Abstract
Watermelon juice was exposed to the enzyme masazyme at varying enzyme concentrations (0.01–0.1 % w/w) and different time (20–120 min) and temperature (30–50 °C) combinations. The effects of the treatments on selected responses (juice recovery, total dissolved solids (TDS), viscosity, turbidity, cloud stability and L value) were determined employing a second order Box Behnken Design in combination with Response Surface Methodology. Enzymatic treatment effectively degraded polysaccharides, resulting in reduced viscosity, turbidity and absorbance value and increased juice recovery, total dissolved solids and lightness. R2 value for all models for the dependent variables were greater than 90 %. The maximum juice recovery (86.27 %), TDS (8.7°Brix) and L value (17.57) while minimum viscosity (0.0020 Pa.s.), turbidity (39.37 NTU) and cloud stability (0.033 abs) were obtained when enzyme treatment was set up with 0.09 % w/w enzyme concentration at 46.90 °C and 117.45 min.
Keywords: Enzyme, Watermelon, Juice recovery, Turbidity, Optimization
Introduction
Watermelon is one of the abundant and cheap fruits that is available in India. Its production is high during summer because of its tropical nature and its availability is almost throughout the year. Watermelon production occupies 6–7 % of overall fruit production (Reddy et al. 2008). Watermelon juice is becoming increasingly popular due to its sensorial, physical and nutritional properties (Edwards et al. 2003; Huor et al. 1980). Watermelon juice contains small amounts of phenolics as well as low vitamin C content compared with other fruits (Gil et al. 2006). On the other hand, watermelon juice is a rich natural source of lycopene, a compound responsible for its red colour (Perkins-Veazie et al. 2001). Intake of lycopene containing-products has been associated with a reduced incidence of coronary heart disease and some types of cancer (Fraser and Bramley 2004; Giovannucci 2002). Watermelon juice is a wonderful diuretic agent and may be of importance in managing diseases such as jaundice, typhoid and nephritis.
Pectinases are upcoming commercial enzymes, particularly in fruit juice industry. Pectic enzymes are employed in fruit processing to obtain better juice recovery, improve filtration rate and produce clear juices of high quality for concentration. Pectinolytic enzymes or pectinase act upon the negatively charged high molecular weight pectic substances (Rastogi 1998). Maximizing yield from fruits is a key demand for juice makers, because fluctuations in the supply of raw fruit in the context of consistently growing demand can increase price and decrease the availability of quality fruit juice. Enzymes have been employed in the fruit industry for the following purposes: a) to break down all polymeric carbohydrates such as pectin, hemicelluloses and starches, thus increasing the yield of juice by enabling better pressing of the pulp; b) to improve the yield of substances contained in fruit, e.g. acids, colouring or aroma substances and c) to clarify the juice and to liquefy the entire fruit pulp for maximum yield (Gerhartz 1990). Pectin is a major hindrance to filtration of juice as it forms a highly viscous gel-type layer on the membrane surface. Therefore, the juice is treated with enzyme to degrade the viscous pectin (Sahin and Bajindirli 1993; Rai et al. 2004). Pectinolytic enzymes break down the pectin molecules, which facilitate the formation of pectin-protein flocs, leaving a clear supernatant and significantly removing the colloidal formulations in the juices (Yusuf and Ibrahim 1994; Alvarez et al. 1998).
Increase in yield and clarity and decreases in viscosity in fruit juices have been reported by use of pectin degrading enzymes in dates (Kulkarni et al. 2010), peach (Santin et al. 2008), banana (Lee et al. 2006), mosambi (Rai et al. 2004), kiwi fruit (Dawes et al. 1994) and pineapple (Sreenath et al. 1994). The enzymatic hydrolysis of pectic substances depends on incubation time, temperature and enzyme concentration (Abdullah et al. 2007; Lee et al. 2006; Sin et al. 2006). The present study was conducted to ascertain the effect of a pectin-degrading enzyme (masazyme) treatment (at different time, temperature and enzyme concentration) on watermelon juice (juice recovery, TDS, viscosity, turbidity, cloud stability and L value) and optimize the process condition by response surface methodology.
Materials and methods
Materials
Fresh watermelon fruit (crimson sweet) were purchased from local market. Masazyme encompassing a balanced blend of enzyme complex with activity on celluloses, pectins, beta-glucans, xylan and other hemicellulosic polymer associated with fruit pulp was obtained from M/s Advanced Enzyme Technologies Ltd, Mumbai, India.
Enzyme treatment
For each experiment, about 200 g of juice pulp was subjected to different enzyme treatment conditions as cited in Table 1. Based on preliminary experiments, the range on the variables for enzymatic treatment was selected. These were: concentration of enzyme, X1 (0.01–0.1 % w/w), incubation time, X2 (20–120 min) and incubation temperature, X3 (30–50 °C). The temperature of enzyme treatment was adjusted to the desired level using a constant temperature water bath (Narang Scientific Works Pvt Ltd, New Delhi, India). The pH of the watermelon juice was kept at its natural pH value. The enzyme was inactivated after the requisite treatment by heating the suspension at 90 °C for 5 min in a water bath. After that the juice was filtered through muslin cloth and the filtrate was retained for further analysis.
Table 1.
Experimental design matrix and physical attributes scores of clarified watermelon juice by enzymatic method
| Independent variables | Dependent variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RUN | Enzyme concentration (w/w %) X1 (x1) | Incubation time (min) X 2 (x 2) | Incubation temperature (°C) X3 (x3) | Juice recovery (%) Y1 | TDS a (°Brix) Y2 | Viscosity (Pa.s.) Y3 | Turbidity (NTU) Y4 | Cloud stability (abs) Y5 | Lightness (L value) Y6 |
| 1 | 0.1(+1) | 70(0) | 30(−1) | 84.85 | 7.8 | 0.0025 | 75.8 | 0.0524 | 17.45 |
| 2 | 0.01(−1) | 70(0) | 50(+1) | 76.23 | 7.3 | 0.0035 | 109 | 0.1176 | 13.98 |
| 3 | 0.1(+1) | 120(+1) | 40(0) | 88.54 | 8.5 | 0.0018 | 38 | 0.033 | 17.96 |
| 4 | 0.055(0) | 20(−1) | 50(+1) | 78.56 | 7.9 | 0.0033 | 76 | 0.0519 | 15.98 |
| 5 | 0.055(0) | 70(0) | 40(0) | 80.86 | 7.6 | 0.0034 | 93 | 0.0653 | 16.87 |
| 6 | 0.055(0) | 70(0) | 40(0) | 79.65 | 7.6 | 0.0035 | 92 | 0.0699 | 16.98 |
| 7 | 0.055(0) | 70(0) | 40(0) | 80.65 | 7.7 | 0.0035 | 89 | 0.0705 | 17.23 |
| 8 | 0.055(0) | 120(+1) | 50(+1) | 76.45 | 8.1 | 0.0032 | 65 | 0.0514 | 15.47 |
| 9 | 0.055(0) | 20(−1) | 30(−1) | 77.03 | 7.3 | 0.0034 | 104.7 | 0.0698 | 14.65 |
| 10 | 0.1(+1) | 20(−1) | 40(0) | 88.45 | 8.3 | 0.0023 | 55.98 | 0.0412 | 18.01 |
| 11 | 0.01(−1) | 20(−1) | 40(0) | 78.80 | 7.2 | 0.0035 | 116 | 0.1397 | 13.98 |
| 12 | 0.01(−1) | 70(0) | 30(−1) | 77.32 | 7.1 | 0.0035 | 121.32 | 0.1543 | 13.57 |
| 13 | 0.055(0) | 120(+1) | 30(−1) | 78.45 | 7.4 | 0.0034 | 92.89 | 0.064 | 15.2 |
| 14 | 0.055(0) | 70(0) | 40(0) | 81.65 | 7.7 | 0.0034 | 90 | 0.0702 | 17.28 |
| 15 | 0.055(0) | 70(0) | 40(0) | 80.45 | 7.7 | 0.0033 | 90 | 0.0706 | 17.36 |
| 16 | 0.1(+1) | 70(0) | 50(+1) | 87.80 | 8.8 | 0.0017 | 40 | 0.036 | 17.57 |
| 17 | 0.01(−1) | 120(+1) | 40(0) | 77.56 | 7.2 | 0.0035 | 107 | 0.1298 | 14.01 |
aTotal dissolved solid
Response measurement techniques
Juice recovery was reported as the % of clear juice obtain in g per 200 g of pulp. Total dissolved solids (TDS) content was determined using Atago digital refractometer (RP-101, Tokyo, Japan) at 20 °C with a scale ranging 0–45°Brix. Viscosity was measured using Brookfield Viscometer (DV-II + Pro, Brookfield Engineering Laboratory, Inc., Middleboro, USA) at 100 rpm with LV-2 spherical spindle. Turbidity was determined in term of Nephelometric Turbidity Unit (NTU) using a portable turbidity meter (Systronics Digital NTU-132, Ahmadabad, India). Cloud stability was determined by measuring absorbance at 660 nm using a Systronic Double Beam Spectrophotometer (Systronic UV–vis-2203, Ahmadabad, India). Distilled water was used as the reference. L value of the clarified juice was measured using Hunter colorimeter Ultrascan (SN 7877, Hunter Associates Laboratory, Inc., Virginia, USA). The Commission International de L’Eclairage (CIE) system reference measures the L value on a numerical scale, where white is equal to 100 and black is 0.
Experimental design
The experimental design and statistical analysis were performed with Stat-Ease software (Design Expert version 7.1.6 2009). In the present research, a 3-level variables second order Box Bhenken Design with quadratic model (Myers 1971; Khuri and Cornell 1989) was employed (Table 1). The factors (independent variables) were concentration of enzyme used (X1), incubation time (X2) and incubation temperature (X3) of enzyme treatment. The experimental design in the coded (x) and actual (X) levels of variables is shown in Table 1. The range of factors was chosen based on literature and preliminary results. In each experiment, juice recovery (Y1), TDS (Y2), viscosity (Y3), turbidity (Y4), cloud stability (Y5) and L value (Y6) were determined as the responses (dependent variables). The complete design consisted of 17 combinations (including 5 replicates of the center point) of independent variables. All the experiments were carried out in random order (Table 1). The variance for each factor assessed was partitioned into linear, quadratic and interactive components and were represented using a second order polynomial (1) for 3 factors, as follows:
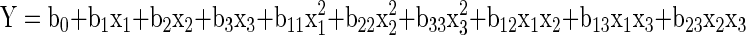 |
1 |
The coefficients of the polynomial were represented by b0 (constant term) b1, b2 and b3 (linear effects), b11, b22 and b33 (quadratic effects) and b12, b13 and b23 (interaction effects). Analysis of variance (ANOVA) tables were generated and the effects of individual linear quadratic and interactive terms were been determined (Khuri and Cornell 1989). The significance of all the terms in the polynomial was judged statistically by computing the F-value at a probability (p) of 0.001, 0.01 or 0.05. Terms that were not significant were deleted one at a time (stepwise deletion) and the polynomial was recalculated.
Result and discussion
Statistical analysis
The results of the complete three-factor three level factorial experiment designs with five-center points presented in Table 1. Replications of runs at the center points were used to estimate the experimental error and to allow for checking the adequacy of the second-order model. Model analysis, which included checking the validity of the model with the help of various relevant statistical aids, such as—F-value, coefficient of determination (R2) and coefficient of variation (c.v.) revealed that all the models were statistically adequate (Chakraborty et al. 2011). The result of the regression analysis and analysis of variance (ANOVA) for all the models is reported in Table 2. The probability values of all regression models were less than 0.05, indicating that models terms are significant at a confidence level of 95 %. The F- value of lack of fit for all models implies the lack of fit is not significant relative to the pure error. The R2 values provide a measure of how much of variability in the observed response value can be explained by the experimental factors and their interactions. A good model (R2 values above 90 % are considered very well) explains most of the variation in the response. R2 values for juice recovery, TDS, viscosity, turbidity, cloud stability and L value were 98.89, 99.42, 96.67, 99.50, 99.45 and 99.12 %, respectively. Coefficient of variation (C.V.) indicates the relative dispersion of the experimental points from the prediction of the model. It is desirable to have a C.V. of less than 10 %. In the present c.v. for juice recovery, TDS, viscosity, turbidity, cloud stability and L value were 0.82, 0.68, 3.46, 3.07, 5.44 and 1.37 %, respectively.
Table 2.
Analysis of regression and variance of the second order polynomial models for dependent variables of clarified watermelon juice by enzymatic process
| Source | Juice recovery | TDS | Viscosity | Turbidity | Cloud stability | Lightness |
|---|---|---|---|---|---|---|
| Regression | ||||||
| Model | 80.65* | 7.66* | 3.420* | 90.80* | 0.069* | 17.14* |
| Linear | ||||||
| X1 | 4.97* | 0.58* | −7.125* | −30.44* | −0.047* | 1.93* |
| X 2 | −0.023N.S. | 0.063** | −7.500 N.S. | −6.22* | −3.050 N.S. | 2.50 N.S. |
| X3 | 0.17 N.S. | 0.31** | −1.375** | −13.09* | 0.010* | 0.27** |
| Interactive | ||||||
| X1 * X 2 | 0.33 N.S. | 0.050N.S. | −1.250 N.S.. | −2.24 N.S. | 4.250 N.S. | −0.020 N.S. |
| X1 * X3 | 1.01** | 0.20* | −2.000** | −5.87** | 5.075*** | −0.072 N.S. |
| X 2 * X3 | −0.88*** | 0.025 N.S. | −2.500 N.S. | 0.20 N.S. | 1.325 N.S. | −0.26*** |
| Quadratic | ||||||
| X21 | 3.31* | 0.11** | −5.850* | −4.84** | 0.024* | −0.42** |
| X22 | −0.62 N.S. | 0.032 N.S. | −6.000N.S. | −6.72* | −7.088** | −0.74* |
| X23 | −2.41* | −0.017 N.S. | −3.500 N.S. | 0.57 N.S. | −2.937 N.S. | −1.08* |
| ANOVA | ||||||
| R2, % | 98.89 | 99.42 | 96.67 | 99.50 | 99.45 | 99.12 |
| R2(adj), % | 97.46 | 98.80 | 96.96 | 98.85 | 98.74 | 97.99 |
| R2(Pre), % | 93.07 | 96.25 | 85.42 | 93.59 | 92.56 | 92.24 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Coefficient of variation, % | 0.82 | 0.68 | 3.46 | 3.07 | 5.44 | 1.39 |
| Adequate precision | 24.87 | 43.84 | 22.18 | 43.24 | 37.006 | 26.60 |
| Lack-of-fit | 0.6290 N.S. | 0.541 N.S. | 0.1985 N.S. | 0.0867 N.S. | 0.051 N.S. | 0.3783N.S. |
X1 = Enzyme concentration; X2 = incubation time; X3 = incubation temperature, N.S. non significant, * Significant at 0.001 level, ** Significant at 0.01 level., *** Significant at 0.05 level
Juice recovery
Perusal of Table 2 reveals that enzyme concentration had a positive effect on juice recovery at linear and quadratic level, showing a highly significant level (p < 0.001). The juice recovery could have increased because an enzyme that hydrolysed protopectin might have liberated water soluble pectin. The commercial pectin enzyme is added to aid in liquefaction of plums in varying concentration of pectinase enzyme at constant temperature and time (Chang et al. 1995). It is evinced from Table 2 that at quadratic level incubation temperature significantly (p < 0.001) decreased juice recovery. From the Fig. 1a, the interaction effect between enzyme concentration and temperature significantly increased with increase in enzyme concentration and temperature up to optimum level after that temperature increased it decreases the juice recovery. The interaction effect between incubation time and incubation temperature was significant (p < 0.05) and its effect were negative on juice recovery. Incubation temperature and incubation time also increases enzyme activity by increasing the kinetic energy within molecules i.e. faster reaction or catabolism or anabolism. Although at extreme temperature and time, the enzyme denatures. The findings of Zhanga et al. (2007) is comparable with the result of present study who reported that juice recovery varied curvilinearly with increase of α-amylase dosage, alcalase dosage and hydrolysis time. Litchi pulp treated with pectinase enzyme facilitated extraction of juice (Vijayanand et al. 2010). The use of fungal enzyme in fruit juice extraction had shown significant increases in juice recovery as compared to cold and hot extraction (Joshi et al. 1991). Kaur et al. (2009) reported that enzyme concentration was the most significant factor that affects the juice recovery due to the degradation of pectin substances. Diwan and Shukla (2006) also reported similar behaviour in guava juice. An increase in pineapple juice yield by 52.9 % was observed using xylalase enzyme (Pal and Khanum 2011). Our results are, therefore, in corroboration with the result of other researches that enzyme concentration boots the juice recovery.
Fig.1.
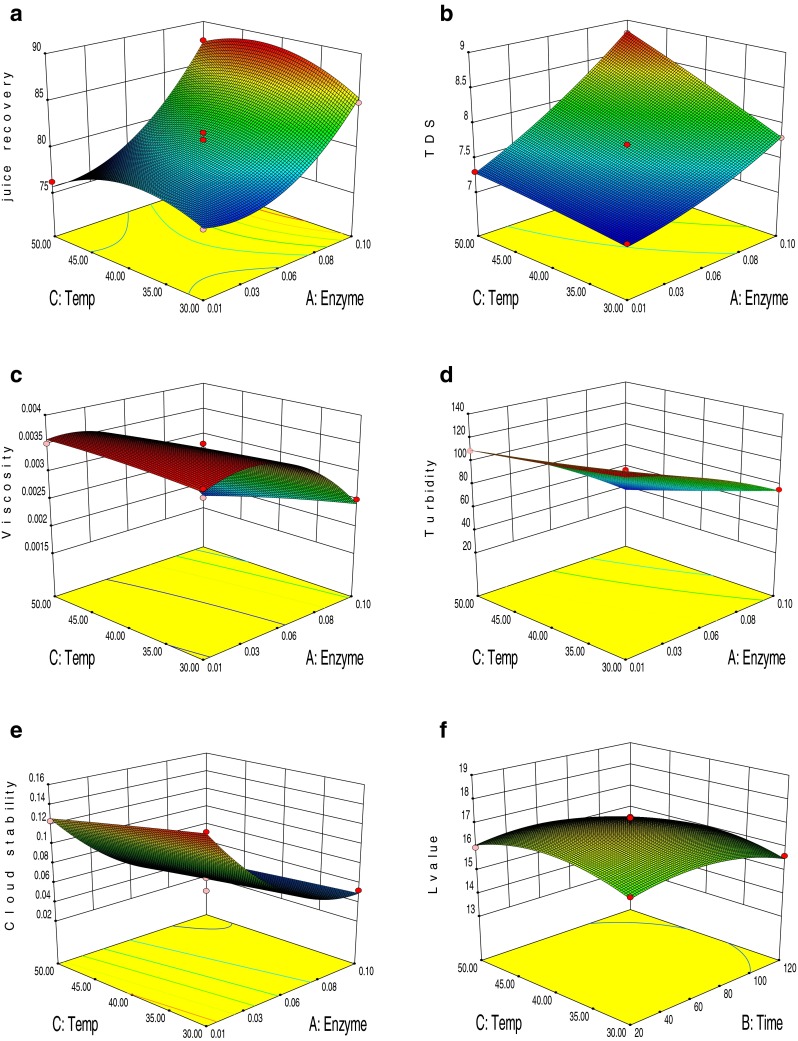
Response surface plots for different parameters as a function of enzyme concentration, incubation time and incubation temperature
Total dissolved solid (TDS)
A simple Brix measurement shows all of the dissolved solids in the sample which includes soluble minerals, carbohydrates (sugars and starches) and other soluble chemicals such as amino acids, enzyme and so on. For fruits, high total dissolved solids are desirable.
It can be inferred from Table 2 that enzyme concentration significantly (p < 0.001) affected the TDS at linear level. Incubation time and incubation temperature also affected the TDS at linear level with a positive effect but to a lesser extent (p < 0.01). Enzyme concentration, incubation time and incubation temperature showed significantly positive effect at linear level, but at quadratic level only enzyme concentration showed significant (p < 0.001) positive effect. A significant (p < 0.001) positive interactive effect showed by enzyme concentration and incubation temperature. This might be due to the action of enzyme on the pectic substances of juice pulp causing hydrolysis of these substances and release of dissolve material. Influence of enzyme concentration and incubation temperature on TDS is shown in Fig. 1b at constant time. According to Norjana and Noor Aziah (2011), TDS content of the enzyme treated pulps increased slightly with increase in incubation time. The present result was in agreement with observation of Shah (2007) who achieved significantly higher TDS by enzyme-assisted process for juice extraction and clarification from litchis.
Viscosity
Fruit and vegetable products exist in various forms from simple newtonian fluids (clarified juices), disperse systems (juices and pulps) to solids (fresh and processed fruits and vegetable). Fluids, however viscous, yield in time to stress and begin to flow; but solids, however plastic, require a certain magnitude of stress before they begin to flow. The science of rheology is devoted to the study of flow and deformation.
Table 2 showed the effect of the independent variables on the viscosity. Enzyme concentration was significantly (p < 0.001) affect the viscosity at linear as well as quadratic level. The effect of incubation temperature was also significant (p < 0.01) at linear level. Enzyme concentration and incubation temperature showed a negative effect on linear and quadratic level. It was clear from the Fig. 1c that viscosity decreased with the increase in enzyme concentration and temperature at constant time. This phenomenon may be due to the breakdown of cohesive network of pectinaceous substances because of enzymatic action, which decreases the water holding capacity (WHC) of juice pulp and therefore available water released to the juice to further reduce the viscosity. The interaction effect between enzyme concentration and incubation temperature was significant (p < 0.01) and its effect was negative on viscosity as evident from Table 2, meaning that the action of enzyme was dependent on the incubation temperature during enzyme treatment. Lee et al. (2006) reported that the rate of enzymatic action increase with increase in temperature. Abdullah et al. (2007) and Sin et al. (2006) also reported that enzymatic action upon the pectin, leading to a reduction of WHC, releasing free water into the system and reducing the viscosity of the juice
Turbidity
In preparation of fruit juices, haziness is the major problem due to the presence of pectins. Pectin can be associated with plant polymers and the cell wall debris which are fibrous molecular structures that principally consist of cellulose and protein with small amount of hemicelluloses and hydroxyproline-rich protein (Smock and Neubert 1950). Turbidity in fruit juice can be a positive or negative attributes dependending on the expectation of the consumers (Hutchings 1994).
The result presented in Table 2 showed that enzyme concentration, incubation time and incubation temperature significantly (p < 0.001) reduced the turbidity at linear level whereas at quadratic level enzyme concentration (p < 0.01) and incubation time (p < 0.001) showed negative effect on turbidity. Interaction between enzyme concentration and incubation temperature showed significant (p < 0.01) negative effect at constant time (Fig. 1d). Juice turbidity may be decrease due to the action of pectinase on the pectin layers encapsulating the protein core of proteinaceous pectin particles in suspension. This action result in an electrostatic agglomeration of oppositvely charged particles that may lead to transient turbidity increase and subsequently results in precipitation of agglomerated complex resulting in decreased turbidity. Similar result were achieved by Grassin and Fauquembergue (1999) that increases enzyme concentration, incubation time and incubation temperature might decrease the turbidity due to polygalacturonase action that corresponding to hydrolysis of pectin substances and causing pectin protein complex to flocculate.
Cloud stability
For clarified fruit a juice, a juice that has an unstable cloud is considered ‘muddy’ is unacceptable to be marketed as clear juices (Floribeth et al. 1981). Figure 1e shows the surface diagram of the independent variables on the juice cloud stability. From Table 2, it may be observed that cloud stability depends on the enzyme concentration as its linear effect was negative at p < 0.001 whereas quadratic effect was positive at p < 0.001. Hence overall effect was curvilinear in nature (Fig. 1e). Lower absorbance values indicated a good stability of cloud. Increase in enzyme concentration may increase the rate of clarification by exposing the positively charged protein beneath, thus reducing electrostatic repulsion between cloud particles which clumps together and theses clumps are eventually settle out. The similar result was reported by Sin et al. (2006). Incubation temperature also showed significant (p < 0.001) effect on linear level. At quadratic level incubation temperature showed non-significant negative effect whereas incubation time showed significant (p < 0.01) negative effect. Figure 1e showed significantly (p < 0.01) positive interaction effect between enzyme concentration and incubation time at constant temperature. Ahmad et al. (2009) also reported that cloud stability decreased significantly (p < 0.01) with increase in levels of enzymes and incubation time. In general, time required to obtain a clear juice inversely proportional to the concentration of enzyme used at constant temperature (Kilara 1982).
Lightness
L value represents the lightness index of a juice. In clarified juice value should be very high. A dark colour product is usually less appealing to the consumers as it may indicate deterioration. An attractive red colour is one of the main characteristics of watermelon juice. Most of the undesirable changes on the attractive red colour and flavour of watermelon juice are catalyzed by enzymes such as peroxides, lipoxygenase, pectin methyl esterase and polygalacturonase (Robinson 1991).
All the independent variables showed a positive effect on lightness at linear level whereas the effect was negative at quadratic level and interactive level on lightness. From the Table 2, it was observed that enzyme concentration significantly affect the lightness at linear (p < 0.001) and quadratic (p < 0.01) level. Incubation time and temperature also significantly (p < 0.001) affected the L value at quadratic level whereas at the linear level only incubation temperature showed significant (p < 0.01) positive effect. The interaction between incubation time and temperature deteriorated the colour significantly (p < 0.05) at constant enzyme concentration. It is evince from Fig. 1f that L value decreased with increase incubation time and temperature up to particular level at constant enzyme concentration. The changes in L value may be due to the formation of brown pigment. When maillard reaction takes place between amino acid and carbonyl compounds, the subsequent formation of 5 hydroxymethyl furfural (HMF) and other decomposed products causes undesirable brown colour development. Mackinney and Chichester (1952) also reported that colour deterioration in strawberry fruit was due to the formation of brown pigments. Heating can creates an opportunity for oxidative reactions, which cause a degradation of the pigments.
Optimization of enzyme treatments for clarified watermelon juice and model validation
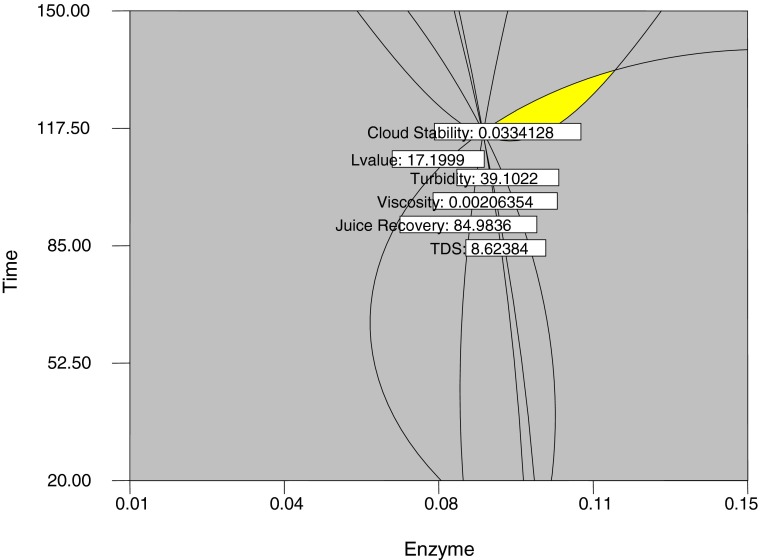
The optimum formulation was determined by superimposing the contour plots of all the responses. The final condition would be considered optimum if the juice recovery, TDS and L value were as high as possible while turbidity, cloud stability and viscosity were as low as possible. Figure 2 shows the optimum condition of clarification process to generate maximum juice recovery, L value and TDS and minimum absorbance value, turbidity and viscosity, respectively. The goal that was set for obtaining the best result combination is illustrated in Table 3 regarding to the efficiency and it’s cost-effectiveness. Criteria set in Table 3 produced an optimum region in the superimposed plot shown in Fig. 2. The data were analyzed in Design Expert version 7.1.6 (2009) and a solution obtained for optimization as detailed in Table 4, which gives the optimum parameters for responses juice recovery, TDS, viscosity, turbidity, cloud stability and L value along with predicted response values. The juice recovery, TDS, viscosity, turbidity, cloud stability and L value were 86.27 %, 8.6°Brix, 0.0020 Pa.s., 39.37 NTU, 0.033 abs and 17.57 L value, respectively when enzyme treatment was set up with 0.09 % w/w enzyme concentration at 46.90 °C incubation temperature and 117.45 min incubation time. The calculated values of ‘t’ for all responses were found non significant at 5 % level of significance. It is, therefore, confirmed that the selected combination is the best in term of juice recovery, TDS, viscosity, turbidity, cloud stability and lightness (Table 4).
Fig. 2.
Superimposed diagram for optimum condition as a function of enzyme concentration and incubation time at 46.90 °C
Table 3.
Optimization parameter in the response optimizer for clarification of watermelon juice by enzymatic method
| Name | Goal | Lower limit | Upper limit |
|---|---|---|---|
| Enzyme (%) | Minimize | 0.01 | 0.1 |
| Incubation time (min) | Is in range | 20 | 120 |
| Incubation temperature (°C) | Is in range | 30 | 50 |
| Juice recovery (%) | Maximize | 76.23 | 88.54 |
| TDS (°Brix) | Maximize | 7.1 | 8.8 |
| Viscosity (Pa.s.) | Minimize | 0.0017 | 0.0035 |
| Turbidity (NTU) | Minimize | 38 | 121.27 |
| Cloud stability (abs) | Minimize | 0.033 | 0.154 |
| Lightness (L value) | Maximize | 13.57 | 18.01 |
Lower and upper weights values were 1 and Importance values were 3 for all variables
Table 4.
Optimized level of process parameter for clarification of watermelon juice by enzymatic method along with the predicted values for the response
| Responses | Predicted value# (μo) | Experimental value@ (μ1) | % deviation | tcal |
|---|---|---|---|---|
| Juice recovery (%) | 84.71 | 86.27 | 1.35 | 3.245 |
| TDS (°Brix) | 8.6 | 8.7 | 1.14 | 1.724 |
| Viscosity (Pa.s.) | 0.0020 | 0.0020 | 0.0 | 0.000 |
| Turbidity (NTU) | 39.70 | 39.37 | 0.83 | −0.520 |
| Cloud stability (abs) | 0.033 | 0.033 | 0.0 | 0.000 |
| Lightness (L value) | 17.18 | 17.57 | 2.21 | 0.586 |
The recommended enzyme clarification condition was 0.09 % enzyme concentration at 46.90 °C for 117.45 min, Ho: μo = μ1, tcal < ttable at p < 0.1, ‘Ho’ was accepted., @ mean of five replications., # the desirability for this result was 0.64
Conclusion
The methodology of the experimental design was shown to be very useful for the evaluation of enzyme hydrolysis for watermelon juice clarification. The different conditions (incubation time, incubation temperature and concentration of the enzyme) for enzyme treatment revealed that all these variables markedly affected the different physical parameters (juice recovery, TDS, viscosity, turbidity, cloud stability and L value) and can be related to the enzyme treatment conditions by second order polynomials. The recommended enzyme clarification condition was 0.09 % enzyme concentration at 46.90 °C for 117.45 min.
References
- Abdullah LAG, Sulaiman NM, Aroua MK, Megat Mohd Noor MJ. Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J Food Eng. 2007;81(1):65–71. doi: 10.1016/j.jfoodeng.2006.10.013. [DOI] [Google Scholar]
- Ahmad I, Jha YK, Anurag RK. Optimization of enzymic extraction process for higher yield and clarity of guava juice. J Food Sci Technol. 2009;46(4):307–311. [Google Scholar]
- Alvarez S, Alvarez R, Riera FA, Coca J. Influence of depectinization on apple juice ultrafiltration. Colloids Surf A. 1998;138(2–3):377–382. doi: 10.1016/S0927-7757(98)00235-0. [DOI] [Google Scholar]
- Chakraborty SK, Kumbhar BK, Chakraborty S, Yadav P. Influence of processing parameters on textural characteristics and overall acceptability of millet enriched biscuits using response surface methodology. J Food Sci Technol. 2011;48(2):167–174. doi: 10.1007/s13197-010-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Siddiq M, Sinha NK, Cash JN. Commercial pectinase and the yield quality of stanley plum juice. J Food Process Preserv. 1995;19(2):89–101. doi: 10.1111/j.1745-4549.1995.tb00280.x. [DOI] [Google Scholar]
- Dawes H, Struebi P, Keene J. Kiwi fruit juice clarification using a fungal proteolytic enzyme. J Food Sci. 1994;59(4):859–861. doi: 10.1111/j.1365-2621.1994.tb08144.x. [DOI] [Google Scholar]
- Design Expert version 7.1.6 (2009) Stat-Ease, Inc., MN, USA
- Diwan A, Shukla SS. Process development for the production of clarified banana juice. J Food Sci Technol. 2006;42(3):245–249. [Google Scholar]
- Edwards AJ, Vinyard BT, Wiley ER, Brown ED, Collins JK, Perkins-Veazie P, Baker RA, Clevidence BA. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J Nutr. 2003;133(4):1043–1050. doi: 10.1093/jn/133.4.1043. [DOI] [PubMed] [Google Scholar]
- Floribeth V, Lastreto C, Cooke RD. A study of the production of clarified banana juice using pectinolytic enzymes. J Food Sci Technol. 1981;16(2):115–125. [Google Scholar]
- Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43(3):228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gerhartz W. Industrial uses of enzymes. In: Gerhartz W, editor. Enzyme in industry production and application. Weinheim: VCH Verlagsgesellschaft MBH publishers; 1990. pp. 77–92. [Google Scholar]
- Gil MI, Aguayo E, Kaer AA (2006) Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. J Agric Food Chem 54(12):4284–4296 [DOI] [PubMed]
- Giovannucci E. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp Biol Med (Maywood) 2002;227(10):852–859. doi: 10.1177/153537020222701003. [DOI] [PubMed] [Google Scholar]
- Grassin C, Fauquembergue P. Enzymes, fruit juice processing. In: Flickinger MC, Drew SW, editors. Encyclopedia of bioprocess technology: fermentation, biocatalysis, bioseparation. New York: Wiley; 1999. pp. 1030–1061. [Google Scholar]
- Huor S, Ahmed EM, Rao PV, Cornell JA. Formulation and sensory evaluation of a fruit punch containing watermelon juice. J Food Sci. 1980;45(4):809–813. doi: 10.1111/j.1365-2621.1980.tb07455.x. [DOI] [Google Scholar]
- Hutchings JB (1994) Food colour and appearance. 2nd edn. In: An Aspen publication, Gaitherburg, USA, pp19–20
- Joshi VK, Chauhan SK, Lal BB. Extraction of juices from peaches, plums and apricots by pectinolytic treatment. J Food Sci Technol. 1991;28(1):65.1–66.1. [Google Scholar]
- Kaur S, Sarkar BC, Sharma HK, Singh C. Optimization of enzymatic hydrolysis process parameters for maximum juice recovery from guava using response surface methodology. Food Bioprocess Tech. 2009;2(1):96–100. doi: 10.1007/s11947-008-0119-1. [DOI] [Google Scholar]
- Khuri AI, Cornell JA (1989) Response surfaces: designs and analyses. In: Marcel Dekker publication, New York, USA
- Kilara A. Enzymes and their uses in the processed apple industry: a review. Process Biochem. 1982;17(4):35–41. [Google Scholar]
- Kulkarni SG, Vijayanand P, Shubha L. Effect of processing of dates into date juice concentrate and appraisal of its quality characteristics. J Food Sci Technol. 2010;47(2):157–161. doi: 10.1007/s13197-010-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Yusof S, Hamid NSA, Baharin BS. Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM) J Food Eng. 2006;73(1):55–63. doi: 10.1016/j.jfoodeng.2005.01.005. [DOI] [Google Scholar]
- Mackinney G, Chichester CO. Color deterioration in strawberry preserves. Canner. 1952;114(12):13. [Google Scholar]
- Myers RH. Response surface methodology. Boston: Allyn and Bacon Inc; 1971. [Google Scholar]
- Norjana I, Noor Aziah AA. Quality attributes of durian (Durio zibethinus Murr) juice after pectinase enzyme treatment. Int Food Res J. 2011;18(3):1117–1122. [Google Scholar]
- Pal A, Khanum F. Efficacy of xylanase purified from Aspergillus niger DFR-5 alone and in combination with pectinase and cellulose to improve yield and clarity of pineapple juice. J Food Sci Technol. 2011;48(5):560–568. doi: 10.1007/s13197-010-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Veazie P, Collins JK, Pair SD, Roberts W. Lycopene content differs among red-fleshed watermelon cultivars. J Sci Food Agric. 2001;81(10):983–987. doi: 10.1002/jsfa.880. [DOI] [Google Scholar]
- Rai P, Majumdar GC, Gupta DS, De S. Optimizing pectinase usage in pretreatment of mosambi juice for clarification by response surface methodology. J Food Eng. 2004;64(3):397–403. doi: 10.1016/j.jfoodeng.2003.11.008. [DOI] [Google Scholar]
- Rastogi G. Vishal’s objective botany. Meerut: Vishal publishers; 1998. [Google Scholar]
- Reddy LV, Reddy YHK, Reddy LPA, Reddy OVS. Wine production by novel yeast biocatalyst prepared by immobilization on watermelon (Citrullus vulgaris) rind pieces and characterization of volatile compounds. Proc Biochem. 2008;43(7):748–752. doi: 10.1016/j.procbio.2008.02.020. [DOI] [Google Scholar]
- Robinson DS. In: Peroxidases and catalases in foods. Oxidative enzymes in foods. Robinson DS, Eskin NAM, editors. London: Elsevier Applied Science; 1991. pp. 1–47. [Google Scholar]
- Sahin S, Bajindirli L. The effect of depectinization and clarification on the filtration of sour cherry juice. J Food Eng. 1993;19(3):237–245. doi: 10.1016/0260-8774(93)90045-L. [DOI] [Google Scholar]
- Santin MM, Treichel H, Valduga E, Cabral LMC, Luccio MD. Evaluation of enzymatic treatment of peach juice using response surface methodology. J Food Agric Environ. 2008;88(3):507–512. doi: 10.1002/jsfa.3114. [DOI] [Google Scholar]
- Shah N. Optimization of an enzyme assisted process for juice extraction and clarification from litchis (Litchi chinensis sonn.) Int J Food Eng. 2007;3(3):1–17. doi: 10.2202/1556-3758.1073. [DOI] [Google Scholar]
- Sin HN, Yusuf S, Sheikh AHN, Rahman RA. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J Food Eng. 2006;73(4):313–319. doi: 10.1016/j.jfoodeng.2005.01.031. [DOI] [Google Scholar]
- Smock RM, Neubert AM. Apples and apples products. New York: Inter Science publishers; 1950. [Google Scholar]
- Sreenath HK, Sudarshan KKR, Santhanam K. Improvement of juice recovery from pine pulp residue using celluloses and pectinases. J Ferment Bioeng. 1994;78(6):486–488. doi: 10.1016/0922-338X(94)90054-X. [DOI] [Google Scholar]
- Vijayanand P, Kulkarni SG, Prathibha GV. Effect of pectinase treatment and concentration of litchi juice on quality characteristics of litchi juice. J Food Sci Technol. 2010;47(2):235–239. doi: 10.1007/s13197-010-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Ibrahim N. Quality of sour soup juice after pectinase enzyme treatment. Food Chem. 1994;51:83–88. doi: 10.1016/0308-8146(94)90052-3. [DOI] [Google Scholar]
- Zhanga H, Wang Z, Xua SY. Optimization of processing parameters for cloudy ginkgo (Ginkgo biloba Linn.) juice. J Food Eng. 2007;80(4):1226–1232. doi: 10.1016/j.jfoodeng.2006.09.021. [DOI] [Google Scholar]