Abstract
Waxy rice starch was modified with vinyl acetate at levels of 4, 6, 8, and 10 % with degree of substitution of 0.021, 0.023, 0.032 and 0.056. The modified starches were studied for physicochemical, morphological, thermal and infra red spectral properties. Waxy starch acetates had high water holding capacity and did not sediment. Scanning electron microscopy revealed surface damage of the granules and their fusion. X ray diffractography showed that crystalline peak intensity had increased on acetylation. Differential scanning calorimetry studies showed changes in thermal properties. While gelatinization temperatures of modified starches were higher than the native starch, their transition enthalpies were lower than the native starch. IR spectra of the starch acetates did not show the peak typical for acetyl group. Thus, modification of waxy rice starch with vinyl acetate caused changes in the starch properties. The high water holding capacity of starch acetates can be exploited for specific applications.
Keywords: Vinyl acetylation, Waxy rice starch, Physicochemical properties, SEM, XRD, FT-IR
Introduction
The rise in the textural expectations of the consumers is making researchers to give more attention to the functional properties of starch. The need to improve the functionality of starch and expand its usefulness brought about the search for ways to modify the properties of native starch. These modifications disrupt hydrogen bonding and overcome the limitations of native starch in providing controlled hydration, water holding capacity, gel formation, clarity and stability during aging (Fennema 2005). Modified starches may be defined by source, prior treatment, amylose and amylopectin content or ratio, degree of polymerization or molecular weight, degree of substitution, physical form, type of derivative and associated constituent (Marshall and Wadsworth 1994).
Acetylation, hydroxypropylation and cross-linking are the well known methods of starch modification. Cross-linking makes the starch resistant to shear, pH and heat while hydroxypropylation improves cold storage stability. Acetylation of starch to the maximum allowed in foods (degree of substitution (DS) 0.09) lowers the gelatinization temperature, improves paste clarity, and provides stability to retrogradation and freeze-thaw process and functional properties such as hydrophobic, cationic or anionic characters (Wurzburg 1964; French 1984). Derivatization of starches with monofunctional reagents reduces intermolecular associations, the tendency of the starch paste to gel, and the tendency for precipitation to occur. Hence this modification is often called stabilization, and the products are called stabilized starches (Wurzburg 1964). In modified food starches, only very few of the hydroxyl groups are modified. Normally ester or ether groups are attached at very low DS (degree of substitution) values. DS values are usually <0.1 and generally in the range 0.002–0.2. Thus, there is, on average, one substituent group on every 500 D-glucopyranosyl units. According to the United States Code of Federal Regulations (1994), all acetylated starches with an acetyl content of 2.5 % can be used in foods.
Acetylation of starch has been the subject of much research (Wang and Wang 2002; Raina et al. 2006; Yadav et al. 2007; Chi et al. 2008; Mirmoghtadaie et al. 2009). Acetylation with acetic anhydride or vinyl acetate introduce stabilizing groups in starch which at low DS has versatile uses as film forming, binding, adhesion, thickening and texturing in food systems (Wurzburg and Szymanski 1970; Matti et al. 2004). Miyazaki et al. (2006) have observed that the three free OH groups at C2, C3, and C6 have different reactivities. The primary OH at C6 is more reactive and is acetylated more readily than the secondary ones at C2 and C3 due to steric hindrance. The primary OH located at the exterior surface of the starch molecules reacts readily with the acetic groups, while the two secondary ones located within the interior surface of starch form hydrogen bonds with the OH groups on the neighbouring glucose unit. As the degree of substitution increases from near 0 to 3.0, the nature of the starch acetate changes from hydrophilic to more hydrophobic and, simultaneously, the inter-particulate bonding capacity increases greatly (Korhonen et al. 2002).
The source of starch for modification has generally been corn or potato starch. However, rice starch because of its small granule size that is bland in taste and gives no mouth feel can also be an alternative source of starch modification (Raina et al. 2006). The brokens from waxy rice milling, a byproduct with low price character can be used for modification to alter the physical and chemical behavior for use in a food system. The detection of structural changes in modified starch can be used for industrial applications (French 1984).
Acetylation of starches from different sources has generally been done with acetic anhydride (Wu and Seib 1990; Vasanthan et al. 1995; Betancur-Ancona et al. 1997; Liu et al. 1997; Gonzalez and Perez 2002). Acetylation of waxy rice starch with vinyl acetate and the characterization of the modified starch have not been reported. The objectives of the present study were to prepare acetylated waxy rice starch of low DS with vinyl acetate and to know their water holding capacity, to measure and analyze the structure of the native and acetylated starches by FT-IR, X-ray diffractometer, differential scanning calorimeter and scanning electron microscope. Study of the pasting properties of acetylated starches was not included as suitable equipment could not be accessed.
Materials and methods
A commercial variety of waxy milled rice was locally procured from Tezpur market. Analytical grade chemicals from Merck, Germany were used in the study.
Isolation of rice starch
Starch was isolated from waxy rice as described by Mahanta et al. (1989). Milled waxy rice was soaked in water for 4 h and homogenized in a Philips blendor for 3 min at medium speed. The slurry was successively passed once through a 150-mesh and twice through a 200-mesh sieve and filtered through a Buchner funnel. The starch was then deproteinized, by utilizing the difference in density between starch and protein in a salt solution. The residue on the funnel was blended with 3.6 M sodium chloride and left overnight. The suspension was centrifuged at 1,000 rpm and the top layer was scraped off. The process was repeated twice after suspending the sediment in water until no off-white top layer was seen. The sediment was washed repeatedly with distilled water and filtered through Buchner funnel till chloride free as tested with silver nitrate. The cake was then dried in the vacuum oven at 40 °C at 500 mmHg for 8 h, powdered and kept in air tight container for further use.
Analysis of rice starch
Rice starch was analyzed for moisture, protein and ash (AOAC 2010). Apparent amylose content was estimated by the method of Sowbhagya and Bhattacharya (1979).
Preparation of acetylated waxy rice starch
The method described by Raina et al. (2006) was followed for acetylation. For acetylation, rice starch slurry was prepared by adding 162 g (db) of waxy rice starch (referred to as native starch hereafter) to 220 mL deionised water at room temperature. The mixture was stirred with magnetic stirrer until homogenous slurry was obtained. The pH was adjusted to 8.0 by adding drop wise 3 % aqueous sodium hydroxide solution. The required amount of vinyl acetate (4, 6, 8 and 10 % on starch, db) was added drop wise, while simultaneously, 3 % sodium hydroxide was added to maintain the pH between 8.0 and 8.4 with continuous stirring. The reaction was terminated by bringing the pH to 4.5 with the addition of 0.5 N hydrochloric acid. The slurry was filtered under vacuum through Buchner funnel. The filtered cake was washed with five volumes of deionised water. The resultant cake was vacuum dried at 40 °C at 500 mmHg pressure for about 24 h to less than 12 % (Wurzburg 1964). Starch acetates were pulverized and stored in air tight container for future use.
Determination of acetyl group (AG) and degree of substitution (DS)
The AG expressed as percentage on dry basis and DS of starch were determined according to Smith (1967). Starch slurry from 5 g of acetylated starch and 50 mL distilled water was made in a conical flask. A few drops of phenolphthalein indicator were added and titrated with 0.1 N sodium hydroxide to permanent pink colour. Then 25 mL of 0.45 N NaOH was added and slurry was vigorously shaken for 30 min. The stopper and neck of flask was flushed with small quantity of deionised water. The excess alkali in the slurry was treated with 0.2 N HCl until pink color disappeared. AG and DS were calculated.
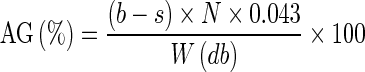 |
where b is the volume of 0.2 N HCl used to titrate blank (mL), s is the volume of 0.2 N HCl used to titrate sample (mL), N is the normality of HCl (0.2 N) and W is the mass of the sample (g).
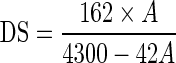 |
where A = % acetylated group (db)
Sediment volume
The sediment volume of native starch and starch acetates was determined (Tessler 1978). Starch slurry was made with 1 g of starch and 95 mL of deionised water. The pH of the slurry was adjusted to 7 with 5 % sodium hydroxide or hydrochloric acid followed by cooking of the slurry in a vigorously boiling water bath for 15 min. The total weight of the cooked starch slurry was made up to 100 g with deionised water. The mixture was then stirred thoroughly and transferred to a 100 mL graduated cylinder. The cylinder was stoppered and kept undisturbed for 24 h at ambient temperature. The volume of the sediment was then measured.
Morphological properties
Scanning electron micrograph of native and starch acetates was obtained with a scanning electron microscope (Jeol, JSM-6390LV, Jeol Ltd., Japan). Starch samples were sprinkled on a thick tape fixed on aluminum stub and treated with platinum for coating up to 12 nm thickness before examination. The coating was done in Jeol JFC1600, auto fine coater.
X-ray diffraction
X-ray diffractograms were studied to determine the crystallinity of both the native starch and starch acetates. Samples were dried at 40 °C to constant weight in a vacuum oven prior to X-ray scanning. X-ray diffractograms were obtained with an X-ray diffractometer (Rigaku, Japan; Model Miniflex). Data were collected from 2θ of 5° to 30° with a step width of 0.02° and at a scanning rate of 5.0°/min. The relative crystallinity (RC) of the starch granules was calculated as described by Rabek (1980).
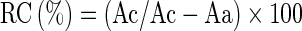 |
where Ac is the crystalline area and Aa is the amorphous area on the X-ray diffractograms
Thermal properties
Gelatinization and melting characteristics of native and starch acetates were studied using a differential scanning calorimeter (DSC-60, Shimadzu, Japan). The instrument was first calibrated with indium and purged with nitrogen gas at 30 mL/min. Gelatinization characteristics were determined with 4 mg of starch that were accurately weighed into an aluminum sample pan. Up to 8 mg of distilled water was added and the pan was hermetically sealed and allowed to equilibrate for 1 h before analysis. Onset temperature (To), peak temperature (Tp), concluding temperature (Tc) and enthalpy of transition (ΔH, J/g) were computed automatically.
Fourier transform infrared (FT-IR) spectroscopy
The IR spectra of native and starch acetates were obtained from samples in KBr pellets using a Nicolet FT-IR spectrometer (Impact 410, Madison, WI).
Statistical analysis
All analyses were done in triplicates. Duncan’s least significant test was used to compare means (significance level p < 0.05).
Results and discussion
Analysis of extracted starch
The starch extracted from waxy rice was analyzed to determine the extent of purity and to establish the major fraction of the starch present. The isolated starch had 10.5 % moisture, 0.4 % protein and 0.5 % ash. The rice starch had 0.16 % apparent amylose.
Acetyl content (%) and degree of substitution (DS)
Native waxy starch was acetylated at four different levels of 4, 6, 8 and 10 % with vinyl acetate. The effect of addition of vinyl acetate on acetyl content (%) and DS is shown in Table 1.
Table 1.
Physicochemical characteristics of starch acetates containing different levels of vinyl acetate
| Characteristics | Native starch | Levels of vinyl acetate, % | |||
|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | ||
| Acetyl content (%) | 0.00 | 0.50a ± 0.03 | 0.61b ± 0.03 | 0.88c ± 0.04 | 1.46d ± 0.06 |
| Degree of substitution | 0.00 | 0.021a ± 0.004 | 0.023b ± 0.005 | 0.032c ± 0.003 | 0.056d ± 0.003 |
| Sediment volume (mL) | 55.0 | Nil | Nil | Nil | Nil |
aValues in the same row followed by different letter are significantly different (p < 0.05; n = 3)
The percentage of acetyl groups in starch was calculated to be 0.58, 0.61, 0.88, and 1.46 % after acetylation with 4, 6, 8 and 10 % vinyl acetate on weight of starch (db). The acetyl groups increased with the increase in the level of vinyl acetate from 4 % to 10 %. The DS increased progressively with increase in acetyl content. The DS was 0.021 for 4 % acetylation, 0.023 for 6 % acetylation, 0.032 for 8 % acetylation and 0.056 for 10 % acetylation. Native rice starch had sparkling white colour whereas modified starches had slightly dull white appearance. They all were observed to be odourless.
Sediment volume
Table 1 gives the sediment volume of both native and acetylated starches. Sediment volume of native starch was found to be 55 mL. The suspensions of modified starch did not form any sediment. The absence of any sediment shows the superior water holding capacity of acetylated waxy rice starches to form very stable gels. In comparison, the sediment volume of native low amylose rice starches was reported to be between 19 mL and 20.5 mL which increased to 25 mL on acetylation (Raina et al. 2006). It has been established that cereal amylopectins have a reduced rate of retrogradation compared to tuber starches which is linked to their shorter average chain length (Kalichevsky et al. 1990). Further, acetyl groups incorporated into the amorphous regions around the amylopectin structures known as blocklets of the granule may have prevented the intra- and inter-molecular reassociation of the linear portions of the amylopectin molecules which could explain the absence of any sediment formation. These blocklets span the growth rings that probably arise due to the structural weakness in the amylopectin molecule (Baker et al. 2001). The decreased associative forces between molecular chains as a result of the acetyl group present (Lee and Yoo 2009) must have resulted in increased water retention capacity.
Appearance and colour
Native starch had sparkling white color whereas acetylated starches had slightly dull white appearance as observed visually. Native and starch acetates were found to be odorless. The dull white appearance of acetylated starches may be attributed to the incorporation of vinyl acetate.
Morphological properties
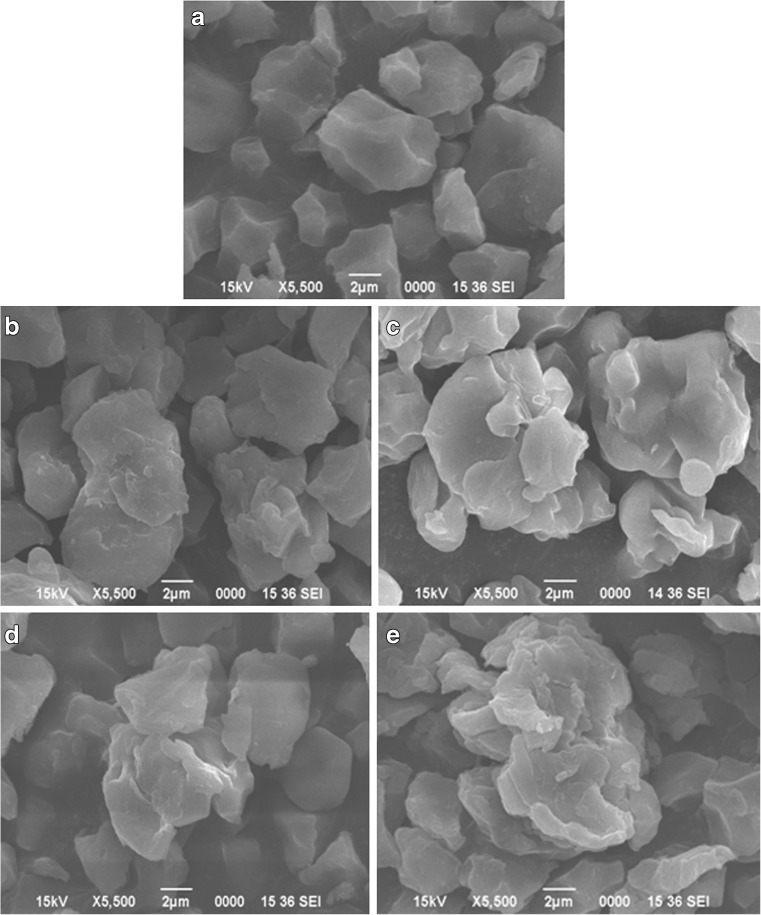
The granule structures of native and acetylated starches are shown in Fig. 1. The granules in native starch were observed to be irregularly shaped and polygonal and varied from 2 to 9 μm in size. The polygonal edges and cavities (indentations) observed in some granules originate from plastic deformation caused by other granules and/or constituents. Similar observation was also reported by Lii and Chang (1991), Juliano (1992), Ong and Blanshard (1995), Champagne (1996), and Gonzalez and Perez (2002). Acetylation caused changes in granular structure and the changes became magnified with increase in the level of acetylation. Modification affected the size and shape of the starch acetate granules even at the low degree of substitution that ranged from 0.186 to 0.056 %. Acetylating with vinyl acetate caused granule fusion which was also observed in corn and potato starch granules acetylated with acetic anhydride (Singh et al. 2004) and in rice starch acetate (Gonzalez and Perez 2002). The fusion of starch granules after acetylation at the low levels was explained by Singh et al. (2004) due to the introduction of hydrophilic groups to the starch molecules that enabled coalescing with other granules through hydrogen bonding. The extent of fusion in rice starch granules was found to increase with degree of acetylation. The surface of the fused starch granules showed extensive damage and erosion which can be attributed to the effect of sodium hydroxide treatment during the process of acetylation. The magnitude of the degradation being highest at 10 % level of acetylation. SEM pictures of acetylated starches clearly revealed that acetylation extensively changed the granule morphology and the severity of the change was highest at 10 % level acetylation. The apparent increased damage as DS increased may be because of the steric effects in starch granules induced by acetylation. The fused and surface damaged granules absorbed more water and at the same time, they were broken and swelling might have taken place.
Fig. 1.
Scanning electron micrographs of native and acetylated waxy rice starch a Native starch, b Modified with 4 % vinyl acetate, c Modified with 6 % vinyl acetate, d Modified with 8 % vinyl acetate, and e Modified with 10 % vinyl acetate
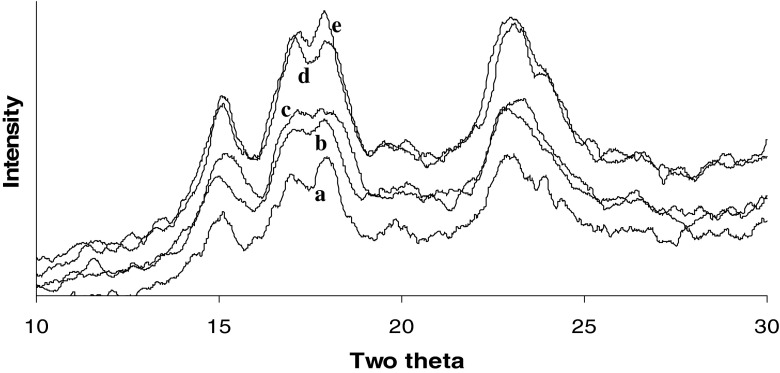
X-ray diffraction
A fairly close triplet at around 2θ values of 18°, 17° and 15° along with a peak around 23° signified A type of cereal starches (Zobel 1964). Similar peak values were seen in native starch (Fig. 2). Modification with vinyl acetate did not cause loss of crystalline pattern of the starch. No new crystalline pattern was formed. The crystallinity of the acetylated starch was enhanced as revealed by the increase in the peak height as well as width at 2θ values around 15°, 17°, 18° and 23°. The relative crystallinity value of native, 4 %, 6 %, 8 % and 10 % acetylated starches was 39.1 %, 63.8 %, 63.0 %, 62.3 % and 66.1 %. Probably the acetyl groups were introduced in the blocklets (Baker et al. 2001) that must have reinforced the crystallinity in the acetylated starch. The diffraction peaks of 4 % and 6 % acetylated starches did not have much difference in peak heights. Similarly, diffraction peaks of 8 % and 10 % acetylated starches were closer. Even though scanning electron microscopy revealed substantial damage to the granules, the effect of the damage was not observed on granule crystallinity. Wide peaks at 2θ values of 9° and 20° were assigned to acetylated starches (Chi et al. 2008) which however were not seen in waxy starch acetates. Wang and Wang (2002) had acetylated waxy maize starch with acetic anhydride and observed no changes in X-ray diffraction pattern or diffraction peaks among the acetylated starches and unmodified waxy maize starch. The authors explained that acetylation occurred primarily in the amorphous regions and the residual catalysts did not change the crystalline structure of acetylated starches. However, the authors did not mention whether the amorphous areas were the spaces between blocklets or the amorphous lamellae within the amylopectin molecule.
Fig. 2.
X-ray diffractograms of a Native starch, b Modified with 4 % vinyl acetate, c Modified with 6 % vinyl acetate, d Modified with 8 % vinyl acetate, and e Modified with 10 % vinyl acetate
Differential scanning calorimetry (DSC)
The DSC characteristics of native starch and starch acetates are shown in Table 2. Native starch gelatinized with a peak value at 69.1 °C and an enthalpy value of 9.1 J/g. Transition temperatures increased on modification while the transition enthalpy decreased. In the starch acetates, To ranged from 76.6 °C to 89.6 °C, Tp ranged from 84.2 °C to 94.8 °C and Tc from 87.6 °C to 103.5 °C. The transition enthalpies varied from 3.1 to 5.1 J/g. The increase in crystallinity as suggested by X-ray diffractography and granule fusion shown by SEM may both have made the acetylated starch resist gelatinization. The lowering of the transition enthalpies of the starch acetates may be explained as to be the severe effect of alkali during the acetylation process. The high melting point of the crystalline regions of amylopectin molecule and the increased energy requirement for initiation of gelatinization has been justified by (Singh et al. 2003) as to be due to high energy required to initiate melting in the absence of amylose rich amorphous regions. Contrary to the findings, the DSC endotherms of starch acetates acetylated with acetic anhydride are reported to lower the gelatinization peak (Singh et al. 2004; Mirmoghtadaie et al. 2009) of potato, corn and oat starches which were ascribed to the destabilization of granular starches. The peak temperature also increased as degree of acetylation increased. However, the transition enthalpies were found to decrease with increase in acetylation levels again due to the effect of alkali during acetylation. SEM had earlier shown extensive granule surface erosion (Fig. 1).
Table 2.
Gelatinization endotherms of native and acetylated starchesa
| Vinyl acetate (g/100 g of starch) | Gelatinization endotherm | |||
|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | |
| Native starch | 65.6a ± 0.55 | 69.1a ± 0.26 | 75.4a ± 0.61 | 9.1a ± 0.15 |
| 4 | 89.6d ± 0.46 | 94.8d ± 0.35 | 103.5d ± 0.49 | 5.1b ± 0.14 |
| 6 | 76.6b ± 0.36 | 88.2c ± 0.42 | 90.6c ± 0.67 | 4.5c ± 0.35 |
| 8 | 82.5c ± 0.38 | 86.3c ± 0.40 | 89.9c ± 0.50 | 3.8d ± 0.28 |
| 10 | 76.8b ± 0.42 | 84.2b ± 0.56 | 87.6b ± 0.55 | 3.1e ± 0.21 |
To is Onset temperature, Tp is peak temperature, Tc is concluding temperature, ΔH is enthalpy of transition. aNumbers in the same column followed by different letters are not significantly different (p < 0.05, n = 3)
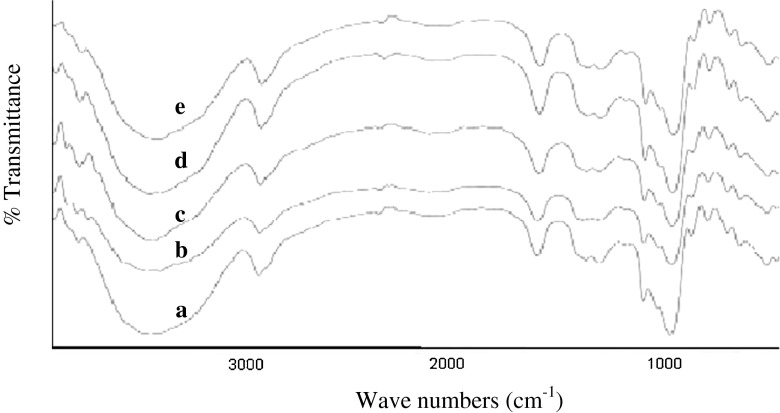
FT-IR spectroscopy
The infrared spectra for native starch and starch acetates with different degree of substitutions are shown in Fig. 3. The characteristic bond stretches involved at particular spectral peaks were interpreted in the light of earlier investigations (Yadav et al. 2007; Chi et al. 2008; Ruan et al. 2009). The IR spectra of native and acetylated starches were found to have similar overall stretches and functional groups. Several discernible absorbancies in both native and acetylated starches ranging from 928 to 929 cm−1, 1,016 to 1,017 cm−1 and 1,153 to 1,155 cm−1 which are attributed to C = O bond stretching were observed. A very broad band from 3,412 to 3,438 cm−1 appeared due to vibration of the hydrogen bonded hydroxyl groups (O-H) was seen. Another characteristic peak occurred at 1,640–1,644 cm−1 that was assigned to O-H stretching and bending vibration. The band at 2,928–2,930 cm−1 characteristic of the C-H stretching vibration was also found. The introduction of acetyl moiety, through a band at 1,733 cm−1 (Singh et al. 2004) in acetic anhydride acetylated potato and corn starch and at 1,754 cm−1 (Chi et al. 2008) in acetic anhydride acetylated corn starch was reported that however was not seen in the present study. The absence of such a peak at around 1,733 cm−1 in the present study is paradoxical when estimation of acetyl content and degree of substitution, thermal and crystalline properties and SEM data clearly showed the effect of introduction of acetyl group into the starch. It is difficult to explain the absence of a band at 1,733 cm−1 for acetyl group in the waxy starch acetates. The paradox might be due to three reasons, the very low level of substitution, the smaller molecular size of vinyl acetate than acetic anhydride and the very bushy nature of amylopectin molecule that could incorporate acetyl moiety deeply into the blocklets (Baker et al. 2001) of its structure i.e., in between the crystalline lamellae within the amylopectin molecule. This may also explain the increased crystallinity of waxy starch on acetylation.
Fig. 3.
FT-IR spectrogram of a Native starch, b Modified with 4 % vinyl acetate, c Modified with 6 % vinyl acetate, d Modified with 8 % vinyl acetate, and e Modified with 10 % vinyl acetate
Conclusion
Waxy rice starch was modified with vinyl acetate and studied for physicochemical, morphological, thermal and IR spectral properties. The DS of starch acetates studied ranged between 0.019 and 0.056. The absence of any sediment indicated high water holding capacity of the starch acetates. SEM showed damage to the granule morphology during acetylation but this did not cause any change in the X-ray diffraction peaks. The diffraction peaks were intensified. Modification brought changes in the thermal properties of starch acetates. While transition temperatures increased, transition enthalpies decreased as compared to native starch suggesting that both kinetics and thermodynamic properties of the acetylated starch-water mixture had changed. The findings indicate possible use of vinyl acetylated waxy starch in food models that are required to withstand fair amount of heat and where gelling is required at a higher temperature. Such uses indicate that acetylated starches can be used for gelling, thickening and binding purposes. FT-IR spectroscopy did not show the characteristic vibration of the acetyl group in starch acetates at around 1,724 cm−1to 1,733 cm−1. The study revealed that the high water holding capacity of acetylated waxy rice starch in addition to its delayed gelatinization temperature will help in trapping and retaining more water in food systems which are easily susceptible to quick retrogradation. The industrial use of acetylated waxy rice starch in bread and cake making can be further explored and exploited.
Acknowledgments
We acknowledge Dr. Binoy K. Saikia, Mr. Nipu Dutta, Mr. Jayanta Borah and Mr. Ratan Baruah of Tezpur University for technical assistance.
References
- AOAC (2010) Official methods of analysis. Washington DC, USA
- Baker AA, Miles MJ, Helbert W. Internal structure of the starch granule revealed by AFM. Carbohydr Res. 2001;330(2):249–256. doi: 10.1016/S0008-6215(00)00275-5. [DOI] [PubMed] [Google Scholar]
- Betancur-Ancona D, Chel-Guerrero L, Canizares-Homandez E. Acetylation and characterization of Canavalia ensiformis starch. J Agric Food Chem. 1997;45:362–378. [Google Scholar]
- Champagne ET. Rice starch: composition and characteristics. Cereal Foods World. 1996;41:833–838. [Google Scholar]
- Chi H, Kun X, Xiuli W, Qiang C, Donghua X, Chunlei S, Wende Z, Wang P. Effect of acetylation on the properties of corn starch. Food Chem. 2008;106:923–928. doi: 10.1016/j.foodchem.2007.07.002. [DOI] [Google Scholar]
- Fennema OR. Food chemistry. New York: Marcel Dekker Inc; 2005. pp. 123–127. [Google Scholar]
- French D (1984) Organization of starch granules. In: Whistler RL, BeMiller JN, Paschall EF (eds) Starch-chemistry and technology. London Academic Press, pp 183–247
- Gonzalez Z, Perez E. Effect of acetylation on some properties of rice starch. Starch-Starke. 2002;54:148–154. doi: 10.1002/1521-379X(200204)54:3/4<148::AID-STAR148>3.0.CO;2-N. [DOI] [Google Scholar]
- Juliano BO. Structure, chemistry and function of the rice grain and its fractions. Cereal Foods World. 1992;37:772–774. [Google Scholar]
- Kalichevsky MT, Oxford PD, Ring SG. The retrogradation and gelation of amylopectins from various botanical sources. Carbohydr Res. 1990;198:49–55. doi: 10.1016/0008-6215(90)84275-Y. [DOI] [Google Scholar]
- Korhonen O, Pohja S, Peltonen S, Suihko E, Vidgren M, Paronen P, Ketolinen J. Effects of physical properties for starch acetate powders on tableting. AAPS PharmSciTech. 2002;3(4):68–76. doi: 10.1208/pt030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Yoo B. Dynamic rheological and thermal properties of acetylated sweet potato starch. Starch-Starke. 2009;61:407–413. doi: 10.1002/star.200800109. [DOI] [Google Scholar]
- Lii CY, Chang YH. Study of starch in Taiwan. Food Rev Int. 1991;7:185–203. doi: 10.1080/87559129109540907. [DOI] [Google Scholar]
- Liu H, Ramsden L, Corke H. Physical properties and enzymatic digestibility of acetylated ae, wx, and normal maize starch. Carbohydr Polym. 1997;34:283–289. doi: 10.1016/S0144-8617(97)00130-6. [DOI] [Google Scholar]
- Mahanta CL, Ali SZ, Bhattacharya KR, Mukherjee PS. Nature of starch crystallinity in parboiled rice. Starch-Starke. 1989;41:171–176. doi: 10.1002/star.19890410504. [DOI] [Google Scholar]
- Marshall WE, Wadsworth JI. Rice science and technology. New York: Marcel Dekker Inc; 1994. pp. 405–420. [Google Scholar]
- Matti E, Tomas A, Pasi S, Reino L, Soili P, Sari H. Determination of the degree of substitution of acetylated starch by hydrolysis, 1H NMR and TGA/IR. Carbohydr Polym. 2004;57:261–267. doi: 10.1016/j.carbpol.2004.05.003. [DOI] [Google Scholar]
- Mirmoghtadaie L, Kadivar M, Shahedi M. Effects of cross-linking and acetylation on oat starch properties. Food Chem. 2009;116:709–713. doi: 10.1016/j.foodchem.2009.03.019. [DOI] [Google Scholar]
- Miyazaki M, Hung PV, Maeda T, Morita N. Recent advances in application of modified starches for breadmaking. Trends Food Sci Technol. 2006;17:591–599. doi: 10.1016/j.tifs.2006.05.002. [DOI] [Google Scholar]
- Ong MH, Blanshard JMV. Texture determinants in cooked parboiled rice: rice starch amylose and the fine structure of amylopectin. J Cereal Sci. 1995;21:251–260. doi: 10.1006/jcrs.1995.0028. [DOI] [Google Scholar]
- Rabek JF. Experimental methods in polymer chemistry: applications of wide-angle X-ray diffraction (WAXD) to the study of the structure of polymers. Chichester: Wiley Interscience; 1980. pp. 124–126. [Google Scholar]
- Raina CS, Singh S, Bawa AS, Saxena DC. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. Eur Food Res Technol. 2006;223:561–570. doi: 10.1007/s00217-005-0239-z. [DOI] [Google Scholar]
- Ruan H, Chen QH, Fu ML, Xu Q, He GQ. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009;114:81–86. doi: 10.1016/j.foodchem.2008.09.019. [DOI] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal, and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Singh N, Chawla D, Singh J. Influence of acetic anhydride on physico-chemical, morphological and thermal properties of corn and potato starch. Food Chem. 2004;86:601–608. doi: 10.1016/j.foodchem.2003.10.008. [DOI] [Google Scholar]
- Smith RJ. Characteristics and analysis of starches. In: Whistler RL, Paschal EF, editors. Starch: chemistry and technology, industrial aspects vol. 2. New York: Academic; 1967. pp. 569–631. [Google Scholar]
- Sowbhagya CM, Bhattacharya KR. Simplified determination of amylose in milled rice. Starch-Starke. 1979;31:159–163. doi: 10.1002/star.19790310506. [DOI] [Google Scholar]
- Tessler MM (1978) Process for preparing cross-linked starches. US Patent 4098997
- United States Code of Federal Regulations . Food additives permitted in food for human consumption. Washington: US Government Printing Office; 1994. [Google Scholar]
- Vasanthan T, Sosulski FW, Hoover R. The reactivity of native and autoclaved starches from different origins towards acetylation and cationization. Starch-Starke. 1995;4:135–143. doi: 10.1002/star.19950470404. [DOI] [Google Scholar]
- Wang YJ, Wang L. Characterization of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline-earth hydroxides. Starch-Starke. 2002;54:25–30. doi: 10.1002/1521-379X(200201)54:1<25::AID-STAR25>3.0.CO;2-T. [DOI] [Google Scholar]
- Wu Y, Seib P. Acetylated and hydroxypropylated distarch phosphates from waxy barley: paste properties and freeze- thaw stability. Cereal Chem. 1990;67:202–208. [Google Scholar]
- Wurzburg OB. In: Methods in carbohydrate chemistry. Whistler RL, editor. New York: Academic; 1964. pp. 286–288. [Google Scholar]
- Wurzburg OB, Szymanski CD. Modified starches for the food industry. J Agric Food Chem. 1970;18:997–1001. doi: 10.1021/jf60172a030. [DOI] [Google Scholar]
- Yadav AR, Mahadevamma S, Tharanathan RN, Ramteke RS. Characteristics of acetylated and enzyme-modified potato and sweet potato flours. Food Chem. 2007;103:1119–1126. doi: 10.1016/j.foodchem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Zobel HF. X-ray analysis of starch granules. In: Whistler RL, editor. Methods in carbohydrate chemistry. New York: Academic Press Inc; 1964. pp. 109–111. [Google Scholar]