Abstract
Banana is a highly nutritious fruit crop consumed by many people’s worldwide while endangered species are consumed by limited peoples and their health benefits are not explored. The unripe fruits and flowers of wild and commercial banana are consumed by peoples after cooking only. Hence, the present study was undertaken to evaluate and compare the effect of pressure cooking on antioxidant activity of wild and commercial banana species. The raw and processed samples were extracted with 70 % acetone. Except wild flower, thermal processing enhanced the content of phenolics, tannins, flavonoids, DPPH, ABTS, FRAP, hydroxyl and peroxidation activity than raw. Wild species presented higher phenolics, tannins, DPPH, ABTS and FRAP activity than commercial ones. Except few samples, wild species and commercial species exhibit similar activity in superoxide, hydroxyl and peroxidation activity. FRAP (r2 = 0.922; 0.977) and hydroxyl (r2 = 0.773; 0.744) activities were dependent on phenolics and tannin content whereas tannins may be responsible for DPPH scavenging activity (r2 = 0.745). Thermal processing enhanced the antioxidant activity might be due to the release of bound phenolics from cell wall and oxidation and polymerisation of compounds present in it. This wild species may be an alternative to commercial ones and will be valuable to consumers for protecting from chronic diseases.
Keywords: Banana, Polyphenolics, ABTS, Thermal processing, Correlation
Introduction
Fruits and vegetables are important sources of essential dietary nutrients such as vitamins, minerals and fibre (Sagar and Suresh Kumar 2010). Among fruits, banana (Musaceae family) is the world’s fourth most valued fruit crop for its nutritive value with high carbohydrates, fiber, protein, less fat and water. There are more than 300 kinds of banana but only a few are commercially important. Many wild species of banana have many medicinal qualities and it will not explore to the present population. In search of rare and endangered species, Ensete superbum (Cliff banana), an Indian wild banana species was selected for this present study. It can withstand severe drought and it occupies as staple food of almost 10 million people in Ethiopia. The seeds were reported to cure various ailments like diabetes, leucorrhoea, kidney stone and dysuria (Yesodharan and Sujana 2007). Indigenous communities consume these fruits, flowers and pseudostem as vegetable (Monica et al. 2008). This species are observed to be rare and endangered (Saroj Kumar et al. 2010). Commercial species of Musa paradisiaca var. Monthan was compared with wild species. The ripening period of Ensete superbum and Musa paradisiaca is 12–15 days. M. paradisiaca fruit is reported to have strong ability to protect itself from the oxidative stress caused by intense sunshine and high temperature (Kanazawa and Sakakibara 2000). After processing only, rural peoples in India include these banana flower and unripe fruits in their diet. Thus, it is important to investigate the changes in processing. Many health benefits of plants are mainly due to the presence of antioxidant compounds. Reactive oxygen species or free radicals (hydroxyl, superoxide anion, hydrogen peroxide, singlet oxygen, nitric oxide, hypochlorite and various lipid peroxides) in biological system can be formed by peroxidative enzyme systems, lipid oxidation, irradiation, inflammation, smoking, air pollutants and glycoxidation (Halliwell 1997). These free radicals are capable of reacting with membrane lipids, nucleic acids, proteins and enzymes, and other small molecules, resulting in cellular damage and lead to a number of degenerative diseases like atherosclerosis, diabetes mellitus, ischemia/reperfusion (I/R) injury, Alzheimer’s disease, inflammatory diseases, carcinogenesis, neurodegenerative diseases, hypertension, ocular diseases, pulmonary diseases and haematological diseases (Maxwell 1995). The consumption of fruits and vegetables rich in antioxidants may neutralize free radicals and prevent these diseases. To date, no one had studied the antioxidant activity of Ensete superbum. It is also important to investigate whether processing increases or decreases the antioxidant activity in fruits and flowers. Hence, this present study deals with the influence of processing in wild (Ensete superbum) and commercial banana (Musa paradisiaca var. Monthan) unripe fruit and flower.
Materials and methods
Plant material
The whole unripe fruits and flowers of banana, Ensete superbum and Musa paradisiaca (var. Monthan) were collected from their natural vicinity at Idukki, Kerala, India.
Preparation of plant extracts
The whole unripe fruits and flowers were chopped into pieces. Then, the samples were divided into two portions, 600 g for processing and 400 g as fresh without any treatment. Unripe fruits and flowers of E. superbum and M. paradisiaca (600 g) were autoclaved separately using sample: water in the ratio of 1:5 (W/V) at a 15 lbs pressure for 15 min. The pressure-cooked and fresh fruits and flowers were frozen before freeze drying. The samples were lyophilized (4KBTXL-75; VirTis Benchtop K, New York, NY, USA) (flower lyophilised at −70 °C for 8–12 h and fruit lyophilised at −70 °C for 15–18 h) and then grounded into small particles using laboratory blender. Then, the powdered samples were stored in an air tight container at 4 °C until extraction. Both raw and processed samples of fruit and flower of E. superbum and M. paradisiaca were subjected to extraction. Before extraction, the samples (30 g) were defatted with petroleum ether for 24 h. The samples were filtered and sample residues were collected and dried. The sample residues (20 g) were extracted by stirring with 70 % acetone (1:7 w/v) for 48 h at room temperature in a shaker. Then the supernatant was filtered with Whatman No.4 filter paper. The residues were re-extracted with 70 % acetone for 5 h. The solvent of the combined extract was evaporated using reduced pressure in a rotary vacuum-evaporator and the remaining water was removed by lyophilisation. The freeze dried extract was used for analysis.
Chemicals
Butylated hydroxyanisole (BHA), Butylated hydroxytoluene (BHT), potassium ferricyanide, 2,2′-diphenyl-1-picryl-hydrazyl (DPPH˙), β- carotene, linoleic acid, nitro blue tetrazolium (NBT), potassium persulfate, ferrous chloride, ascorbic acid, Tween 20, 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-azinobis (3-ethyl benzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were obtained from Himedia, Merck and Sigma. All chemicals used were of analytical grade. All analysis was performed with UV-visible spectrophometer (Cyberlab-UV 100, USA).
Determination of total phenolics and tannin contents
Total phenolics and tannins were measured as tannic acid equivalents (Makkar et al. 2007) from tannic acid standard curve (3–15 μg range). One mL of the sample extract was transferred to a test tube and 0.5 mL of Folin-Ciocalteu reagent and 2.5 mL of sodium carbonate solution (20 % w/v) were added. After an incubation period of 40 min in dark and the absorbance was recorded at 725 nm against the reagent blank. For tannin estimation, the sample extracts were incubated with polyvinylpolypyrrolidone (PVPP) (100 mg) for 4 h at 4 °C. The supernatant was centrifuged and using the same method of phenolics, tannins were estimated. The phenolics and tannins were expressed as mg tannic acid equivalents (TAE)/g extract.
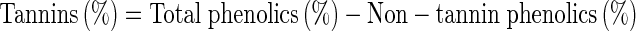 |
Estimation of total flavonoids
Total flavonoid content was measured according to the method of Zhishen et al. (1999). Sample was added with 0.3 mL of 5 % sodium nitrite and well mixed. After 5 min of incubation, 0.3 mL of 10 % aluminium chloride solution was added. Then, after 6 min, 2 mL of 1 M sodium hydroxide was added to the mixture and made up the volume to 10 mL with water. The absorbance was measured at 510 nm. The flavonoids were expressed as mg rutin/g extract.
Free radical scavenging activity on 2, 2-diphenyl-1-picrylhydrazyl (DPPH˙)
The antioxidant activity of extracts and standards (BHA, rutin and ascorbic acid) was measured in terms of hydrogen donating ability using a stable, commercially available organic nitrogen radical DPPH˙ by the method of Brand-Williams et al. (1995) with slight modifications. Sample extracts were prepared in methanol was mixed with 3.9 mL of DPPH˙ (0.025 g/L) and incubated in dark for 30 min. The absorbance was measured at 515 nm. The trolox standard was prepared in the range of 0–2.5 mM. The concentration of DPPH was calculated from trolox standard graph and expressed as mmol trolox equivalents/g extract.
Antioxidant activity by the ABTS˙+ assay
The ABTS˙+ radical cation decolourization assay was performed to evaluate the radical scavenging ability of crude extracts by the method of Re et al. (1999). ABTS radical cation (ABTS˙+) was generated by adding 2.45 mM potassium persulphate to 7 mM ABTS and incubated in dark at room temperature for 12–16 h. This stock solution of ABTS˙+ was diluted with ethanol to give an absorbance of 0.70 (±0.02) at 734 nm, which act as a positive control. 10 μL of crude extract (prepared in ethanol) was mixed with 1.0 mL of diluted ABTS˙+ solution and incubated at 30 °C for 30 min. The absorbance value was estimated at 734 nm. Trolox standards were also prepared (in ethanol: 0–1.5 mM) to get the concentration response curve. The unit of Trolox equivalent antioxidant activity (TEA) was defined as the concentration of Trolox having the equivalent antioxidant activity expressed as mmol/g of extracts. The TEA of ascorbic acid, BHA and rutin were also measured by ABTS˙+ method for comparison.
Ferric reducing antioxidant power assay (FRAP)
FRAP assay can be used to evaluate the electron donating ability of antioxidants according to the method of Benzie and Strain (1996). An aliquot of 30 μL sample was mixed with 90 μL of water and 900 μL of FRAP reagent (2.5 mL of 20 mmol/L of TPTZ in 40 mM of HCl, 2.5 mL of 20 mmol/L of ferric chloride, 25 mL of 0.3 mol/L of acetate buffer (pH–3.6)) and incubated at 37 °C for 30 min. After incubation the absorbance values were recorded at 593 nm. Known ferrous sulphate concentrations ranging from 400 to 2,000 μmol were used to generate the calibration curve. From the graph, the ferrous ions reduced by the sample were calculated using regression equation. The antioxidant activity is expressed as amount of extract required to reduce 1 mmol of ferrous ions. The antioxidant activity of sample was compared with the following standards: rutin, BHA and ascorbic acid.
Superoxide anion radical scavenging assay
This assay is used to evaluate the superoxide radical scavenging ability of plant extracts according to the method of Beauchamp and Fridovich (1971) as described by Zhishen et al. (1999). All the solutions used for this assay should be prepared in phosphate buffer, 0.05 M, pH 7.8. Samples (250 μg/mL) prepared in phosphate buffer 0.05 M, pH 7.8 were mixed with 1 mL of NBT (10−4 mol/L), 1 mL of methionine (10−2 mol/L) and 3 mL of riboflavin (10−6 mol/L) solution. The mixtures were kept in an aluminium foil lined box with two 20 W fluorescent lamps. The reactants were kept in such a way that the light should reach the contents with approximately 4,000 lux intensity. Control was also (assay mixture without sample) treated as above. All the samples and standards (rutin, catechin, BHA and trolox) were run in triplicates and in both illuminated and non-illuminated conditions. The absorbance was recorded at 560 nm. The differences in sample absorbance (A1) and control (A) between the illuminated and non-illuminated condition was recorded in order to avoid interferences.
The degree of superoxide radical scavenging activity was calculated as,
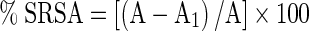 |
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging ability of extract and standard like catechin was measured according to the method of Klein et al. (1981). Sample extracts and standard (200 μg/mL) were mixed with 1 mL of iron–EDTA solution (0.13 % ferrous ammonium sulphate in 0.26 % EDTA), 0.5 mL of 0.018 % EDTA and 1 mL of DMSO solution (0.85 % in phosphate buffered saline 0.1 M, pH 7.4). The reaction was initiated by the addition of 0.5 mL of 0.22 % ascorbic acid and incubated at 80–90 °C in water bath for 15 min. The reaction was terminated by the addition of 1 mL of ice cold TCA (17.5 w/v). 3 mL of Nash reagent was added to the above mixture and allowed to stand at room temperature for 15 min and the absorbance values were recorded at 412 nm. Sample control was also run with the substitution of phosphate buffer instead of ascorbic acid. Reaction mixture without samples was used as control. The % hydroxyl radical scavenging activity (HRSA) was calculated using the following formula,
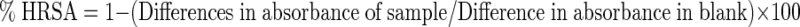 |
β-carotene linoleic acid system
The antioxidant activity of sample extracts and standards (BHA, rutin and trolox) were analyzed according to the method of Taga et al. (1984) with slight modifications. Two milligram of β-carotene was dissolved in 1 mL of chloroform containing 40 mg of linoleic acid and 400 mg of Tween 40. The chloroform was removed by rotary vacuum evaporator at 45 °C for 4 min, and 100 mL of distilled water was added slowly to the semisolid residue with vigorous agitation to form an emulsion. A 5 mL aliquot of the emulsion was added to a tube containing sample extract (250 μg/mL) and the absorbance was measured at 470 nm, immediately, against a blank, consisting of the emulsion without β-carotene. The tubes were placed in a water bath at 50 °C, and the absorbance measurements were conducted again at 30 min intervals up to 120 min. All determinations were carried out in triplicates. The antioxidant activity (AA) of the extracts was evaluated in terms of bleaching of β-carotene using the following formula: AA = [1−(A0−At)/(A′0−A′t)] × 100, where A0 and A′0 are the absorbance of values measured at zero time of the incubation for test sample and control, respectively and At and A′t are the absorbances measured in the test sample and control, respectively, after incubation for 120 min.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD) of at least three independent experiments. Differences are estimated by the analysis of variance (ANOVA) followed by Duncan’s multiple-range test. Differences were considered to be significant at P < 0.05. Correlation analysis was performed between phenolics and tannins with antioxidant activity using Pearson correlation-Two tailed. All statistical analyses were performed using the statistical software SPSS 13.0 version (SPSS Inc., Chicago, Illinois, USA).
Results and discussion
Total phenolics and tannins
Polyphenols form a large group of phytochemicals, which are produced by plants as secondary metabolites to protect them from photosynthetic stress, reactive oxygen species (Das et al. 2012). Phenolics acting as primary antioxidants or free radical terminators that are much stronger than those of Vitamin C and E. Tannins or polymeric polyphenolics are also potent antioxidants than simple monomeric phenolics and thus may be important dietary antioxidants (Hagerman et al. 1998). So, it is reasonable to determine the phenolics and tannins in selected plant extracts. Wild species expressed highest phenolic and tannin contents than commercial species (Table 1). Phenolics and tannins were resistant to thermal processing and the phenol content of raw samples was 27.95–352.55 mg TAE/g extract and processing samples were 95.45–402.42 mg TAE/g extract respectively. The values of tannins were within the range of 15.05–298.79 mg TAE/g extract. All processed samples except wild flower were observed to contain higher amount of phenolics and tannins than their respective raw samples. Processing increased 1.6–3.5 times of phenolics and 1.2–5.2 times of tannins than their raw samples. Among each species, the processed fruit in wild species and processed flower in commercial species showed higher phenolics and tannins. Similar increase in phenolic content during thermal processing were observed in banana peel (Gonzalez-Montelongo et al. 2010), leafy vegetables and corn (Adefegha and Oboh 2011; Kwon et al. 2007). The present report showed higher phenolic content than Ensete glauca ripe fruit (1.27 mg/g) (Kubola et al. 2011) and Musa sapientum fruit (0.51 mg/g) (Patthamakanokporn et al. 2008). Hence, this increase could be due to the release of bound phenolic acids from the breakdown of cellular constituents and cell walls during processing, disassociation of conjugate phenolic forms and formation of by-products (Chism and Haard 1996). Other reasons could also be due to Maillard reaction (non-enzymatic browning), caramelization, chemical oxidation of phenols and maderisation (Eichner and Ciner-Doruk 1981).
Table 1.
Total phenolics, tannins and flavonoid contents of raw and processed samples of Ensete superbum and Musa paradisiaca (var. Monthan)
| Samples | Total phenolics (mg TAE/g extract)A | Tannins (mg TAE/g extract)A | Flavonoids (mg rutin/g extract) |
|---|---|---|---|
| ERFA | 249.4c ± 34.02 | 249.2 ± 34.02 b | 530.9 ± 28.32 c |
| EPFA | 402.4 a ± 16.11 | 298.8 ± 14.94 a | 648.0 ± 32.38 b |
| ERFLA | 352.6 b ± 30.28 | 276.2 ± 35.13 ab | 519.1 ± 49.02 c |
| EPFLA | 335.2 b ± 39.92 | 239.6 ± 37.07 b | 311.8 ± 22.05 d |
| MRFA | 27.9 e ± 2.69 | 15.1 ± 0.98 e | 585.6 ± 40.29 bc |
| MPFA | 95.5 d ± 8.99 | 77.9 ± 9.38 d | 1670.6 ± 121.41 a |
| MRFLA | 77.9 d ± 2.39 | 53.9 ± 3.20 de | 176.4 ± 10.34 e |
| MPFLA | 237.2 c ± 36.61 | 148.7 ± 13.17 c | 391.8 ± 22.55 d |
A mg Tannic acid equivalents
Values are means of triplicate determination ± standard deviation. Mean values followed by different superscript in the same column are significantly (P < 0.05) different. ERFA E. superbum raw fruit acetone extract; EPFA E. superbum processed fruit acetone extract; ERFLA E. superbum raw flower acetone extract; EPFLA E. superbum processed flower acetone extract; MRFA M. paradisiaca raw fruit acetone extract; MPFA M. paradisiaca processed fruit acetone extract; MRFLA M. paradisiaca raw flower acetone extract; MPFLA M. paradisiaca processed flower acetone extract
Flavonoids
Flavonoids, mainly present as colouring pigments in plants also function as potent antioxidants at various levels and could protect membrane lipids from oxidation (Terao et al. 1994). Variation in flavonoid content was shown in Table 1. As like phenolics and tannins, except wild flower, all other processed samples showed higher flavonoid content up to 1.22–2.85 times than raw. The increase might be due to the release of bound flavonoid compounds and loss is due to the thermal degradation of compounds. The present report showed higher flavonoid content than Ensete glauca ripe fruit (Kubola et al. 2011). Similar increases in flavonoid content were shown in leafy vegetables after cooking (Adefegha and Oboh 2011) and oak acorns during thermal treatment (Rakic et al. 2007). Except wild flower, commercial species have higher flavonoid content than wild species.
Free radical scavenging activity on DPPH˙
DPPH˙ is a free stable radical and it is an easy and efficient method to determine the antioxidant capacity of samples. In its radical form, DPPH˙ absorbs at 515 nm, but upon reduction by an antioxidant or a radical species, the absorption disappears. Sample extract react very quickly with DPPH˙, reducing a number of DPPH˙ molecules equal to their number of available hydroxyl groups. The DPPH activity of all samples was presented in Table 2. All samples showed lower scavenging activity than standards because the interference of many compounds in the crude extract and purity of standards used. All processed samples other than wild flower registered higher DPPH activity than raw. Antioxidant activity toward DPPH was found to correlate with their tannin content (r2 = 0.745 and P < 0.05) (Table 3). Tannins, which are highly polymerized and have many phenolic hydroxyl groups with molecular weights of between 500 and 30,000 Da. It is much more potent antioxidants than are simple monomeric phenolics and scavenge DPPH efficiently (Hagerman et al. 1998). Wild species observed to have higher scavenging activity than commercial ones. The present results showed higher DPPH scavenging activity than Musa sapientum fruit extracts, stem and flower of Musa paradisiaca (Lim et al. 2007; Loganayaki et al. 2010). The number of DPPH˙ radical is reduced by available hydroxyl groups in sample extract.
Table 2.
DPPH˙, ABTS˙+ and Ferric reducing power assay of raw and processed samples of Ensete superbum and Musa paradisiaca (var. Monthan)
| Samples | DPPHA | ABTSA | FRAPB |
|---|---|---|---|
| mmol TEA/g extract | mmol TEA/g extract | mmol Fe(II)/g extract | |
| BHA | 814172.8 ± 187.06 b | 654356.1 ± 617.42 c | 350278.7 ± 735.79 b |
| RUT | 748175.2 ± 598.32 c | 432942.7 ± 233.01 d | 172898.6 ± 272.78 c |
| ASC | 841240.9 ± 273.72 a | 597916.7 ± 104.88 c | 730676.3 ± 911.82 a |
| ERFA | 420194.7 ± 126.02 f | 43657.1 ± 258.46 e | 59072.5 ± 458.51 d |
| EPFA | 483941.6 ± 394.77 d | 2259047.6 ± 174.57 b | 66695.7 ± 469.57 d |
| ERFLA | 440997.6 ± 402.01 e | 125714.3 ± 174.57 e | 64347.8 ± 301.72 d |
| EPFLA | 439781.0 ± 291.97 e | 118476.2 ± 412.63 e | 53739.1 ± 487.89 d |
| MRFA | 33625.3 ± 172.48 h | 8266.7 ± 25.91 e | 10391.3 ± 130.43 d |
| MPFA | 37262.8 ± 166.93 h | 28457.1 ± 34.75 e | 34739.1 ± 98.29 d |
| MRFLA | 383090.0 ± 197.26 g | 32495.2 ± 108.19 e | 20086.9 ± 291.76 d |
| MPFLA | 39914.8 ± 486.01 h | 3394285.7 ± 261.86 a | 32463.8 ± 98.89 d |
A mmol of trolox equivalent antioxidant activity
B Ferric reducing antioxidant power assay (mmol Fe (II) equivalent)
Values are means of triplicate determination ± standard deviation. Mean values followed by different superscript in the same column are significantly (P < 0.05) different. BHA Butylated hydroxyanisole; RUT rutin; ASC Ascorbic acid. Refer to Table 1 for abbreviations of samples
Table 3.
Correlation between phenolics, tannins and antioxidant activities
| Parameters | DPPH | FRAP | Hydroxyl |
|---|---|---|---|
| Phenolics | 0.687 | 0.922** | 0.773* |
| Tannins | 0.745* | 0.977** | 0.744* |
** Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
Antioxidant activity by the ABTS˙+ assay
ABTS ˙+ is a radical generated from potassium persulphate and ABTS˙+ in a dark for 16 h and forms a blue-green chromophore. The increase in concentration of extracts decreases the absorbance. The ABTS activity of all samples was presented in Table 2. Similar results were reported in Musa acuminata peel extract and leafy vegetables, the ABTS activity was increased with increase in temperature (Gonzalez-Montelongo et al. 2010; Adefegha and Oboh 2011). Except wild fruit, all other processing samples showed higher activity than raw. Processed samples reported to have high phenolic content and it might be responsible to scavenge ABTS radical efficiently than raw sample. However, when compared to standards, processed wild fruit and commercial flower showed higher ABTS radical scavenging activity. Processed fruit and flower of both the species (10.7–189.6 mmol/g DM) registered higher ABTS activity than reported banana stem and flower (2.6–10.2 mmol/g DM) (Loganayaki et al. 2010).
FRAP assay
FRAP measures the ferric reducing ability of the samples at a low pH, forming an intense blue colour as the ferric tripyridyltriazine (Fe3+-TPTZ) complex is reduced to the ferrous (Fe2+) form and absorbance is measured at 593 nm (Gil et al. 2000). The reductants present in the sample extract causes the reduction of ferric to ferrous. The ferric reducing antioxidant activity of all sample extracts was shown in Table 2 (10391.3–66695.65 mmole Fe (II)/g extract). All sample extracts showed higher reducing power than Musa sapientum fruit residues (Babbar et al. 2011) and Ensete glauca ripe fruit (Kubola et al. 2011). Previous reports showed that the reducing power of bioactive compounds associated with the antioxidant activity (Siddhuraju and Becker 2003). All processed samples other than wild flower exhibited higher reducing activity than raw. The correlation also showed that phenolics (r2 = 0.922 and p > 0.01) and tannins (r2 = 0.977 and p > 0.01) were responsible for reducing activity (Table 3). All sample extract showed lower activity than standards compared. The presence of antioxidants like polyphenols in the samples would result in the reduction of Fe 3+ to Fe 2+ by donating an electron. The reducing power of extracts appears to be more related to the degree of hydroxylation and the extent of conjugation in polyphenols (Blazovics et al. 2003).
Superoxide anion radical scavenging activity
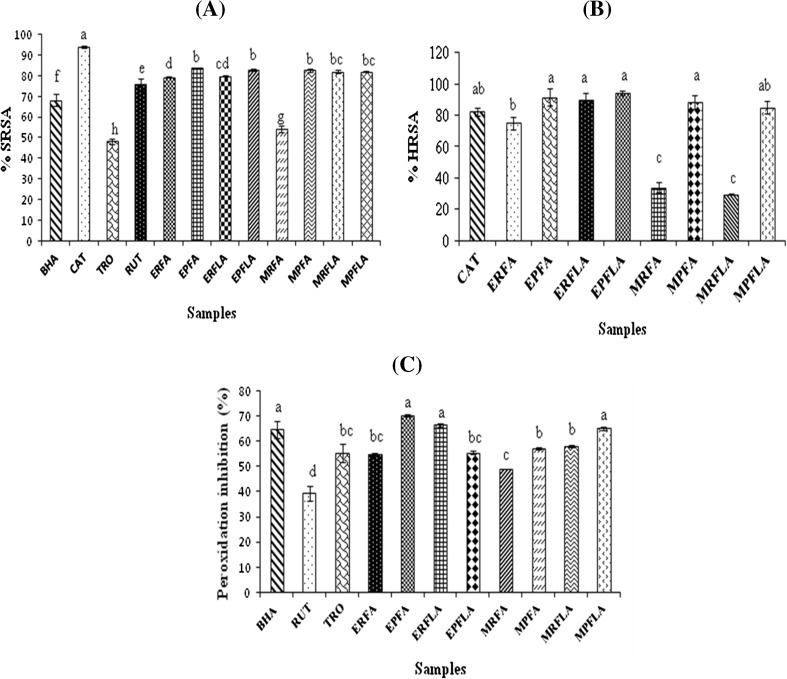
Superoxide anion radical (O・−2) is one of the strongest reactive oxygen species among the free radicals that are generated first after oxygen is taken into living cells. O・−2 changes to other harmful reactive oxygen species and free radicals such as hydrogen peroxide and hydroxyl radical attacks a number of biological molecules and leads to unfavourable alterations of biomolecules including DNA (Al-Mamun et al. 2007). The superoxide anion radical scavenging activity of all sample extracts (at 250 μg) showed 54.2–83.8 % (Fig. 1a). Processing of both unripe fruit and flower of wild and commercial species increases their superoxide radical scavenging activity than raw. When compared to standards, all samples except commercial raw fruit registered higher scavenging activity towards superoxide. Except commercial fruit, wild and commercial species expressed similar activity. The presence of vitamin-C, flavonoids, superoxide dismutase in sample extracts may be responsible for superoxide scavenging activity (Percival 1996).
Fig. 1.
Superoxide anion radical scavenging activity (a), Hydroxyl radical scavenging activity (b) and β-carotene linoleic acid peroxidation activity (c) of Ensete superbum and Musa paradisiaca extract. Values of triplicate determinations (mean ± SD) with different letters are significantly different (P < 0.05). SRSA–Superoxide radical scavenging activity; HRSA–Hydroxyl radical scavenging activity; BHA–Butylated hydroxyanisole; CAT–Catechin; TRO–Trolox; RUT–Rutin. Refer to Table 1 for abbreviations of samples
Hydroxyl radical scavenging activity
Hydroxyl radical is the most toxic radical and nonspecifically oxidizes all classes of biological macromolecules including lipids, proteins, and nucleic acids at virtually diffusion-limited rates and giving rise to many diseases including arthritis, atherosclerosis, cirrhosis, diabetes, cancer, Alzheimer’s disease, emphysema, and ageing (Imlay and Linn 1988). The range of hydroxyl radical scavenging activity was 29.9–94.6 % (Fig. 1b) and the order of activity was EPFLA> EPFA> ERFLA> MPFA> MPFLA> CAT> ERFA> MRFA> MRFLA. At 200 μg, all samples except commercial raw fruit and flower exhibited similar hydroxyl radical scavenging activity than standard, catechin. Processing increases the scavenging activity towards hydroxyl radical but in wild flower, it showed similar activity. Phenolics (r2 = 0.773) and tannins (r2 = 0.744) were correlated with hydroxyl radical scavenging activity at P < 0.05 (Table 3). The presence of phenolics and tannins in sample extract may be responsible to donate active hydrogen groups to hydroxyl radical and stabilizes it (Percival 1996). Tannic acid, has ten galloyl groups and has ability to complex with ferric formed in fenton reaction and converted in to Fe(II)n-TAoxidized. Then this complex may not react to form hydroxyl radical (Lopes et al. 1999). When compared to commercial species, raw samples of wild species expressed higher activity and processed samples showed similar activity.
β-carotene linoleic acid assay
In this method, β-carotene, a highly unsaturated molecule will be degraded by a free radical formed from linoleic acid. This linoleic acid abstracts hydrogen atom from one of its diallylic methylene groups and form free radicals. The presence of antioxidants in sample extracts can protect the extent of β-carotene bleaching by neutralizing the linoleate-free radical and other free radicals formed in the system (Chew et al. 2008). β-carotene degradation depends on the antioxidant activity of the sample extracts. The protection of sample extracts at 250 μg on β-carotene was 49.14–70.39 % (Fig. 1c). In this assay also, except wild flower, all processed samples have more protecting activity towards β-carotene from peroxidation. EPFA, ERFLA and MPFLA showed higher protection activity than standards, rutin and trolox whereas, exhibit similar activity on BHA. The presence of vitamin E, flavonoids and glutathione peroxidase in samples may be responsible for scavenging lipid peroxides generated from oxidation of linoleic acid (Percival 1996).
During processing, the phenolics, tannins and flavonoid contents of wild fruit were increased and in wild flower, it was decreased. In commercial species of banana, processing increased higher amount of phenolics, tannins and flavonoids. The difference might be due to the variations in the composition of samples used. The increase of phenolics, tannins and flavonoids might be due to the following reasons. The disruption of the cell wall through heating or the breakdown of insoluble phenolic compounds could have led to better extractability of these compounds. The increase of phenolics might be due to the release of more bound phenolic acids from the breakdown of cellular constituents (Dewanto et al. 2002a). The decrease might be due to the more solubility nature of compounds in cooking water and discarded it. Overall, except wild flower, the processing increased the antioxidant activity of all other samples. Thermal processing alters the phenolic structure resulting in improved antioxidant function. Other factors for improved antioxidant activity could be due to the additive and synergistic effects between other phytochemicals and thermally altered phenolics, increase in extractability of antioxidant compounds and formation of Maillard reaction products (Bondet et al. 1997). The Maillard reaction is known as a major source of compounds related to enhanced antioxidant activity by heat treatment in various foods (Chen and Kitts 2008). In a recent paper (Rufián-Henares and Delgado Andrade 2009), it has been demonstrated that cooking process releases some Maillard reaction products that contribute to the overall antioxidant activity in breakfast cereals (Nicoli et al. 1997). Also, it has been reported that thermal processing deactivates the oxidative and hydrolytic enzymes which are responsible for destroying the antioxidants in fruits and vegetables and enhances their antioxidant activity (Chism and Haard 1996). The present findings found to be agreement with earlier findings where antioxidant activity increased during processing in following samples, sweet corn (Dewanto et al. 2002a), tomato and carrot purées (Patras et al. 2008) and tomatoes (Dewanto et al. 2002b).
The correlation was made for phenolics, tannins and flavonoids with their antioxidant activity. There was no significant correlation except phenolics and tannins with hydroxyl and FRAP and tannins with DPPH. This could mean that other phytochemicals than phenolics are responsible for antioxidant activity. Certain phenolic and non-phenolic compounds in various parts of banana were identified in previous reports. They are arginine, flavonoids, catcholamines, vitamins (A, B, C and E), sulfhydryl- containing compounds (Cysteine and Glutathione), catechin, epicatechin, lignin, tannins, anthocyanins, lutein, β-cryptoxanthin, total carotenoids, apigenin glycosides, myricetin glycoside, myricetin-3-O-rutinoside, naringenin glycosides, kaempferol-3-O-rutinoside, quercetin-3-O-rutinoside, dopamine and N-acetylserotonin, anthocyanidins, procyanidins, trans-ferulic acid, p-coumaric acid, caffeic acid, vanillic acid, syringic acid, p-hydroxybenzoic acid and leucocyanidin (Lewis et al. 1999; Kanazawa and Sakakibara 2000; Vinaykumar et al. 2010; Someya et al. 2002; Lim et al. 2007; Isabelle et al. 2010; Kanazawa and Sakakibara 2000; Bennett et al. 2010). Processing might increases these compounds and the synergism of these compounds might contribute for the highest antioxidant activity in the present study.
Conclusion
Based on the relative order of potency in the above assays tested, the wild species showed higher polyphenol content than commercial species. They exhibited higher activity against some radicals and similar activity on others. Hence, the wild species can afford to be an alternative source to commercial banana for consumers. In various studies, it may be shown that processing decreases the antioxidant activity. This study may be a contradiction to these reports. All samples except in wild flower, processing increases the polyphenol and antioxidant activity. So, this present investigation may encourage the consumption of processed bananas and this study will be useful to investigate their potential in alleviating diseases and maximizing their use in food industry. The proper conservation of this wild species in their natural habitat will pave a way as alternative species of banana to include in our diet in upcoming days. Certain in vivo and toxicity studies will be focussed to promote this species in health promoting attributes in future.
Acknowledgments
Authors are much thankful to the University Grants Commission (UGC, New Delhi) for the financial assistance. One of the authors, G. S. P, is grateful to the University authority for an award of University Research Fellowship (URF).
References
- Adefegha SA, Oboh G. Cooking enhances the antioxidant properties of some tropical green leafy vegetables. Afr J Biotechnol. 2011;10:632–639. [Google Scholar]
- Al-Mamun M, Yamaki K, Masumizu T, Nakai Y, Saito K, Sano H, Tamura Y. Superoxide anion radical scavenging activities of herbs and pastures in Northern Japan determined using electron spin resonance spectrometry. Int J Biol Sci. 2007;3:349–355. doi: 10.7150/ijbs.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant activity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bennett RN, Shiga TN, Hassimotto NMA, Rosa EAS, Lajolo FM, Cordenunsi BR. Phenolics and antioxidant properties of fruit pulp and cell wall fractions of postharvest banana (Musa acuminata Juss.) cultivars. J Agric Food Chem. 2010;58:7991–8003. doi: 10.1021/jf1008692. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blazovics A, Szentmihályi K, Lugasi A, Hagymási K, Bànyai ÉM, Rapavi E, Héthelyi É. In vitro analysis of the properties of Beiqishen tea. Nutrition. 2003;19:869–875. doi: 10.1016/S0899-9007(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Bondet W, Williams B, Berset C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Lebensm Wiss Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chen XM, Kitts DD. Antioxidant activity and chemical properties of crude and fractionated Maillard reaction products derived from four sugar-amino acid Maillard reaction model systems. Ann N Y Acad Sci. 2008;1126:220–224. doi: 10.1196/annals.1433.028. [DOI] [PubMed] [Google Scholar]
- Chew YL, Lima YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci Technol. 2008;41:1067–1072. doi: 10.1016/j.lwt.2007.06.013. [DOI] [Google Scholar]
- Chism GW, Haard NF. Characteristics of edible plant tissues. In: Fennema OR, editor. Food chemistry. New York: Dekker; 1996. pp. 943–1011. [Google Scholar]
- Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50:4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Eichner K, Ciner-Doruk M. Early indication of the maillard reaction by analysis of reaction intermediates and volatile decomposition products. In: Eriksson C, editor. Progress in food nutrition and science 5. Oxford: Pergammon; 1981. pp. 115–135. [Google Scholar]
- Gil IM, Tomás-Barberán AF, Hess-Pierce B, Holcrft MD, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Montelongo R, Gloria Lobo M, Gonzalez M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119:1030–1039. doi: 10.1016/j.foodchem.2009.08.012. [DOI] [Google Scholar]
- Hagerman AE, Reidl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfield PW, Tiechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Halliwell B (1997) Antioxidants in disease mechanisms and therapy. In: Sies H (ed) Advance in pharmacology, vol.38. Academic Press
- Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Isabelle M, Lee BL, Lim MT, Koh WP, Huang D, Ong CN. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010;123:77–84. doi: 10.1016/j.foodchem.2010.04.002. [DOI] [Google Scholar]
- Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in Cavendish banana. J Agric Food Chem. 2000;48:844–848. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochem. 1981;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Kubola J, Siriamornpun S, Meeso N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 2011;126:972–981. doi: 10.1016/j.foodchem.2010.11.104. [DOI] [Google Scholar]
- Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin; In vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10:266–275. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J Ethnopharmacol. 1999;65:283–288. doi: 10.1016/S0378-8741(99)00005-7. [DOI] [PubMed] [Google Scholar]
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- Loganayaki N, Rajendrakumaran D, Manian S. Antioxidant capacity and phenolic content of different solvent extracts from banana (Musa paradisiaca) and Mustai (Rivea hypocrateriformis) Food Sci Biotechnol. 2010;19:1251–1258. doi: 10.1007/s10068-010-0179-7. [DOI] [Google Scholar]
- Lopes GKB, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/S0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Siddhuraju P, Becker K. Plant secondary metabolites. Methods in molecular biology, vol: 393. USA: Humana; 2007. [DOI] [PubMed] [Google Scholar]
- Maxwell SRJ. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- Monica K, Agarwal SS, Sanjay PN. Characterization of a chroman derivative isolated from the seeds of Ensete superbum Cheesm. Phcog Mag. 2008;4:114–117. [Google Scholar]
- Nicoli MC, Anese M, Parpinel MT, Franceschi S, Lerici CR. Loss and/or formation of antioxidants during food processing and storage. Cancer Lett. 1997;114:71–74. doi: 10.1016/S0304-3835(97)04628-4. [DOI] [PubMed] [Google Scholar]
- Patras A, Brunton N, Pieve SD, Butler F, Downey G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov Food Sci Emerg Technol. 2008;10:16–22. doi: 10.1016/j.ifset.2008.09.008. [DOI] [Google Scholar]
- Patthamakanokporn O, Puwastien P, Nitithamyong A, Sirichakwal PP. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J Food Compos Anal. 2008;21:241–248. doi: 10.1016/j.jfca.2007.10.002. [DOI] [Google Scholar]
- Percival M (1996) Antioxidants. Clinical Nutrition Insights; Advanced Nutrition Publications Inc.
- Rakic S, Petrovic S, Kukic J, Jadranin M, Tesevic V, Povrenovic D, et al. Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 2007;104:830–834. doi: 10.1016/j.foodchem.2007.01.025. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Evans CR. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rufián-Henares J, Delgado Andrade C. Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res Int. 2009;42:394–400. doi: 10.1016/j.foodres.2009.01.011. [DOI] [Google Scholar]
- Sagar VR, Suresh Kumar P. Recent advances in drying and dehydration of fruits and vegetables: a review. J Food Sci Technol. 2010;47:15–26. doi: 10.1007/s13197-010-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj Kumar V, Jaishanker R, Annamalai A, Iyer CSP. Ensete superbum (Roxb.) Cheesman: a rare medicinal plant in urgent need of conservation. Curr Sci. 2010;98:602–603. [Google Scholar]
- Siddhuraju P, Becker K. Studies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino acids through in vitro models. J Sci Food Agric. 2003;83:1517–1524. doi: 10.1002/jsfa.1587. [DOI] [Google Scholar]
- Someya S, Yoshiki Y, Okubo K. Antioxidant compounds from bananas (Musa cavendish) Food Chem. 2002;79:351–354. doi: 10.1016/S0308-8146(02)00186-3. [DOI] [Google Scholar]
- Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc. 1984;61:928–931. doi: 10.1007/BF02542169. [DOI] [Google Scholar]
- Terao J, Piskula M, Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch Biochem Biophys. 1994;308:278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- Vinaykumar T, Sumanth MH, Suman L, Vijayan V, Srinivasarao D, Sharmila A, Naveen M, Ramana B. Reno protective and testicular protective effect of Musa paradisiaca flower extract in streptozotocin induced diabetic rats. J Innov Trends Pharm Sci. 2010;1:106–114. [Google Scholar]
- Yesodharan K, Sujana KA. Ethnomedicinal knowledge among Malamalasar tribe of Parambikulam wildlife sanctuary, Kerala. Indian J Tradit Knowl. 2007;6:481–485. [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavanoid contents on mulberry and their scavenging effects on superoxide radical. Food Chem. 1999;50:6929–6934. [Google Scholar]