Abstract
The kinetics of colour (measured as Hunter ‘a/b’ value) degradation in beetroot puree (Beta vulgaris L.) was studied over a temperature range of 50–120 °C (isothermal process), and also during normal open pan cooking, pressure-cooking and a newly developed and patented fuel-efficient ‘EcoCooker’ (non-isothermal heating process). The degradation of visual colour as measured as Hunter ‘a/b’ value was found to follow a first order kinetics, where the rate constant increased with an increase in the temperature. The temperature dependence of degradation was adequately modeled by Arrhenius equation. A mathematical model has been developed using the kinetic parameters obtained from the isothermal experiments to predict the losses of color in the non-isothermal heating/processing method based on the time-temperature data for each of the methods. The results obtained indicate a colour degradation of similar magnitude in all the three modes of cooking used in the study.
Keywords: Quality kinetics, Colour degradation, Beetroot, Betalin, EcoCooker, Rate constant
Introduction
Determination of stability of quality factors in food requires an understanding of rate orders and the parameters that influence them. Colour plays an important role in visual recognition and assessment of the surface and the subsurface properties of the object. It has a great influence on the appearance, processing and acceptance of food materials (Woolfe 1979).
Colour has been measured in the food industry by subjective visual inspection, including the use of visual color standards. Colour instruments objectively measures colour, are tools that assist the eye. With proper usage, it can provide repeatable, meaningful color data that agree with visual measurement (Loughrey 2000). The colour of any food product can be represented in terms of ‘L’ (lightness), ‘a’ (‘+a’, redness and ‘−a’, greenness), and ‘b’ (‘+b’, yellowness and ‘−b’, blueness) values or combination of these three depending upon the nature of the pigment present in the food material. It is reported that the objective measurement of colour using ‘L’, ‘a’ and ‘b’ system is a good indicator of the total colour change of heat treated peach puree (Avila and Silva 1999), broccoli juice (Weemas et al. 1999), mango puree, red chilli puree & paste, papaya puree and plum puree (Ahmed et al. 2002a, b, c, 2004).
Beet root powder or extracted pigments are used industrially for improving the red color of tomato pastes, sauces, soups, desserts, jams, jellies, ice creams, sweets, and breakfast cereals (Roy et al. 2004; Koul et al. 2002). The red beetroot (Beta vulgaris L.) is a good source of red and yellow pigments known as betalains. Betalains consist of betacyanins (red) and betaxanthins (yellow). The major betacyanin in beetroot is betanin and accounts for 75–95 % of the red pigment (Von Elbe et al. 1972). The degradation of betanin was reported to follow first order reaction kinetics. It is reported that in the presence of excess of oxygen, betanin degradation follows pseudo first order reaction (Attoe and Von Elbe 1982; Patkai and Barta 1996).
Betanins are reported to have some antioxidant activity and are found to be effective in inhibiting lipid peroxidation. Thus it is suggested that red beet products consumed regularly in the diet may provide protection against certain oxidative stress-related disorders in humans and also improve digestion and blood quality (Kanner et al. 2001; Butera et al. 2002; Herbach et al. 2004; Azeredo. 2009). Therefore, the retention of colour pigments as indicated by its colour it is very important with regard to its health benefits as well as visual appeal. Knowledge of kinetic parameters of colour degradation during thermal processing is very important in optimization of the process parameter. A literature survey indicate that similar work on beet root has not been reported.
Therefore, present study was undertaken
To find out visual colour degradation in red beetroot (Beta vulgaris L.), a model food system, over a temperature range of 50–120 °C (isothermal temperature) and to determine kinetic parameters for visual colour degradation in red beetroot (Beta vulgaris L.).
To study the degradation kinetics of colour in different cooking methods (non-isothermal process).
To develop a mathematical model relating the calculated kinetic data from the isothermal temperature and the time temperature profiles of different cooking methods (non-isothermal process).
To apply this model to predict the colour degradation in beetroot puree for non-isothermal heating process, from the time temperature data of the non-isothermal heating process and comparing it with the actual degradation values, which could then be used to predict the changes in the colour quality as a function of method of cooking or heat processing of the food system under consideration.
Materials and methods
Sample preparation
Fresh beetroots, which are deep purple in colour were obtained from a local market of Mumbai City, washed thoroughly and peeled. It was then pureed in a house hold mixer grinder (Sumeet mixer grinder, India). The pH (Oakton D0300 series, Singapore), brix (Hand refractometer, Arico India) and viscosity (Brookfeild DV III Rheometer at 100 rpm using LV-4 (64) spindle) of the puree were measured.
Heat treatment
Heat treatments were carried out at different temperatures (50, 60, 70, 80, 90, 100, 110 and 120 °C) for time periods over a range of 0–60 min. The temperatures were measured with ±0.1 °C accuracy using a thermocouple (Scientific Controls, Mumbai) and the response time was less than a minute. A water bath (Remi Equipmrnts, India) was used as a heating device for temperatures up to 100 °C, while for 110 and 120 °C, an autoclave with a calibrated temperature display which can be set the particular experimental temperatures (110 & 120 °C), was used. The samples were placed in the autoclave when the temperature reached 98–99 °C. It was then closed and subjected to heat treatment.
30 g each of the pureed beetroot samples were taken in a 100 ml beaker, covered with a watch glass and heated at predetermined temperature/time, with frequent stirring. Samples were withdrawn periodically and the Hunter ‘L’, ‘a’ and ‘b’ values were measured immediately to avoid regeneration of color, using Hunterlab DP-9000 D25A colourimeter (Hunter associates laboratory, Reston, Va., U.S.A).
Cooking methods
For cooking studies normal open pan cooking (15 min at a gas flow rate of 15 ml/s), pressure-cooking (10 min at a gas flow rate of 15 ml/s ) and one newly developed slow cooker named ‘EcoCooker’(30 min at a gas flow rate of 6 ml/s and 30 min holding period) were selected as different cooking methods. The principles of ‘EcoCooker’ are based on multiple effect evaporation, minimal flue gas heat losses, insulation, proper selection of burner diameter in relation to vessel diameter and final cooking without further application of heat. The time and flow rates for open pan and pressure cooking were selected as per the protocol used in household practices that ensures required cooking of beetroot. The time and flow rates for ‘EcoCooker’ were selected as per the instructions given for its usage (Joshi and Patel 2002). This cooker saves 75 % of the fuel in comparison with the commonly used cooking methods as the food to be cooked can be stalked in the cooker and the slow cooking ensures the complete cooking of different types foods which require varying cooking time (eg., rice, grams, vegetables, cereals etc) (Joshi and Patel 2002).
Samples were withdrawn at different time intervals and color was measured.
Time-temperature data
Time-temperature data for each cooking methods was monitored using a digital thermocouple having a response time of less than a minute.
Evaluation of colour
Visual colour was evaluated using a HunterLab colourimeter model DP-9000 D25A (Hunter associates laboratory, Reston, Va., U.S.A) in terms of Hunter ‘L’ (lightness), ‘a’ (redness and greenness) and ‘b’ (yellowness and blueness). The instrument was calibrated with standard white and black tiles. A glass cuvette containing the heat-treated puree was place above the light source and covered with the black cover provided with the instrument and Hunter ‘L’, ‘a’ and ‘b’ values were recorded. All the experiments were done in triplicates. The readings were taken within five minutes after the heat treatments.
Kinetic calculations
A general reaction rate expression for the degradation kinetics can be written as follows (Labuza and Riboh 1982; Van Boekel 1996; Nisha et al. 2004).
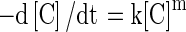 |
1 |
Where ‘C’ is the quantitative value of the component under consideration, ‘k’ is the reaction rate constant, and ‘m’ is the order of the reaction. The pigment degradation of beetroot has been found to follow first order kinetics. Following these evidences, the equation for first order kinetics after integration of Eq. (1) can be written as:
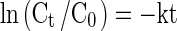 |
2 |
The dependence of the degradation rate constant (kT) on temperature was quantified by the Arrhenius equation, where
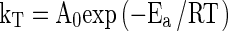 |
3 |
Where
- C0
measured Hunter colour value (L, a, b) at time zero (dimensionless),
- Ct
measured Hunter colour value (L, a, b) or a combination of these at time‘t’.
- t
heating time (s)
- Ea
activation energy of the reaction (kJ.mol−1)
- R
universal gas constant (8.3145 J mol−1 K−1)
- T
absolute temperature (K)
- A0
frequency factor (s−1) is a pre-exponential constant.
Each experiment was done in triplicate, and for each sample four Hunter ‘L’, ‘a’ and ‘b’ values were recorded by rotating the glass cuvette at 0 to 360°. Thus average of 12 readings was taken for the kinetic analysis. Kinetic data were analyzed by linear regression analysis using MS Excel.
The mathematical modeling was developed using the above mentioned equations and the corresponding kinetic parameters calculated
Results and discussion
pH, brix and viscosity of the beet root puree were 5.5. 8.1 and 3830 mPa s respectively.
Effect of temperature on visual colour of beetroot puree
It was observed that the temperature did not show any significant effect on the Hunter ‘L’ value, but there was consistent change in Hunter ‘a’ and ‘b’ values (Table 1). With the increase in the temperature and time, the Hunter ‘a’ values decreased with a corresponding increase in the ‘b’ values. During heat treatment the colour of beetroot puree changed from deep violet red colour to yellowish brown. Different permutation and combinations were tried to explain the colour of beetroot in terms of ‘L’, ‘a’ and ‘b’ values and Hunter ‘a/b’ was found to be the best parameter to express the colour degradation correctly and quantitatively. During the heat processing the puree turned brownish and the ‘a/b’ value for beetroot puree changed from an initial value of 7.4 to 6.207 at 50 °C after 60 min of treatment, corresponding to 16.12 % degradation. At 120 °C, the degradation was 67.4 %, were the ‘a/b’ value changes from an initial value of 7.9 to 2.574 after 60 min. It was also observed that after a particular point, the degradation tends to reach a maximum, after which the ‘a/b’ (∼2.5–2.6, Table 1) value remained constant. At 100 °C, the a/b value was constant after 40 min where as at 110 and 120 °C, it was 30 and 15 min respectively. This indicates that, at any specific temperature, the maximum (asymptotic) degradation is reached after specific time of treatment. This variation is equivalent to an equilibrium conversion in a chemical reaction and hence the estimated kinetic rate constants need to take this to account.
Table 1.
Effect of heat treatment on the ‘L’, ‘a’ and ‘b’ values of beetroot puree
| Temperature (°C) | Time (min) | ‘L’ | ‘a’ | ‘b’ | ‘a/b’ | Temperature (°C) | Time (min) | ‘L’ | ‘a’ | ‘b’ | ‘a/b’ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 0 | 6.91 ± 0.04 | 9.81 ± 0.07 | 1.33 ± 0.05 | 7.4 | 90 | 0 | 7.11 ± 0.06 | 14.16 ± 0.04 | 2.34 ± 0.05 | 6.12 |
| 20 | 7.18 ± 0.07 | 9.66 ± 0.02 | 1.44 ± 0.01 | 6.69 | 20 | 9.44 ± 0.06 | 12.03 ± 0.04 | 2.81 ± 0.04 | 4.28 | ||
| 40 | 6.47 ± 0.03 | 9.65 ± 0.03 | 1.49 ± 0.05 | 6.48 | 40 | 8.83 ± 0.05 | 10.47 ± 0.02 | 3.19 ± 0.07 | 3.29 | ||
| 60 | 6.26 ± 0.06 | 9.37 ± 0.04 | 1.51 ± 0.02 | 6.21 | 60 | 8.56 ± 0.06 | 9.705 ± 0.06 | 3.51 ± 0.04 | 2.77 | ||
| 60 | 0 | 6.02 ± 0.04 | 11.57 ± 0.03 | 1.33 ± 0.04 | 7.4 | 100 | 0 | 8.42 ± 0.08 | 14.16 ± 0.05 | 2.31 ± 0.03 | 6.31 |
| 20 | 6.56 ± 0.06 | 9.50 ± 0.03 | 1.34 ± 0.02 | 7.05 | 10 | 8.54 ± 0.06 | 12.48 ± 0.04 | 3.11 ± 0.05 | 4.02 | ||
| 40 | 6.54 ± 0.03 | 9.04 ± 0.01 | 1.39 ± 0.02 | 6.51 | 20 | 8.78 ± 0.04 | 11.17 ± 0.07 | 3.34 ± 0.03 | 3.35 | ||
| 60 | 6.66 ± 0.01 | 8.76 ± 0.02 | 1.45 ± 0.04 | 6.04 | 40 | 7.98 ± 0.05 | 8.76 ± 0.02 | 3.36 ± 0.02 | 2.61 | ||
| 70 | 0 | 7.75 ± 0.06 | 16.15 ± 0.05 | 2.5 ± 0.02 | 6.46 | 110 | 0 | 7.48 ± 0.07 | 13.19 ± 0.03 | 2.1 ± 0.06 | 6.28 |
| 20 | 7.89 ± 0.04 | 15.26 ± 0.06 | 2.71 ± 0.03 | 5.63 | 10 | 7.55 ± 0.04 | 12.78 ± 0.02 | 3.31 ± 0.5 | 3.86 | ||
| 40 | 7.53 ± 0.05 | 14.33 ± 0.04 | 2.89 ± 0.04 | 4.96 | 20 | 7.77 ± 0.06 | 12.13 ± 0.04 | 3.83 ± 0.04 | 3.17 | ||
| 60 | 8.04 ± 0.02 | 13.85 ± 0.03 | 3.07 ± 0.05 | 4.51 | 30 | 8.12 ± 0.09 | 10.65 ± 0.05 | 4.11 ± 0.05 | 2.59 | ||
| 80 | 0 | 8.93 ± 0.03 | 9.10 ± 0.05 | 1.48 ± 0.07 | 6.16 | 120 | 0 | 6.12 ± 0.11 | 15.17 ± 0.01 | 1.92 ± 0.03 | 7.90 |
| 20 | 8.27 ± 0.07 | 8.36 ± 0.03 | 1.88 ± 0.07 | 4.45 | 5 | 6.21 ± 0.12 | 8.78 ± 0.04 | 2.47 ± 0.04 | 3.56 | ||
| 40 | 7.93 ± 0.08 | 8.90 ± 0.02 | 2.38 ± 0.05 | 3.75 | 10 | 7.3 ± 0.07 | 9.11 ± 0.06 | 3.01 ± 0.05 | 3.03 | ||
| 60 | 7.99 ± 0.04 | 9.28 ± 0.04 | 2.94 ± 0.08 | 3.16 | 15 | 7.34 ± 0.06 | 10.06 ± 0.03 | 3.91 ± 0.07 | 2.57 |
Various combination of Hunter ‘L’, ‘a’ and ‘b’ values were analyzed to find out the best combination to express to the colour changes of various fruits and vegetables. The Hunter (a × b) values were reported to be correlated well with pigment, carotenoids, concentration of papaya puree heated for different times at selected temperatures (Ahmed et al. 2002c). It is reported that a trisimulous combination (L.a.b) explains the degradation of colour in plum than the combinations La/b, L/ab and Lb/a (Ahmed et al. 2004). Rodrigo et al. (2007) reported that the L*a*/b* parameter combination described best the colour change of tomato and strawberry puree during thermal and high pressure thermal treatments. In the present study it was observed that a ratio of Hunter ‘a/b’ indicate the colour change due to thermal treatment more effectively. It is reported that the yellow pigments of beet root, betaxanthins, are more stable than the betacyanins (red pigments), but the degradation of both the pigments are proportional (Gokhale and Lele 2011).
Degradation kinetics of visual colour of beetroot puree
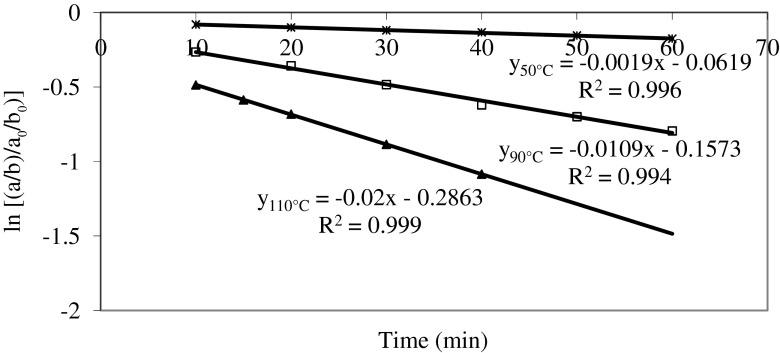
Since the color of beetroot is indicated by ‘a/b’ value, the kinetic study was carried out only with respect to ‘a/b’ values. Using linear regression, the degradation data were analyzed using Eq. (2) to determine the overall order and rate constant for the degradation reaction. Accordingly, ‘ln [(a/b)/(a0/b0)]’ was plotted vs ‘t’, from which rate constant, ‘k’ was calculated as the slope. Figure 1 show the representative values for the first order plots for the degradation of redness (Hunter ‘a/b’ value) for beetroot puree at 50, 90 and 110 °C, respectively. A correlation coefficient >0.95 in all cases confirmed that the degradation of visual colour in beetroot puree indeed follows a first order reaction at all temperatures studied in this work. Table 2 documents the rate constants for the colour degradation measured as Hunter ‘a/b’ value of beetroot puree.
Fig. 1.
First order plot of color (Hunter ‘a/b’) degradation in beet root puree at 50, 90 and 120 °C
Table 2.
Rate constanta,b (k), correlation coefficient (R 2) and half-life (t1/2) for color degradation for beetroot puree
| Temperature (°C) | K (min−1) | R 2 | T1/2 (min) |
|---|---|---|---|
| 50 | 0.0019 | 0.996 | 365 |
| 60 | 0.0039 | 0.993 | 178 |
| 70 | 0.0055 | 0.995 | 126 |
| 80 | 0.0086 | 1.00 | 80 |
| 90 | 0.0109 | 0.994 | 64 |
| 100 | 0.0142 | 0.997 | 49 |
| 110 | 0.0200 | 0.999 | 35 |
| 120 | 0.0324 | 1.00 | 21 |
aCalculated from semi-log plot of ln [(a/b)t/(a/b)0] vs time
bThe standard error in k values were less than 6 × 10−4
The degradation of colour in many food systems during thermal treatments have been reported to follow first order kinetics (Ahmed et al. 2002a, 2004; Ahmed and Ramaswami 2005; Rodrigo et al. 2007). In the present study, it was observed that the degradation of colour, which was measured as Hunter ‘a/b’ value, followed first order degradation kinetics.
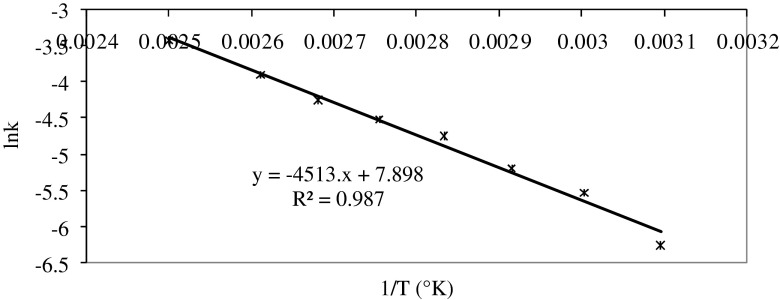
Activation energy Ea, (kJ. mol−1) was calculated as a product of gas constant, R (8.3145 kJ. mol−1 K−1) and the slope of the graph obtained by plotting ‘Ln k’ vs ‘1/T’. Figure 2 shows the Arrhenius plot for color degradation in beetroot puree. The activation energy for red colour degradation in beetroot puree was calculated to be 37.54 (R2 = 0.99) kJ mol−1. The activation energy for thermal degradation of Hunter ‘b’ value (represents yellow colour) in mango puree, tristimulus combination value (L.a.b) for chillee puree, Hunter ‘a’ value (represents red colour) in Hunter (a × b) for papaya and plum puree were reported to be 36.26, 24.8, 32.59 and 25.86 kJ mol−1 respectively (Ahmed et al. 2002a, b, c, 2004).
Fig. 2.
Arrhenius plot for color degradation (Hunter ‘a/b’) in beet root purees
Time-temperature data of the three modes of cooking
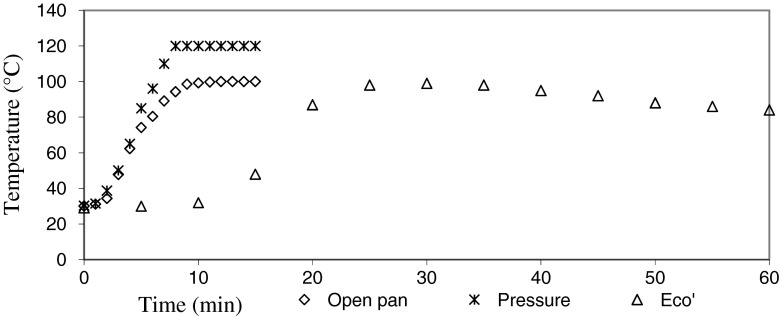
To extend the results obtained from the isothermal experiments to the non-isothermal heating encountered in the three modes of cooking, viz. open pan cooking, pressure cooking and cooking in ‘EcoCooker’, time-temperature data during the processing of each was recorded (Fig. 3).
Fig. 3.
Time-temperature profiles of the different cooking method used. Degradation profile and kinetics of visual colour variation of beetroot puree under the three modes of cooking
Degradation profile and kinetics of visual colour variation of beetroot puree under the three modes of cooking
Colour degradation was followed in each of these modes of cooking similar to the under isothermal conditions. Table 3 documents effect of temperature on Hunter ‘a’, ‘b’ and ‘a/b’ values and the kinetic parameters such as rate constant (k), correlation coefficient (R2), and half-life (t1/2) for color degradation for beetroot puree under different cooking methods. The results documented in Table 3 indicate that there is a loss in colour as can be seen from the decrease in Hunter ‘a/b’ in all the methods of cooking.
Table 3.
The effect of temperature on Hunter ‘L’a, ‘a’a, ‘b’a and ‘a/b’a values and the kinetic parameters (rate constantb,c, k correlation coefficient, R 2 and half life, T1/2) for color degradation for beetroot puree at different cooking methods
| Cooking method | Time (min) | ‘L’ | ‘a’ | ‘b’ | ‘a/b’ | Rate constant, (min−1) | T1/2 (min) |
|---|---|---|---|---|---|---|---|
| Open pan | 0 | 5.88 | 14.16 | 2.34 | 6.05 | 0.0128 (0.991) | 54 |
| 5 | 6.20 | 12.99 | 2.74 | 5.73 | |||
| 10 | 6.22 | 9.95 | 3.05 | 5.34 | |||
| 15 | 6.20 | 8.47 | 3.33 | 5.04 | |||
| Pressure | 0 | 7.12 | 16.15 | 2.5 | 6.46 | 0.0253 (0.994) | 27 |
| 5 | 7.69 | 14.00 | 2.81 | 6.12 | |||
| 10 | 7.72 | 12.10 | 3.05 | 5.36 | |||
| 15 | 7.80 | 8.50 | 3.39 | 4.75 | |||
| EcoCookera | 0 | 6.91 | 17.12 | 2.33 | 7.36 | 0.0104 (0.997) | 66 |
| 10 | 6.54 | 14.90 | 2.57 | 5.78 | |||
| 20 | 6.99 | 14.70 | 2.81 | 5.23 | |||
| 30 | 7.18 | 14.11 | 3.01 | 4.69 |
aThe standard error values were less than 0.05
bCalculated from semi-log plot of Ln [(a/b)t/(a/b)0] vs time
cThe standard error in ‘k’ values were less than 1 × 10−3
dHeld for 30 min as per the protocol recommended for cooking with ‘EcoCooker’
Prediction of colour loss during non-isothermal heating processing
To predict the degradation of color occurring during a given non-isothermal heating process, the arrhenius equation, kT = A0 exp (−Ea/RT) (Eq. 3) was used, where ‘kT’ is the rate constant at any absolute temperature ‘T’ and time ‘t’. ‘Ea’ is the activation energy of the reaction, ‘R’ is the gas constant, and A0 is a pre-exponential constant, which are already calculated for isothermal heating process. The rate constant ‘kT’ at each temperature was calculated using Eqs. (2, 3) substituting for ‘T’ from the time-temperature data of any specific non-isothermal heating process. Knowing the rate constant kT, the rate (dC/dt), the amount degraded (ΔC) during the short time interval zero to t (=ΔtT) and the final concentration ‘Ct+Δt’ can be calculated as follows.
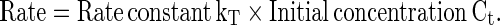 |
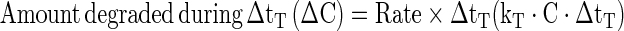 |
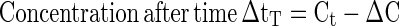 |
These calculations were continued for the entire time period (heating and constant temperatures) at which each of the unsteady cooking process was done. An MS excel based computer program was used to calculate the above parameters.
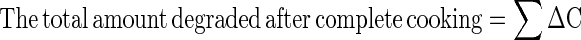 |
The final concentration thus will be = C0−∑ΔC, where C0 is the initial value of red color (‘a0’) and ΔC is cumulative total degradation totaled over all the time intervals considered.
The resulting predictions and the actual degradation, obtained experimentally, are given in Table 4. As seen, an excellent agreement between the actual and the predicted degradation/retention of colour has been obtained. The correlation coefficient ‘R2’ for the regression analysis of actual and predicted value of degradation was 0.78 indicating a good correlation between actual and predicted values (regression equation ‘y = −0.077x + 5.13 R2 = 0.781’). Using this prediction method, the degradation of visual colour can be predicted for any heat processing method, if the time-temperature profile of that processing operation is known.
Table 4.
The actual and predicted retention colour (Hunter ‘a/b’) in beetroot puree in different cooking methods
| Cooking method | Actual retention | Predicted retention |
|---|---|---|
| Open pan | 5.04 | 5.07 |
| Pressure | 4.75 | 4.89 |
| EcoCooker’a | 4.69 | 4.72 |
aHeld for 30 min as per the protocol recommended for cooking with ‘EcoCooker’
Conclusion
The colour degradation in beetroot puree could be calculated by ‘a/b’ value of Hunter system, and it followed first order kinetics. The mathematical model developed could be successfully used for predicting colour degradation during thermal processing of beetroot puree. The slow cooker ‘EcoCooker’ showed no significant difference in the magnitude of retention of colour as compared to normal open pan and pressure-cooking. Therefore based on the colour retention and fuel savings it can be suggested that ‘EcoCooker’ can be favored over normal cooking methods. The information obtained from the study could be used as a guideline for designing thermal processes to reduce the quality degradation of beetroot while processing and preservation.
Acknowledgement
The authors gratefully acknowledge the financial support provided by Land Research Institute, Mumbai in carrying out this work.
References
- Ahmed J, Ramaswamy HS (2005) Effect of temperature on dynamic rheology and colour degradation kineticsof date paste. Food and Bioprod Process 83(C3):198–202
- Ahmed J, Shivhare US, Mandeep Kaur (2002a) Thermal colour degradation kinetics of mango puree 5(2):359–366
- Ahmed J, Shivhare US, Ramaswamy HS. A fraction conversion kinetic model for thermal degradation of color in red chilli puree and paste. Lebensm Wiss u Technol. 2002;35:497–503. doi: 10.1006/fstl.2002.0897. [DOI] [Google Scholar]
- Ahmed J, Shivhare US, Kaur M. Thermal degradation kinetics of carotenoids and visual color of papaya puree. J Food Sci. 2002;67:2692–2695. doi: 10.1111/j.1365-2621.2002.tb08800.x. [DOI] [Google Scholar]
- Ahmed US, Shivhare GS, Raghavan V. Thermal degradation kinetics of anthocyanin and visual colour of plum puree. Eur Food Res Technol. 2004;218:525–528. doi: 10.1007/s00217-004-0906-5. [DOI] [Google Scholar]
- Attoe EL, Von Elbe JH. Degradation kinetics of betanine in solutions as influenced by oxygen. J Agric Food Chem. 1982;30:708–711. doi: 10.1021/jf00112a021. [DOI] [Google Scholar]
- Avila IMLB, Silva CLM. Modelling kinetics of thermal degradation of colour in peach puree. J Food Eng. 1999;39:161–166. doi: 10.1016/S0260-8774(98)00157-5. [DOI] [Google Scholar]
- Azeredo HMC. Betalains. Properties, sources, applications, and stability—a review. Int J Food Sci Technol. 2009;44:2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- Butera D, Tesoriere L, Gaudio F, Bongiorno A, Allegra M, Pintaudi AM, Kohen R, Livrea MA. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains. betanin and indicaxanthin. J Agric Food Chem. 2002;50:6895–6901. doi: 10.1021/jf025696p. [DOI] [PubMed] [Google Scholar]
- Gokhale SV, Lele SS. Dehydration of red beet root (Beta vulgaris) by hot air drying: process optimization and mathematical modeling. Food Sci Biotechnol. 2011;20:955–964. doi: 10.1007/s10068-011-0132-4. [DOI] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Impact of thermal treatment on colour and pigment pattern of red beet (Beta vulgaris L.) preparations. J Food Sci. 2004;69:491–498. doi: 10.1111/j.1365-2621.2004.tb10994.x. [DOI] [Google Scholar]
- Joshi JB, Patel SB (2002) Fuel efficient steam cooking device. Patent WO 01/39640 A3.
- Kanner J, Harel S, Granit R. Betalains—a new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- Koul VK, Jain MP, Koul S, Sharma VK, Tikoo CL, Jain SM. Spray drying of beet root juice using different carriers. Indian J Chem Technol. 2002;9:442–445. [Google Scholar]
- Labuza TP, Riboh D. Theory and application of Arrhenius kinetics to the prediction of nutrient losses in foods. J Food Sci. 1982;36:66–74. [Google Scholar]
- Loughrey K. Measurement of colour. In: Lauro GJ, Francis FJ, editors. Natural food colourants. New York: Marcel Dekker; 2000. pp. 273–288. [Google Scholar]
- Nisha P, Rekha SS, Anirudha BP. A study on the degradation kinetics of visual green colour in spinach (Spinacea oleracea L.) and the effect of salt therein. J Food Eng. 2004;64:135–142. doi: 10.1016/j.jfoodeng.2003.09.021. [DOI] [Google Scholar]
- Patkai G, Barta J. Decomposition of betacyanins and betaxanthins by heat and pH changes. Nahrung. 1996;40:267–270. doi: 10.1002/food.19960400508. [DOI] [Google Scholar]
- Rodrigo D, van Loey A, Hendrickx MC. Combined thermal and high pressure colour degradation of tomato puree and strawberry juice. J Food Eng. 2007;79:553–560. doi: 10.1016/j.jfoodeng.2006.02.015. [DOI] [Google Scholar]
- Roy K, Gullapalli S, Chaudhuri UR, Chakraborty R. The use of anatural colorant based on betalain in the manufacture of sweet products in India. Int J Food Sci Technol. 2004;39:1087–1091. doi: 10.1111/j.1365-2621.2004.00879.x. [DOI] [Google Scholar]
- Van Boekel MAJS. Statistical aspects of kinetic modeling for food science problems. J Food Sci. 1996;61:477–489. doi: 10.1111/j.1365-2621.1996.tb13138.x. [DOI] [Google Scholar]
- Von Elbe JH, Sy SH, Maing IY, Gabelman WH. Quantitative analysis of betacyanins in red beets (Beta vulgaris) J Food Sci. 1972;37:932–934. doi: 10.1111/j.1365-2621.1972.tb03707.x. [DOI] [Google Scholar]
- Weemas CA, Ooms V, Van Loey AM, Hendrickx ME. Kinetics of chlorophyll degradation and colour loss in heated broccoli juice. J Agric Food Chem. 1999;47:2404–2409. doi: 10.1021/jf980663o. [DOI] [PubMed] [Google Scholar]
- Woolfe ML. Pigments. In: Priestley RJ, editor. Effect of heat processing on food stuffs. London: Applied Science Publishers Ltd; 1979. pp. 76–120. [Google Scholar]