Abstract
In this study, extraction of phenolic compounds from nettle by microwave and ultrasound was studied. In both microwave and ultrasound-assisted extractions, effects of extraction time (5–20 min for microwave; 5–30 min for ultrasound) and solid to solvent ratio (1:10, 1:20, and 1:30 g/mL) on total phenolic content (TPC) were investigated. Effects of different powers (50 % and 80 %) were also studied for ultrasound-assisted extraction. In microwave-assisted extraction, the optimum TPC of the extracts (24.64 ± 2.36 mg GAE/g dry material) was obtained in 10 min and at 1:30 solid to solvent ratio. For ultrasound-assisted extraction, the condition that gave the highest TPC (23.86 ± 1.92 mg GAE/g dry material) was 30 min, 1:30 solid to solvent ratio, and 80 % power. Extracts obtained at the optimum conditions of microwave and ultrasound were compared in terms of TPC, antioxidant activity (AA) and concentration of phenolic acids with conventional extraction and maceration, respectively. Microwave reduced extraction time by 67 %. AA of extracts varied between 2.95 ± 0.01 and 4.48 ± 0.03 mg DPPH/g dry material among four methods. Major phenolic compounds were determined as naringenin and chlorogenic acid in nettle.
Keywords: Leaching, Microwave, Nettle, Phenolic, Ultrasound
Introduction
Nettle is a member of Urticaceae family which has sharp leaves (Pinelli et al. 2008) and stinging hairs containing different kinds of acids (Yarnell 1998) in its fresh form. Aerial parts and roots of nettle can be used as tea or cooked in meals. It is used as a folk medicine due to its polyphenol content and their antioxidant characteristics (Gulcin et al. 2004; Yildiz et al. 2008). According to many studies, nettle has been used as diuretic, hypoglycemic, anti-inflammatory (Yarnell 1998), anti-rheumatic, and hypotensive (Ozyurt et al. 2007; Riehemann et al. 1999; Sezik et al. 2001; Yildiz et al. 2008). The anti-inflammatory characteristics, which come from mainly caffeic acid, malic acid, and chlorogenic acid (Yarnell 1998), are used for treatments of some allergic rhinitis (Pinelli et al. 2008). Nettle leaves and seeds have become important traditional medicine for preventing and curing the cancer disease by their antioxidant characteristics (Ozyurt et al. 2007; Pinelli et al. 2008). It is also an important source of vitamin C (Al-Ismail et al. 2007; Gulcin et al. 2004; Ozyurt et al. 2007). There are various studies in literature about water extraction of nettle in order to investigate its antioxidant activity, total phenolic contents, and specific phenolic acids (Gulcin et al. 2004; Matsingou et al. 2001; Pinelli et al. 2008; Hudec et al. 2007).
Leaching of phenolic compounds from plant matrices is complex and challenging because of wide variations in structures and polarities of phenolic compounds. There are different methods for leaching of phenolic compounds such as supercritical fluid extraction, microwave and ultrasound-assisted extraction. Supercritical fluid extraction is a separation method that uses the advantage of unique properties of gases above their critical points to extract soluble components from the solid matrix. Supercritical CO2 is used as a solvent in supercritical fluid extraction because of its low critical temperature and pressure (31.1 °C, 72.8 atm), its non-toxic and non-flammable properties and its availability in high purity with low cost (Sonsuzer et al. 2004). Leaching of phenolic antioxidants from grape pomace has been studied using supercritical fluid extraction method (Pinelo et al. 2007).
Microwave assisted extraction enhances the extraction efficiency as compared to conventional extraction since microwaves interact with the polar molecules in the extraction media, heat is generated and the internal pressure of the solid material is increased. Microwave-assisted extraction of phenolic compounds from different plants has been studied before by different researchers (Beejmohun et al. 2007; Proestos and Komaitis 2008). Gallo et al. (2010) extracted phenolic compounds from four different spices, namely Cinnamomum zeylanicum, Coriandrumsativum, Cuminum cyminum, and Crocus sativus. They found that microwave-assisted extraction has reduced the extraction time significantly. In another study, Tsubaki et al. (2010) studied with green, oolong and black tea residues. They extracted the tea residues in water under autohydrolytic conditions and stated that microwave-assisted extraction enhanced the obtained phenolic compounds.
Cavitation phenomena and mechanical mixing effect are the main mechanisms in ultrasound-assisted extraction which increases extraction efficiency and reduces extraction time. In addition, thermal decomposition of heat sensitive compounds is avoided in ultrasound since it is a non-thermal process (Ma et al. 2008). Ultrasound extraction of phenolic compounds and antioxidants from citrus (Ma et al. 2009), coconut shell powder (Rodrigues and Pinto 2007), acacia flowers and buds (Tung et al. 2011), grape seeds (Ghafoor et al. 2009), pomegranate seed (Abbasi et al. 2008), strawberry (Herrera and Castro 2004); and olive leaves (Japon-Lujan et al. 2006) were studied by various researchers.
In literature, there is no study on extraction of phenolic compounds from nettle by using microwave and ultrasound. Therefore, the objective of this study was to determine the best extraction conditions to obtain phenolic compounds from nettle by using these extraction methods. In addition, effects of different extraction methods on antioxidant activity and concentration of phenolic compounds would be compared.
Materials and methods
Reagents and materials
Aerial parts of dry nettle were obtained from local markets. They were used in their original dried form without doing any crushing or grinding. The moisture content of dried nettle was 9 %.
Standards for phenolic compounds (gallic acid, caffeic acid, chlorogenic acid, p-coumaric acid, naringenin, and naringin), DPPH, Folin&Ciocalteu’s phenol reagent (2N), sodium carbonate, and methanol (HPLC grade) were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Extraction of phenolic compounds
Water was used as solvent for extraction and all extraction experiments were replicated twice. Laboratory scale microwave oven (Milestone Ethos D, Sorisole, Italy) was used for microwave-assisted extraction. Cavity of the oven was approximately 45 L in volume. Power was chosen as constant (407 W, which was measured by IMPI-2 L test). Extraction flask (1 L) which was connected to the condenser was placed into the microwave oven and extraction was performed for different times (5, 10, 15, and 20 min). Effects of different solid to solvent ratios of 1:10, 1:20, and 1:30 g/mL was studied by mixing 5 g of sample with appropriate amount of water.
Conventional extraction was also performed for comparison with microwave-assisted extraction. In conventional extraction, heating was achieved with hot plate instead of microwave. Extraction was performed with a solid to solvent ratio of 1:30 g/mL for different times.
In ultrasonic extraction, Sonic Ruptor400 Ultrasonic Homogenizer (Omni Sonic Ruptor400 Ultrasonic Homogenizer, Kennesaw, USA) with a standard probe of diameter 2.54 cm was used for leaching of phenolic compounds. It has a maximum power of 300 W and 20 kHz frequency. Two power levels were chosen, which were 50 % and 80 %. Ultrasound was operated at 50 % pulser mode. Extraction temperature was kept constant at 40 ± 1 °C using water bath. As in the case of microwave-assisted extraction, 1:10, 1:20, and 1:30 g/mL solid to solvent ratios were used. 10 g of sample was placed into a 600 mL beaker with the appropriate amount of distilled water. The beaker was placed into water bath and ultrasonic probe was dipped at most 1.5 cm depth into the extraction media. Extractions were performed at different times (5, 10, 20, and 30 min).
Maceration was carried out at 40 ± 1 °C for comparison with ultrasound-assisted extraction. Sample (10 g) and distilled water at 40 ± 1 °C were placed into the beaker to obtain 1:30 g/mL solid to solvent ratio. They were mixed for a few seconds in order to soak all the solid particles. Beakers were covered with aluminum foil and kept at 40 ± 1 °C for 24 h using incubator.
After each extraction process, extracts were roughly filtered through a piece of cloth and centrifuged (Sigma 2-16PK Centrifuge; Buckinghamshire, England) at 10,000 rpm (8,720 g) for 10 min. The volume and mass of the extract were recorded. Extracts to be analyzed were kept in 20 mL dark colored bottles in refrigerator at most for 2 days for the analysis.
Analysis
Determination of total phenolic content (TPC)
Folin-Ciocalteu method was used (Singleton et al. 1999; Singleton and Rossi 1965) for the determination of TPC. Two hundred μL diluted sample was mixed with 1 mL of 0.2 N Folin&Ciocalteu’s reagent and the mixture was left to stand at dark for 5 min. Then, 800 μL sodium carbonate solution (75 g/L) was added and the mixture was kept in dark for 1 h at room temperature for the reaction to take place. Afterwards, the absorbance was measured at 760 nm. The results were expressed in mg GAE (gallic acid equivalent)/g dry material. The color change due to the reaction between Folin&Ciocalteu’s reagent and the phenolic compounds indicated the concentration of the phenolic compounds.
Determination of antioxidant activity (AA)
DPPH● method was used for determination of antioxidant activity (Elmastas et al. 2006). For this determination, 0.025 g DPPH● / L methanol was prepared. Absorbance values were measured at 515 nm immediately after the addition of 1.95 mL DPPH● solution into the cuvette containing 0.05 mL extract (at t = 0) and after 2 h of waiting in dark (at t = 2 h). Calibration curve was prepared with different concentrations of DPPH● in methanol. The antioxidant activity and also DPPH scavenging activity were calculated using the following formulas:
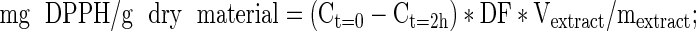 |
2.1 |
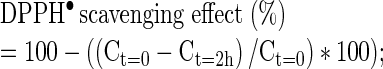 |
2.2 |
where Ct=0 is the concentration of DPPH● initially and Ct=2h is the concentration of DPPH● after 2 h, DF is the dilution factor, Vextract is the volume of extract in mL, and mextract is the weight of dried nettle in g. Corresponding color change in 2 h showed the concentration difference of unreacted DPPH●, and hence the reacted DPPH● as well as the amount of antioxidants in the sample. DPPH● scavenging activity was calculated in order to compare the results easily with the literature values.
Determination of phenolic compounds by HPLC
Agilent Zorbax SB-C18 (Santa Clara, USA) reversed phase column (250 × 4.6 mm, 5 μm particle size) was used in Shimadzu UFLC equipment (Columbia, USA). The model of degasser was GDU-20A5, pump was LC-20AD, autosampler was SIL-20A HT, column oven was CTO-20A, and the diode array detector was SPD-M20A.
Two mobile phases which were 0.2 % CH3COOH distilled water (A) and 90 % aqueous methanol solution (B) were used. Standards were prepared in 90 % methanol solution. Calibration curves were obtained for each phenolic acid and had R2 values greater than 0.98. All standards, samples, and mobile phases were filtered through 0.45 μm filter before injection. Standards were scanned in the range of 190 and 800 nm and the peak values were obtained. The wavelength that gave the highest value was chosen specifically for each standard. Gallic acid, naringenin and naringin were analyzed at 280 nm, p-coumaric acid was analyzed at 310 nm and caffeic acid and chlorogenic acids were analyzed at 330 nm.
Gradient program included increasing of mobile phase B from 0 % up to 50 % with a 1 mL/min flow rate at 40 °C in a 55 min time period. Wavelengths changed in the range of 280 and 330 with respect to the type of phenolic compound.
Statistical analysis
Statistical Analysis Software (SAS 9.1) was used. Two-way analysis of variance (ANOVA) was performed to determine if there is significant difference between microwave extraction conditions on affecting TPC content. In order to find out if there is significant difference among ultrasound power, time and solid to solvent ratio on affecting TPC, three way ANOVA was used. One-way ANOVA was applied for comparison of extraction methods. If significant difference was found (p ≤ 0.05), means were compared using Duncan’s multiple comparison method. Extraction experiments were duplicated and all other analyses were replicated three times.
Results and discussion
Effects of microwave-assisted and conventional extraction on total phenolic content of nettle extract
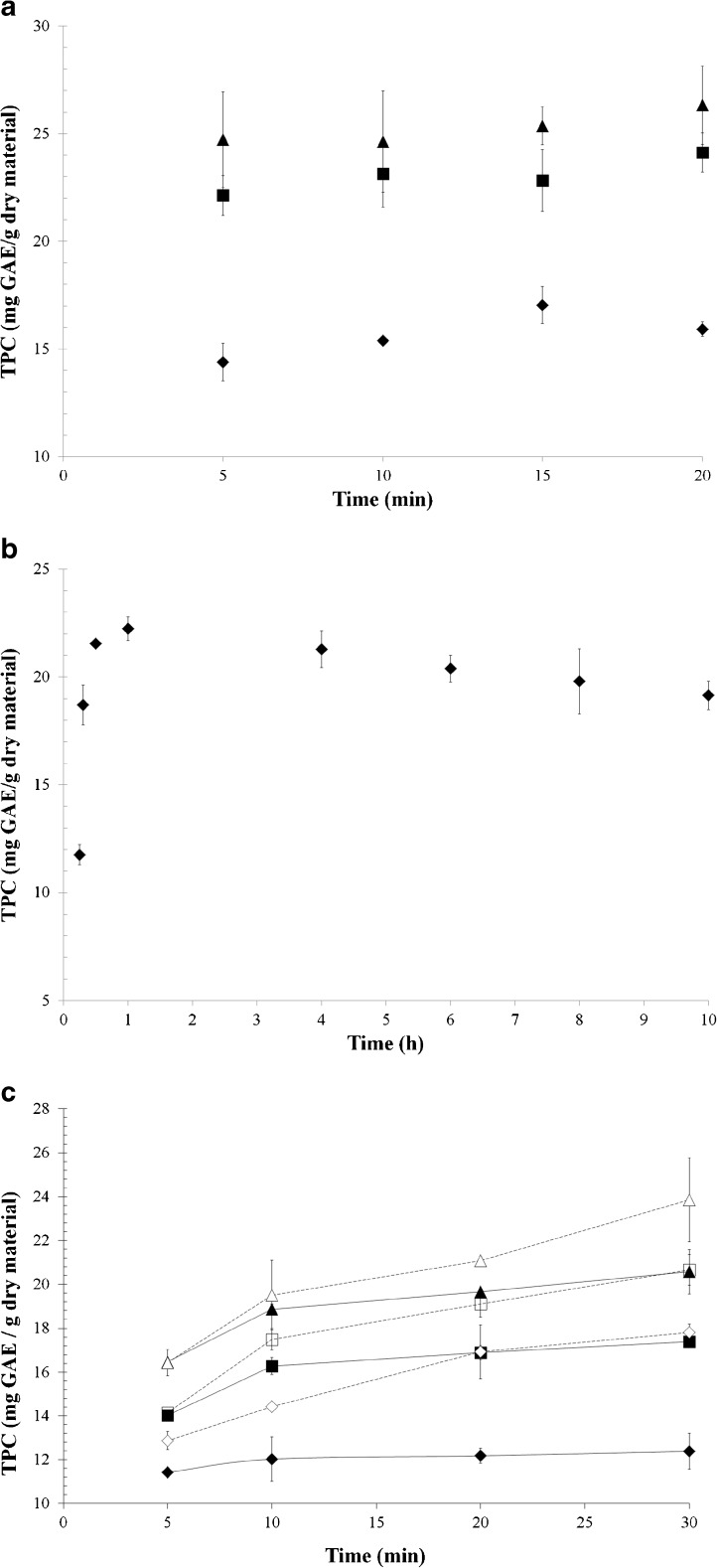
In microwave-assisted extraction, it can be seen that solid to solvent ratio had an important effect on total phenolic content (Fig. 1a). Solid to solvent ratio of 1:30 provided significantly higher concentration of phenolic compounds. The reason of this was the increase in concentration gradient with increase in solvent amount. There was no significant difference between 10, 15 and 20 min in terms of TPC. However, TPC of the extracts obtained at these times were significantly greater than TPC of the extract obtained at 5 min according to the statistical analysis. Therefore, the shortest time for complete leaching of phenolic compounds from nettle, which was 10 min, was chosen as the optimum. This showed that all the extractable phenolics diffused to the solvent in 10 min. Diffusion resistance to mass transfer was negligible since the aerial parts of nettle were very thin. The best conditions in microwave extraction were selected as 10 min extraction time and 1:30 solid to solvent ratio.
Fig. 1.
Total phenolic contents (TPC) of nettle extract obtained a by microwave-assisted extraction at different solid to solvent ratios (♦) 1:10, (■) 1:20, (▲) 1:30, for different extraction times, b conventionally at 1:30 solid to solvent ratio for different extraction times, and c by ultrasound-assisted extractions at different conditions (♦) 50 % power and 1:10 solid to solvent ratio, (■) 50 % power and 1:20 solid to solvent ratio, (▲) 50 % power and 1:30 solid to solvent ratio, (◊) 80 % power and 1:10 solid to solvent ratio, (□) 80 % power and 1:20 solid to solvent ratio, (Δ) 80 % power and 1:30 solid to solvent ratio (n = 2)
To determine the optimum time in conventional extraction, 1:30 solid to solvent ratio was used. Figure 1b shows that extraction of phenolic substances in nettle was nearly completed at 30 min. When extraction was performed for 15 and 20 min, it can be clearly seen that time was not sufficient to extract all the phenolic substances. After 30 min, concentration of total phenolic content remained almost constant. This value was also determined by the statistical analysis of TPC of extracts according to extraction time, TPC at 30 min showed significant difference from TPC at 15 min and 20 min, but there was no significant difference between TPC at 30 min and 60 min. So, the required time for conventional extraction was determined as 30 min.
When microwave-assisted extraction was used, conventional extraction time was reduced by 67 %. The reduction of extraction time was due to the heating mechanism of microwave. Microwaves increase the internal pressure of the solid media and enhance the extraction, thus phenolic compounds can be leached in a shorter time by microwave as compared to conventional extraction (Bayramoglu et al. 2008).
Effect of ultrasound-assisted extraction on total phenolic content of nettle extract
In ultrasound-assisted extraction, power had a significant effect on TPC of the extracts. Higher power level gave higher TPC for nettle extracts (Fig. 1c). Increasing power level of ultrasound supplies a faster and stronger mixing effect that reduces external resistance and enhances the mass transfer, so increasing power enhances the extraction and increases the efficiency (Chemat et al. 2004; Ma et al. 2009; Shalmashi 2009). By means of ultrasound, mixing occurs in the solid-liquid interface. As a result, the thickness of the boundary layer decreases.
Time had also a significant effect on TPC of extracts (p < 0.0001). TPC of extracts increased with respect to time. Longer extraction times increased the extracted amount of phenolics. This is in accordance with other leaching studies (Chemat et al. 2004; Rostagno et al. 2003; Shalmashi 2009). TPC of extracts at 1:30 solid to solvent ratio was significantly higher than 1:20 and 1:10 solid to solvent ratios. The reason for this can be the increase in concentration gradient with increase in solvent amount as discussed before. It was previously shown that concentration of total soluble phenolic substances increases with the amount of the solvent (Alekovski et al. 1998; Bi et al. 2011; Cacace and Mazza 2003; Sayyar et al. 2009).
For ultrasound extraction of nettle leaves, the best conditions were determined as 80 % power level, 30 min and 1:30 solid to solvent ratio.
Comparison of different extraction methods according to total phenolic content, antioxidant activities and concentration of phenolic compounds
TPC of extract obtained by microwave at the best condition was 24.64 ± 2.36 mg GAE/g dry material. TPC of the extract obtained conventionally using the same solid to solvent ratio (1:30) for 30 min was 21.54 ± 0.01 mg GAE/g dry material. TPC of microwave extract was not significantly different than TPC of extract obtained conventionally.
Antioxidant activities of extracts obtained by different methods showed significant difference statistically. When microwave-assisted extraction was used, antioxidant activity of the nettle extract (4.16 ± 0.02 mg DPPH/g dry material) was significantly higher than that obtained by conventional method (3.86 ± 0.01 mg DPPH/g dry material). They were also expressed as DPPH radical scavenging activity and 57.19 % and 63.82 % were calculated for the extracts from microwave and conventional, respectively. In both cases, values were significantly different from each other statistically. There was no correlation between antioxidant activity and total phenolic content of extracts obtained by microwave and conventional heating. Similar results were obtained before by other researchers (Arbianti et al. 2007; Sengul et al. 2009). In addition, values of TPC and DPPH scavenging activity were compared with the values from literature in Table 1. They are in accordance with each other. The fluctuations might be due to the different extraction methods of nettle.
Table 1.
Total phenolic content (TPC) and antioxidant activity (AA) values obtained from nettle in this study and in literature
| TPC (mg/g dry material) | DPPH● radical scavenging (%) | ||
|---|---|---|---|
| From this study | From literature | From this study | From literature |
| 14.39 ± 0.87–25.67 ± 0.52 | 11.03a | 56.33–72.17 | 59c |
| −25.35b | |||
TPC of ultrasound extract at the best condition was 23.86 ± 1.92 mg GAE / g dry material. It was not significantly different than the TPC of the extract obtained by maceration (25.67 ± 0.52 mg GAE / g dry material). There was a possibility of formation of oxidative radicals during ultrasound extraction in water (Proestos and Komaitis 2006). Proestos and Komaitis (2006) studied ultrasonic treatment of aromatic plants and they have found lower phenolic acid concentration in water extracts. This was explained by the formation of hydrogen peroxide (Paniwnyk et al. 2001). However, this was not the case in this study because aqueous ultrasonic extraction of nettle did not show a considerable degradation in terms of TPC as compared to maceration.
AA values obtained from the extracts by ultrasound and maceration were 2.95 ± 0.01 mg DPPH/g dry material (72.17 % DPPH radical scavenging activity) and 4.48 ± 0.03 mg DPPH/g dry material (56.33 % DPPH radical scavenging activity), respectively. These values showed significant difference statistically and also no correlation between TPC and AA was observed, as in the case of microwave and conventional extractions.
Table 2 shows the concentrations of individual phenolic compounds in the extracts obtained at the best conditions of different methods. The most abundant phenolic acids that could be detected in nettle extract were naringenin and chlorogenic acid. In general, concentrations of phenolic compounds were slightly lower in microwave extraction as compared to conventional extraction (Table 2). Ma et al. (2009) also showed that microwave radiation can easily degrade unstable phenolics due to their structural properties. Phenolic compounds with higher number of hydroxyl group in their aromatic rings are unstable and can easily be degraded under microwave radiation.
Table 2.
Concentrations of main phenolic compounds in nettle extracts obtained by different methods with1:30 solid to solvent ratio and detected by HPLC (mg/g dry material)
| Extraction method | Gallic acid | Caffeic acid | Chlorogenic acid | p-Coumaric acid | Naringenin | Naringin |
|---|---|---|---|---|---|---|
| Microwave, 407 W, 10 min | 1.125 | 1.223 | 4.798 | 1.157 | 5.582 | 0.665 |
| Conventional, 30 min | 1.256 | 1.327 | 5.108 | 1.255 | 6.034 | 0.865 |
| Ultrasound, 80 % power, 30 min | 1.209 | 1.289 | 4.453 | 1.100 | 5.735 | 0.784 |
| Maceration, 24 h | 1.185 | 1.343 | 5.009 | 1.180 | 5.881 | 0.779 |
Similar concentrations of individual phenolic compounds were obtained in ultrasound extraction and maceration (Table 2).
Conclusions
Nettle extracts obtained by microwave and ultrasound-assisted extractions were compared with conventional extraction and maceration, respectively in terms of total phenolic contents, antioxidant activities, and concentration of individual phenolic compounds. As a common trend, decreasing solid to solvent ratio increases the concentration of total phenolic compounds for both microwave and ultrasound-assisted extraction methods. In addition, TPC of the extract obtained using higher power was higher in ultrasonic extraction. TPC of extracts obtained using different methods were not significantly different from each other. Antioxidant activities of extracts obtained by microwave were higher than conventional and ultrasound extractions. In addition, extraction time was significantly reduced in microwave extraction. Thus, microwave extraction method can be recommended for leaching phenolic compounds from nettle.
References
- Abbasi H, Rezaei K, Emamdjomeh Z, Mousavi SME. Effect of various extraction conditions on the phenolic contents of pomegranate seed oil. Eur J Lipid Sci Technol. 2008;110:435–440. doi: 10.1002/ejlt.200700199. [DOI] [Google Scholar]
- Alekovski S, Sovova H, Curapova B, Poposka F. Supercritical CO2 extraction and Soxhlet extraction of grape seeds oil. Bull Chem Technol Maced. 1998;17:129–134. [Google Scholar]
- Al-Ismail K, Herzallah SM, Rustom AS. Antioxidant activities of some edible wild Mediterranean plants. Ital J Food Sci. 2007;19:287–296. [Google Scholar]
- Arbianti R, Utami TS, Kurmana A, Sinaga A (2007) Comparison of antioxidant activity and total phenolic content of Dillenia indica leaves extracts obtained using various techniques. In: 14th Regional Symposium on Chemical Engineering, Gadjah Mada University, Yogyakarta-Indonesia, 4–5 December 2007
- Bayramoglu B, Sahin S, Sumnu G. Solvent-free microwave extraction of essential oil from oregano. J Food Eng. 2008;88:535–540. doi: 10.1016/j.jfoodeng.2008.03.015. [DOI] [Google Scholar]
- Beejmohun V, Fliniaux O, Grand E, Lamblin F, Bensaddek L, Christen P, Kovensky J, Fliniaux MA, Mesnard F. Microwave-assisted extraction of main phenolic compounds in flaxseed. Phytochem Anal. 2007;18:275–282. doi: 10.1002/pca.973. [DOI] [PubMed] [Google Scholar]
- Bi W, Zhou J, Row KH. Decaffeination of coffee bean waste by solid-liquid extraction. Korean J Chem Eng. 2011;28:221–224. doi: 10.1007/s11814-010-0264-x. [DOI] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Chemat S, Lagha A, AitAmar H, Bartels PV, Chemat F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavor Fragr J. 2004;19:188–195. doi: 10.1002/ffj.1339. [DOI] [Google Scholar]
- Elmastas M, Gulcin I, Beydemir S, Kufrevioglu OI, Aboul-Enein HY. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) fruit extracts. Anal Lett. 2006;39:47–65. doi: 10.1080/00032710500423385. [DOI] [Google Scholar]
- Gallo M, Ferracane R, Graziani G, Ritieni A, Fogliano V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules. 2010;15:6365–6374. doi: 10.3390/molecules15096365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafoor K, Choi YH, Jeon JY, Jo IH. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J Agric Food Chem. 2009;57:4988–4994. doi: 10.1021/jf9001439. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Kufrevioglu I, Oktay M, Buyukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica diocia L.) J Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Herrera MC, Castro MDL. Ultrasound-assisted extraction for the analysis of phenolic compounds in strawberries. Anal Bioanal Chem. 2004;379:1106–1112. doi: 10.1007/s00216-004-2684-0. [DOI] [PubMed] [Google Scholar]
- Hudec J, Burdova M, Kobida L, Komora L, Macho V, Kogan G, Turianica I, Kochanova R, Lozek O, Haban M, Chlebo P. Antioxidant capacity changes and phenolic profile of Echinacea purpurea, nettle (Urtica diocia L.), and dandelion (Taraxacum officinale) after application of polyamine and phenolic biosynthesis regulators. J Agric Food Chem. 2007;55:5689–5696. doi: 10.1021/jf070777c. [DOI] [PubMed] [Google Scholar]
- Japon-Lujan R, Luque-Rodriguez JM, Luque de Castro MD. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J Chromatogr A. 2006;1108:76–82. doi: 10.1016/j.chroma.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Ma YQ, Ye XQ, Fang ZX, Chen JC, Xu GH, Liu DH. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J Agric Food Chem. 2008;56:5682–5690. doi: 10.1021/jf072474o. [DOI] [PubMed] [Google Scholar]
- Ma YQ, Chen JC, Liu DH, Ye XQ. Simultaneous extraction of phenolic compounds of citrus peel extracts: effect of ultrasound. Ultrason Sonochem. 2009;16:57–62. doi: 10.1016/j.ultsonch.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Matsingou TC, Kapsokefalou M, Salioglou A. Aqueous infusions of Mediterranean herbs exhibit antioxidant activity towards iron promoted oxidation of phospholipids, linoleic acid, and deoxyribose. Free Radic Res. 2001;34:593–605. doi: 10.1080/10715760100301601. [DOI] [PubMed] [Google Scholar]
- Ozyurt D, Demirata B, Apak R. Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement. Talanta. 2007;71:1155–1165. doi: 10.1016/j.talanta.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Paniwnyk L, Beaufoy E, Lorimer JP, Mason TJ. The extraction of rutin from flower buds of Sophora japonica. Ultrason Sonochem. 2001;8:299–301. doi: 10.1016/S1350-4177(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Pinelli P, Ieri F, Vignolini P, Bacci L, Baronti S, Romani A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica diocia L. J Agric Food Chem. 2008;56:9127–9132. doi: 10.1021/jf801552d. [DOI] [PubMed] [Google Scholar]
- Pinelo M, Ruiz-Rodriguez A, Sineiro J, Senorans FJ, Reglero G, Nunez MJ. Supercritical fluid and solid-liquid extraction of phenolic antioxidants from grape pomace: a comparative study. Eur Food Res Technol. 2007;226:199–205. doi: 10.1007/s00217-006-0526-3. [DOI] [Google Scholar]
- Proestos C, Komaitis M. Ultrasonically assisted extraction of phenolic compounds from aromatic plants: comparison with conventional extraction techniques. J Food Qual. 2006;29:567–582. doi: 10.1111/j.1745-4557.2006.00096.x. [DOI] [Google Scholar]
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci Technol. 2008;41:652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Riehemann K, Behnke B, Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica diocia), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-κB. FEBS. 1999;442:89–94. doi: 10.1016/S0014-5793(98)01622-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Pinto GAS. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J Food Eng. 2007;80:869–872. doi: 10.1016/j.jfoodeng.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Rostagno MA, Palma M, Barroso CG. Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A. 2003;1012:119–128. doi: 10.1016/S0021-9673(03)01184-1. [DOI] [PubMed] [Google Scholar]
- Sayyar S, Abidin ZZ, Yunus R, Muhammed A. Extraction of oil from jatropha seeds-optimization and kinetics. Am J Appl Sci. 2009;6:1390–1395. doi: 10.3844/ajassp.2009.1390.1395. [DOI] [Google Scholar]
- Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci. 2009;22:102–106. [PubMed] [Google Scholar]
- Sezik E, Yesilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol. 2001;75:95–115. doi: 10.1016/S0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- Shalmashi A. Ultrasound-assisted extraction of oil from tea seeds. J Food Lipids. 2009;16:465–474. doi: 10.1111/j.1745-4522.2009.01159.x. [DOI] [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sonsuzer S, Sahin S, Yilmaz L. Optimization of supercritical CO2 extraction of Thymbra spicata oil. J Supercrit Fluids. 2004;30:189–199. doi: 10.1016/j.supflu.2003.07.006. [DOI] [Google Scholar]
- Tsubaki S, Sakamoto M, Azuma J. Microwave-assisted extraction of phenolic compounds from tea residues under autohydrolytic conditions. Food Chem. 2010;123:1255–1258. doi: 10.1016/j.foodchem.2010.05.088. [DOI] [Google Scholar]
- Tung YT, Chang WC, Chen PS, Chang TC, Chang ST. Ultrasound-assisted extraction of phenolic antioxidants from Acacia confusa flowers and buds. J Sep Sci. 2011;34:844–851. doi: 10.1002/jssc.201000820. [DOI] [PubMed] [Google Scholar]
- Yarnell E. Stinging nettle. Altern Complement Ther. 1998;4:180–186. doi: 10.1089/act.1998.4.180. [DOI] [Google Scholar]
- Yildiz L, Sozgen Baskan K, Tutem E, Apak R. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta. 2008;77:304–313. doi: 10.1016/j.talanta.2008.06.028. [DOI] [PubMed] [Google Scholar]