Abstract
Changes in chemical composition, physical properties and antioxidant activities of Kapi were monitored during fermentation for 12 months. DPPH (2, 2-diphenyl-1-picryl hydrazyl), ABTS (2, 2 – azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging activity as well as ferric reducing antioxidant power (FRAP) gradually increased as the fermentation time increased, particularly during the first 8 months (P < 0.05). Thereafter, the decreases in DPPH and ABTS radical scavenging activities were observed (P < 0.05), whereas FRAP remained constant (P > 0.05). The continuous increases in ammonia nitrogen, formaldehyde nitrogen and amino nitrogen contents were noticeable within the first 8 months (P < 0.05), indicating the formation of peptides and free amino acids via the hydrolysis of protein by both microbial and indigenous proteases. Browning intensity most likely caused by the formation of Maillard reaction products (MRPs) were concomitantly observed throughout fermentation, as evidenced by the decreases in lightness (L*-value), but the increases in redness (a*-value) and yellowness (b*-value). Low level of thiobarbituric acid reactive substances in Kapi was found during 12 months. Antioxidant activities of Kapi were more likely governed by the low molecular weight peptides, amino acids as well as Maillard reaction products generated during fermentation.
Keywords: Antioxidant activities, Peptides, Amino acid, Fermentation time, Fermented shrimp paste, Kapi
Introduction
Kapi, a Thai traditional fermented shrimp paste, is generally consumed as a condiment and widely used as a crucial ingredient throughout the south-east Asian region due to its taste and flavour. Kapi is normally produced by fermenting small shrimp (Acetes vulgaris) or krill (Mesopodopsis oreintalis) with solar salt at a ratio of 5:1 (shrimp: salt, w/w). The mixture is sun-dried and thoroughly ground before being compacted in a container usually earthen jar. Fermentation of Kapi is generally taken place until the typical aroma is developed and the fermentation time varies with each manufacturer (Phithakpol 1993). Enzymatic hydrolysis of fish yielded a large amount of short chain peptide and amino acid (Benjakul et al. 2009; Rajapakse et al. 2005), thereby the disruption of the native structure resulted in the unfolding and exposing of active amino acid residues and patches which were capable of reacting with oxidants (Sun et al. 2011). The hydrolysis of peptides and amino acids during fermentation was associated with antioxidant activity (Sachindra and Bhaskar 2008). Generally, peptides with bioactivity could be liberated by both indigenous and microbial enzymes (Tungkawachara et al. 2003; Xu et al. 2008; Choi et al. 2008; Chang et al. 2009). With increased fermentation, the degree of hydrolysis and amino nitrogen content of fish sauce increased (Tungkawachara et al. 2003; Jiang et al. 2007). Fermented fish products had potential antioxidant activities in favor of substantial amount of peptides and amino acids generated throughout fermentation from both endogenous and exogenous enzymes (Rajapakse et al. 2005; Yin et al. 2005). Recently, it has been reported that fermented shrimp and krill products exhibited strong antioxidant activities (Faithong et al. 2010).
Kapi, commercially produced with 3–6 months of fermentation, possessed the high antioxidant activities, compared with that of other fermented shrimp products. Additionally, antioxidative peptides and amino acids found in Kapi were stable over wide pH (2–11) and temperature (30–100 °C) range (Faithong et al. 2010). Apart from the raw materials used, processing and formula exploited and fermentation time might affect the quality of the product. Fermentation not only causes the hydrolysis of protein, but also induces the changes in chemical composition as well as physical properties of fish product such as Kapi. Additionally, antioxidative peptides could be produced during fermentation. Nevertheless, a little information regarding chemical and physical properties as well as antioxidant activities of Kapi during fermentation has been reported. Therefore, the objective of this study was to investigate the changes in Kapi during 12 months of fermentation.
Materials and methods
Chemicals
2,2 – azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picryl hydrazyl (DPPH) and 2,4,6- trinitrobenzenesulfonic acid (TNBS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Potassium persulfate, 2,4,6-tripyridyl-s-triazine(TPTZ), thiobarbituric acid (TBA), ferric chloride hexahydrate, acrylamide, N,N,N′,N′- tetramethylethylenediamine (TEMED) and bis-acrylamide were procured from Fluka Chemical Co. (Buchs, Switzerland). Sodium sulfite and ammonium thiocyanate were obtained from Riedel-deHaen (Seelze, Germany). Ethanol, methanol and trichloroacetic acid were procured from Merck (Darmstadt, Germany).
Preparation of Kapi
Shrimp (Acetes vulgaris) were obtained from the lake of Songkhla, the Gulf of Thailand in January and February 2010 and placed in ice with an ice-to-shrimp ratio of 2:1 (w/w). Upon arrival, shrimp were mixed with ground solar salt at a ratio of 5:1 (w/w). The mixture was kept overnight in plastic woven basket. The exudates were drained continuously through the basket in order to lower moisture content. The mixture was then sun-dried to obtain a moisture content of 50–55 %. Thereafter, the mixture obtained was transferred into a container, covered with the plastic sheet and incubated at room temperature for 7 days. The mixture was then blended thoroughly with hammer mill and transferred into a 2 l earthen jar with the volume up to the brim. The paste was compacted in the jar and the jar was then covered entirely with a polyethylene sheet. The paste contained 47.91 % moisture, 18.46 % protein, 0.97 % fat, 7.20 % carbohydrate, 25.46 % ash as analyzed by AOAC method (1999) with analytical method No. of 35.1.13, 35.1.14, 35.1.25 and 35.1.15, respectively. Salt content in the paste determined by AOAC method (No. 35.1.18) was 20.20 % (w/w). pH was determined using a pH meter equipped with reference electrode (Sartorius, Germany). Kapi was left for fermentation at room temperature (28–35 °C) for 12 months. The sample was collected at months 1, 2, 3, 4, 6, 8, 10 and 12. The collected sample were placed in a polyethylene bag, sealed and stored at −20 °C before analyses.
Chemical analysis
Proximate composition analysis
Moisture, fat, protein and ash contents were determined as per the AOAC method with the analytical number of 35.1.13, 35.1.25, 35.1.14 and 35.1.15, respectively (AOAC 1999). Moisture content was determined by drying the samples at 105 °C until the constant weight was obtained. To measure ash content, the samples were incinerated at 550 °C to pyrolize all organic substances. Fat content was measured by Soxhlet apparatus, while protein content was determined by the micro Kjeldahl method using a conversion factor of 6.25.
Salt content
Salt content in a sample was measured by the method of AOAC (1999) with the analytical number of 35.1.18. Samples (2 g) were mixed with 10 ml of 0.1 N AgNO3 and 10 ml of conc. HNO3. The mixture was boiled gently on a hot plate until all samples except AgCl2 were dissolved (usually 10 min.). The mixture was then cooled to room temperature using running water. Then, 5 ml of ferric alum indicator were added. The mixture was titrated with standardized 0.1 N KSCN until the solution became permanent light brown. The percentage of salt was then calculated and expressed as %(w/w).
Ammonia nitrogen, formaldehyde nitrogen and amino nitrogen contents
Ammonia nitrogen, formaldehyde nitrogen and amino nitrogen contents were determined as described by the Thai Industrial Standard (1992).
Ammonia nitrogen content was determined by the distillation method. Sample (4 g) was mixed with 3 g of MgO and 100 ml of distilled water. The mixture was subjected to distillation apparatus and distilled to release volatile nitrogen into 50 ml of 4 % (w/v) boric acid containing mixed indicator [0.125 g methyl red and 0.082 g bromocresol green in 95 % alcohol (100 ml) and 0.1 % methylene blue in distilled water with ratio of 5:1(v/v)]. The distillate was finally titrated with 0.05 M H2SO4 until the end-point was obtained as indicated by the change of color from green to grey. Ammonia nitrogen content was calculated as follows:
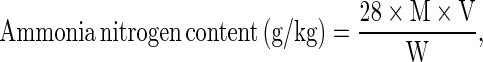 |
where M is the concentration of H2SO4 (M); V is the volume of H2SO4 (ml); and W is the weight of sample (g).
To determine formaldehyde nitrogen content, sample (0.5 g) was mixed with 10 ml of distilled water. The mixture was homogenised at a speed of 11,000 rpm for 2 min using a homogenizer (Polytron, Kinematica, Switzerland). The mixture was then neutralized to pH 7 with 0.1 M NaOH. Ten ml of formaldehyde solution [38 % (v/v), pH 9.0] was then added to the neutralized sample. Titration was conducted with 0.1 M NaOH until the pH of 9.0 was obtained. Formaldehyde nitrogen content was calculated as follows:
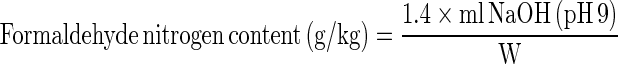 |
where W is the weight of sample (g).
Amino nitrogen content was calculated using the following formula:
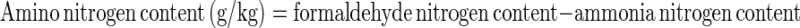 |
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of Kapi were analysed using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) according to the method of Laemmli (1970) using 12 % separating gel and 4 % stacking gel. Kapi samples were solubilised using 5 % SDS and homogenised for 1 min at a speed of 10,000 rpm. The homogenates were then incubated at 85 °C for 30 min. The mixtures were centrifuged at 7,500 xg for 10 min. The supernatants were mixed with sample buffer (0.5 M Tris–HCl, pH 6.8 containing 4 % (w/v) SDS, 20 % (v/v) glycerol and 10 % (v/v) β-ME) at the ratio of 1:1 (v/v). Fifteen μg of protein were loaded onto the gel. Electrophoresis was conducted using the Protean II xi vertical cell and the 1,000 powerpac (Bio-Rad laboratories, Hercules, CA) at a constant current of 15 mA. Gels were stained using 0.05 % Coomassie Brilliant Blue R250 dissolved in 15 % (v/v) methanol and 5 % (v/v) acetic acid and de-stained with 30 % (v/v) methanol and 10 % (v/v) acetic acid.
Determination of thiobarbituric acid reactive substances (TBARS)
TBARS was determined according to the method of Pearson (1970) with slight modification. Sample (20 g) was homogenised with 50 ml of distilled water using a homogenizer at speed 10,000 rpm for 2 min. Homogenate was transferred to a 500 ml Kjeldahl flask which was connected to a distillation unit. Before heating, 2.5 ml of 4 N HCl and 47.5 ml distilled water were added. Distillation was conducted until the volume of distillate reached 50 ml (within 10 min). Five milliliter of distillate were pipetted into a test tube and 5 ml of TBA reagent (0.2883 g of thiobarbituric acid in 90 % acetic acid) were added. The mixture was subjected to heating in boiling water for 35 min. The mixture was centrifuged at speed of 3,000 rpm for 10 min. Thereafter, the absorbance at 532 nm of the supernatant was read against a blank using a spectrophotometer (UV1701, Shimadzu, Kyoto, Japan). A calibration curve was constructed using malondialdehyde as a standard. TBARS content was expressed as milligrams MDA per Kg of sample.
Physical analyses
Nonenzymatic browning and fluorescence intensity
Nonenzymatic browning of samples was determined by measuring melanoidin pigment formation following the method of Klomklao et al. (2006) with a slight modification. One gram of sample was mixed with 50 ml of 50 % (v/v) ethanol and the mixture was stirred for 1 h at 25 °C. The sample was then centrifuged at 7,700 × g for 30 min. The absorbance of supernatant was read at 420 nm. A420 was used as an index of browning intensity. The fluorescence intensity was measured at an excitation wavelength of 347 nm and emission wavelength of 415 nm using a RF-1501 spectrofluorophotometer (Shimadzu Co., Kyoto, Japan).
Colour determination
Colour was measured using a Hunter Lab Miniscan XE plus colorimeter (Reston, VA, USA). Results were expressed according to the CIELAB system with reference to illuminant D65 and a visual angle of 10°. The parameters determined were L* (lightness), a* (redness/greenness) and b* (yellowness/blueness).
Determination of antioxidant activity of soluble fraction
Preparation of soluble fraction
One gram of Kapi was mixed with distilled water (100 ml) and the mixture was homogenised at a speed of 10,000 rpm for 2 min using a homogenizer. The homogenate was stirred at room temperature for 30 min. The mixture was then centrifuged at 3,000xg for 10 min at room temperature using a Beckman Coulter Model Avanti J-E refrigerated centrifuge (Beckman Coulter, CA, USA) to remove undissolved debris. The supernatant was used for determination of antioxidative activity.
DPPH radical scavenging activity
DPPH radical scavenging activity was determined by DPPH assay as described by Faithong et al. (2010). Sample (1.5 ml) was added with 1.5 ml of 0.15 mM 2,2-diphenyl-1-picryl hydrazyl (DPPH) in 16.3 M ethanol. The mixture was mixed vigorously and allowed to stand at room temperature in dark for 30 min. The absorbance of the resulting solution was measured at 517 nm using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). The blank was prepared in the same manner, except the distilled water was used instead of the sample. A standard curve was prepared using Trolox in the range of 10–60 μM. The activity was expressed as μmol Trolox equivalents (TE)/g sample.
ABTS radical scavenging activity
ABTS radical scavenging activity was determined by ABTS assay as per the method of Faithong et al. (2010). The stock solutions included 7.4 mM ABTS solution and 2.6 mM potassium persulphate solution. The working solution was prepared by mixing the two stock solutions in equal quantities. The mixture was allowed to react for 12 h at room temperature in dark. The solution was then diluted by mixing 1 ml of ABTS solution with 50 ml of methanol in order to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using a spectrophotometer. Fresh ABTS solution was prepared for each assay. Sample (150 μl) was mixed with 2,850 μl of ABTS solution and the mixture was left at room temperature for 2 h in dark. The absorbance was then measured at 734 nm using a spectrophotometer. A standard curve of Trolox ranging from 50 to 600 μM was prepared. The activity was expressed as μmol Trolox equivalents (TE)/g sample.
FRAP (Ferric reducing antioxidant power)
FRAP was assayed as per the method of Benzie and Strain (1996). Stock solutions included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3. 6 H2O. A working solution was prepared freshly by mixing 25 ml of acetate buffer (pH 3.6), 2.5 ml of TPTZ solution and 2.5 ml of FeCl3.6 H2O solution. The mixed solution was incubated at 37 °C for 30 min and was referred to as FRAP solution. A sample (150 μl) was mixed with 2,850 μl of FRAP solution and kept for 30 min in dark. The ferrous tripyridyltriazine complex (coloured product) was measured by reading the absorbance at 593 nm. The standard curve was prepared using Trolox ranging from 50 to 600 μM. The activity was expressed as μmol Trolox equivalents (TE)/g sample.
Statistical analysis
The experiments were run using three different lots of samples. All analyses were performed in triplicate. The data was subjected to Analysis of Variance (ANOVA) and the differences between means were evaluated by Duncan’s Multiple Range Test (Steel and Torrie 1980). Statistical package program (SPSS version 14, SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results and discussion
Changes in chemical composition of Kapi during fermentation
Ammonia, formaldehyde and amino nitrogen contents
Changes in ammonia, formaldehyde and amino nitrogen contents during fermentation of Kapi for 12 months are shown in Fig. 1. Ammonia and formaldehyde nitrogen contents of Kapi increased with increasing fermentation time up to 8 months (P < 0.05). Thereafter, both nitrogen contents remained constant until the end of fermentation. Formaldehyde nitrogen content has been used to measure the degree of protein hydrolysis (Klomklao et al. 2006). Generally, the increases in formal nitrogen content were obtained with increasing fermentation time of fish fermented products (Tungkawachara et al. 2003; Klomklao et al. 2006; Jiang et al. 2007; Xu et al. 2008). The results indicated that the degradation of protein took place continuously during fermentation as evidenced by the increase in formaldehyde nitrogen content. The decomposition or deamidation of nitrogenous compound also occurred during fermentation as indicated by the increase in ammonia nitrogen content. Prolonged fermentation resulted in the breakdown of amino acids and conversion of nitrogen compounds into ammonia by both metabolic activity of microorganisms and enzymatic decomposition (Xu et al. 2008). The combined effect of autolysis by indigenous proteases and microbial degradation of fish muscle led to substantial increase in peptides (Yin et al. 2005). After 8 months of fermentation, protease might be inactivated or hydrolysed proteins could not serve as the substrate for further hydrolysis. Therefore, the fermentation time had the impact on protein hydrolysis as well as decomposition.
Fig. 1.
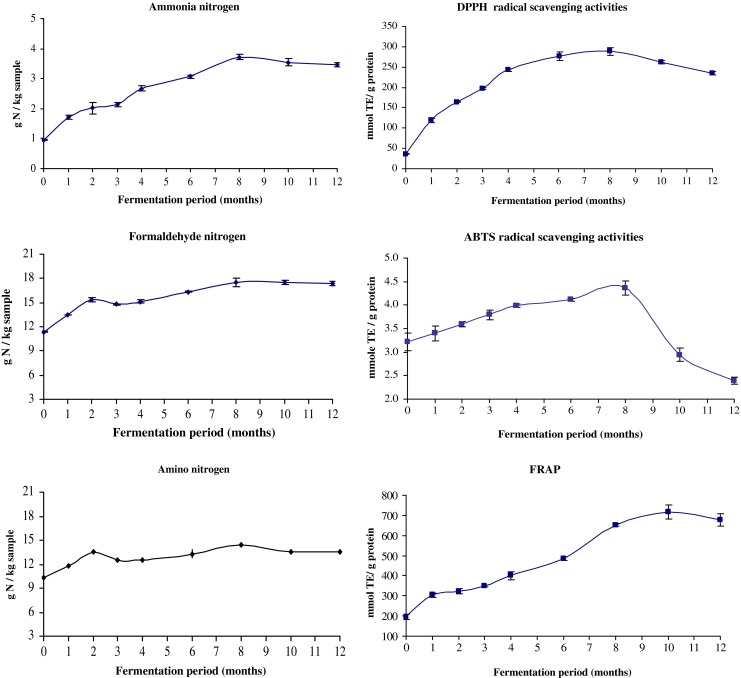
Chemical changes in Kapi during fermentation, (n = 3)
Changes in amino nitrogen content in Kapi at various time of fermentation are depicted in Fig. 1. A similar result was found, compared with that of ammonia and formaldehyde nitrogen contents. The results suggested that the fermentation of Kapi resulted in considerable increases in amino acids. Similar results were also found during hydrolysis of fish and fish products including minced mackerel fermented with LAB (Yin et al. 2005), and fish viscera during fish sauce fermentation (Klomklao et al. 2006). Enzymatic fermentation of small shrimp mediated by indigenous proteinases yields the short chain peptide and free amino acid (Steinkraus 2002). During fermentations, myosin heavy chain and actin in Kapi underwent degradation completely and the low molecular weight proteins, less than 20 kDa, were formed (Faithong et al. 2010). Those small peptide or free amino acids might contribute to the taste and flavour of Kapi. The cleavage of proteins could yield free amino groups, which could undergo glycation with carbonyl compounds via Maillard reaction (Lertittikul et al. 2007). Increasing breakdown of protein indicated by the increase in formaldehyde nitrogen and amino nitrogen contents (Fig. 1.) was in accordance with the browning intensity developed. Since fermentation of Kapi was taken place in sunlight with the temperature ranging from 28 to 35 °C, such a high temperature could enhance the Maillard reaction (Lertittikul et al. 2007).
Changes in antioxidant activities
Changes in antioxidant activities of the soluble fraction of Kapi obtained at various fermentation times as tested by DPPH, ABTS radical scavenging activity and FRAP are illustrated in Fig. 1. DPPH, ABTS radical scavenging activities increased continuously up to 8 months, followed by a slight and sharp decrease in DPPH and ABTS radical scavenging activity, respectively, until 12 months of fermentation. The decreases in both DPPH and ABTS radical scavenging activities after 8 months of fermentation might be due to the changes in antioxidative peptides generated within the first 8 months. Further hydrolysis or polymerisation of those peptides might lead to the decrease in their antioxidant activities. The decrease in DPPH radical scavenging activities after 8 months was coincidental with the decrease in browning intensity (Table 1), suggesting that Maillard reaction final brown products were partially involved in antioxidant activity. It was noted that ABTS radical scavenging activity markedly decreased after 8 months of fermentation. For FRAP, the continuous increase was observed up to 10 months (P < 0.05). The differences in the changes in activities determined by different assays after 8 months suggested that the peptides with different modes of action were present in Kapi. Peptide prevalently generated might undergo cross-linking via Maillard reaction, thereby losing its activity. ABTS radical scavenging assay can determine both hydrophilic and lipophilic antioxidants (Sun and Tanumihardjo 2007). Low molecular weight peptides and amino acids generated, especially during months 10–12 might have different modes of action or lost in activity differently. The hydrolysis combined with LAB fermentation led to the production of substantial active peptides which could interact with free radical and terminate the chain reaction of auto-oxidation (Yin et al. 2005). Our previous study revealed antioxidant activities including DPPH, ABTS radical scavenging activity and FRAP of commercially available Kapi (Faithong et al. 2010). Hydrolysis was progressed throughout the prolonged fermentation, leading to the accumulation of hydrolysed peptides and amino acids. Low molecular weight peptides and amino acids have been reported to possess antioxidant activity (Faithong et al. 2010; Benjakul et al. 2009; Binsan et al. 2008; Sachindra and Bhaskar 2008; Rajapakse et al. 2005)
Table 1.
Thiobarbituric acid value (TBA), fluorescence intensity, browning intensity (A 420 nm) and color of Kapi during fermentation of 12 months
| Month | TBA (mg MDA/kg sample) | Fluorescence intensity | Browning intensity (A 420 nm) | Color | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| 0 | 0.21 ± 0.01*, a # | 503.8 ± 50.06 a | 0.109 ± 0.00 h | 49.3 ± 0.22 a | 9.8 ± 0.15 d | 12.5 ± 0.41 d |
| 1 | 0.18 ± 0.02 b | 511.7 ± 59.52 a | 0.164 ± 0.00 g | 42.7 ± 0.52 b | 11.1 ± 0.19 c | 14.4 ± 1.08 c |
| 2 | 0.13 ± 0.01 c | 335.5 ± 28.87 b | 0.185 ± 0.00 g | 40.3 ± 0.35 c | 11.3 ± 0.13 b | 14.3 ± 0.21 c |
| 3 | 0.13 ± 0.00 c | 326.2 ± 15.17 b | 0.238 ± 0.01 f | 39.4 ± 0.15 d | 11.3 ± 0.16 bc | 14.8 ± 0.33 c |
| 4 | 0.12 ± 0.02 d | 312.9 ± 13.37 c | 0.321 ± 0.00 e | 36.7 ± 0.47 e | 11.6 ± 0.11 a | 16.5 ± 0.21 b |
| 6 | 0.14 ± 0.01 c | 256.9 ± 5.94 d | 0.459 ± 0.01 d | 35.4 ± 0.14 f | 11.4 ± 0.11 a | 16.4 ± 0.31 b |
| 8 | 0.15 ± 0.01 c | 135.5 ± 13.97 e | 0.766 ± 0.01 a | 32.9 ± 0.36 g | 11.3 ± 0.16 a | 16.9 ± 0.49 b |
| 10 | 0.16 ± 0.01 c | 156.2 ± 12.99 e | 0.724 ± 0.01 b | 29.7 ± 0.24 h | 11.7 ± 0.21 a | 17.7 ± 0.34 a |
| 12 | 0.14 ± 0.02 c | 152.4 ± 12.38 e | 0.661 ± 0.02 c | 29.5 ± 0.27 h | 11.1 ± 0.23 c | 18.2 ± 0.24 a |
* Mean ± SD (n = 3)
# Different lower case superscripts in the same column indicate the significant difference (p < 0.05)
Protein patterns
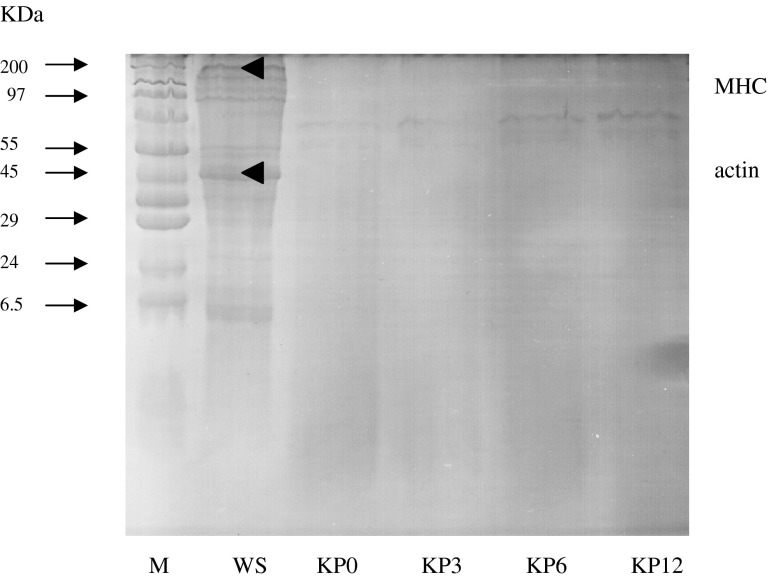
After prior salting and drying for a total of 7 days (KP0), the degradation of proteins was pronounced, as evidenced by the almost complete disappearance of myosin heavy chain (MHC) and actin (Fig. 2). Other muscle proteins were also degraded to a high extent. For the whole shrimp (WS), it was found that actin was the dominant protein. This was plausible due to the susceptibility of MHC after capture and handling. It was reported that actin in fish muscle was more resistant to autolysis than MHC (Benjakul et al. 1997). During the subsequent fermentation of 12 months, no marked changes in protein pattern were observed. Thus, the hydrolysis of proteins took place mainly during salting/drying. However, further hydrolysis still occurred to small degree during the fermentation. Those peptides or free amino acids could have served as the antioxidative components and underwent various reactions during fermentation, especially browning reaction.
Fig. 2.
SDS-PAGE pattern of proteins of whole shrimp (Acetes vulgaris) and Kapi at different fermentation times. M: Molecular weight standard; WS: whole shrimp; KP0, KP3, KP6 and KP12: Kapi obtained at 0, 3, 6 and 12 month of fermentation, respectively
TBARS
Thiobarbituric acid reactive substances (TBARS) of Kapi ranged from 0.12 to 0.21 mg MDA/kg sample during fermentation of 12 months (Table 1). Due to the low fat content in raw material (0.52 % w/w), autoxidation occurred at the low extent as indicated by relatively low level of TBARS value. A gradual decrease in TBARS value was found during the first 2 months of fermentation (P < 0.05). Thereafter, no differences in TBARS value were observed until the end of fermentation, except for month 4, when the lowest TBARS value was obtained. Malondialdehyde, the secondary products from autoxidation, is generally produced during the propagation stage of lipid oxidation. This compound could also act as a reactant for the Maillard reaction (Fernandez et al. 1997). As a consequence, TBARS in the sample was gradually decreased. MRPs have been reported to be capable of retarding lipid oxidation of many food systems (Zamora and Hidalgo 2005). Moreover, the proliferation of short chain peptides and amino acids generated from protein hydrolysis showed potential effects on inhibition of lipid oxidation via scavenging free radical and reduction of hydroperoxide (Elias et al. 2008).
Changes in physical properties of Kapi during fermentation
Browning intensity and fluorescence intensity
Continuous decrease in fluorescence intensity of Kapi was observed during the first 8 months (P < 0.05) and remained unchanged during 8 and 12 months of fermentation (P > 0.05), as shown in Table 1. Fluorescence intensity has been used to monitor the occurrence of intermediate products, which subsequently undergo polymerization to form the brown pigments (Morales and Perez 2001). Concomitantly, the browning intensity (A420) of Kapi increased continuously with increasing fermentation time (Table 1). Melanoidin generated during fermentation might play an important role in browning (Zamora and Hidalgo 2005). Maillard reaction was found to be responsible for the brown colour in fish sauce (Tungkawachara et al. 2003; Klomklao et al. 2006). Therefore, prolonged fermentation resulted in substantial increases in browning intensity, thereby influencing the colour development in Kapi. However, A420 tended to decrease during 10–12 months. During extended fermentation, Maillard reaction products might interact with proteins and other compounds, leading to the formation of flavour and odour. The results were in accordance with the decreases in antioxidant activities during month 10 and 12 (Fig. 1).
Colour
Changes in colour of Kapi during fermentation of 12 months are shown in Table 1. Lightness (L*) of Kapi decreased throughout fermentation of 12 months (P < 0.05). On the other hand, b*-value gradually increased with increasing fermentation time (P < 0.05). For a*-value, the highest value was obtained during 4–10 months of fermentation (P < 0.05), suggesting that a slight increase in redness occurred. The results indicated that the colour of Kapi was developed extensively as fermentation time increased. During extended fermentation, the autolysis might cause the increased release of carotenoids from carotenoprotein. Astaxanthin, protein-pigment complexes in shrimp, has a red-orange in colour via denaturation of protein due to the separation of pigment from protein moiety (Schiedt et al. 1993). Processing as well as the fermentation of Kapi causing the degradation of protein might enhance the progressive release of certain pigments. As a result, Kapi tended to possess the increased redness. Furthermore, the decrease in lightness was in agreement with the increased browning index (A420) (Table 1). When Maillard reaction occurred, Kapi mostly turned to reddish brown in colour.
Correlation between antioxidant activities, nitrogen content and browning intensity of Kapi during fermentation
DPPH radical scavenging activity and FRAP of water soluble fraction in Kapi showed the close correlation with ammonia and formaldehyde nitrogen contents (r = 0.941, 0.861 and r = 0.92, 0.749, respectively) as shown in Table 2. Fermentation of Kapi for 12 months brought about the considerable amount of water soluble active peptides, which were capable of quenching free radicals as evidenced by their high correlation. It was notable that there were lower correlations between DPPH radical scavenging activity or FRAP and amino nitrogen content (r = 0.652 and 0.407, respectively). However, no correlation between ABTS radical scavenging activity with nitrogen content and browning intensity were noticeable (Table 2). Within the first 8 months of fermentation, the degradation took place progressively in Kapi as indicated by the increases in formaldehyde nitrogen and amino nitrogen contents (Fig. 1). The concomitant generation of antioxidative peptides with both DPPH and ABTS radical scavenging activities was found. However, after 8 months, the sharp decrease in ABTS radical scavenging activity was obtained and this more likely resulted in the negligible correlation between ABTS radical scavenging activity and nitrogen content or browning. Browning intensity correlated with DPPH radical scavenging activity (r = 0.818) and FRAP (r = 0.965) (Table 2). Fluorescence intensity has been used as an effective index to follow the formation of MRPs (Morales and Perez 2001). Since the correlation between browning intensity and DPPH radical scavenging activity and FRAP was established, browning of Kapi was more likely related with antioxidant activity in Kapi. The result was in accordance with Peralta et al. (2008) who reported that the increased antioxidant ability of Philippines salt-fermented shrimp paste with prolonged fermentation was related to the formation of Maillard reaction products. Antioxidant activities of Kapi including DPPH, ABTS radical scavenging activity and FRAP seemed to be governed by the formation of a low molecular weight of peptides, amino acids, and Maillard reaction products.
Table 2.
Correlation between antioxidant activities, nitrogen content, browning intensity (A 420) and fluorescence intensity (FI) of Kapi during 12 months of fermentation
| Antioxidant activities | Ammonia nitrogen content | Formaldehyde nitrogen content | Amino nitrogen content | Browning intensity (A 420) | Fluorescence intensity |
|---|---|---|---|---|---|
| DPPH radical scavenging activities | 0.941a | 0.861a | 0.652a | 0.818a | -0.784a |
| ABTS radical scavenging activities | 0.020 | −0.118 | −0.076 | −0.033 | −0.517a |
| FRAP | 0.920a | 0.749a | 0.407b | 0.965a | −0.456b |
a, bsignificant at 0.01 and 0.05 level, respectively
Conclusions
Naturally prolonged fermentation of Kapi, salt fermented shrimp paste, could enhance antioxidant activity throughout the first 8 months. The cleavage of protein led to the accumulation of short chain peptides and amino acids as well as the formation of Maillard reaction products, which were most likely responsible for enhancing antioxidant activities. However, the excessive fermentation brought about the decreases in antioxidant activities. Additionally, the storage condition and packaging that may affect the quality of this product should be further studied.
Acknowledgments
This study was supported by a grant from the Research and Development Office, Prince of Songkla University. The authors would like to express their sincere thanks to Lance D. Terry for language revision.
References
- AOAC (1999) Official methods of analytical chemistry, 16th edn. Arlington: The Association of Official Analytical Chemists, Washington DC
- Benjakul S, Seymour TA, Morrissey MT, An H. Physicochemical changes in Pacific Whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Benjakul S, Binsan W, Visessanguan W, Osako K, Tanaka M. Effects of flavourzymes on yield and some biological activities of Mungoong, an extract paste from the cepholothorax of white shrimp. J Food Sci. 2009;74:S73–S80. doi: 10.1111/j.1750-3841.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [PubMed] [Google Scholar]
- Chang CT, Hsu CK, Chou ST, Chen YC, Huang FS, Chung YC. Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int J Food Sci Technol. 2009;44:799–806. doi: 10.1111/j.1365-2621.2009.01907.x. [DOI] [Google Scholar]
- Choi HK, Lim YS, Kim YS, Park SY, Lee CH, Hwang KW, Kwon DY. Free-radical-scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various times. Food Chem. 2008;106:564–568. doi: 10.1016/j.foodchem.2007.06.024. [DOI] [Google Scholar]
- Elias RJ, Kellerby S, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- Faithong N, Benjakul S, Phatcharat S, Binsan W. Chemical composition and antioxidative activity of Thai traditional fermented shrimp and krill products. Food Chem. 2010;119:133–140. doi: 10.1016/j.foodchem.2009.06.056. [DOI] [Google Scholar]
- Fernandez J, Alvarez JAP, Lopez JAF. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59:345–353. doi: 10.1016/S0308-8146(96)00114-8. [DOI] [Google Scholar]
- Jiang JJ, Zeng QX, Zhu ZW, Zhang LY. Chemical and sensory changes associated Yu-lu fermentation process – a traditional Chinese fish sauce. Food Chem. 2007;104:1629–1634. doi: 10.1016/j.foodchem.2007.03.024. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Visessanguan W, Kishimura H, Simpson B. Effects of the addition of spleen of skipjack tuna (Katsuwonus pelamis) on the liquefaction and characteristics of fish sauce made from sardine (Sardinella gibbosa) Food Chem. 2006;98:440–452. doi: 10.1016/j.foodchem.2005.06.013. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lertittikul W, Benjakul S, Tanaka M. Characteristic and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chem. 2007;100:669–677. doi: 10.1016/j.foodchem.2005.09.085. [DOI] [Google Scholar]
- Morales FJ, Perez SJ. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72:119–125. doi: 10.1016/S0308-8146(00)00239-9. [DOI] [Google Scholar]
- Pearson D. The chemical analysis of foods. 6. London: J&A Churchill; 1970. [Google Scholar]
- Peralta EM, Hatate H, Kawabe D, Kuwahara R, Wakamatsu S, Yuki T, Murata H. Improving antioxidant activity and nutritional components of Philippine salt-fermented shrimp paste through prolonged fermentation. Food Chem. 2008;111:72–77. doi: 10.1016/j.foodchem.2008.03.042. [DOI] [Google Scholar]
- Phithakpol B. Fish fermentation in Thailand. In: Lee CH, Steinkraus KH, Reilly PJ, editors. Fish fermentation technology. Tokyo: United Nations University Press; 1993. pp. 155–168. [Google Scholar]
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Sachindra NM, Bhaskar N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresource Technol. 2008;99:9013–9016. doi: 10.1016/j.biortech.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Schiedt K, Bischof S, Glinz E, Packer L. Metabolism of carotenoids and in vivo racemization of (3S, 3 S)-astaxanthin in the crustacean Penaeus. Method Enzymol. 1993;214:148–168. doi: 10.1016/0076-6879(93)14062-N. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principle and procedure of statistics. 2. New York: McGraw-Hill; 1980. [Google Scholar]
- Steinkraus KH. Fermentations in world food processing. Compr Rev Food Sci. 2002;F1:23–32. doi: 10.1111/j.1541-4337.2002.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Sun T, Tanumihardjo SA. An integrated approach to evaluate food antioxidant capacity. J Food Sci. 2007;72(9):R159–R165. doi: 10.1111/j.1750-3841.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Sun Q, Shen H, Luo Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J Food Sci Technol. 2011;48:53–60. doi: 10.1007/s13197-010-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai Industrial Standard . Kapi standard. Bangkok: Department of Industry; 1992. [Google Scholar]
- Tungkawachara S, Park JW, Choi YJ. Biochemical properties and consumer acceptance of Pacific Whiting fish sauce. J Food Sci. 2003;63:855–860. doi: 10.1111/j.1365-2621.2003.tb08255.x. [DOI] [Google Scholar]
- Xu W, Yu G, Xue C, Xue Y, Ren R. Biochemical changes associated with fast fermentation of squid processing by-products for low salt fish sauce. Food Chem. 2008;107:1597–1604. doi: 10.1016/j.foodchem.2007.10.030. [DOI] [Google Scholar]
- Yin LJ, Tong YL, Jiang ST. Improvement of the functionality of minced mackerel by hydrolysis and subsequent lactic acid bacteria fermentation. J Food Sci. 2005;70:M172–M178. doi: 10.1111/j.1365-2621.2005.tb07146.x. [DOI] [Google Scholar]
- Zamora R, Hidalgo FJ. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit Rev Food Sci. 2005;45:49–59. doi: 10.1080/10408690590900117. [DOI] [PubMed] [Google Scholar]