature and packaged conditions (4 °C) retained a considerably higher amount of water, with values of 66.99 % for Chinese yam after 45 day. This result implied that only 0.97 % moisture was lost during storage. Those tubers had a fresh moisture appearance, without appearing shrunk or rotten. Analyses of variance indicated that storage conditions and storage time of the tubers significantly affected (P ≤ 0.05) the moisture content of Dioscorea opposita after harvest.
Table 1.
Changes in moisture, allantoin, starch, total sugars, reducing sugars, amylase activities and protein of Chinese yam tubers during storage at ambient conditions and at cold temperature and packaged conditions (n = 3)
| Storage period (days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 18 | 24 | 30 | 36 | 45 | |
| Moisture content (%) | ||||||||||
| Ambient conditions | 68.0 ± 0.00a | 65.1 ± 0.14b | 63.7 ± 0.52c | 61.6 ± 0.54g | 61.3 ± 0.53gh | 61.3 ± 0.58gh | 60.6 ± 0.42h | 58.3 ± 0.40d | 57.4 ± 0.43e | 56.5 ± 0.30f |
| 4 °C and packaged conditions | 68.0 ± 0.00a | 67.9 ± 0.02d** | 67.8 ± 0.00df** | 67.6 ± 0.03ef** | 67.5 ± 0.12e** | 67.5 ± 0.04e** | 67.2 ± 0.25b** | 67.1 ± 0.20bc** | 67.0 ± 0.11c** | 66.9 ± 0.12a** |
| Allantion content (mg/g) | ||||||||||
| Ambient conditions | 2.0 ± 0.12a | 3.4 ± 0.14fg | 5.4 ± 0.13b | 3.1 ± 0.18g | 3.6 ± 0.15fh | 6.0 ± 0.19c | 3.8 ± 0.22f | 4.2 ± 0.25d | 4.7 ± 0.16e | 4.7 ± 0.10e |
| 4 °C and packaged conditions | 2.0 ± 0.15a | 5.5 ± 0.19b** | 2.4 ± 0.22d** | 2.7 ± 0.25df* | 6.0 ± 0.20c** | 3.0 ± 0.14f** | 2.8 ± 0.25f** | 3.4 ± 0.18e** | 3.7 ± 0.22e** | 3.7 ± 0.14e** |
| Starch content (%) | ||||||||||
| Ambient conditions | 45.0 ± 1.60cb | 53.0 ± 1.20d | 56.9 ± 1.70e | 59.3 ± 1.50e | 58.0 ± 1.70e | 57.4 ± 1.90e | 53.9 ± 1.60d | 47.0 ± 1.20c | 43.8 ± 2.00b | 42.0 ± 1.30a |
| 4 °C and packaged conditions | 45.0 ± 1.20df | 46.0 ± 1.60fg** | 47.8 ± 1.00eg** | 49.8 ± 1.70e** | 47.4 ± 1.50ef** | 43.5 ± 1.50cd** | 41.7 ± 1.60c** | 36.8 ± 1.90b** | 34.7 ± 1.30ab** | 33.0 ± 1.00a** |
| Total sugars content (%) | ||||||||||
| Ambient conditions | 6.5 ± 0.29a | 8.1 ± 0.21b | 8.9 ± 0.29c | 9.3 ± 0.41cd | 9.4 ± 0.60cd | 9.5 ± 0.23cd | 9.5 ± 0.44d | 9.6 ± 0.33d | 9.7 ± 0.38d | 9.8 ± 0.40d |
| 4 °C and packaged conditions | 6.5 ± 0.29a | 12.0 ± 0.70b** | 12.9 ± 1.39bc** | 13.0 ± 1.25bc** | 13.5 ± 0.76cd** | 14.0 ± 0.45cd** | 14.1 ± 0.46cd** | 14.3 ± 0.73cd** | 14.6 ± 0.74d** | 14.9 ± 1.00d** |
| Reducing sugars content (%) | ||||||||||
| Ambient conditions | 1.5 ± 0.15a | 1.6 ± 0.19ab | 1.7 ± 0.14ad | 1.8 ± 0.12bd | 1.9 ± 0.16de | 2.0 ± 0.18e | 2.1 ± 0.17ce | 2.1 ± 0.14ce | 2.2 ± 0.15ce | 2.3 ± 0.10c |
| 4 °C and packaged conditions | 1.5 ± 0.15a | 2.6 ± 0.22b** | 3.3 ± 0.32c** | 3.6 ± 0.19d** | 3.9 ± 0.2df** | 4.2 ± 0.18fg** | 4.4 ± 0.21gh** | 4.5 ± 0.15eg** | 4.6 ± 0.17eh** | 4.7 ± 0.16e** |
| Amylase activities (mg·min−1·g−1) | ||||||||||
| Ambient conditions | 1.3 ± 0.10a | 1.5 ± 0.09b | 1.9 ± 0.12c | 1.9 ± 0.14c | 1.8 ± 0.15cd | 1.8 ± 0.14cd | 1.6 ± 0.13bd | 1.6 ± 0.15b | 1.6 ± 0.12ab | 1.6 ± 0.10a |
| 4 °C and packaged conditions | 1.3 ± 0.12a | 2.9 ± 0.09b** | 3.7 ± 0.20c** | 4.6 ± 0.23d** | 4.8 ± 0.26de** | 4.6 ± 0.22d** | 4.4 ± 0.21f** | 4.1 ± 0.20fg** | 3.9 ± 0.19cg** | 3.6 ± 0.16c** |
| Protein content (%) | ||||||||||
| Ambient conditions | 13.0 ± 0.20a | 13.5 ± 0.40ab | 14.0 ± 0.38bc | 14.5 ± 0.40cd | 15.0 ± 0.50d | 14.9 ± 0.34d | 14.8 ± 0.30d | 14.7 ± 0.36d | 14.6 ± 0.31d | 14.6 ± 0.21cd |
| 4 °C and packaged conditions | 13.0 ± 0.20a | 14.5 ± 0.37b** | 16.0 ± 0.45c** | 17.0 ± 0.50e** | 17.8 ± 0.53d** | 17.7 ± 0.42d** | 17.5 ± 0.39d** | 17.3 ± 0.47d** | 17.0 ± 0.30e** | 16.9 ± 0.34e** |
a–hDifferent letters in the same row mean significant differences at p < 0.05 as measured by Duncan’s multiple test
*Correlation is significant at the 0.05 level. ** Correlation is significant at the 0.01 level
Fig. 1.
Changes in moisture, allantoin, starch, total sugars, reducing sugars, amylase activities and protein of Chinese yam tubers during storage at ambient conditions and at cold temperature and packaged conditions.(■) at ambient conditions (10 °C to 18 °C); (▲) at cold temperature and packaged conditions (n = 3)
Changes in the allantoin content of Chinese yam tubers during storage
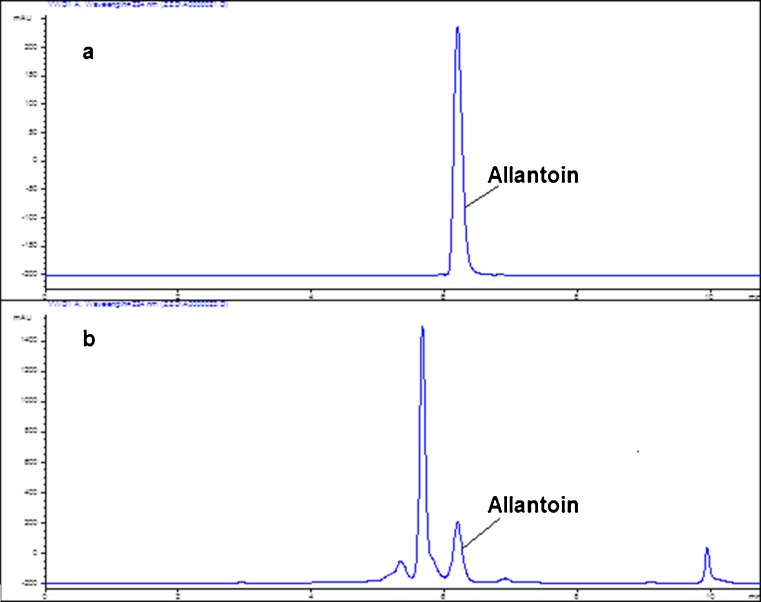
The high performance liquid chromatography HPLC chromatograms of allantoin standard and isolated from sample are exhibited in Fig. 2. Interesting changes in allantoin content after 45 day of storage are shown in Fig. 1. Two peaks appeared, and all values were higher than the initial value of 1.98 mg/g. The allantoin content increased, reaching the first peak (5.37 mg/g) after 6 day of storage under ambient temperature conditions, and then decreased to 3.12 mg/g after 3 day. The second peak of allantoin content appeared at the 18th d when the value reached 5.97 mg/g but did not increase further during the remaining storage period. After 6 day, the allantoin content dropped to 3.75 mg/g. However, the content increased again and remained at approximately 4.70 mg/g, larger than the post-harvest content. The changes during cold temperature and packaged storage conditions were similar to those at ambient temperature. The development of the first (5.49 mg/g) and second (5.98 mg/g) peaks advanced at/in 3 and 6 day, respectively. The allantoin content also increased slowly after the last drop. At cold temperature and packaged conditions, it remained stable at approximately 3.70 mg/g, which was slightly lower than that at ambient temperature.
Fig. 2.
a High Performance Liquid Chromatography chromatograms of allantoin standard at the concentration of 0.00324 mg/mL; retention time = 6.18 min. b High Performance Liquid Chromatography chromatograms of yam extract at the concentration of; retention time = 6.19 min
Roots are considered to be the main site of ureides synthesis (Thomas and Schrader 1981). As shown in Fig. 1, the allantoin content changed in volatility after 45 day of storage. At the end of the storage period, the content was obviously higher than that in postharvest samples. The continued accumulation of allantoin indicated that the synthesis and metabolism of allantoin were still active in yams after harvest. The trend was similar for refrigerated yams, although the allantoin content was lower than in yams stored at ambient temperature conditions. However, the refrigeration caused the earlier arrival of peaks. This result indicated that a low temperature affected the related enzyme activities, such as allantoinase and allantoicase, and then affected the synthesis and metabolism of allantoin in Chinese yam tubers. In conclusion, we can achieve different purposes by employing different storage methods. For example, the tubers can be stored under cold temperature and packaged conditions (4 °C) to obtain the largest content in the shorter time.
Changes in the starch content of Chinese yam tubers during storage
The trend of changes in the starch content of Chinese yam tubers is different from the results obtained by other teams (Medoua et al. 2007; Afoakwa and Sefa-Dedeh 2001). They found that the starch content decreased during storage. In this study, during the early days of storage, the starch content increased instead of exhibiting any signs of decrease in different storage conditions, even if the increase was short-lived. In fact, the increase developed in two phases: from 0th to 9th d and from 10th to 45th d. In the first phase, the starch content increased from 44.99 % to 59.28 % at ambient conditions and from 44.99 % to 49.79 % at cold temperature and packaged conditions. In the second phase, the content decreased rapidly from 59.28 % to 43.76 % at ambient conditions and from 49.79 % to 34.67 % at cold temperature and packaged conditions. At the end of the storage period, the starch content was comparatively lower in tubers stored at ambient temperature than in those stored at 4 °C. However, the low-temperature storage considerably increased the rate of starch loss compared with the rate observed in tubers stored under ambient conditions.
Changes in the total alcohol-soluble and reducing sugars content of Chinese yam tubers during storage
Different storage temperatures caused significant increases in both total alcohol-soluble and reducing sugar contents of the Chinese yam studied in this work. The rate of increase in total sugars was relatively higher at ambient conditions (6.49 % to 17.9 %) than that at cold temperature and packaged conditions (6.49 % to 9.81 %). The reducing sugars ranged from 1.45 % to 2.27 % at ambient conditions and from 1.45 % to 4.71 % at cold temperature and packaged conditions. Notably, the lower temperature storage of the tubers maximized the rate of increase in total sugars and reducing sugars during storage.
Changes in the amylase activities of Chinese yam tubers during storage
Changes in the amylase activities of Chinese yam tubers during storage are shown in Fig. 1. Prior to the occurrence of a slow decrease with storage time, a rapid increase occurred in the amylase activities of samples (i.e., from 1.24 mg/min/g to 1.89 mg/min/g) at ambient temperature conditions during the early days of storage (0 day to 9 day). The amylase activities of the samples began to decrease from 1.89 mg/min/g to 1.27 mg/min/g. The shift from an increase to a decrease is related to the shift in amylase inhibitors from a decrease to an increase, as observed by Medoua et al. (2007). Similar trends were observed at cold temperature and packaged conditions (4 °C). The amylase activities underwent a rapid increase from 1.24 mg/min/g to 4.77 mg/min/g and a gradual decrease from 4.77 mg/min/g to 3.63 mg/min/g. However, the amylase activities at 4 °C were significantly higher than those at ambient temperature.
A series of complicated transformation reactions occurred between starch and sugars (Sefa-Dedeh and Emmanuel 2002). The amylase activities increased rapidly, and starch hydrolyzed into sugars. Hence, the total and reducing sugar content increased greatly, whereas the starch content of tubers decreased. In the case of respiration and synthesis of cellulosic and hemicellulosic fibers from sugars, the total sugars and reducing sugars increased slowly. More significant changes were investigated with higher sugar content, lower starch content, and higher amylase activities at cold temperature and packaged conditions (4 °C). These results indicate that a low-temperature sweetening occurs in Chinese yam tubers, which has been reported in potato tubers stored at cold temperature and packaged conditions (Cottrell et al. 1993). In the case of potato tubers stored at low temperature conditions, starch hydrolyze and reducing sugars accumulate in a phenomenon known as low-temperature sweetening (Kumar 2009). When fried, reducing sugars in low-temperature sweetening tubers may react with free amino acids in edible oil (known as the Maillard reaction), which could produce brown and slightly bitter substances, and severely reduce the quality of processed products. Yam tubers and potato tubers are both rich in starch and sugars. Their similarity in composition may cause a similar phenomenon. The low-temperature sweetening may be observed in yam tubers stored at low temperature conditions. As a result, the effects of storage and the taste of Chinese yam tubers are affected.
Changes in the protein content of Chinese yam tubers during storage
Changes in the protein concentration in Chinese yam samples observed during their storage are exhibited in Fig. 1. Two phases occurred at ambient and cold temperature conditions: from 0th to 12th d and from 13th to 45th d. In the first phase, the protein content of samples at ambient temperature conditions increased from 13.02 % to 15.13 %. In the second phase, it slowly reduced to 14.55 % and remained constant at this level. These results are in agreement with the findings by Chou et al. (2006). This team observed that the crude protein content of Taiwanese yam tubers increased from 2.58 % to 2.70 %, 2.58 % to 2.68 %, and 2.58 % to 2.73 % at room temperature (20 °C ± 8 °C), 17 °C, and 10 °C, respectively, over a storage period of two months. These changes showed a similar trend but more marked at cold temperature and packaged conditions. In the first stage, the protein content increased from 13.12 % to 17.8 % and then slowly decreased to 16.89 %. The increase in the protein content of tubers was due to the increase in the content of enzymes, such as amylase, which may be attributed to the strong enzyme activity at the cold temperature and packaged conditions.
From the 9th to the 12th d of storage, the content of main nutrients such as sugars, protein, starch, and medicinal compositions markedly increased. The enzyme activity also increased. These results revealed that the metabolism remained vigorous after harvest, especially during the early period of storage, when the nutrient substances continued to be accumulated. After the enhancing, starch and protein contents, as well as enzyme activity, decreased, whereas total sugars and reducing sugars increased slowly. This phenomenon indicates that after-ripening occurs in Chinese yam tubers, an activity common in some fruits, vegetables, and seeds (Sanchez et al. 1993). The tuber is rich in nutrients and is the edible part of Chinese yams. The tubers cut from mature Chinese yam plants will transit from harvest maturity to edible maturity, after which the tuber can be used for food. The after-ripening period of Chinese yam tubers is approximately 9 day to 12 day after the tubers are stored under ambient temperature conditions (10 °C to 18 °C). Cold temperature and packaged conditions can slightly affect the length of the after-ripening period but can greatly affect the nutrient content and medicinal compositions.
Conclusions
Dioscorea opposita tubers underwent nutrient and medicinal composition changes during storage. Moisture rapidly decreases during storage, whereas total sugars, reducing sugars, and protein increase with the tuber storage duration. Starch and enzyme activity increase during the early days of storage and progressively decrease afterward. Allantoin changes in volatility during storage. More significant changes were observed at cold temperature and packaged conditions. In general, these results suggest that after-ripening of tubers positively affects their nutritional potential by a significant increase in nutrients during the early days of storage. Low-temperature sweetening greatly affects the tubers’ nutritional potential by a series of complicated changes between starch and sugars at cold temperature conditions (4 °C).
Acknowledgments
The authors would like to thank the referees for their valuable comments and suggestions. This work was supported by the National Natural Science Foundation of China (No. 30472148). We are also thankful to the research center of Tianjin University of Traditional Chinese Medicine for providing the use of experimental instruments and materials for a low charge.
References
- Afoakwa EO, Sefa-Dedeh S. Chemical composition and quality changes occurring in Dioscorea dumetorum pax tubers after harvest. Food Chemistry. 2001;75:85–91. doi: 10.1016/S0308-8146(01)00191-1. [DOI] [Google Scholar]
- Afoakwa EO, Sefa-Dedeh S. Changes in rheological properties and amylase activities of trifoliate yam, Dioscorea dumetorum, starch after harvest. Food Chemistry. 2002;77:285–291. doi: 10.1016/S0308-8146(01)00344-2. [DOI] [Google Scholar]
- Ahuja KK, Goyal GK (2012) Combined effect of vacuum packaging and refrigerated storage on the chemical quality of paneer tikka. Journal of Food Science and Technology. doi:10.1007/s13197-012-0688-x [DOI] [PMC free article] [PubMed]
- Alam T, Goyal GK. Effect of MAP on microbiological quality of Mozzarella cheese stored in different packages at 7 ± 1 °C. Journal of Food Science and Technology. 2011;48(1):120–123. doi: 10.1007/s13197-010-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 13. Arlington: Association of Official Analytical Chemists; 1980. [Google Scholar]
- Azene M, Workneh TS, Woldetsadik K (2011) Effect of packaging materials and storage environment on postharvest quality of papaya fruit. Journal of Food Science and Technology. doi:10.1007/s13197-011-0607-6 [DOI] [PMC free article] [PubMed]
- Bajwa U, Sandhu KS (2011) Effect of handling and processing on pesticide residues in food- a review. Journal of Food Science and Technology. doi:10.1007/s13197-011-0499-5 [DOI] [PMC free article] [PubMed]
- Bernfeld P. Amylase α and β. Methods in enzymology. NewYork: Academic Press Inc; 1955. pp. 149–154. [Google Scholar]
- Cheng XG, Nomura M, Sato T, Fujikake H, Ohyama T, Tajima S. Effect of exogenous NH4+-N Nsupplyon distribution of ureide content in various tissues of alfalfa plants, Medicago sativa. Soil Science and Plant Nutrition. 1999;45:921–927. doi: 10.1080/00380768.1999.10414341. [DOI] [Google Scholar]
- China Flora Editing Group . Flora Republicae Popularis Sinicae. Beijing: Science press; 2004. pp. 54–55. [Google Scholar]
- Chou ST, Chiang BH, Chung YC, Chen PC, Hsu CK. Effects of storage temperatures on the antioxidative activity and composition of yam. Food Chemistry. 2006;98:618–623. doi: 10.1016/j.foodchem.2005.06.039. [DOI] [Google Scholar]
- Cottrell JE, Duffus CM, Paterson L, Mackay GR, Allison MJ, Bain H. The effect of storage temperature on reducing sugar concentration and the activities of three amylolytic enzyme in tuber of the cultivated potato, Solanum Tuberosun L. Potato Research. 1993;36:107–117. doi: 10.1007/BF02358725. [DOI] [Google Scholar]
- Dhall RK, Sharma SR, Mahajan BVC (2011) Effect of shrink wrap packaging for maintaining quality of cucumber during storage. Journal of Food Science and Technology. doi:10.1007/s13197-011-0284-5 [DOI] [PMC free article] [PubMed]
- Efiuvwevwere BJQ, Nwachukwu E. Incidence of yam (Dioscorea rotundata Poir) rots, inoculation-induced quality changes, and control by chemical fungicides and modified atmospheres. Postharvest Biology and Technology. 1998;14:235–243. doi: 10.1016/S0925-5214(98)00038-6. [DOI] [Google Scholar]
- Fu YC, Ferng LHA, Huang PY. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chemistry. 2006;94:541–549. doi: 10.1016/j.foodchem.2004.12.006. [DOI] [Google Scholar]
- Hussain SA, Garg FC, Pal D (2012) Effect of different preservative treatments on the shelf-life of sorghum malt based fermented milk beverage. Journal of Food Science and Technology. doi:10.1007/s13197-012-0657-4 [DOI] [PMC free article] [PubMed]
- Jain SK, Verma RC, Murdia LK, Jain HK, Sharma GP. Optimization of process parameters for osmotic dehydration of papaya cubes. Journal of Food Science and Technology. 2011;48(2):211–217. doi: 10.1007/s13197-010-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Dhillon WS, Mahajan BVC (2011) Effect of different packaging materials and storage intervals on physical and biochemical characteristics of pear. Journal of Food Science and Technology. doi:10.1007/s13197-011-0317-0 [DOI] [PMC free article] [PubMed]
- Kudachikar VB, Kulkarni SG, Keshava Prakash MN. Effect of modified atmosphere packaging on quality and shelf life of ‘Robusta’ banana (Musa sp.) stored at low temperature. Journal of Food Science and Technology. 2011;48(3):319–324. doi: 10.1007/s13197-011-0238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D. Changes in antioxidant system of potato tuber during low temperature storage in relation to sweetening development. Journal of Food Science and Technology. 2009;46(4):392–394. [Google Scholar]
- Lane JH, Eynon L. Determination of sugars by fehling solution with methylene blue as an internal indicator. Journal of the Society Chemical India. 1923;42:32–34. doi: 10.1002/jctb.5000420208. [DOI] [Google Scholar]
- Lee HJ, Park HJ, Jeong JW, Kim D, Chinnan MS. Effect of electrolyzed water treatments on the quality of hand- and machine-peeled yams (Dioscorea spp.) during cold storage. Food Science and Technology-LEB. 2007;40:646–654. doi: 10.1016/j.lwt.2006.05.006. [DOI] [Google Scholar]
- Mahalingaiah L, Venateshaiah BV, Kulkarni, Jayaraj Rao K (2012) Studies on the effect of preservatives on physico-chemical microbiological and sensory quality of kunda. Journal of Food Science and Technology. doi:10.1007/s13197-011-0564-0 [DOI] [PMC free article] [PubMed]
- Majidi H, Minaei S, Almassi M, Mostofi Y (2012) Tomato quality in controlled atmosphere storage, modified atmosphere packaging and cold storage. Journal of Food Science and Technology. doi:10.1007/s13197-012-0721-0 [DOI] [PMC free article] [PubMed]
- Matsumoto T, Yatazawa M, Yamamoto Y. Distribution and change in the contents of allantoin and allantoic acid in developing nodulating and non-nodulating soybean plants. Plant & Cell Physiology. 1977;18:353–359. [Google Scholar]
- Medoua GN, Mbome IL, Agbor-Egbe T, Mbofung CMF. Study of the hard-to-cook property of stored yam tubers (Dioscorea dumetorum) and some determining bilchemical factors. Food Research International. 2005;38:143–149. doi: 10.1016/j.foodres.2004.09.005. [DOI] [Google Scholar]
- Medoua GN, Mbome IL, Agbor-Egbe T, Mbofung CMF. Antinutritional factors changes occurring in trifoliate yam (Dioscorea dumetorum) tubers after harvest. Food Chemistry. 2007;102:716–720. doi: 10.1016/j.foodchem.2006.06.005. [DOI] [Google Scholar]
- Mishra B, Khatkar BS, Garg MK, Wilson LA. Permeability of edible coatings. Journal of Food Science and Technology. 2010;47(1):109–113. doi: 10.1007/s13197-010-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S, Acholo M, McNamara N, Oliver R. Control of storage insects as a means of limiting yam tuber fungal rots. Journal of Stored Product Research. 2000;36:37–45. doi: 10.1016/S0022-474X(99)00025-9. [DOI] [Google Scholar]
- Nie GH, Zhou KF, Dong XH, Zhang C. Research advance of yam. Chinese Traditional and Herbal Drugs. 1993;24:158–160. [Google Scholar]
- Oliveira DM, Kwiatkowski A, Rosa CILF, Clemente E (2012) Refrigeration and edible coatings in blackberry (Rubus spp.) conservation. Journal of Food Science and Technology. doi:10.1007/s13197-011-0702-3 [DOI] [PMC free article] [PubMed]
- Omonigho SE, Ikenebomeh MJ. Effects of different preservative treatments on the chemical changes of pounded white yam (Dioscorea rotundata) in storage at 28 ± 2 °C. Food Chemistry. 2000;68:201–209. doi: 10.1016/S0308-8146(99)00183-1. [DOI] [Google Scholar]
- Oyebanji AO, Olayemi FF, Okoye WI. Varietal effect on spoilage of yam (Dioscorea rotundata) tubers during storage. Tropical Science. 2003;43:199–200. doi: 10.1002/ts.118. [DOI] [Google Scholar]
- Pisalkar PS, Jain NK, Jain SK. Osmo-air drying of aloe vera gel cubes. Journal of Food Science and Technology. 2011;28(2):183–189. doi: 10.1007/s13197-010-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha CR, Vijayalakshmi G (2011) Influence of processing parameters on the quality of soycurd (tofu). Journal of Food Science and Technology. doi:10.1007/s13197-011-0245-z [DOI] [PMC free article] [PubMed]
- Sahore DA, Nemlin GJ, Kamenan A. Changes in nutritional properties of yam (Dioscorea spp.), plantain (Musa spp.) and cassava (Manihot esculenta) during storage. Tropical Science. 2007;47(2):81–88. doi: 10.1002/ts.200. [DOI] [Google Scholar]
- Sanchez VM, Sundstrom FJ, McClure GN, Lang NS. Fruit maturity, storage and postharvest maturation treatments affect bell pepper (Capsicum annuum L.) seed quality. Scientia Horticulturae. 1993;54:191–201. doi: 10.1016/0304-4238(93)90087-7. [DOI] [Google Scholar]
- Sedmak JJ, Grossberg SE. A rapid, sensitive and versatile assay for protein using coomassie brilliant blue G250. Analytical Biochemistry. 2005;79:544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sefa-Dedeh S, Emmanuel OA. Biochemical and textural changes in trifoliate yam Dioscorea dumetorum tubers after harvest. Food Chemistry. 2002;79:27–40. doi: 10.1016/S0308-8146(02)00172-3. [DOI] [Google Scholar]
- Shakerardekani A, Karim R (2012) Effect of different types of plastic packaging films on the moisture and aflatoxin contents of pistachio nuts during storage. Journal of Food Science and Technology. doi:10.1007/s13197-012-0624-0 [DOI] [PMC free article] [PubMed]
- Shittu TA, (2011) Functional effects of dried okra powder on reconstituted dried yam flake and sensory properties of ojojo-a fried yam (Dioscorea alata L.) snack. Journal of Food Science and Technology. doi:10.1007/s13197-011-0513-y [DOI] [PMC free article] [PubMed]
- Stasiewicz M, Lipinski K, Cierach M (2012) Quality of meat products packaged and stored under vacuum and modified atmosphere conditions. Journal of Food Science and Technology. doi:10.1007/s13197-012-0682-3 [DOI] [PMC free article] [PubMed]
- Sudha S, Naik MK, Ajithkumar K (2011) An integrated approach for the reduction of aflatoxin contamination in chilli (Capsicum annuum L.). Journal of Food Science and Technology. doi:10.1007/s13197-011-0471-4 [DOI] [PMC free article] [PubMed]
- Thomas RJ, Schrader LE. Urede metabolism in higher plants. Phytochemistry. 1981;20:361–371. doi: 10.1016/S0031-9422(00)84147-3. [DOI] [Google Scholar]
- Wijewardane RMNA, Guleria SPS (2011) Effect of pre-cooling, fruit coating and packaging on postharvest quality of apple. Journal of Food Science and Technology. doi:10.1007/s13197-011-0322-3 [DOI] [PMC free article] [PubMed]
- Xu CJ, Chen WJ, Chen KS, Zhang SL. A simple method for determining the content of starch – iodine colorimety. Biotechnology. 1998;8(2):41–43. [Google Scholar]
- Yang DJ, Lin JT. Effects of different storage conditions on steroidal saponins in yam (Dioscorea pseudojaponica Yamamoto) tubers. Food Chemistry. 2008;110:670–677. doi: 10.1016/j.foodchem.2008.02.061. [DOI] [PubMed] [Google Scholar]
- Yuan SL. Research advances on chemical compositions and bioactivities of Dioscorea opposite Thumb. (Chinese yam) Food Research and Development. 2008;29(3):176–179. [Google Scholar]
- Zhang L, Bai B, Liu XH, Wang Y, Li MJ, Zhao DB. α–Glucosidase inhibitors from Chinese yam (Dioscorea opposita Thunb.) Food Chemistry. 2011;126:203–206. doi: 10.1016/j.foodchem.2010.10.100. [DOI] [Google Scholar]
- Zhou YF, Wu Y, Zhang YM, Yan YH. The manufacture and utilization of Chinese yam. Anhui Agriculture Science Bulletin. 2004;10:65–66. [Google Scholar]