Abstract
With the aim of reducing phytic acid content of wheat bran, particle size reduction (from 1,200 to 90 μm), hydrothermal (wet steeping in acetate buffer at pH 4.8 at 55 °C for 60 min) and fermentation (using bakery yeast for 8 h at 30 °C) and combination of these treatments with particle size reduction were applied and their effects on some properties of the bran were studied. Phytic acid content decreased from 50.1 to 21.6, 32.8 and 43.9 mg/g after particle size reduction, hydrothermal and fermentation, respectively. Particle size reduction along with these treatments further reduced phytic acid content up to 76.4 % and 57.3 %, respectively. Hydrothermal and fermentation decreased, while particle size reduction alone or in combination increased bran lightness. With reducing particle size, total, soluble and insoluble fiber content decreased from 69.7 to 32.1 %, 12.2 to 7.9 % and 57.4 to 24.3 %, respectively. The highest total (74.4 %) and soluble (21.4 %) and the lowest insoluble fiber (52.1 %) content were determined for the hydrothermaled bran. Particle size reduction decreased swelling power, water solubility and water holding capacity. Swelling power and water holding capacity of the hydrothermaled and fermented brans were lower, while water solubility was higher than the control. The amount of Fe+2, Zn+2 and Ca+2 decreased with reducing particle size. Fermentation had no effect on Fe+2and Zn+2 but slightly reduced Ca+2. The hydrothermal treatment slightly decreased these elements. Amongst all, hydrothermal treatment along with particle size reduction resulted in the lowest phytic acid and highest fiber content.
Keywords: Fermentation, Hydrothermal treatment, Particle size reduction, Phytic acid, Wheat bran
Introduction
The health benefits of dietary fibers (e.g. cellulose, hemicelluloses, β-glucans, lignins, pectins and gums) are well demonstrated (Arvanitoyannis and Van Houwelingen-Koukaliaroglou 2005; Dhingra et al. 2012). Therefore, increasing the dietary fiber of foods particularly staple foods such as bakery products have been suggested and there is a strong shift towards these healthier foods. Apart from the health benefits of dietary fiber, it also has distinctive physicochemical properties such as water swelling, water holding capacity and fermentability and has been used in production of low calorie foods, to retain consistency and texture of the food and also to return mouthfeel that is lost when sugar or fat is removed (Sandrou and Arvanitoyannis 2000).
Wheat bran is known as a low-cost and abounded source of dietary fiber (36–52 %) (Vitaglione et al. 2008; Zhu et al. 2010). It also contains high quality proteins, vitamins and minerals and has strong antioxidant activity (Adom et al. 2003; Kaur et al. 2012). Wheat bran has been used traditionally to increase the fiber content of bread and other bakery products. However, the presence of phytic acid (myo-inositol hexaphosphate) in the bran (up to 5 %) and its negative effects on bioavailability of minerals such as iron, calcium, magnesium and zinc has limited its application (Palacios et al. 2008). Phytic acid is highly charged with six phosphate groups extending from the central inositol ring structure and therefore, is a superb chelator of mineral ions. It also reduces the bioavailability of food proteins and vitamins. Mineral deficiency (particularly iron and zinc) is considered highly prevalent in developing countries, where the diet is based on cereals and legumes, and also in vulnerable population groups in industrial countries. The negative effect of phytate on mineral bioavailability may have impact in some age groups, particularly children and women in developing countries (Prentice and Bates 1994). Thus, development of foods with improved mineral availability is of great importance.
To reduce the phytic acid content of wheat bran, different methods have been established including reduction of the bran particle size, fermentation, malting, soaking and hydrothermal processing (Liu et al. 2007; Sanz Penella et al. 2008; Mosharraf et al. 2009; Noort et al. 2010). Although either of these methods can reduce the phytic acid content of the bran, they may also affect physicochemical and nutritional properties of the bran. These possible changes may impact the properties of the foods in which the bran is included. Therefore, determination of such effects is of great importance for further applications of bran in food products.
The main aim of this study was to determine the effects of the three common methods used for reducing the phytic acid content of wheat bran including size reduction, hydrothermal and fermentation processes and to investigate the effects of these treatments on some physicochemical characteristics of wheat bran.
Materials and methods
Coarse wheat bran with average particle size of about 1,200 μm was gifted by Sepidan milling factory, Zarghan, Fars province, Iran. Active dried bakery yeast (Saccharomysis cervicea) was purchased from the local market. The bran had 10.9 ± 0.03 % moisture content, 11.5 ± 0.05 % protein, 9.1 ± 0.10 % fat, 0.5 ± 0.05 % ash and 12.5 % crude fiber as determined by the Approved Methods of the AACC (2000). Chemicals used for analytical tests were obtained from Merck, Darmstadt, Germany.
Bran grinding
To obtain different particle sizes of the bran, it was first ground in a laboratory mill (Retsch GmbH, Model SK1, Germany) and then sieved manually to obtain average particle sizes of 420, 280, 170 and 90 μm. The brans of varying particle sizes with no further treatment were known as control.
Hydrothermal treatment
Hydrothermal process was performed according to the method described by Mosharraf et al. (2009) with slight modification. Bran of different particle sizes (300 g) was wet-steeped in 2 volumes of acetate buffer (pH 4.8) at 55 °C for 60 min during which the buffer was replaced by new buffer solution twice. Afterwards the bran was incubated at 55 °C for 24 h and then it was washed several times with distilled water to reach the initial pH (6.20 ± 0.2) and dried in an oven at 50 °C to reach moisture content of 10.9 ± 0.03 %.
Yeast fermentation
Wheat bran was fermented by compressed bakery yeast according to the method described by Servi et al. (2008) with slight modification. The fermentation continued for 8 h at 30 °C in a temperature controlled water bath. The brans were then washed several times with distilled water to reach the initial pH (6.20 ± 0.2) and were dried in an oven at 50 °C to reach moisture content of 11.0 ± 0.02 %.
Determination of phytic acid
The phytic acid content of the bran of different particle sizes was determined using the method described by García-Estepa et al. (1999) based on complexometric titration of residual iron (III) after phytic acid precipitation.
Determination of the bran color
The color parameters of the brans were evaluated using a modified method of Afshari-Jouibari and Farahnaky (2011).
Dietary fiber content of the bran
Total, soluble and insoluble dietary fiber content of the control and treated brans were determined using the Approved Methods of the AACC (2000).
Hydration properties of the bran
Hydration properties of wheat bran samples in terms of water holding capacity (WHC) and swelling power (SP) were determined according to the methods described by Chen et al. (1988) and Robertson et al. (2000), respectively.
Water solubility index (WSI)
The WSI of brans was determined by slight modification of the method described by Anderson et al. (1969). Samples (2.5 g) were dispersed in 30 mL of distilled water, using a glass rod, and cooked at 90 °C for 15 min in a water bath. The cooked paste was cooled at room temperature and centrifuged at 3,000 g for 10 min. The supernatant was decanted for determination of its solid content into a tarred evaporating dish. The weight of dry solids was recovered by evaporating the supernatant overnight at 110 °C. WSI was calculated by Eq. 1.
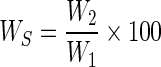 |
1 |
Where W2 = weight of dissolved solids in supernatant and W1 = weight of dry solids
Determination of mineral content
The amount of Fe+2, Zn+2 and Ca+2 were measured according to atomic absorption method (AOAC 2000).
Statistical analysis
All experiments were conducted in triplicates. Data were analyzed using analysis of variance (ANOVA) procedure by means of the statistical software of SPSS 16. The mean comparison was performed using the Multiple Range Duncan’s test (p <0.05).
Results and discussion
Effects of different treatments on phytic acid content of the bran are presented in Table 1. The results showed that the methods used in this study could significantly reduce the phytic acid content of the bran. The phytic acid of the largest particle size (i.e. 1,200 μm) was 50.1 mg/g. This value and other values obtained for different particle sizes of bran (without any further treatments) were close to the values reported by García-Estepa et al. (1999). They determined the phytic acid content of the wheat bran obtained from different varieties using the same method used in the current study and reported that most of the samples had a phytic acid content in the range of 34–47 mg/g. In other studies the phytic acid content was in the range of 42–54 mg/g as determined by colorimetric method (Harland and Oberleas 1986), and 46–67 mg/g as determined by HPLC method (Camire and Clydesdale 1982).
Table 1.
Effect of size reduction, hydrothermal and fermentation treatments on phytic acid content and its reduction in wheat bran compared to the phytic acid content of wheat bran with original particle size (1,200 μm) (n = 3)
| Average particle size of the bran (μm) | Control | Hydrothermaled | Fermented |
|---|---|---|---|
| Phytic acid content (mg/g) | |||
| 200 | 50.1 ± 2.85Aa | 32.8 ± 0.57Ac | 43.9 ± 2.85Ab |
| 420 | 43.8 ± 2.85Ba | 31.8 ± 0.57Bc | 35.9 ± 0.75Bb |
| 280 | 35.6 ± 2.85Ca | 27.1 ± 0.45Cc | 32.1 ± 0.49Cb |
| 170 | 29.0 ± 2.85Da | 21.4 ± 1.25Dc | 27.8 ± 1.24Db |
| 90 | 21.6 ± 1.42Ea | 11.8 ± 1.40Eb | 21.3 ± 2.98Ea |
| Phytic acid reduction (%) | |||
| 420 | 12.5 ± 0.20Dc | 36.5 ± 0.32Da | 28.4 ± 0.40Db |
| 280 | 28.9 ± 0.41Cc | 45.8 ± 0.40Ca | 35.9 ± 0.40Cb |
| 170 | 42.1 ± 0.30Bb | 57.2 ± 0.22Ba | 44.5 ± 0.22Bb |
| 90 | 56.9 ± 0.20Ab | 76.4 ± 0.31Aa | 57.3 ± 0.31Ab |
Different capital letters in each column and small letters in each row indicate the significant difference between the samples (p < 0.05)
By reducing bran particle size from 1,200 to 90 μm, phytic acid content reduced from 50.1 to 21.6 mg/g that showed 12.5–56.9 % phytic acid reduction compared to the initial bran particle size (i.e. 1,200 μm) (Table 1). In wheat kernel, phytic acid is largely distributed in external layers of pericarp and aleurone layer (Wu et al. 2009). Larger particle sizes of bran are obtained from outer bran layers and hence contain higher phytic acid content compared to the smaller particle sizes. It is also possible that the reduction in phytate with decreasing particle size may be attributed to the effect of particle size on extractability of phytate and possibility the treatment applied were more effective when the particle size was reduced. Similar results were presented by García-Estepa et al. (1999).
For the largest particle size used (i.e. 1,200 μm), hydrothermal treatment decreased the phytic acid content from 50.1 mg/g to 32.8 mg/g that showed 34.0 % reduction in phytic acid. For the same sample, bran fermentation also caused phytic acid reduction from 50.1 to 43.9 mg/g that was 0.12 % reduction. Combination of particle size reduction with either of these treatments further reduced the phytic acid content. Combination of hydrothermal treatment and particle size reduction reduced phytic acid content from 32.8 to 11.8 mg/g that showed 36.5 to 76.4 % reduction compared to the control. Yeast fermentation along with particle size reduction decreased the phytic acid content from 43.9 to 21.3 mg/g that showed 28.4 to 57.3 % reduction compared to the control (Table 1). Thus hydrothermal treatment along with particle size reduction was the most effective treatment for phytic acid reduction. Zielinski et al. (2001) and Mosharraf et al. (2009) observed a significant reduction in phytic acid content of wheat brans by hydrothermal treatment. The effect of hydrothermal process on phytic acid reduction may be related to the acetic condition (pH 4.5) used in this treatment which can enhance the endogenous phytase of the bran resulting in the degradation of phytic acid. Moreover, several washing steps during this process may remove phytic acid. High phytic acid loss in the fine bran is likely explained by its larger surface area so that phytic acid is more readily leached into the water. Different mechanisms have been explained for the effects of yeast on phytic acid content of the bran. For instance, it has been claimed that yeasts contribute phytase activity and thus enhance phytic acid degradation (Türk et al. 2000). Despite this claim, it has been indicated that pH, temperature and the amount of inorganic phosphorus accumulated during fermentation may have disfavored yeast phytase activity. Accordingly, the contribution of yeast phytase to the phytic acid reduction in the wheat bran would be ignorable (Leenhardt et al. 2005; Servi et al. 2008). The contribution of yeasts to the reduction of phytic acid may be related to the production of carbon dioxide and organic acids during fermentation which can reduce the pH of the medium and hence increase the phytic acid solubility and phytase activity (Sandberg and Svanberg 1991; Sandberg 2002).
According to the results (Table 2) decreasing bran particle size increased the lightness (L-value), while reduced the redness (a-value) and blueness (b-value) of the samples. The inner layers of the bran which are closer to the endosperm are generally ground into smaller particle sizes during milling. These layers contain more starchy materials and hence are lighter in color than the outer layers. The L-value of the control was higher than the hydrothermaled and fermented brans. Moreover, the hydrothermaled samples were lighter than the fermented ones. Fermentation had no significant effect on the a-value, while it significantly increased the b-value of the bran compared to the control and hydrothermaled counterparts. Hydrothermal treatment reduced the a-value of the samples with particle sizes greater than 170 μm, while it had no significant effect on the a-value of the other samples. The b-value of the control and hydrothermal samples were the same for particle sizes larger than 170 μm, while for smaller particle sizes, the b-value of the hydrothermaled samples was higher than the control. Chemical composition of the bran, chemicals used for the hydrothermal process, acids and enzymes produced during fermentation as well as drying step may affect bran color.
Table 2.
Effect of size reduction, hydrothermal and fermentation treatments on color characteristics of wheat bran (L-value: lightness; a: redness-greenness; b: blueness-yellowness) (n = 3)
| Average particle size of the bran (μm) | Control | Hydrothermaled | Fermented |
|---|---|---|---|
| L-value | |||
| 1200 | 39.3 ± 0.54Da | 37.1 ± 0.52Cb | 35.4 ± 1.00Cc |
| 420 | 41.3 ± 2.08Ca | 38.6 ± 2.51Cb | 36.3 ± 5.77Cc |
| 280 | 54.6 ± 3.78Ba | 53.3 ± 1.52Bb | 47.3 ± 1.52Bc |
| 170 | 60.3 ± 1.52Aa | 60.0 ± 1.00Aa | 57.0 ± 3.00Ab |
| 90 | 63.3 ± 0.57Aa | 61.3 ± 1.15Ab | 59.0 ± 1.00Ab |
| a-value | |||
| 1200 | 4.8 ± 0.00Aa | 4.6 ± 0.00Ab | 4.9 ± 0.00Aa |
| 420 | 4.6 ± 0.57Ba | 4.3 ± 0.57Bb | 4.6 ± 0.57Ba |
| 280 | 4.3 ± 0.57Ca | 3.0 ± 0.00Cb | 3.4 ± 0.57Ca |
| 170 | 2.3 ± 0.57Da | 2.3 ± 0.57Da | 2.3 ± 0.57Da |
| 90 | 1.3 ± 0.57Ea | 1.3 ± 0.57Ea | 1.3 ± 0.57Ea |
| b-value | |||
| 1200 | 37.0 ± 1.00Ab | 36.0 ± 1.00Ab | 39.0 ± 1.00Aa |
| 420 | 32.0 ± 3.00Ba | 31.3 ± 1.15Ba | 34.0 ± 1.00Ba |
| 280 | 25.6 ± 1.52Cb | 27.0 ± 3.00Cab | 28.3 ± 1.15Ca |
| 170 | 18.6 ± 2.30Dc | 26.6 ± 1.52Cb | 28.0 ± 2.64Ca |
| 90 | 14.0 ± 1.73Ec | 21.6 ± 1.52Db | 23.3 ± 3.51Da |
Different capital letters in each column and small letters in each row indicate the significant difference between the samples (p < 0.05)
Effects of hydrothermal and fermentation treatments on total, soluble and insoluble fiber of bran are given in Table 3. In general, reducing bran particle size (alone or along with hydrothermal or fermentation) decreased total, soluble and insoluble fiber content of the samples. By reducing the particle size from 1,200 μm to 90 μm (with no more treatment) about 54 % reduction in total, 35 % in soluble and 57 % in insoluble fiber contents of the bran were determined. This can be related to the fact that the fine bran particle sizes are obtained from inner layers of the bran fraction which contain less fiber than the outer layers (de Kock et al. 1999). Larger particle sizes showed higher content of insoluble and lower content of soluble fiber in comparison with smaller particle sizes. This is in agreement with previous results reported by Shenoy and Prakash (2001). Hydrothermal and fermentation treatments increased the total and soluble fiber contents of the bran, while reduced the insoluble fiber content. Accordingly, the hydrothermaled bran had the highest amount of soluble and the lowest amount of insoluble fibers followed by the fermented samples. Acidic buffer together with heating used in hydrothermal treatment and acids produced during fermentation and as well as activation of some enzymes may increase the solubility of the fiber. The washing steps used in both of these treatments may remove some of the water soluble materials (e.g. proteins and polysaccharides) and hence the remaining materials had higher fiber content compared to the control.
Table 3.
Effect of size reduction, hydrothermal and fermentation treatments on total, soluble and insoluble fiber content of wheat bran (n = 3)
| Average particle size of the bran (μm) | Control | Hydrothermaled | Fermented |
|---|---|---|---|
| Total fiber (%) | |||
| 1200 | 69.7 ± 0.20Ac | 74.4 ± 0.10Aa | 72.3 ± 0.10Ab |
| 420 | 68.2 ± 0.20Ac | 71.3 ± 0.40Aa | 69.9 ± 1.40Ab |
| 280 | 61.2 ± 0.40Bc | 67.3 ± 0.30Ba | 63.6 ± 1.60Bb |
| 170 | 59.1 ± 0.30Cb | 64.1 ± 0.20Ca | 55.7 ± 1.50Cc |
| 90 | 32.1 ± 0.30Db | 37.2 ± 0.30Da | 32.3 ± 1.10Db |
| Soluble fiber (%) | |||
| 1200 | 12.2 ± 0.06Ac | 21.4 ± 1.00Aa | 15.8 ± 0.00Ab |
| 420 | 12.1 ± 0.10Ac | 21.2 ± 0.20Aa | 15.3 ± 1.20Ab |
| 280 | 11.3 ± 0.00Bc | 19.3 ± 0.40Ba | 13.0 ± 1.50Bb |
| 170 | 10.8 ± 0.20Cc | 18.4 ± 0.20Ca | 11.4 ± 1.00Cb |
| 90 | 7.9 ± 0.30Dc | 16.3 ± 0.20Da | 8.1 ± 0.20Db |
| Insoluble fiber (%) | |||
| 1200 | 57.4 ± 0.30Aa | 52.1 ± 0.00Ac | 56.3 ± 0.00Ab |
| 420 | 56.2 ± 0.10Aa | 50.2 ± 0.30Bc | 54.5 ± 0.10Bb |
| 280 | 49.9 ± 0.50Ba | 48.0 ± 0.50Cb | 50.6 ± 0.60Ca |
| 170 | 46.7 ± 0.80Ca | 45.7 ± 0.00Db | 44.3 ± 0.50Dc |
| 90 | 24.3 ± 0.60Da | 20.8 ± 0.50Eb | 24.1 ± 0.90Ea |
Different capital letters in each column and small letters in each row indicate the significant difference between the samples (p < 0.05)
Interactions of the bran molecules with water described by SP, WSI and WHC are presented in Table 4. The results showed that by decreasing the particle size of all the samples, SP and WHC of the brans decreased, significantly, while WSI increased. These parameters are related to the amount of hydrocolloids (e.g. cellulose, pentosans and proteins) present in the bran. Previous results (Table 3) indicated that the insoluble fiber content of the bran with larger particle sizes was more than that of the bran with smaller particle sizes. The porous matrix structure of the insoluble fiber chains can hold large amounts of water through hydrogen bonds (Kethireddipalli et al. 2002) and increase the SP of the samples. Noort et al (2010) reported that by size reduction of the bran from 1,000 to 75 μm, WHC decreased from 500 to 250 %. The hydrothermaled and fermented samples had less insoluble fiber content than the control and hence they showed lower SP and WHC. On the other hand, WSI shows the amount of water soluble materials of the samples. Water soluble materials such as sugars, proteins particularly albumins and globulins and soluble polysaccharides such as soluble fibers are accounted for the bran water soluble materials. The amount of these components reduced as the particle size of the bran decreased. As mentioned before hydrothermal and fermentation treatments can increase the soluble fiber content of the bran resulting in higher WSI. According to the results (Table 4), brans with smaller particle sizes are less effective in holding water and swelling in aqueous media. Accordingly, they may be less effective in holding water in faeces and in promoting rapid transit of digesta through the gut. Therefore, coarse bran and foods supplemented with them are suitable choice for patients with divertiticular disease and people needing of high fiber diet to encourage colonic health (Sidhu et al. 1999). On the other hand, higher swelling of the bran may have some effects on the water uptake and quality of the products in which bran is included. For instance, Bonnand-Ducasse et al. (2010) reported significant correlations between bread dough peak time and peak bandwidth as determined by a mixograph. They also found that the insoluble fibers can retain water and compete for water with other constituents of the dough and hence may reduce dough development time. Insoluble fibers also lead to an increase of dough consistency and resistance to expansion.
Table 4.
Effect of size reduction, hydrothermal and fermentation treatments on swelling power, water solubility index and water holding capacity of wheat bran (n = 3)
| Average particle size of the bran (μm) | Control | Hydrothermaled | Fermented |
|---|---|---|---|
| Swelling power (mL/g) | |||
| 1200 | 9.5 ± 0.10Aa | 8.3 ± 0.00A b | 8.6 ± 0.20A b |
| 420 | 8.3 ± 0.10Ba | 7.1 ± 0.30Bb | 7.4 ± 0.10Bb |
| 280 | 7.7 ± 0.10Ca | 6.8 ± 0.10Cb | 6.9 ± 0.10Cb |
| 170 | 6.8 ± 0.10Da | 5.3 ± 0.20Db | 5.4 ± 0.20Db |
| 90 | 5.7 ± 0.10Ea | 4.7 ± 0.10Eb | 4.8 ± 0.30Eb |
| Water solubility index (%) | |||
| 1200 | 8.3 ± 0.11Ac | 13.1 ± 0.10A a | 9.8 ± 0.11A b |
| 420 | 7.1 ± 0.10Bc | 12.2 ± 0.31Ba | 9.0 ± 0.10Bb |
| 280 | 6.2 ± 0.12Cc | 11.8 ± 0.10Ca | 7.6 ± 0.10Cb |
| 170 | 5.3 ± 0.11Dc | 11.1 ± 0.20Ca | 6.4 ± 0.21Db |
| 90 | 4.7 ± 0.10Ec | 10.5 ± 0.11Da | 5.6 ± 0.30Eb |
| Water holding capacity (%) | |||
| 1200 | 25.2 ± 0.10Aa | 24.3 ± 0.00A b | 24.0 ± 0.20A b |
| 420 | 21.1 ± 0.10Ba | 20.9 ± 0.30Bb | 20.8 ± 0.10Bb |
| 280 | 20.1 ± 0.10Ca | 19.7 ± 0.10Cb | 19.8 ± 0.10Cb |
| 170 | 18.5 ± 0.10Da | 18.3 ± 0.20Da | 18.1 ± 0.20Da |
| 90 | 13.1 ± 0.10Ea | 13.3 ± 0.10Ea | 13.1 ± 0.30Ea |
Different capital letters in each column and small letters in each row are significantly different (p < 0.05)
Previous studies have reported that the type of cultivar and environmental conditions have great influence on the mineral content of the whole grain and its bran. The amount of Zn+2, Fe+2 and Ca+2 have been reported in the range of 42.0–120.9, 54.0–146.0 and 443–1,680 μg/g for 27 wheat cultivars grown in six different locations (Peterson et al. 1986). The results showed (Table 5) that the level of these elements decreased with reducing bran particle size possibly because of the presence of more starchy materials in the finer brans. The Fe+2 decreased from 0.026 to 0.016 %, Zn+2 decreased from 0.006 to 0.003 % and Ca+2 reduced from 0.080 to 0.046 % when particle size decreased from 1,200 to 90 μm. Comparing to the control, the Fe+2 and Zn+2 content of the bran remained unchanged after fermentation, but a small decrease in the Ca+2 content was observed. Nevertheless, hydrothermal treatment slightly reduced of these elements so that the Fe+2 decreased from 0.025 to 0.014 %, Zn+2 decreased from 0.006 to 0.002 % and Ca+2 reduced from 0.080 to 0.040 % that might be related to several washing steps used in this treatment (Liang et al. 2009).
Table 5.
Effect of size reduction, hydrothermal and fermentation treatments on mineral content of wheat bran (n = 3)
| Average particle size of the bran (μm) | Control | Hydrothermaled | Fermented |
|---|---|---|---|
| Fe+2(%) | |||
| 1200 | 0.026 ± 0.0010Aa | 0.025 ± 0.0020Ab | 0.026 ± 0.0010Aa |
| 420 | 0.023 ± 0.0010Ba | 0.022 ± 0.0010Bb | 0.023 ± 0.0010Ba |
| 280 | 0.018 ± 0.0020Ca | 0.017 ± 0.0020Cb | 0.017 ± 0.0010Ca |
| 170 | 0.016 ± 0.0010Da | 0.015 ± 0.0010Db | 0.016 ± 0.0010Ca |
| 90 | 0.016 ± 0.0010Da | 0.014 ± 0.0020Db | 0.014 ± 0.0020Db |
| Zn+2(%) | |||
| 1200 | 0.006 ± 0.0001Aa | 0.006 ± 0.0001Ab | 0.006 ± 0.0001Aa |
| 420 | 0.005 ± 0.0002Ba | 0.005 ± 0.0001Bb | 0.005 ± 0.0001Ba |
| 280 | 0.004 ± 0.0001Ca | 0.004 ± 0.0001Cb | 0.004 ± 0.0002Ca |
| 170 | 0.004 ± 0.0001Ca | 0.003 ± 0.0001Db | 0.003 ± 0.0002Da |
| 90 | 0.003 ± 0.0002Da | 0.002 ± 0.0001Eb | 0.003 ± 0.0002Da |
| Ca+2(%) | |||
| 1200 | 0.080 ± 0.0010Aa | 0.080 ± 0.0010Ab | 0.082 ± 0.0090Aa |
| 420 | 0.070 ± 0.0020Ba | 0.067 ± 0.0090Bb | 0.068 ± 0.0200Bb |
| 280 | 0.063 ± 0.0020Ca | 0.056 ± 0.0090Cb | 0.058 ± 0.0090Bb |
| 170 | 0.055 ± 0.0090Da | 0.051 ± 0.0090Cb | 0.052 ± 0.0100Cb |
| 90 | 0.046 ± 0.0100Ea | 0.040 ± 0.0080Db | 0.042 ± 0.0100Db |
Different capital letters in each column and small letters in each row are significantly different (p < 0.05)
Conclusion
The results of this study showed that particle size reduction, hydrothermal and fermentation treatments were effective methods for reducing the phytic acid content of wheat bran. The hydrothermal and fermentation treatments along with reducing bran particle size appeared to be more effective compared to either of these methods, individually. Amongst them, particle size reduction along with hydrothermal treatment resulted in the lowest phytic acid content. Phytic acid reduction of up to 56.9 %, 76.4 and 57.3 were obtained for particle size reduction alone and along with hydrothermal and fermentation treatments, respectively. Effects of these methods on the functional and nutritional properties of the bran were not similar. Decreasing barn particle size had positive effect on barn lightness, while hydrothermal and fermentation treatments had negative effect on this parameter. Particle size reduction had negative effect on total, soluble and insoluble fiber content. Applying hydrothermal and fermentation treatments increased total and soluble fiber contents, while reduced insoluble fiber content. The highest amount of total and soluble fiber content obtained for the hydrothermaled bran (37.2–74.4 % and 16.3–21.4 %, respectively). The highest SP and WHC obtained for the samples of larger particle size that followed no further treatments. The highest WSI obtained for hydrothermal followed by the fermentation treatment. Reducing barn particle size had negative effect on Fe+2, Ca+2 and Zn+2 content of the bran. Except Ca+2 content that decreased slightly upon fermentation, other minerals tested in this study remained unchanged. The hydrothermal treatment decreased the amount of all minerals determined in this study. In general, it can be concluded that hydrothermal treatment was the most effective method in reducing phytic acid content with highest amount of total and soluble fiber content. Further studies are required to improve these methods in order to obtain lower phytic acid content and to determine the properties of different food products such as cakes and bread containing low phytic acid wheat bran.
References
- AACC (2000) Approved methods of the AACC (10th ed). American Association of Cereal Chemists, St. Paul, MN. Method number 44–19 for moisture; 46–10 for protein; 30–10 for fat; 08–01 for ash and 32–05 for crude fiber and 32–07 for soluble fiber content
- Adom KK, Sorrells ME, Liu RH. Phytochemical profiles and antioxidant activity of wheat varieties. J Agric Food Chem. 2003;51:7825–7834. doi: 10.1021/jf030404l. [DOI] [PubMed] [Google Scholar]
- Afshari-Jouibari H, Farahnaky A. Evaluation of Photoshop software potential for food colorimetry. J Food Eng. 2011;106:170–175. doi: 10.1016/j.jfoodeng.2011.02.034. [DOI] [Google Scholar]
- Anderson RA, Conway HF, Pfeifer VF, Griffin E. Gelatinization of corn grits by roll and extrusion cooking. Cereal Sci Today. 1969;14:11–12. [Google Scholar]
- AOAC (2000) Official methods of analysis (16th ed), Association of official analytical chemists (methods 975.03, 986.11). Washington DC
- Arvanitoyannis IS, Van Houwelingen-Koukaliaroglou M. Functional foods: a survey of health claims, pros and cons and current legislation. Crit Rev Food Sci Nutr. 2005;45:385–404. doi: 10.1080/10408390590967667. [DOI] [PubMed] [Google Scholar]
- Bonnand-Ducasse M, Della Valle G, Lefebvre J, Saulnier L. Effect of wheat dietary fibers on bread dough development and rheological properties. J Cereal Sci. 2010;52:200–206. doi: 10.1016/j.jcs.2010.05.006. [DOI] [Google Scholar]
- Camire AL, Clydesdale FM. Analysis of phytic acid in foods by HPLC. J Food Sci. 1982;47:575–578. doi: 10.1111/j.1365-2621.1982.tb10126.x. [DOI] [Google Scholar]
- Chen H, Rubenthaler GL, Schanus EG. Effect of apple fiber and cellulose on the physical properties of wheat flour. J Food Sci. 1988;53:304–305. doi: 10.1111/j.1365-2621.1988.tb10242.x. [DOI] [Google Scholar]
- De Kock S, Taylor J, Taylor JRN. Effect of heat treatment and particle size of different brans on loaf volume of brown bread. LWT Food Sci Technol. 1999;32:349–356. doi: 10.1006/fstl.1999.0564. [DOI] [Google Scholar]
- Dhingra D, Michael M, Rajput H, Patil RT. Dietary fiber in foods: a review. J Food Sci Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Estepa RM, Guerra-Hernández E, García-Villanova B. Phytic acid content in milled cereal products and breads. Food Res Int. 1999;32:217–221. doi: 10.1016/S0963-9969(99)00092-7. [DOI] [Google Scholar]
- Harland BF, Oberleas D. Anion-exchange method for determination of phytate in foods: collaborative study. J Assoc Off Anal Chem. 1986;69:667–670. [PubMed] [Google Scholar]
- Kaur KD, Jha A, SabikhI L, Singh AK (2012) Significant of coarse ceresals in health and nutrition: a review. J Food Sci Technol. doi:10.1007/s13197-011-0612-9 [DOI] [PMC free article] [PubMed]
- Kethireddipalli P, Hung YC, Phillips RD, McWatters KH. Evaluating the role of cell material and soluble protein in the functionality of cowpea (Vigna unguiculata) pastes. J Food Sci. 2002;67:53–59. doi: 10.1111/j.1365-2621.2002.tb11358.x. [DOI] [Google Scholar]
- Leenhardt F, Levart-Verny MA, Chanliaud E, Remesy C. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J Agric Food Chem. 2005;53:98–102. doi: 10.1021/jf049193q. [DOI] [PubMed] [Google Scholar]
- Liang J, Han BZ, Nout MJR, Hamer RJ. Effect of soaking and phytase treatment on phytic acid, calcium, iron and zinc in rice fractions. Food Chem. 2009;115:789–794. doi: 10.1016/j.foodchem.2008.12.051. [DOI] [Google Scholar]
- Liu ZH, Wang HY, Wang XE, Zhang GP, Chen PD, Liu DJ. Phytase activity, phytate, iron, and zinc contents in wheat pearling fractions and their variation across production locations. J Cereal Sci. 2007;45:319–326. doi: 10.1016/j.jcs.2006.10.004. [DOI] [Google Scholar]
- Mosharraf L, Kadivar M, Shahedi M. Effect of hydrothermaled bran on physicochemical, rheological and microstrctural characteristics of Sangak bread. J Cereal Sci. 2009;49:398–404. doi: 10.1016/j.jcs.2009.01.006. [DOI] [Google Scholar]
- Noort MWJ, Haaster DV, Hemery Y, Schols HA, Hamer RJ. The effect of particle size of wheat bran fractions on bread quality - evidence for fiber - protein interactions. J Cereal Sci. 2010;52:59–64. doi: 10.1016/j.jcs.2010.03.003. [DOI] [Google Scholar]
- Palacios MC, Haros M, Rosell CM, Sanz Y. Selection of phytate-degrading human bifidobacteria and application in whole wheat dough fermentation. Food Microbiol. 2008;25:169–176. doi: 10.1016/j.fm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Peterson CJ, Johnson VA, Mattern PJ. Influence of cultivar and environment on mineral and protein concentrations of wheat flour, bran and grain. Cereal Chem. 1986;63:183–186. [Google Scholar]
- Prentice A, Bates CJ. Adequacy of dietary mineral supply for human bone growth and mineralization. Eur J Clin Nutr. 1994;48:S161–S177. [PubMed] [Google Scholar]
- Robertson JA, Monredon FD, Dysseler P, Guillon F, Amadò R, Thibault JF. Hydration properties of dietary fiber and resistant starch: a European Collaborative Study. LWT Food Sci Technol. 2000;33:72–79. doi: 10.1006/fstl.1999.0595. [DOI] [Google Scholar]
- Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002;88:S281–S285. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- Sandberg AS, Svanberg U. Phytate hydrolysis by phytase in cereals. Effects on in vitro estimation of iron availability. J Food Sci. 1991;56:1330–1333. doi: 10.1111/j.1365-2621.1991.tb04765.x. [DOI] [PubMed] [Google Scholar]
- Sandrou DK, Arvanitoyannis IS. Low-fat/calorie foods: current state and perspectives. Crit Rev Food Sci Nutr. 2000;40:427–447. doi: 10.1080/10408690091189211. [DOI] [PubMed] [Google Scholar]
- Sanz Penella JM, Collar C, Haros M. Effect of wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. J Cereal Sci. 2008;48:715–721. doi: 10.1016/j.jcs.2008.03.006. [DOI] [Google Scholar]
- Servi S, Ozkaya H, Colakoglu AS. Dephytinization of wheat bran by fermentation with bakers’ yeast, incubation with barley malt flour and autoclaving at different pH levels. J Cereal Sci. 2008;48:471–476. doi: 10.1016/j.jcs.2007.10.011. [DOI] [Google Scholar]
- Shenoy AH, Prakash J. Wheat bran (Triticum aestivum): composition, functionality and incorporation in unleavened bread. J Food Qual. 2001;25:197–211. [Google Scholar]
- Sidhu JS, Al-Hooti SN, Al-Saqer JM. Effect of adding wheat bran and germ fractions on the chemical composition of high-fiber toast bread. Food Chem. 1999;67:365–371. doi: 10.1016/S0308-8146(99)00123-5. [DOI] [Google Scholar]
- Türk M, Sandberg AS, Carlsson NG, Andlid T. IP6 hydrolysis by bakers’ yeast. Capacity, kinetics and degradation. J Agric Food Chem. 2000;48:100–104. doi: 10.1021/jf9901892. [DOI] [PubMed] [Google Scholar]
- Vitaglione V, Napolitano A, Fogliano V. Cereal dietary fiber: a natural functional ingredients to deliver phenolic compounds into the gut. Trends Food Sci Technol. 2008;19:451–463. doi: 10.1016/j.tifs.2008.02.005. [DOI] [Google Scholar]
- Wu P, Tian JC, Walker CE, Wang FC. Determination of phytic acid in cereals–a brief review. Int J Food Sci Technol. 2009;44:1671–1676. doi: 10.1111/j.1365-2621.2009.01991.x. [DOI] [Google Scholar]
- Zhu K, Haung S, Peng W, Qian H, Zhou H. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res Int. 2010;43:943–948. doi: 10.1016/j.foodres.2010.01.005. [DOI] [Google Scholar]
- Zielinski H, Kozłowska H, Lewczuk B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov Food Sci Emerg Technol. 2001;2:159–169. doi: 10.1016/S1466-8564(01)00040-6. [DOI] [Google Scholar]


