Abstract
Tomato fruit at the mature green stage were treated with salicylic acid at different concentration (0, 1 and 2 mM) and analyzed for chilling injury (CI), electrolyte leakage (EL), malondialdehyde (MDA) and proline contents and phospholipase D (PLD) and lipoxygenase (LOX) activities during cold storage. PLD and LOX activities were significantly reduced by salicylic acid treatment. Compared with the control fruit, salicylic acid treatment alleviated chilling injury, reduced electrolyte leakage, malondialdehyde content and increased proline content. Our result suggest that the reduce activity of PLD and LOX, by salicylic acid may be a chilling tolerance strategy in tomato fruit. Inhibition of PLD and LOX activity during low temperature storage could ameliorate chilling injury and oxidation damage and enhance membrane integrity in tomato fruit.
Keywords: Salicylic acid, Tomato, Chilling injury, Postharvest
Introduction
Cold storage is one of the most effective postharvest technologies to control quality of fruit and vegetables from the time of harvest until final preparation for human consumption in food chain (Bourne 2006). However, there is a great risk of cold storage for postharvest produces to suffer chilling injury (CI), especially the cold sensitive crops. Tomato fruits are originated from tropical region and are typically cold sensitive. They easily get CI at cold storage (Hong and Gross 2006). Chilled tomato fruits develop several symptoms, such as surface pitting, diseases caused by pathogen, and losing their ability to develop full color (Wang 1993), which lead to substantial degradation of fruit quality.
Cell membranes are primary sites for development of CI (Rui et al. 2010). Phase transitions from a flexible liquid crystalline to a solid gel structure occur in cell membrane in chilling temperature (Lyons 1973). Maintenance of membrane integrity at low temperature has been reported to be important in the resistance to chilling temperature (Wonsheree et al. 2009). As indicators of membrane damage, electrolyte leakage and malondialdehyde (MDA) content are generally considered to be indirect measurements of membrane integrity and can reflect the occurrence of CI and loss of membrane integrity (Shewfelt and Purvis 1995). Lipolytic cascade in membrane lipids deterioration during senescence and CI was achieved by the concerted activities of a variety of membranous lipolytic enzymes such as phospholipase D (PLD) and lipoxygenase (LOX) (Pinhero et al. 1998). LOX and PLD catalyze peroxidation of polyunsaturated fatty acids and are believed to be major contributors to membrane damage and thus CI in plant (Pinhero et al. 1998; Wang 2001).
Salicylic acids (SA) as an endogenous signaling molecule plays a pivotal role in plant development and response to environmental stress (Asghari and Aghdam 2010; Paul and Pandey 2011). SA can alleviate postharvest chilling injury in fruit and vegetables via different mechanism such as enhance alternative oxidase (AOX) gene expression as a ROS avoidance gene in tomato fruit (Fung et al. 2006), increase ascorbate peroxidase (APX) and glutathione reductase (GR) activities and reduced-to-oxidized ascorbate ratio (AsA/DHAsA) and reduced-to-oxidized glutathione ratio (GSH/GSSG) in peach fruit (Wang et al. 2006) and enhance heat shock proteins (HSPs) in tomato (Ding et al. 2001) and peach fruit (Wang et al. 2006).
However, no information has been reported on the possible impact of SA on PLD and LOX activity in tomato fruit under low temperature storage. The objective of this study was to determine the effects of SA on PLD and LOX activity and their relation to CI in tomato fruit.
Material and methods
Tomato plants (Lycopersicon esculentum cv. Newton) were grown in a greenhouse (28 °C with a 16 h light/8 h dark photoperiod) in Ahar, Iran. Fruit were harvested in July 2011 according to the number of days post anthesis (DPA) or based on their surface color using ripening stages set by USDA. Mature green fruit (40–42 DPA) at the MG4 stage were used for salicylic acid treatment. The MG4 stage was confirmed by cutting fruit open and observing entirely liquefied locular gel and no cut seeds (Smith and Gross 2000). About 500 fruit were manually picked and immediately transferred to the laboratory. Tomato fruit with defects were discarded, and 270 fruit were selected and divided into 3 lots of 90 for the following treatments in triplicate (30 fruit per replicate): control (0) and salicylic acid at 1 or 2 mM. For each treatment and replicate, fruit were dipped in a fresh 10 L solution for 5 min. Following treatments, fruit were allowed to completely dry at room temperature before storage at 1 °C and 85–90 % RH for 3 weeks. Samples of 15 fruits from each treatment were removed on 1, 2 or 3 weeks from cold storage for PLD and LOX enzyme activity, MDA and proline analyses. Mesocarp from sampled fruit equator area was cut into small pieces, frozen in liquid nitrogen and stored at −80 °C. Other samples of 15 fruits from each treatment were removed weekly from cold storage and held at 20 °C for 3 days to simulate shelf conditions for CI evaluation. Each treatment was replicated three times.
Chilling injury index of fruits was evaluated at 20 °C for 3 d after 1, 2 or 3 weeks in cold storage. The fruits were returned to ambient temperature (20 °C) for development of CI symptoms. Symptoms were manifested as surface pitting according to the method of Ding et al. (2002). The severity of the symptoms was assessed visually in a 4-stage scale: 0=no pitting; 1=pitting covering <25 % of the fruit surface; 2=pitting covering <50 %, but >25 % of surface; 3=pitting covering <75 %, but >50 % of surface and 4=pitting covering >75 % of surface. The average extent of cold damage was expressed as a CI index, which was calculated using the following formula:
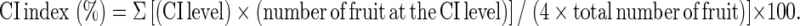 |
Electrolyte leakage was measured according to the method of Zhao et al. (2009). 3 mm thick of mesocarp tissue were excised from equator part of 5 fruits. Disks were put into aqueous 0.1 M mannitol under constant shaking. The conductivity of the solution (L1) was measured with a conductivity meter. Solutions were boiled for 10 min and then cooled to 20 °C. The conductivity of tissues (L2) was measured. The percentage of electrolyte leakage was calculated using the following formula: 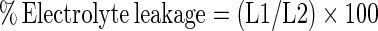 .
.
MDA content was measured using the thiobarbituric acid method described by Zhao et al. (2009). Absorbance at 532 nm was recorded and corrected for nonspecific absorbance at 600 nm. The amount of MDA expressed as μmol MDA per gram of pulp.
Proline content was measured using the acid ninhydrin method described by Zhang et al. (2010). Proline in tissues was extracted with 30 mL L−1 sulfosalicylic acid at 100 °C for 10 min with shaking. The extract was mixed with an equal volume of glacial acetic acid and acid ninhydrin reagent and boiled for 30 min. After cooling, the reaction mix was partitioned against toluene and the absorbance of the organic phase was recorded at 520 nm. The resulting values were compared with a standard curve constructed using known amounts of proline and expressed as μg proline g−1 fresh weight (FW).
For PLD and LOX, 5 g of tissue was ground with 5 mL of 50 mmol L−1 Tris–HCl (pH 8), containing 10 mmol L−1 KCl, 500 mmol L−1 sucrose and 0.5 mmol L−1 phenylmethylsulfonylfluoride. The extracts were then homogenized and centrifuged at 12 000 × g for 10 min at 4 °C. The supernatants were used for the enzyme assays.
PLD assay was determined according to Rui et al. (2010). The reaction mixture consisted of 1.8 mL of 50 mmol L−1 calcium acetate (pH 5.6) mixed with 27.4 mmol L−1 nitrophenylphosphorylcholine, 0.2 mL (0.4 U) of acid phosphatase dissolved in 50 mmol L−1 calcium acetate (pH 5.6) and 0.6 mL of enzyme extract. After 60 min at 37 °C, 0.1 mL of 50 mmol L−1 NaOH was added and the D-nitrophenol content determined at 400 nm. One unit of PLD was defined as the amount of enzyme that catalysed the formation of 1 nmol D-nitrophenol h−1.
LOX activity was assayed using the method of Rui et al. (2010). The standard assay mixture contained 200 μL Tween 20 and 40 μL of linoleic acid in 40 mL of 0.1 mol L−1 phosphate, pH 7.0. To 1.0 mL of the standard assay mixture in a cuvette was added 0.2 mL of LOX extract. One unit of LOX is defined as the amount of enzyme which causes an increase in absorption of 0.01 min−1 at 234 nm and 25 °C when linoleic acid is used as the substrate.
The experiment was arranged as split plots in time in the basis of completely randomized design with three replications. Analysis of variance (ANOVA) was carried out with SPSS software. Mean comparison were performed by Duncan’s multiple range tests. Differences at P < 0.05 were considered significant. The results of the ANOVA are shown in Table 1.
Table 1.
ANOVA for dependent variables for treatment applied, storage time and their interactions for tomato fruit
| Time | Treatment | Time×Treatment | |
|---|---|---|---|
| Chilling injury index | 16.188a | 10.388a | 3.221a |
| Electrolyte leakage | 189.46a | 131.525a | 72.91ns |
| Malondialdehyde content | 60.101b | 640175b | 13.57 ns |
| Proline content | 2.56 ns | 22.994a | 2.532 ns |
| Phospholipase D activity | 7.992 × 10−4a | 0.14a | 2.1 × 10−4a |
| Lipoxygenase activity | 8.27 × 10−3a | 3.042 × 10−2a | 1.224 × 10−3a |
a and b represent significance at the 0.01, and 0.05 levels, respectively, and ns represents non-significance at P < 0.05
Results and discussion
Chilling injury and electrolyte leakage
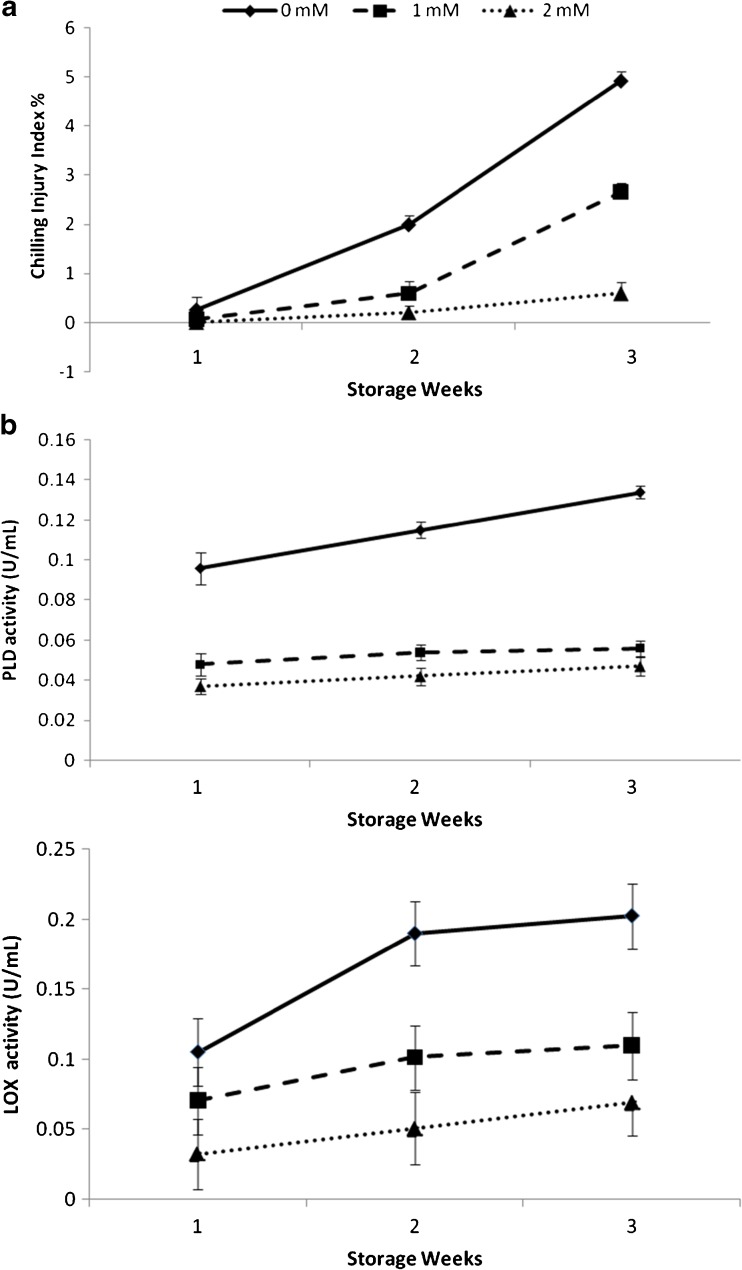
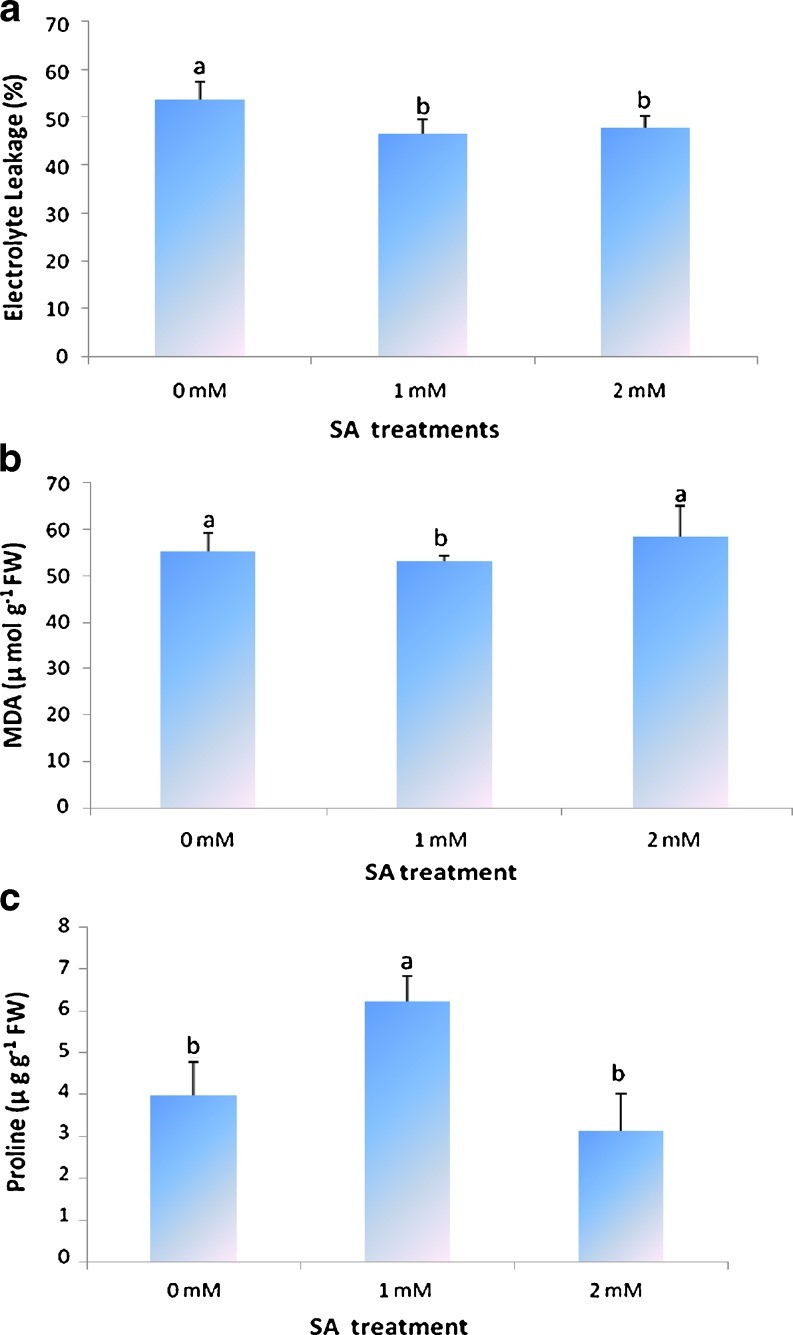
In our present study, we found that salicylic acid treatment could effectively reduce chilling injury in tomato fruit, and 2 mM was the most effective concentration (P < 0.01; Fig. 1a). Generally, CI occurs primarily at the cell membrane with changes in the fatty acid phospholipids composition (Lurie et al. 1987; Stanley 1991; Mirdehghan et al. 2007) and the membrane damages initiate a cascade of secondary reactions leading to disruption of cell structures. This membrane damage can be measured by the electrolyte leakage, which in this study was significantly higher in the control fruit than in salicylic acid treated fruit (Fig. 2a). These results show a role of salicylic acid in maintaining membrane integrity, as has been reported for loquat fruit (Cai et al. 2006) and pomegranate fruit (Sayyari et al. 2011). With respect to electrolyte leakage, application of salicylic acid led to significantly lower EL than control fruits, without significant differences concentrations used (P < 0.01; Fig. 2a). We report herein that salicylic acid treatment alleviate symptoms and severity of CI, and maintain membrane integrity, manifested as reduced electrolyte leakage, without significant differences between 1 and 2 mM concentrations. Similar results have been reported in peach (Wang et al. 2006) and pomegranate fruits (Sayyari et al. 2009). In peach fruit alleviation of CI was achieved at 1 mM but failed at 0.7 mM or lower SA concentrations and in pomegranate fruit SA treatments, especially at 2 mM concentration, were highly effective in reducing CI and electrolyte leakage in the husk of pomegranate. Kumar et al. (2011) reported that the application of salicylic acid led to reduce the litchi fruit pericarp browning, relative leakage rate, and decay percentage. It was effective in reduction of polyphenol oxidase (PPO) activity and improvement of anthocyanin pigments of the fruit pericarp.
Fig. 1.
Effect of salicylic acid treatment at 0, 1 and 2 mM concentrations on (a) Chilling injury during storage at 1 °C plus 3 days of shelf life at 20 °C, (b) Phospholipase D and (c) Lipoxygenase activities of tomato fruit during storage at 1 °C (n = 3)
Fig. 2.
Effect of salicylic acid treatment at 0, 1 and 2 mM concentrations on (a) Electrolyte leakage, (b) Malondialdehyde content and (c) Proline content of tomato fruit (n = 3)
Malondialdehyde and proline content
As shown in Fig. 2b, significantly (P < 0.05) lower MDA content were observed only in 1 mM salicylic acid treated fruit and in tomato fruit treated with 2 mM salicylic acid were not observed significantly differences compared with control fruit. It suggested that MDA content level was a reflection of fruit cold tolerance. Membrane lipid peroxidation may be one of the first events in the manifestation of CI, in which phase MDA was produced and damaged to cell membrane (Imahori et al. 2008). Posmyk et al. (2005) suggested that the MDA levels in a tissue can be a good indicator of the membranes integrity of plants subjected to low temperature. Lipid peroxidation is one of the adverse effects of CI on plant cells leading to MDA accumulation. It has been reported that MDA accumulation is prevented after SA treatment (Asghari and Aghdam 2010). With respect to proline content, application of salicylic acid at 1 mM concentration, without significant differences between fruit treated with salicylic acid at 2 mM concentration and control fruit, led to significantly higher proline content than control fruits (P < 0.01; Fig. 2c). Proline accumulation has been shown in different abiotic stressed plants. It has been suggested that proline protects plants by functioning as a cellular osmotic regulator between cytoplasm and vacuole, and by detoxifying of ROS, thus protecting membrane integrity and stabilizing antioxidant enzymes (Sharp et al. 1990; Bohnert and Jensen 1996). Positive correlations between accumulation of endogenous proline and improved cold tolerance have been found mostly in chilling-sensitive plants (Zhao et al. 2009; Korkmaz et al. 2009). Our data, which showed a definite increase in proline content of salicylic acid treated fruit along with amelioration of CI, confirm this finding. In plants, proline is synthesized from either glutamate or ornithine via Δ-pyrroline-5-carboxylate synthetase (P5CS) or ornithine δ-aminotransferase (OAT), respectively. Meanwhile, the metabolism and accumulation of proline also depend on its degradation, which is catalyzed by proline dehydrogenase (PDH) (Verbruggen and Hermans 2008). Fabro et al. (2004) reported that the P5CS2 can be activated by salicylic acid which triggers proline accumulation. Zhao et al. (2009) reported that the EL, MDA and proline content were effective indicators for CI analysis in postharvest tomato fruits.
Phospholipase D and lipoxygenase activity
Activities of PLD and LOX increased during storage, salicylic acid treatment slow down the increases in activities of both enzymes and maintained lower enzyme activities throughout the storage (P < 0.01; Fig. 1b, c). To our knowledge, there is no report on the response of phospholipase D activity to salicylic acid treatment in tomato fruit. Therefore, we examined the possible role that PLD and LOX may play in response to chilling stress of tomato fruit in this study. Accompanying the development of CI in tomato fruit, we observed a significant increase in PLD and LOX activities in control fruit (Fig. 1b, c), indicating that uncontrolled activation of membranous lipolytic enzymes in the absence of simultaneous phospholipid biosynthesis might cause irreversible membrane damage and finally the occurrence of CI (Mao et al. 2007). We report herein that salicylic acid treatment significantly reduced the development of CI symptoms and inhibited the activities of PLD and LOX in tomato fruit. We suggest that the reduction of CI by salicylic acid may be related to the inhibition of the activities of PLD and LOX. Cao et al. (2009) reported that the 1-methylcyclopropene (1-MCP) treatment significantly reduced the development of CI symptoms and inhibited the activities of PLC and PLD in loquat fruit after 7 and 21 days of cold storage respectively. These results suggested that PLC and PLD might be involved in the induction of CI in loquat fruit. Cao et al. (2009) suggested that the reduction of CI by 1-MCP may be related to the inhibition of the activities of PLC and PLD. Previous studies showed that the lipolytic cascade in membrane lipids deterioration during senescence and CI was achieved by the concerted activities of membranous lipolytic enzymes such as PLD and LOX (Pinhero et al. 1998). LOX and PLD catalyse peroxidation of polyunsaturated fatty acids and are believed to be major contributors to chilling-induced membrane damage and thus CI in plant tissue (Pinhero et al. 1998; Wang 2001). Mao et al. (2007) showed that the development of CI in cucumber fruit was accompanied with increases in PLD and LOX activities when exposed to chilling stress, and that the enhanced tolerance to CI by heat treatment was related to the reduction in activities of both enzymes. Rui et al. (2010) reported that the heat treatment decreased LOX and PLD activity in response to chilling stress in loquat fruit and the reduction of internal browning (IB) by heat treatment was associated with the reduction in PLD and LOX activities and to increase in unsaturated/saturated fatty acid ratio. This result suggested that these two enzymes might be associated with the initiation of CI by being involved in membrane deterioration and signalling pathway in response to chilling stress. Changes in membrane structure and composition are considered as the primary event of CI and lead to a loss of permeability control and metabolic dysfunctioning (Lyons 1973).
In summary, our results suggest that postharvest application of salicylic acid effectively enhanced chilling tolerance and reduced CI of the tomato fruit. The reduction in CI by salicylic acid appears to be mainly related to its effect in enhancing membrane integrity due to decreased PLD and LOX activity and MDA content. The increase of proline content by the salicylic acid treatment also play an important role in inducing acclimatization to chilling stress in tomato fruit.
Acknowledgment
Financial support for this work was provided by the Young Researchers Club, Ahar Branch, Islamic Azad University, Ahar, Iran
References
- Asghari M, Aghdam MS. Impact of salicylic acid on postharvest physiology of horticultural crops. Trend Food Sci Technol. 2010;21:502–509. doi: 10.1016/j.tifs.2010.07.009. [DOI] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- Bourne MC. Selection and use of postharvest technologies as a component of the food chain. J Food Sci. 2006;69:43–46. doi: 10.1111/j.1365-2621.2004.tb15491.x. [DOI] [Google Scholar]
- Cai C, Li X, Chen K. Acetyl salicylic acid alleviates chilling injury of postharvest loquat (Eriobotrya japonica Lindl.) fruit. Eur Food Res Technol. 2006;223:533–539. doi: 10.1007/s00217-005-0233-5. [DOI] [Google Scholar]
- Cao SF, Zheng YH, Wang KT, Rui HJ, Tanga SS. Effects of 1-methylcyclopropene on oxidative damage, phospholipases and chilling injury in loquat fruit. J Sci Food Agric. 2009;89:2214–2220. doi: 10.1002/jsfa.3710. [DOI] [Google Scholar]
- Ding CK, Wang CY, Gross KC, Smith DL. Reduction of chilling injury and transcript accumulation of heat shock proteins in tomato fruit by methyl jasmonate and methyl salicylate. Plant Sci. 2001;161:1153–1159. doi: 10.1016/S0168-9452(01)00521-0. [DOI] [Google Scholar]
- Ding CK, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- Fabro G, Kovács I, Pavet V, Szabados L, Alvarez ME. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol Plant Microbe Interact. 2004;17:343–350. doi: 10.1094/MPMI.2004.17.4.343. [DOI] [PubMed] [Google Scholar]
- Fung RW, Wang CY, Smith DL, Gross KC, Tao Y, Tian M. Characterization of alternative oxidase (AOX) gene expression in response to methyl salicylate and methyl jasmonate pre-treatment and low temperature in tomatoes. J Plant Physiol. 2006;163:1049–1060. doi: 10.1016/j.jplph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hong JH, Gross KC. Maintaining quality of fresh-cut tomato slices through modified atmosphere packaging and low temperature storage. J Food Sci. 2006;66:960–965. doi: 10.1111/j.1365-2621.2001.tb08219.x. [DOI] [Google Scholar]
- Imahori Y, Takemura M, Bai J. Chilling-induced oxidative stress and antioxidant responses inmume (Prunus mume) fruit during low temperature storage. Postharvest Biol Technol. 2008;49:54–60. doi: 10.1016/j.postharvbio.2007.10.017. [DOI] [Google Scholar]
- Korkmaz A, Korkmaz Y, Demirkiran AR. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ Exp Bot. 2009;67:495–501. doi: 10.1016/j.envexpbot.2009.07.009. [DOI] [Google Scholar]
- Kumar D, Mishra DS, Chakraborty B, Kumar P (2011) Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J Food Sci Technol. doi:10.1007/s13197-011-0384-2 [DOI] [PMC free article] [PubMed]
- Lurie S, Sonego L, Ben-Arie R. Permeability, microviscosity and chemical changes in the plasma membrane during storage of apple fruit. Sci Hortic. 1987;32:73–83. doi: 10.1016/0304-4238(87)90018-5. [DOI] [Google Scholar]
- Lyons JM. Chilling injury in plants. Ann Rev Plant Physiol. 1973;24:445–466. doi: 10.1146/annurev.pp.24.060173.002305. [DOI] [Google Scholar]
- Mao LC, Pang HG, Wang GZ, Zhu CG. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol Technol. 2007;44:42–47. doi: 10.1016/j.postharvbio.2006.11.009. [DOI] [Google Scholar]
- Mirdehghan SH, Rahemi M, Martínez-Romero D, Guillén F, Valverde JM, Zapata PJ, Serrano M, Valero D. Reduction of pomegranate chilling injury during storage after heat treatment: role of polyamines. Postharvest Biol Technol. 2007;44:19–25. doi: 10.1016/j.postharvbio.2006.11.001. [DOI] [Google Scholar]
- Paul V, Pandey R (2011) Role of internal atmosphere on fruit ripening and storability—a review. J Food Sci Technol. doi:10.1007/s13197-011-0583-x [DOI] [PMC free article] [PubMed]
- Pinhero RG, Paliyath G, Yada RY, Murr DP. Modulation of phospholipase D and lipoxygenase activities during chilling. Relation to chilling tolerance of maize seedlings. Plant Physiol Biochem. 1998;36:213–224. doi: 10.1016/S0981-9428(97)86878-7. [DOI] [Google Scholar]
- Posmyk MM, Bailly C, Szafranska K, Jana KM, Corbineau F. Antioxidant enzymes and isoflavonoids in chilled soybean (Glycinemax L. Merr) seedling. J Plant Physiol. 2005;162:403–412. doi: 10.1016/j.jplph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Rui H, Cao S, Shang H, Jin P, Wang K, Zheng Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J Sci Food Agric. 2010;90:1557–1561. doi: 10.1002/jsfa.3993. [DOI] [PubMed] [Google Scholar]
- Sayyari M, Babalar M, Kalantari S, Serrano M, Valero M. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol Technol. 2009;53:152–154. doi: 10.1016/j.postharvbio.2009.03.005. [DOI] [Google Scholar]
- Sayyari M, Castillo S, Valero D, Díaz-Mula HM, Serrano M. Acetyl salicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol Technol. 2011;60:136–142. doi: 10.1016/j.postharvbio.2010.12.012. [DOI] [Google Scholar]
- Sharp RE, Hsiao TC, Silk WK. Growth of maize primary root at low water potential’s. Plant Physiol. 1990;93:1337–1346. doi: 10.1104/pp.93.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewfelt RL, Purvis AC. Toward a comprehensive model for lipid peroxidation in plant tissue disorders. Hortscience. 1995;30:213–218. [Google Scholar]
- Smith DL, Gross KC. A family of at least seven β-galactosidase genes is expressed during tomato fruit development. Plant Physiol. 2000;123:1173–1183. doi: 10.1104/pp.123.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DW. Biological membrane deterioration and associated quality losses in food tissues. Crit Rev Food Sci Nutr. 1991;30:487–553. doi: 10.1080/10408399109527554. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- Wang CY. Approaches to reduce chilling injury of fruits and vegetables. Hortic Rev. 1993;15:83–95. [Google Scholar]
- Wang LJ, Chen SJ, Kun WF, Li SH, Archbold DD. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peach during cold storage. Postharvest Biol Technol. 2006;41:244–251. doi: 10.1016/j.postharvbio.2006.04.010. [DOI] [Google Scholar]
- Wang X. Plant phospholipases. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- Wonsheree T, Kesta S, van Doorn WG. The relationship between chilling injury and membrane damage in lemon basil (Ocimum citriodourum) leaves. Postharvest Biol Technol. 2009;51:91–96. doi: 10.1016/j.postharvbio.2008.05.015. [DOI] [Google Scholar]
- Zhang X, Shen L, Li F, Zhang Y, Menga D, Sheng J. Up-regulating arginase contributes to amelioration of chilling stress and the antioxidant system in cherry tomato fruits. J Sci Food Agric. 2010;90:2195–2202. doi: 10.1002/jsfa.4070. [DOI] [PubMed] [Google Scholar]
- Zhao DY, Shen L, Fan B, Liu KL, Yu MM, Zheng Y, Ding Y, Sheng JP. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J Food Sci. 2009;74:348–352. doi: 10.1111/j.1750-3841.2009.01156.x. [DOI] [PubMed] [Google Scholar]