Abstract
Browning in raw and processed yams resulting from enzymes, polyphenol oxidase (PPO) and peroxidase (POD), activities is a major limitation to the industrial utilization of Dioscorea varieties of yams. Two elite cultivars of D. rotundata species were selected to study the spatial distribution of total phenols and enzymes (PPO and POD) activities. The intensities of tissue darkening in fresh yam chips prepared from the tuber sections of cultivars during frozen storage were also studied. Total phenolic content was observed to be highest in the head and mid sections of the cultivars than at the tail end. PPO activity did not have any specific distribution pattern whereas POD activity was found to be more concentrated in the head than in the middle and tail regions. Browning was found to be most intense in the head regions of the two cultivars studied; and was observed to correlate with total phenol and dry matter contents of tubers. Between the two enzymes, POD activity appeared to be more related to browning than PPO.
Keywords: Peroxidase, Polyphenol oxidase, Browning, Enzyme activity, D. rotunda
Introduction
Yam, Dioscorea sp., is a multi-species tuber crop cultivated in Africa, Asia, parts of South America, as well as the Caribbean and the South Pacific islands (Asiedu and Satie 2010). It is a major staple in West Africa where it is cultivated extensively (Akissoe et al. 2011).
Yam is mostly consumed directly after roasting, frying in oil, grilling or boiling. Yam tissues may also be cooked into pottage with added protein sources and vegetable oil; or boiled and pounded (kneaded) into thick dough (called ‘fufu’ or ‘pounded yam’) that is consumed with soup. Commercially, few products based on dry flakes or flours from yams are produced in West Africa for export and sale in urban areas (Ayernor 1976; Akissoe et al. 2003; Asiedu and Satie 2010). Yam shares many characteristics with potatoes (Solanum tuberosum) as both are tubers. They both have starch as their major storage form of reserves and their main food value is dietary energy due to the high starch reserves. French-fries are hugely popular all over the world, and frozen potato chips have evolved into a multi-billion dollar business. Yams have the potential of similarly evolving into an extended market. To achieve this, research on the post-harvest physiology, management and expanded utilization of the crop needs to be stepped up. Perhaps, the greatest limitation to the utilisation of yam is the high rate of browning of products made from it (Ayernor 1976; Anosike and Ikediobi 1985; Akissoe et al. 2003; Krishnan et al. 2010). The discoloration phenomenon, a major cause of low consumer acceptability of yam products’, has long been studied and has been suggested to occur in three ways- by the non-enzymatic (Maillard) mechanism (Hodge and Osman 1976); by the autodegradation of sugars when heated (Hodge and Osman 1976); and by the enzymatic mechanism. Enzymatic browning is by far the most prominent cause of discolouration during yam processing (Osagie and Opoku 1984; Almenteros and Del Rosatio 1985; Asemota et al. 1992; Krishnan et al. 2010).
Enzymatic browning, a surface phenomenon requiring molecular oxygen and specific phenolic substrates (Palmer 1963; Sheen and Calvert 1969), occurs in numerous crops and has been reported in all edible yams examined so far (Omidiji and Okpuzor 1996). It results in the development of off-colours and the formation of off-flavours that cause a decrease in the acceptability of food. Polyphenol oxidase (PPO) and peroxidase (POD) are the two major enzymes implicated. Polyphenol oxidase catalyses the oxidation of polyphenols to o-quinones (Mayer and Harel 1979; Chilaka et al. 2002) which in turn spontaneously polymerise to produce high molecular weight compounds or brown pigments (Ihekoronye and Nggody 1985). In litchi fruit (Litchi chinensis), PPO has been associated with pericarp browning, a major post-harvest problem which reduces the fruit’s commercial value (Neog and Saikia 2010; Kumar et al. 2011). Peroxidase, a ubiquitous enzyme in plant physiological activities with functions related to morphogenic changes in cell division, growth and differentiation (Goleniowski et al. 2001) catalyses the oxidation of a number of aromatic compounds and has been implicated in darkening of fresh and processed vegetables and fruits (Finger 1994; Chisari et al. 2008; Tian et al. 2011). PPO and POD kinetics and phenolics has been studied (Martinez and Whitaker 1995; Omidiji and Okpuzor 1996; Akissoe et al. 2005; Omigiji et al. 2006). Omidiji and Okpuzor (1996) observed that 86 % of the browning in D. cayenensis, D. alata and D. esculenta was associated with peak PPO activity whereas only 9 % and 40 % of browning in D. dumetorum and D. rotundata respectively was PPO related. POD has been reported to contribute up to 40 % of enzyme-mediated browning in D. esculenta (Omigiji et al. 2006). Both PPO and POD utilise similar substrates, mostly phenols (Martinez and Whitaker 1995; Omigiji et al. 2006). Phenol content has also been reported to vary in different yams (Akissoe et al. 2003).
Morphologically, the yam tuber can be divided into three sections- the head, the middle and the tail (Oluoha 1988; Degras 1993). This study sought to investigate the distribution of phenols (enzyme substrate) and enzymes (PPO and POD) activities across the three regions of two elite cultivars of D. rotundata. Browning development in yam chips processed from these different sections of tubers from the two cultivars of D. rotundata during frozen storage was also studied. The specific gravities and dry matter contents of the different sections of the yam tubers were also determined and correlated with enzyme activity and browning. This formed part of a larger study seeking to develop frozen yam chips similar to frozen potato chips used in preparing French Fries.
Materials and methods
Plant material
Two white yam (Diosocorea rotundata) cultivars, locally known as “Puna” and “Bayere fitaa” (BF), were obtained at full maturity (12 months) from the Agricultural farms of the Crop Research Institute of Ghana, Fumesua, near Kumasi, Ghana and stored on a wooden platform at ambient temperatures (27–29 °C) and relative humidities (85–95 %) until used.
Experimental design
A 2 × 3 factorial design with the factors being yam cultivar (Puna and BF) and tuber section- head (apical), middle (medial) and tail (distal) was employed to study the distribution of phenols and oxidative enzymes: polyphenol oxidase and peroxidase.
Sample preparation
Yam tubers were washed with tap water and cut into the three morphological sections head, middle (mid) and tail sections (Oluoha 1988; Degras 1993). The sections were peeled and yam chips of about 1 cm2 cross sections and 6–7 cm lengths prepared. All cutting operations including chips preparation were carried out at ambient temperature (27–29 °C). Cut samples were however submerged immediately under tap water. Samples were taken for enzyme activity assay.
The rest of the prepared chips were frozen in a General Electric FUM21SVRWW freezer at set temperature of −12 °C for 10 days. Samples were taken after 5, 7 and 10 days for determination of brown index.
Analytical methods
Dry matter content
Dry matter was determined using the Association of Official Analytical Chemists’ Method Number 32.082. Moisture contents of samples were determined and dry matter calculated following the established procedures (AOAC 1984).
Specific gravity
Specific gravity (SG) of tuber sections were measured by using the weight-in-air–weight-in-water method (Kleinkopf et al. 1987), where 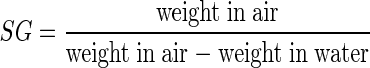
Total phenol content
Total phenolics were determined using the Folin-Ciocalteau reagent (Singleton and Rossi 1965). Mashed samples (2 g) were homogenized in 80 % (v/v) aqueous ethanol at room temperature (27–29 °C) and centrifuged at 10 000 g at 4 °C for 15 min and the supernatant was saved. The residue was re-extracted twice with 80 % (v/v) ethanol and supernatants were pooled, put into evaporating dishes and evaporated to dryness at room temperature. The residue was dissolved in 5 ml distilled water. One-hundred microlitres of this extract was diluted to 3 ml with water and 0.5 ml of Folin-Ciocalteau reagent was added. After 3 min, 2 ml of 20 % (w/v) sodium carbonate was added and the contents were mixed thoroughly. The absorbance of the blue product was measured at 650 nm in a Jenway 6,505 UV/Vis spectrophotometer after 60 min of colour development using catechol as a standard. The results were expressed as mg catechol/100 g of fresh weight material.
Polyphenol oxidase (PPO) activity
The method proposed by Montgomery and Sgarbieri (1975) was followed. Five grams of tissue were homogenized with 0.6 g of polyvinyl polypyrolidone (PVPP) and 20 ml of 50 mM (pH 7) phosphate buffer. This was then filtered and centrifuged at 10,000 × g at 4 °C for 15 min. The supernatant was used as the enzymatic extract. The activity was measured with 2.85 ml of 0.2 mM (pH 7) phosphate buffer, 50 μl of catechol (60 mM) as a substrate and 100 μl of enzymatic extract. The mixture was maintained at 25 °C and the change in absorbance was read over 3 min at 420 nm using a Jenway 6,505 UV/Vis spectrophotometer. Activity was expressed as units of activity (UA) of enzyme per 100 g fresh weight of sample (UA/100 g) in which one unit of activity (UA) of PPO enzyme was defined as the change in one unit of absorbance per second.
Peroxidase (POD) activity
Crude extracts of the enzyme were obtained from 6 g of homogenized tissue with 0.6 g of polyvinyl-polypyrolidone (PVPP) and 20 ml of a phosphate buffer (50 mM, pH 7). The homogenized mixture was centrifuged (10,000 × g, 15 min at 4 °C) and the supernatant liquid was used as an extract of the enzyme. The enzyme activity was measured using methylene blue as substrate (Magalhaes et al. 1996). The assay mixture contained 2.2 ml of the diluted supernatant, 0.1 ml of 1.2 mM methylene blue and 0.6 ml of 0.5 M sodium tartrate buffer (pH 4.0). The reaction was started by the addition of 0.1 ml of 2.7 mM hydrogen peroxide. The conversion of the dye to Azure C was monitored by the measurement of the decrease in absorbance at 664 nm. The results were expressed as units of activity (UA) of enzyme per 100 g fresh weight of sample (UA/100 g) in which one unit of activity (UA) of POD enzyme was defined as the change in one unit of absorbance per second.
Determination of brown index (BI)
Yam chips were packed tightly together to fill the base of cylinder accompanying the Minolta CR- 310 Tristimulus Colour meter (Minolta Camera Co. Ltd, Osaka, Japan). The colorimeter was then affixed to cylinder and the colour space parameter Lightness (L) of samples measured. Quadruple readings per replicate experiment were taken and the means reported.
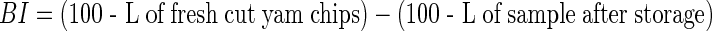 |
Data analysis
Data were analysed using Statgrahics centurion version 15, and Microsoft Office Excel 2007. Analysis of variance, multiple range tests and correlations were carried out. Significance levels were set at p ≤ 0.05. All experiments were carried out in triplicates. Standard errors of means were used in placing error bars on charts.
Results and discussion
Specific gravity and dry matter content of yam cultivars
Table 1 shows the specific gravity and dry matter content of the two D. rotundata cultivars used in study. The results show that specific gravity neither varies within cultivars nor between them. Specific gravity determination serves as a good approximation of the total solids or starch content of tubers (Onayemi et al. 1987). The dry matter content of the two D. rotundata cultivars used in the study ranged from 36–40 % in Puna to 25–34 % in Bayere fitaa (BF). The dry matter content of D. rotundata has been reported to be around 30 % (Akissoe et al. 2003). Between the cultivars Puna also had a significantly (p ≤ 0.05) higher mean dry matter than BF. Additionally, all sections of the Puna cultivar had higher dry matter content than the corresponding BF sections. Within each cultivar, dry matter decreased from head (apical) to tail (distal) region. Whereas dry matter did not vary significantly (p ≤ 0.05) between the head and mid as well as mid and tail sections of the tubers, higher dry matter contents were observed in the head than in the tail of both cultivars. Yam grows from the head towards the tail. The head region is the oldest part of the tuber and starch reserves are deposited there as the tuber grows towards the tip region. Dry matter thus aggregates more in the relatively older sections as the tuber grows.
Table 1.
Specific gravity and dry matter content across tuber sections of D. rotundata cultivars
| Yam section | Specific gravity (SG) | Dry matter (DM)/% | ||
|---|---|---|---|---|
| Puna SG ± SD | BF SG ± SD | Puna DM ± SD | BF DM ± SD | |
| Head | 0.96 ± 0.04a | 1.01 ± 0.07 a | 41.0 ± 0.99 b | 34.1 ± 3.51 b |
| Mid | 0.99 ± 0.04 a | 0.99 ± 0.02 a | 37.8 ± 4.13 a | 32.2 ± 0.69 b |
| Tail | 1.04 ± 0.17 a | 1.00 ± 0.04 a | 36.5 ± 2.10 a | 25.2 ± 7.88 a |
| Mean | 1.00 ± 0.10 | 1.00 ± 0.04 | 38.4 ± 3.16 | 30.5 ± 6.06 |
Values with different superscripts within the same column are significantly different at p ≤ 0.05. n = 3
BF Bayere fitaa
Distribution of total phenolic contents across tuber sections of D. rotundata cultivars
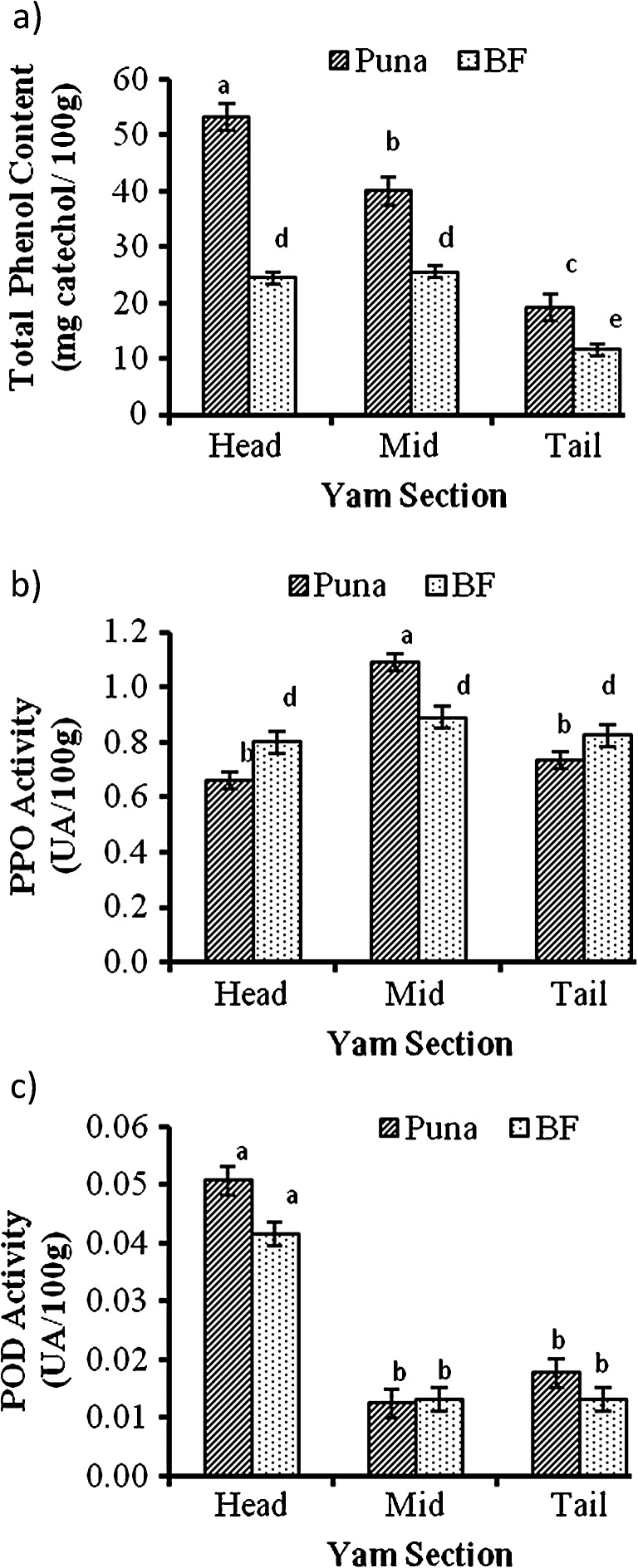
Significant differences (p ≤ 0.05) in total phenols were observed between the two cultivars with Puna having a higher concentration (37mg catechol/100g) of phenols than Bayere fitaa (20mg catechol/100g). Within each cultivar also, significant differences across the tuber sections- head, mid and tail regions were observed (Fig. 1a). In the Puna cultivar, the head section had the highest concentration of phenols followed by the mid and then the tail. In the BF cultivar however, phenol content within the head and mid-section did not differ from each other but were both significantly higher than that observed in the tail. As explained earlier, deposition of starch reserves starts from the head section of yam and with storage inclusions, secondary metabolites like phenols appear to be also deposited with a gradient starting from the head down to the tail and this explains why the upper parts had higher phenolic content than the tail. Akissoe et al. (2003) also reported variations in phenol content in different yam cultivars. Table 2 shows the correlations among the various variables. Total phenol content is observed to correlate strongly (0.89) with dry matter content signifying that the higher the dry matter content the higher the concentration of phenolic substances within a tuber.
Fig. 1.
Distribution of (a) Total Phenol Content, (b) PPO and (c) POD activities across sections of two D. rotundata cultivars. Each observation = mean ± SE; n = 3; Bars with different alphabets are significantly different (p ≤ 0.05). BF- Bayere fitaa; UA- Units of activity; SE- standard error
Table 2.
Pearson’s correlations between pairs of variables
| Total phenol content | Brown index | PPO activity | POD activity | Dry matter | Specific gravity | |
|---|---|---|---|---|---|---|
| Total phenol content | – | 0.76* | −0.19 | 0.57 | 0.82* | −0.44 |
| Brown index | 0.76* | – | 0.05 | 0.32 | 0.68* | −0.21 |
| PPO activity | −0.19 | 0.05 | – | −0.44 | −0.44 | −0.25 |
| POD activity | 0.57 | 0.32 | −0.44 | – | 0.53 | −0.32 |
| Dry matter | 0.82* | 0.68 | −0.44 | 0.53 | – | −0.28 |
| Specific gravity | −0.44 | −0.21 | −0.25 | −0.32 | −0.28 | – |
* Significant at p ≤ 0.05
Distribution of polyphenol oxidase (PPO) and peroxidase (POD) activities across tuber sections of D. rotundata cultivars
Results for PPO and POD activities are shown in Fig. 1(b–c). In Puna, PPO activity was found to be significantly higher (p ≤ 0.05) in the mid-section than in the head and tail portions which did not differ from each other. There were no significant differences (p > 0.05) in PPO activity across the tuber sections of BF. Between the two cultivars it was observed that except for the mid-section where PPO activity was higher in Puna than BF, for both head and tail sections PPO activity was higher in BF than Puna. Besides catalyzing enzymatic browning reactions no other use of PPO in plant tissues has been reported. Differences in PPO activity between the two cultivars can be attributed to sub-varietal (cultivar) differences. Any differences regarding the sections of the two cultivars with respect to PPO activity may be explained in terms of physiological states of the two cultivars. It must be noted that tubers are active underground stems and physiological differences may be apparent but not necessarily permanent.
Conversely, POD has functions related to morphogenic changes in cell division, growth and cell differentiation (Goleniowski et al. 2001). POD activity (Fig. 1c) was highest in the head regions of both yam cultivars than the mid and tail regions. Between the cultivars however, comparable activities were measured in each section. As POD activity is related to growth it will have more activity in the head region as it is the region where growth in yams are initiated.
Browning across tuber sections of yam cultivars
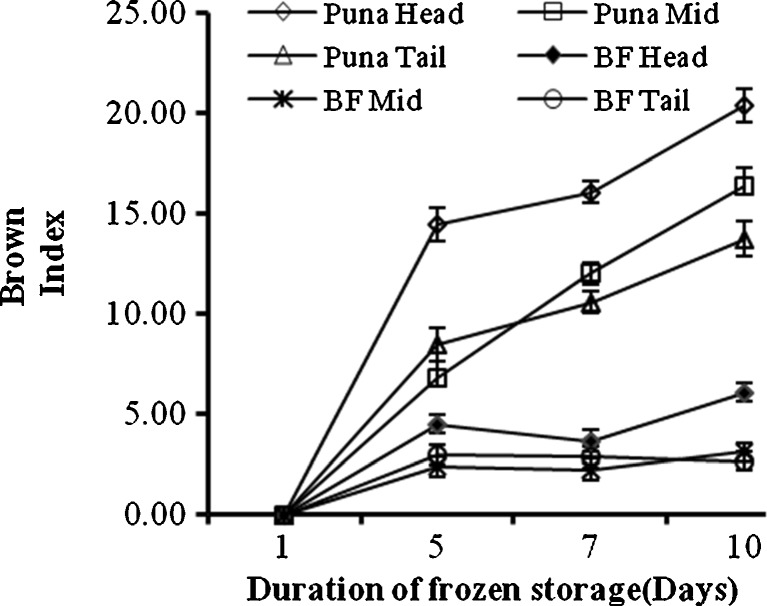
Browning in yam products is the major limitation to the use of yams in product development (Krishnan et al. 2010). Brown indices of yam chips during frozen storage are shown in Fig. 2. Generally, higher indices for browning were observed in frozen yam chips prepared from all sections of Puna than BF. In individual cultivars, the head regions recorded higher brown indices (Fig. 2) and also showed higher browning intensities than the mid and tail sections. The observed patterns in brown indices show that browning may be more associated with POD activity but cannot be limited to it. This is because although individual sections of both cultivars had comparable POD activity they differed in browning intensities which would not be the case if browning was limited to only POD activity. Again comparing the head sections of the two cultivars, BF had higher PPO activity than Puna but browning was still higher in Puna than BF which would not be the case if browning was only PPO dependent.
Fig. 2.
Browning across tuber sections of two D. rotundata cultivars during frozen (−12 °C) storage. Each observation = mean ± SE; n = 3; BF- Bayere fitaa; UA- Units of activity; SE- standard error
Significant (p ≤ 0.05) correlation (Table 2) was found between brown index and total phenol and dry matter contents of yam chips. The observed correlation between phenol content and brown index confirms the findings of Osagie and Opoku (1984) who concluded that the concentration of substrate (phenols) is the most important determinant of browning as well as reinforces the importance of phenols as substrates for enzymes (PPO and POD) activities and browning (Martinez and Whitaker 1995; Omigiji et al. 2006).
Conclusion
There are differences in the contents dry matter, total phenols, and enzymes activities across the sections of yam tubers. These sections also brown to different intensities during storage. Frozen storage time also had effect on the browning of products. The spatial distribution of the activities of enzymes and incidence of browning along the length of the tuber has been confirmed in this study. This information is important in raw material preparation based on sections of the yam tuber for industrial production of yam products.
Acknowledgment
The authors wish to acknowledge the contributions of the following individuals whose assistance facilitated this work: Mr. Ayeh of the Federation of Associations of Ghanaian Exporters (FAGE) and Dr Paa Nii Johnson, Director, Food Research Institute (FRI), Ghana.
References
- Akissoe N, Hounhouigan J, Mestres C, Nago M. How blanching and drying affect the colour and functional characteristics of yam (Dioscorea cayenensis-rotundata) flour. Food Chem. 2003;82:257–264. doi: 10.1016/S0308-8146(02)00546-0. [DOI] [Google Scholar]
- Akissoe N, Mestres C, Hounhouigan J, Nago M. Biochemical origin of browning during the processing of fresh yam (Dioscorea spp.) into dried product. Agric Food Chem. 2005;53(7):2552–2557. doi: 10.1021/jf040265n. [DOI] [PubMed] [Google Scholar]
- Akissoe N, Mestres C, Handschin S, Gibert O, Hounhouigan J, Nago M. Microstructure and physico-chemical bases of textural quality of yam products. LWT Food Sci Technol. 2011;44:321–329. doi: 10.1016/j.lwt.2010.06.016. [DOI] [Google Scholar]
- Almenteros VP, Del Rosatio RR. Phenolic content and polyphenoloxidase activity related to browning in yam (Dioscorea alata Linn.) Philipp Agric. 1985;68:449–452. [Google Scholar]
- Anosike EO, Ikediobi CO. The biochemistry of the browning of yam tubers. In: Osuji G, editor. Advances in yam research vol 1. Nsukka: Anambra State University of Technology; 1985. pp. 145–160. [Google Scholar]
- AOAC . Official methods of analysis. Washington: Association of Official Analytical Chemists; 1984. [Google Scholar]
- Asemota HN, Wellington MA, Odutuga AA, Ahmad MH. Effect of short-term storage on phenolic content, o-diphenolase and peroxidase activities of cut yam tubers (Dioscorea sp.) J Sci Food Agric. 1992;60:309–312. doi: 10.1002/jsfa.2740600306. [DOI] [Google Scholar]
- Asiedu R, Satie A. Crops that feed the world 1. Yams. Yams for income and food security. Food Secur. 2010;2:305–315. doi: 10.1007/s12571-010-0085-0. [DOI] [Google Scholar]
- Ayernor GS. Particulate properties and rheology of pregelled yam (Dioscorea rotundata) products. J Food Sci. 1976;41:180–182. doi: 10.1111/j.1365-2621.1976.tb01130.x. [DOI] [Google Scholar]
- Chilaka FC, Sabinus E, Anyadiegwu C, Uvere PO. Browning in processed yams: peroxidase or polyphenol oxidase? J Sci Food Agric. 2002;82:899–903. doi: 10.1002/jsfa.1119. [DOI] [Google Scholar]
- Chisari M, Barbagallo RN, Spagna G. Characterization and role of polyphenol oxidase and peroxidase in browning of fresh-cut melon. J Sci Food Agric. 2008;56(1):132–138. doi: 10.1021/jf0721491. [DOI] [PubMed] [Google Scholar]
- Degras L. The yam: a tropical root crop. London: Macmillan Press Ltd; 1993. p. 129. [Google Scholar]
- Finger A. In vitro studies of the effect of polyphenol oxidase and peroxidase on the formation of polyphenolic black tea constituents. J Sci Food Agric. 1994;66:293–305. doi: 10.1002/jsfa.2740660306. [DOI] [Google Scholar]
- Goleniowski M, Del Longo O, De Forchetti SM, Arguello JA. Relationship between peroxidase and in vitro bulbification in garlic (Allium sativum L.). In vitro Cell Dev. Biol Plant. 2001;37:683–686. [Google Scholar]
- Hodge JE, Osman EM. Carbohydrates. In: Fennema OR, editor. Food Chem. New York: Marcel Dekker; 1976. p. 47. [Google Scholar]
- Ihekoronye AF, Nggody PO (1985) Integrated food science and technology in the tropics. Macmillan Publishers Ltd. pp. 18, 19
- Kleinkopf GE, Westerman DT, Duelle RB. Dry matter production and nitrogen utilisation by six potato cultivars. Agron J. 1981;73:799–802. doi: 10.2134/agronj1981.00021962007300050013x. [DOI] [Google Scholar]
- Krishnan JG, Padmaja G, Moorthy SN, Suja G, Sajeev MS. Effect of pre-soaking treatments on the nutritional profile and browning index of sweet potato and yam flours. Innov Food Sci Emer Technol. 2010;11:387–393. doi: 10.1016/j.ifset.2010.01.010. [DOI] [Google Scholar]
- Kumar D, Mishra DS, Chakraborty B, Kumar P (2011) Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J Food Sci Technol. doi:10.1007/s13197-011-0384-2 [DOI] [PMC free article] [PubMed]
- Magalhaes DB, de Carvalho MEA, Bon E, Neto JSA, Kling SH. Colorimetric assay for lignin peroxidase activity determination using methylene blue as substrate. Biotechnol Tech. 1996;10(4):73–276. doi: 10.1007/BF00184028. [DOI] [Google Scholar]
- Martinez MV, Whitaker JR. The biochemistry and control of enzymatic browning. Trends Food Sci Technol. 1995;6:195–200. doi: 10.1016/S0924-2244(00)89054-8. [DOI] [Google Scholar]
- Mayer AM, Harel E. Polyphenol oxidase in plants. Phytochem. 1979;18:193–215. doi: 10.1016/0031-9422(79)80057-6. [DOI] [Google Scholar]
- Montgomery MW, Sgarbieri VC. Isoenzymes of banana polyphenol oxidase. Phytochem. 1975;14:1245–1249. doi: 10.1016/S0031-9422(00)98602-3. [DOI] [Google Scholar]
- Neog M, Saikia L. Control of post-harvest pericarp browning of litchi (Litchi chinensis Sonn) J Food Sci Technol. 2010;47(1):100–104. doi: 10.1007/s13197-010-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluoha U. Delimitation of physiological regions in yam tubers (Dioscorea sp.) and distribution pattern of saccharide degrading enzymes, cell sap pH and protein in these regions. Biol Plant (PRAHA) 1988;30(3):210–218. doi: 10.1007/BF02878761. [DOI] [Google Scholar]
- Omidiji O, Okpuzor JC. Time-course of polyphenol-related browning of yams. J Sci Food Agric. 1996;70:190–196. doi: 10.1002/(SICI)1097-0010(199602)70:2<190::AID-JSFA481>3.0.CO;2-2. [DOI] [Google Scholar]
- Omigiji O, Okpuzor JE, Otubu O (2006) The contribution of an ionic peroxidase isozyme to enzyme-mediated browning in Dioscorea esculenta tubers. Pak J Nutr Vol 5. Iss. 5. Asian Network for Scientific Information. pp. 478–480
- Onayemi O, Babalola O, Badanga A. Textural properties of cooked tropical yam (Dioscorea spp.) J Text Stud. 1987;18:19–29. doi: 10.1111/j.1745-4603.1987.tb00567.x. [DOI] [Google Scholar]
- Osagie AU, Opoku AR. Enzymatic browning of yams (Dioscorea species) Niger J Biochem. 1984;1:25–29. [Google Scholar]
- Palmer JK. Banana polyphenol oxidase: purification and properties. Plant Physiol. 1963;38:508–513. doi: 10.1104/pp.38.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen SJ, Calvert J. Studies of polyphenol content, activities and isoenzymes of polyphenol oxidase and peroxidase during air-curing in three tobacco types. Plant Physiol. 1969;11:199–204. doi: 10.1104/pp.44.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am J Ecol Hort. 1965;16:144–158. [Google Scholar]
- Tian W, Lv Y, Cao J, Jiang W (2011) Retention of iceberg lettuce quality by low temperature storage and postharvest application of 1-methylcyclopropene or gibberellic acid. J Food Sci Technol. doi:10.1007/s13197-011-0587-6 [DOI] [PMC free article] [PubMed]