Abstract
Understanding the water sorption characteristics of cereal is extremely essential for optimizing the drying process and ensuring storage stability. Water relation of rough rice was studied at 20, 30, 40 and 50 °C over relative humidity (RH.) between 0.113 and 0.976 using the gravimetric technique. The isotherms displayed the general sigmoid, Type II pattern and exhibited the phenomenon of hysteresis where it was more pronounced at lower temperatures. The sorption characteristics were temperature dependence where the sorption capacity of the paddy increased as the temperature was decreased at fixed (RH). Among the models assessed for their ability to fit the sorption data, Oswin equation was the best followed by the third order polynomial, GAB, Smith, Chung-Pfost, and Henderson models. The monolayer moisture content was higher for desorption than adsorption and tend to decrease with the increase in temperature. Given the temperature dependence of the sorption isotherms the isosteric heats of sorption were calculated using Claussius-Clapeyron equation. The net isosteric heats decreased as the moisture content was increased and heats of desorption were greater than that of adsorption.
Keyword: Malaysian paddy, Sorption isotherms, Hysteresis, Mathematical models, Isosteric heat of sorption
Introduction
Rice (Oryza sativa) is the world’s most widely cultivated crop after wheat and represents a staple food of above 50 % of the total world population (FAO 2004). The post production system is a vital component of the rice industry, and proper harvesting, threshing and drying are integral parts of the system. Poor postharvest or inadequate drying can lead to heat burns or discoloration and yellowing of rice kernels (Reddy et al. 2008), increased respiration (Magan et al. 2010), fungal invasion, which results in mycotoxin production and loss of quality (Reddy et al. 2008). If the surrounding relative humidity (RH) is not carefully controlled throughout the storage facilities, the hygroscopic characteristics of the paddy may lead to substantial gain in their moisture content, and the risk of deterioration becomes high. Understanding the water sorption characteristics is very essential for the design of drying process and the estimation of shelf-life of the foods (Al-Muhtaseb et al. 2002).
Large numbers of sorption equation exist for the characterization of the moisture sorption behavior of foods. A number of these models are entirely empirical or semi-empirical while some rely on theories of sorption (Al-Muhtaseb et al. 2002). In fact, sorption isotherms represent the overall hygroscopic characteristics of the various components and the binding of water molecules to polymers in food matrixes (Kaymak-Ertekin and Gedik 2004). Thus, there is no single sorption model whether empirical or theoretical that is able to predict precisely the sorption isotherm of all food matrixes and at different ranges of water activity (aw) (Chirife and Iglesias 1978). Rice varieties vary in their protein and amylose contents (Bett-Garber et al. 2001), which may lead to differences in equilibrium relative humidity (ERH) and equilibrium moisture content (EMC) relationships (Bianco et al. 1997), and mycotoxin accumulation by the invading fungi (Reddy et al. 2010). Cultivars with higher amylose and protein contents, tend to equilibrate at lower water content at a given relative humidity and temperature (Axberg et al. 1998). Therefore, it is necessary to determine the most appropriate EMC/ERH equation for different varieties or products. Several researchers have developed valued sorption isotherm for different cereals (Iguaz and Vírseda 2007; Martin et al. 2001; Li et al. 2011).
Employing basics of thermodynamics to experimental sorption data can strengthen the understanding of sorption process and provide worth information on energy requirements for drying process. Thermodynamical indices attainable from the sorption process other than the isosteric heat of sorption are including sorption entropy, net integral enthalpy and net integral entropy (McMinn and Magee 2003). Isosteric heat of sorption usually is predicted using the Clausius Clapeyron function. This equation assumes that heat of sorption is independent of temperature, which allows reasonable estimation of the isosteric heat from the data of isotherms (McMinn and Magee 2003). Isosteric heat of sorption also provides valued information on the nature of the water present in the material. When the isosteric heat of soprtion exceeds the heat of vaporization of pure water, it is normally considered as an indication on bound water in food (McLaughlin and Magee 1998). Knowledge of isosteric heat of sorption is pre-requested for the overall description of the state of water in the food matrixes as well as for designing of drying operations (Al-Muhtaseb et al. 2002).
To our knowledge, previous studies have not attempted to characterize the water sorption properties of Malaysian paddy (MR219). The objective of the present study was to determine the sorption isotherms of paddy (MR219) at 20, 30, 40 and 50 °C, to evaluate ability of different sorption models in describing the sorption relation of paddy at different temperature and to determine the net isosteric heat of sorption of the Malaysian paddy (MR219).
Materials and methods
Material
MR219 is a rice variety, introduced by the Malaysian Agricultural Research and Development Institute (MARDI) in January, 2001 and today is widely cultivated through Malaysia. The main characteristics of this improved variety (MR219) that it has a short maturation period, high resistant to the pests, high yield potential and low amylase content, which contributes to the soft texture of cooked rice (MARDI 2011).
Experimental procedure
Rough rice MR219 variety was supplied by Bernas (Sekinchan, Malaysia). Initially, the paddy had aw and moisture content of 0.667 and 0.122 kg kg−1 dry basis (d.b.), respectively. The paddy was stored at 4 °C in cold room until needed. Static gravimetric technique was used to study the EMC and aw relation of the paddy. As a preliminary step prior to start of adsorption experiments, the paddy has been dried in desiccators with calcium sulphate (Merck, Germany) at room temperature at least for a minimum of 3 weeks. At contrast, desorption has been studied on paddy hydrated in sealed glass containers over distilled water at the ambient temperature (30 °C ) up to 32 % (d.b.) moisture before running the desorption study. Around 2 g portions of the paddy were placed in small baskets. All baskets, including paddy were then placed in sealed containers along with saturated salt solutions (LiCl, MgCl2, K2CO3, Mg(NO3)2, KI, NaCl, KCl, K2SO4), employed to create fixed relative humidity (Fontana 2007). Salts used were all of reagent grade. A small bottle with small volume of toluene was located in all jars to suppress microbial deterioration of the paddy (Bell et al. 2000). Three replicates of each experiment were prepared and all jars were then incubated at 20, 30, 40 and 50 °C. The weights of samples were measured every 3 days. Samples were considered at equilibrium when four successive weight measurements displayed a variation lesser than 0.001 g. The moisture content of each paddy sample was determined by drying in an oven (105 °C for 8–10 h) (AOAC 1980) in triplicates and aw values of all samples were verified using a fast water activity meter (GBX scientific FA-st, France).
Fitting sorption data to various isotherm equations
Sorption equations, presented in Table 1, were employed to describe the experimental data and their performance was assessed. The coefficients of sorption equation were determined applying the Levenberg–Marquardt algorithm option of the non-linear regression function of SPSS 17.0 (SPSS Inc., Chicago). The package is based on reducing the residual sum of squares (RSS) between the model and data. The quality of the performance of the sorption models was assessed using the relative percentage deviation modulus (P), known as
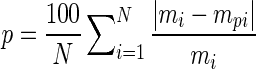 |
8 |
Where N is the number of experimental data and, mi and mpi are the experimental and predicted values, respectively. The mean relative percentage deviation modulus P (%) is broadly employed in literature (Al-Muhtaseb et al. 2004; Jena and Das 2011), where a model with (P) value below 10 % is considered of an acceptable fitting quality.
Table 1.
Models used to describe the sorption isotherm
| Model | Mathematical expression | |
|---|---|---|
| Oswin (Oswin 1946) |
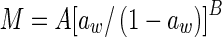
|
(1) |
| Henderson (Henderson 1952) |
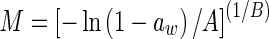
|
(2) |
| Smith (Smith 1947) |
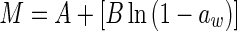
|
(3) |
| Chung Pfost (Chung and Pfost 1967) |
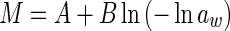
|
(4) |
| Polynomial (Alam and Shove 1973) |
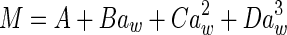
|
(5) |
| Halsey (Halsey 1948) |
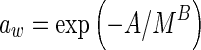
|
(6) |
| GAB (Guggenheim–Anderson–de Boer) (Quirijns et al. 2005) |
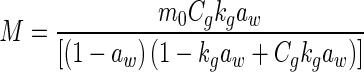
|
(7) |
M is the moisture content (kg kg−1, dry based), aw is the water activity, A, B and C are constants (dimensionless), m0 is the monolayer moisture content (kg kg−1), Cg is Guggenheim constant (dimensionless) and kg is constant (correction factor for water multilayer relative to bulk water, dimensionless)
Determination of the net isosteric heat of sorption
The net isosteric heats (Qst, kJ mol−1) have been calculated using Clausius–Clapeyron equation. At constant water content, the increase in temperature leads to raise in aw value. Thus, predicting aw as a function of temperature and plotting ln (aw) versus 1/T provides a straight line at fixed moisture content (m) where the isosteric heat can be calculated from the slope of the line which is equal to (Qst/R).
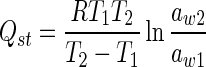 |
9 |
Where R is the universal gas constant (8.314 × 10−3 kJ mol−1 K−1), aw1 and aw2 are the water activity values at their corresponding temperatures T1 and T2.
Results and discussion
Moisture sorption isotherm and sorption hysteresis
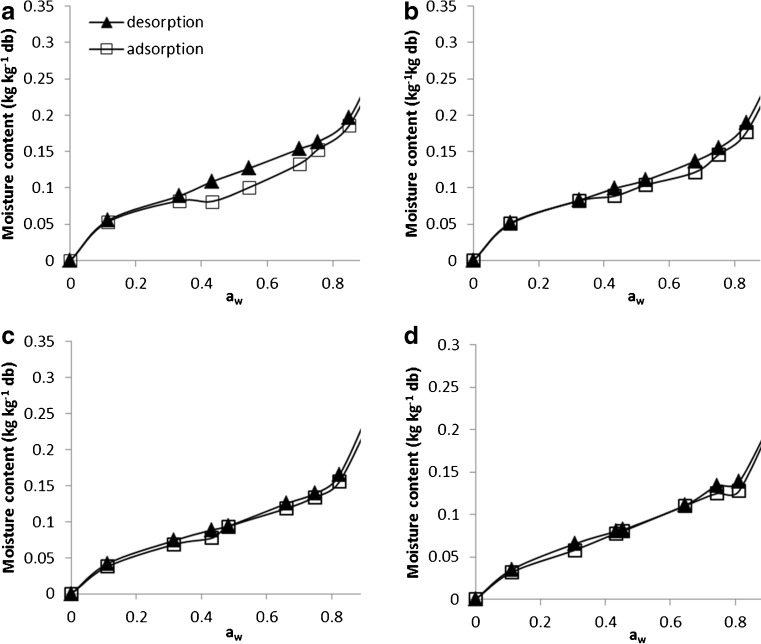
Experimental data obtained for adsorption and desorption isotherms are presented in Fig. 1 where aw plotted versus EMC. This figure demonstrate that at a constant temperature, there is a positive correlation between EMC and the aw of the paddy; where any increase in aw was accompanied by an increase in the EMC. These results imply that, the sorption relation of rice can be reasonably elucidated by a sigmoidal shape, characteristic of a type II isotherm (Al-Muhtaseb et al. 2002). This is in accordance with several studies which verified this behavior in starchy foods including rice (Brett et al. 2009; Erbas et al. 2005). The adsorption and desorption isotherms from our study exhibited the hysteresis phenomenon, where at particular ERH, the EMC of desorption was above the EMC of adsorption. This phenomenon has been observed in rice (Benado and Rizvi 1985; Togrul and Arslan 2006). Closer inspection of Fig. 1 indicated that magnitude of hysteresis is more clear in the aw range of 0.40–0.74. Furthermore, the extent of the hysteresis was obviously diminished when the temperature was increased and almost eliminated at 50 °C. Similarly, Benado and Rizvi (1985), McLaughlin and Magee (1998), Yan et al. (2008) found a decline of the hysteresis magnitude with increasing temperature for rice, potato and banana, respectively. While there is no concrete explanation for this phenomenon, it is commonly believed that there should be some irreversible thermodynamic reactions responsible for the changes in sorption characteristic of food. The most common arguments used to interpret this thermodynamical process, suggested that alteration brought by the dehydration process might render the hydrophobic functional groups on the surface of the food are not easily available to bind to the water molecules compared to samples under wet conditions. Thus, the foods tend to exhibit a lesser water-holding capacity with any further successive cycles of adsorption (Mohsenin 1986). Obtaining valuable reversible thermodynamic parameters might become a tedious duty due to hysteresis (Benado and Rizvi 1985). Elimination of hysteresis throughout consecutive cycling of adsorptions and desorptions of water from the food matrix can facilitate the estimation of reversible thermodynamic sorption indexes (Benado and Rizvi 1985).
Fig. 1.
Sorption isotherm of paddy at different temperatures a 20 °C, b 30 °C, c 40 °C and d 50 °C
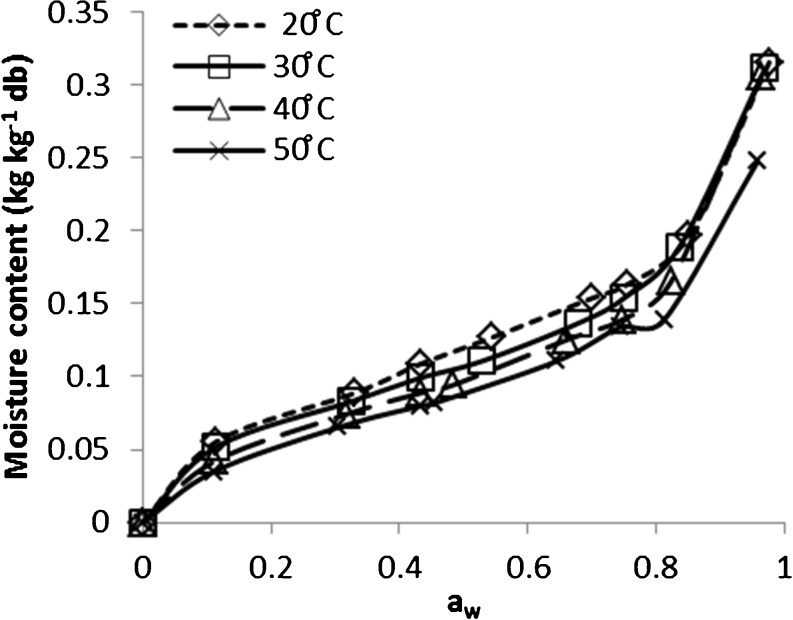
The effect of temperature on sorption behavior is demonstrated in Fig. 2 where the values for the EMC of rice decreased as the temperature of surrounding environment was increased at constant ERH. The impact of temperature on the sorption capacity of rice was more obvious in the desorption data compared to the adsorption. The increase in EMC at the lower temperature is probably because the kinetic energy that the water molecules are holding is insufficient to free them from their corresponding sorption sites (Quirijns et al. 2005). Moreover, water molecules form hydrogen bounds along with hydrophilic groups of the biopolymer such as starch, proteins. The formations of hydrogen bonds are exothermic reactions in nature (Levine 1995). This reaction is possibly suppressed as the temperature is increased. The impact of temperature on the equilibrium moisture can play a decisive role on biological and biochemical reactions associated with spoilage (Al-Muhtaseb et al. 2004). An increase in temperature causes an increase in the water activity at the same moisture content, which serve to enhance the deterioration reactions and loss of the quality.
Fig. 2.
Desorption isotherms of the paddy at 20, 30, 40, and 50 °C
Modeling sorption isotherm
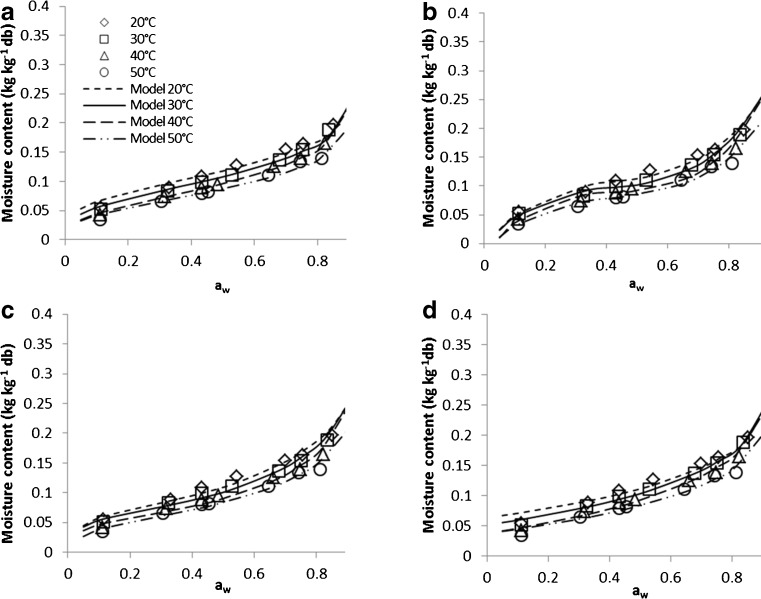
The parameters of the sorption models for the adsorption and desorption of paddy along with their r2 and the mean relative percentage deviation modulus P (%) are presented in Tables 2 and 3, respectively. The r2 values obtained for all models are above 0.964. However, when the p values were considered, it can be found that all models were in good agreement with desorption and adsorption experimental data of paddy except Halsey equation. Halsey is the only model which had an average P value of above 10 %. The results in Tables 2 and 3 also imply that the performance of Oswin model in predicting the adsorption and desorption experimental data within the whole range of water activity was the best. Next to Oswin, other models were ranked as follows: polynomial, GAB, Smith, Chung-Pfost and finally Henderson. The Oswin equation obtained P values between 2.276 % to 7.381 %, with overall values of 4.099 % for desorption and 5.286 % for adsorption. This is compared with average P values for the desorption of 4.754 % and 5.689 % and adsorption of 5.349 % and 5.499 % for polynomial and GAB models, respectively. The major limitation of the polynomial model is that its parameters have no physical meaning compared to GAB model. Figure 3 shows the experimental desorption data along with top ranked four fitted sorption equation, Oswin, polynomial, GAB and Smith. This figure shows that these models were able to provide related estimate of sotption data at their respective temperature.
Table 2.
Estimated parameters and P (%) values of sorption models fitted to the adsorption isotherm data of paddy
| Models | Constant | 20 °C | 30 °C | 40 °C | 50 °C |
|---|---|---|---|---|---|
| Oswin | A | 0.103 | 0.101 | 0.091 | 0.083 |
| B | 0.292 | 0.312 | 0.351 | 0.349 | |
| r2 | 0.995 | 0.996 | 0.998 | 0.990 | |
| P (%) | 6.371 | 3.243 | 4.419 | 7.381 | |
| Chung-Pfost | A | 0.082 | 0.082 | 0.072 | 0.066 |
| B | −0.056 | −0.058 | −0.060 | −0.053 | |
| r2 | 0.987 | 0.982 | 0.979 | 0.976 | |
| P (%) | 8.151 | 8.652 | 11.293 | 7.23515 | |
| Smith | A | 0.050 | 0.048 | 0.036 | 0.034 |
| B | −0.068 | −0.071 | −0.074 | −0.066 | |
| r2 | 0.994 | 0.997 | 0.994 | 0.983 | |
| P (%) | 4.880 | 3.714 | 5.698 | 8.633 | |
| Henderson | A | 25.912 | 23.866 | 19.825 | 26.134 |
| B | 1.568 | 1.527 | 1.384 | 1.453 | |
| r2 | 0.982 | 0.974 | 0.979 | 0.971 | |
| P (%) | 9.897 | 10.672 | 10.767 | 9.559 | |
| Halsey | A | 0.005 | 0.004 | 0.008 | 0.007 |
| B | 2.072 | 2.195 | 1.857 | 1.849 | |
| r2 | 0.986 | 0.987 | 0.978 | 0.964 | |
| P (%) | 5.590 | 9.983 | 12.882 | 16.439 | |
| GAB | m0 | 0.056 | 0.055 | 0.051 | 0.048 |
| Cg | 69.963 | 86.822 | 42.923 | 23.129 | |
| K | 0.830 | 0.838 | 0.852 | 0.837 | |
| r2 | 0.997 | 0.994 | 0.991 | 0.978 | |
| P (%) | 2.839 | 5.017 | 6.033 | 8.099 | |
| Polynomial | A | −0.016 | −0.003 | −0.019 | −0.021 |
| B | 0.421 | 0.595 | 0.624 | 0.568 | |
| C | −0.953 | −1.333 | −1.372 | −1.190 | |
| D | 0.833 | 1.065 | 1.086 | 0.919 | |
| r2 | 0.995 | 0.996 | 0.987 | 0.975 | |
| P (%) | 3.174 | 3.452 | 6.675 | 8.095 |
Table 3.
Estimated parameters and P (%) values of sorption equation fitted to desorption isotherm data of paddy
| Models | Constant | 20 °C | 30 °C | 40 °C | 50 °C |
|---|---|---|---|---|---|
| Oswin | A | 0.118 | 0.108 | 0.096 | 0.087 |
| B | 0.271 | 0.307 | 0.352 | 0.335 | |
| r2 | 0.992 | 0.996 | 0.999 | 0.994 | |
| P (%) | 5.880 | 3.285 | 2.28 | 4.956 | |
| Chung-Pfost | A | 0.096 | 0.087 | 0.077 | 0.070 |
| B | −0.058 | −0.061 | −0.063 | −0.053 | |
| r2 | 0.997 | 0.990 | 0.974 | 0.987 | |
| P (%) | 2.747 | 6.146 | 9.848 | 5.666 | |
| Smith | A | 0.064 | 0.052 | 0.038 | 0.038 |
| B | −0.069 | −0.075 | −0.079 | −0.066 | |
| r2 | 0.989 | 0.997 | 0.993 | 0.999 | |
| P (%) | 6.836 | 3.792 | 5.839 | 7.759 | |
| Henderson | A | 29.175 | 22.587 | 18.052 | 27.931 |
| B | 1.734 | 1.552 | 1.378 | 1.516 | |
| r2 | 0.987 | 0.982 | 0.973 | 0.981 | |
| P (%) | 6.779 | 9.208 | 12.197 | 7.827 | |
| Halsey | A | 0.006 | 0.006 | 0.006 | 0.005 |
| B | 2.145 | 2.079 | 1.991 | 2.013 | |
| r2 | 0.977 | 0.987 | 0.983 | 0.976 | |
| P (%) | 12.128 | 9.889 | 12.821 | 13.439 | |
| GAB | m0 | 0.068 | 0.061 | 0.053 | 0.051 |
| Cg | 40.674 | 46.903 | 30.929 | 22.546 | |
| K | 0.802 | 0.829 | 0.857 | 0.823 | |
| r2 | 0.984 | 0.995 | 0.991 | 0.988 | |
| P (%) | 5.259 | 4.234 | 6.934 | 6.330 | |
| Polynomial | A | 0.004 | −0.004 | −0.012 | 0.012 |
| B | 0.633 | 0.610 | 0.703 | 0.518 | |
| C | −1.298 | −1.323 | −1.573 | −1.071 | |
| D | 1.002 | 1.057 | 1.235 | 0.844 | |
| r2 | 0.988 | 0.995 | 0.989 | 0.987 | |
| P (%) | 5.077 | 3.918 | 5.457 | 4.569 |
Fig. 3.
Desorption isotherm of paddy with fitted sorption models, a Oswin, b polynomial, c GAB and d Smith
Wang and Brennan (1991) previously reported that the sorption isotherms of starch based foods can be reasonably described using Oswin model. Moreover, Jain et al. (2010) considered the Oswin as a superior model in describing the sorption characteristics of osmotically dehydrated papaya cubes. A number of studies have shown that GAB model has been successful in providing reasonable fit to sorption isotherms of several foods (Reddy and Chakraverty 2004; Erbas et al. 2005; Togrul and Arslan 2006; Alam and Singh 2011; Rakshit et al. 2011; Koua et al. 2012).
The results have shown that Smith model was in good agreement with the experimental sorption data of paddy throughout the whole water activity range. Yazdani et al. (2006) also reported that Smith model was able to describe the sorption isotherms of pistachio within water activity values between 0.11 and 0.90 and temperature between 15 and 40 °C. However, Al-Muhtaseb et al. (2004) and Togrul and Arslan (2006) found that Smith model can be successfully employed to describe the adsorption and desorption properties of starch and rice given that the water activity was higher than 0.35 and 0.4, respectively. Former studies have reported that the Chung-Pfost (Samapundo et al. 2007) and Henderson (Peng et al. 2007; Samapundo et al. 2007) models were good predictors of sorption characteristics of starch based materials . The Halsey was the poorest model in simulating the sorption data of paddy obtaining overall p value of 11.224 % and 12.0269 % for adsorption and desorption, respectively. Previous studies have shown that Halsey model is unsuitable for describing the water sorption relations of starchy materials (Al-Muhtaseb et al. 2004; Wang and Brennan 1991; Samapundo et al. 2007) while it is fitted to sorption data of coconut presscake (Jena and Das 2011).
Interpretation of GAB parameters provides valued information on sorption behaviour of the food materials. Closer inspection of the GAB parameters presented in Tables 2 and 3 displayed that the Guggenheim constants Cg are higher whereas the monolayer moisture contents are lower for adsorption than desorption. This indicates while the active sorption sites are less accessible for adsorption, they bind more tightly to the water molecules followed by multi-layer gradually deviating from the free water (Quirijns et al. 2005). The availability of active hydrophilic sites on the surface of food is reflected on the amount of monolayer m0 (McMinn and Magee 2003). Our results also showed that the m0 values tended to decrease as the temperature was increased. The modifications generated on the structure of food polymers such as starch at the elevated temperature are probably responsible for the reduction of the monolayer (McMinn and Magee 2003). The availability of hydrophilic site and ability of polymers to form hydrogen bounding is diminished at the higher temperature thus reducing the amount of monolayer (Quirijns et al. 2005). Moreover, when the temperature of the product was increased, water molecules were gaining greater kinetic energy, which enables them to get away from their binding positions (Quirijns et al. 2005). Similar observations were obtained by McLaughlin and Magee (1998), Al-Muhtaseb et al. (2004) and Erbas et al. (2005). The constants Cg and K give indications on energies of interaction between food and water molecules. The results did not display any clear correlation between the constants Cg and Kg and temperature.
Isosteric heat of sorption
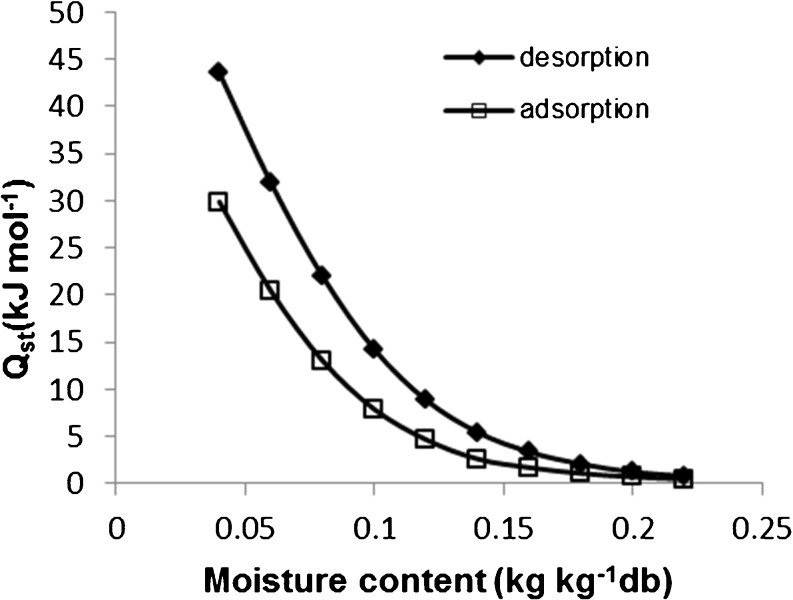
Clausius-Clapeyron equation was used to estimate the isosteric heat of sorption at different levels of moisture contents. The estimation was based on data generated using the best-fitting model (Oswin). The isosteric heats of desorption, and adsorption are plotted versus moisture content in Fig. 4. The result showed that the heat of adsorption is less than of desorption, and both of them were continually decreased as moisture content was increased. Similar findings have been reported for rice (Togrul and Arslan 2006), and corn (Samapundo et al. 2007). The variations in heats of sorption between desorption, and adsorption was found to diminish as moisture content increases. The negative correlation between the moisture content and the isosteric heat of sorption is due to the fact that the sorption is most likely taking place on the exposed active site of food surface thus providing an increase in sorption energy. Once the hydrophilic sites are not available anymore, binding takes place with the lesser active site providing lower heats of sorption (Quirijns et al. 2005).
Fig. 4.
Isosteric heat of sorption of the paddy
The heat of adsorption and desorption almost approached zero at 0.22 kg kg−1 (d.b.) moisture content, implying that total heat of sorption at this point is identical to heat of vaporization of free water. At this stage water molecules act as it is in the liquid state. Therefore, the greater the moisture content of the material, the lesser the energy needed during drying process. The variation in heats of sorption between adsorption, and desorption is possibly due to alteration in the polymer structure through the sorption process which may influence the availability of sorption sites on the surface of the food (McLaughlin and Magee 1998).
The corresponding heat of sorption of the paddy at moisture content of 0.10 kg kg−1 (d.b.) were 7.889 kJ mol−1 and 14.237 kJ mol−1 for adsorption and desorption, respectively. This result is in a similar range as it was reported in literature for paddy and rice flour (Benado and Rizvi 1985; Brett et al. 2009). Haque et al. (2007) and Iguaz and Vírseda (2007) reported that the isosteric heat of sorption of paddy was 9.980 kJ mol−1 and 9.230 kJ mol−1, respectively, which is lesser than the values found in our study. These differences rationalize the necessity to estimate the isosteric heat of sorption for each individual product. At particular moisture content, the extent of isosteric heat sorption is a good measure for the adsorption state of water, thus an indicator on the microbiological, chemical and physical stability of the food system (McMinn and Magee 2003).
Conclusions
The moisture sorption behavior of Malaysian paddy exhibits the common sigmoid pattern of Type II. Sorption behavior of the paddy was found to be temperature dependant where at fixed relative humidity, the equilibrium moisture content decrease with the increase in temperature. Hysteresis was evident, and the magnitude of the hysteresis loop was inversely related with temperature. Among the models evaluated for their ability to describe the experimental sorption data, Oswin was the superior and Hasley was the inferior equation. Net isosteric heat of desorption was greater than of adsorption, and both of them were found to be inversely correlated with moisture content.
References
- Alam A, Shove GC. Hygroscopicity and thermal properties of soybeans. Trans ASAE. 1973;16(4):707–709. doi: 10.13031/2013.37606. [DOI] [Google Scholar]
- Alam M, Singh A. Sorption isotherm characteristics of aonla flakes. J Food Sci Technol. 2011;48(3):335–343. doi: 10.1007/s13197-011-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhtaseb AH, McMinn WAM, Magee TRA. Moisture sorption isotherm characteristics of food products: a review. Food Bioprod Process. 2002;80(2):118–128. doi: 10.1205/09603080252938753. [DOI] [Google Scholar]
- Al-Muhtaseb AH, McMinn WAM, Magee TRA. Water sorption isotherms of starch powders: part 1: mathematical description of experimental data. J Food Eng. 2004;61(3):297–307. doi: 10.1016/S0260-8774(03)00133-X. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Washinghton, DC: Association of Official Analytical Chemists Inc.; 1980. [Google Scholar]
- Axberg K, Jansson G, Hult K. Ochratoxin A in rice cultivars after inoculation of Penicillium verrucosum. Nat Toxins. 1998;6(2):73–84. doi: 10.1002/(SICI)1522-7189(199804)6:2<73::AID-NT18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bell LN, Labuza TP, Chemists AAoC . Moisture sorption: Practical aspects of isotherm measurement and use. St. Paul: American Association of Cereal Chemists; 2000. [Google Scholar]
- Benado AL, Rizvi SSH. Thermodynamic properties of water on rice as calculated from reversible and irreversible isotherms. J Food Sci. 1985;50(1):101–105. doi: 10.1111/j.1365-2621.1985.tb13286.x. [DOI] [Google Scholar]
- Bett-Garber KL, Champagne ET, McClung AM, Moldenhauer KA, Linscombe SD, McKenzie KS. Categorizing rice cultivars based on cluster analysis of amylose content, protein content and sensory attributes. Cereal Chem. 2001;78(5):551–558. doi: 10.1094/CCHEM.2001.78.5.551. [DOI] [Google Scholar]
- Bianco A, Pollio M, Resnik S, Boente G, Larumbe A. Comparison of water sorption behaviour of three rice varieties under different temperatures. J Food Eng. 1997;33(3–4):395–403. doi: 10.1016/S0260-8774(97)00029-0. [DOI] [Google Scholar]
- Brett B, Figueroa M, Sandoval A, Barreiro J, Müller A. Moisture sorption characteristics of starchy products: oat flour and rice flour. Food Biophys. 2009;4(3):151–157. doi: 10.1007/s11483-009-9112-0. [DOI] [Google Scholar]
- Chirife J, Iglesias HA. Equations for fitting water sorption isotherms of foods: part 1 — a review. Int J Food Sci Tech. 1978;13(3):159–174. doi: 10.1111/j.1365-2621.1978.tb00792.x. [DOI] [Google Scholar]
- Chung DS, Pfost H. Adsorption and desorption of water vapor by cereal grains and their products. Trans ASAE. 1967;10(4):549–557. doi: 10.13031/2013.39726. [DOI] [Google Scholar]
- Erbas M, Ertugay MF, Certel M. Moisture adsorption behaviour of semolina and farina. J Food Eng. 2005;69(2):191–198. doi: 10.1016/j.jfoodeng.2004.07.017. [DOI] [Google Scholar]
- FAO (2004) Rice is life, international year of rice. In: Proceeding of the FAO rice conference 53 Rome, Italy
- Fontana AJ. Water activity of saturated salt solutions. In: Barbosa-Cánovas GV, Fontana AJ, Schmidt SJ, Labuza TP, editors. Water activity in foods: Fundamentals and applications. 1. Oxford: Wiley-Blackwell; 2007. pp. 391–393. [Google Scholar]
- Halsey G. Physical adsorption on non uniform surfaces. J Chem Phys. 1948;16:931–937. doi: 10.1063/1.1746689. [DOI] [Google Scholar]
- Haque A, Shimizu N, Kimura T, Bala B. Net isosteric heats of adsorption and desorption for different forms of hybrid rice. Int J Food Prop. 2007;10(1):25–37. doi: 10.1080/10942910600613228. [DOI] [Google Scholar]
- Henderson S. A basic concept of equilibrium moisture. Agric Eng. 1952;33(1):29–32. [Google Scholar]
- Iguaz A, Vírseda P. Moisture desorption isotherms of rough rice at high temperatures. J Food Eng. 2007;79(3):794–802. doi: 10.1016/j.jfoodeng.2006.03.002. [DOI] [Google Scholar]
- Jain SK, Verma RC, Sharma GP, Jain HK. Studies on moisture sorption isotherms for osmotically dehydrated papaya cubes and verification of selected models. J Food Sci Technol. 2010;47(3):343–346. doi: 10.1007/s13197-010-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena S, Das H (2011) Moisture sorption studies on vacuum dried coconut presscake. J Food Sci Technol:1–5. doi:10.1007/s13197-011-0306-3 [DOI] [PMC free article] [PubMed]
- Kaymak-Ertekin F, Gedik A. Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. Lebensm-Wiss u-Technol. 2004;37(4):429–438. doi: 10.1016/j.lwt.2003.10.012. [DOI] [Google Scholar]
- Koua BK, Koffi PME, Gbaha P, Toure S (2012) Thermodynamic analysis of sorption isotherms of cassava (Manihot esculenta). J Food Sci Technol doi:10.1007/s13197-012-0687-y [DOI] [PMC free article] [PubMed]
- Levine I. Physical Chemistry. 4. New York: McGraw-Hill; 1995. [Google Scholar]
- Li X, Cao Z, Wei Z, Feng Q, Wang J. Equilibrium moisture content and sorption isosteric heats of five wheat varieties in China. J Stored Prod Res. 2011;47:39–47. doi: 10.1016/j.jspr.2010.10.001. [DOI] [Google Scholar]
- Magan N, Aldred D, Mylona K, Lambert RJW. Limiting mycotoxins in stored wheat. Food Addit Contam. 2010;27(5):644–650. doi: 10.1080/19440040903514523. [DOI] [PubMed] [Google Scholar]
- MARDI (2011) Yielding Rice Varieties, . Retrieved on 3 December 2011 from http://www.mardigovmy/c/document_library/get_file?uuid=29d5002c-afd5-4892-b118-838f828e0006&groupId=10138
- Martin MBS, Mate J, Fernandez T, Virseda P. Modelling adsorption equilibrium moisture characteristics of rough rice. Drying Technol. 2001;19(3):681–690. doi: 10.1081/DRT-100103944. [DOI] [Google Scholar]
- McLaughlin CP, Magee TRA. The determination of sorption isotherm and the isosteric heats of sorption for potatoes. J Food Eng. 1998;35(3):267–280. doi: 10.1016/S0260-8774(98)00025-9. [DOI] [Google Scholar]
- McMinn WAM, Magee TRA. Thermodynamic properties of moisture sorption of potato. J Food Eng. 2003;60(2):157–165. doi: 10.1016/S0260-8774(03)00036-0. [DOI] [Google Scholar]
- Mohsenin NN. Physical properties of plant and animal materials. New York: Gordon and Breach; 1986. [Google Scholar]
- Oswin CR. The kinetics of package life. III. The isotherm. J Soc Chem Ind. 1946;65(12):419–421. doi: 10.1002/jctb.5000651216. [DOI] [Google Scholar]
- Peng G, Chen X, Wu W, Jiang X. Modeling of water sorption isotherm for corn starch. J Food Eng. 2007;80(2):562–567. doi: 10.1016/j.jfoodeng.2006.04.063. [DOI] [Google Scholar]
- Quirijns EJ, Van Boxtel AJB, van Loon WKP, Van Straten G. Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agric. 2005;85(11):1805–1814. doi: 10.1002/jsfa.2140. [DOI] [Google Scholar]
- Rakshit M, Moktan B, Hossain S, Sarkar P (2011) Moisture sorption characteristics of wadi, a legume-based traditional condiment. J Food Sci Technol:1–7. doi:10.1007/s13197-011-0491-0 [DOI] [PMC free article] [PubMed]
- Reddy B, Chakraverty A. Equilibrium moisture characteristics of raw and parboiled paddy, brown rice, and bran. Drying Technol. 2004;22(4):837–851. doi: 10.1081/DRT-120034266. [DOI] [Google Scholar]
- Reddy K, Reddy C, Abbas H, Abel C, Muralidharan K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Rev. 2008;27(3–4):287–317. doi: 10.1080/15569540802432308. [DOI] [Google Scholar]
- Reddy KRN, Reddy C, Salleh B. Varietal differences in accumulation of aflatoxin B1 in Indian rice cultivars. World Mycotoxin J. 2010;3(3):251–256. doi: 10.3920/WMJ2010.1226. [DOI] [Google Scholar]
- Samapundo S, Devlieghere F, Meulenaer BD, Atukwase A, Lamboni Y, Debevere JM. Sorption isotherms and isosteric heats of sorption of whole yellow dent corn. J Food Eng. 2007;79(1):168–175. doi: 10.1016/j.jfoodeng.2006.01.040. [DOI] [Google Scholar]
- Smith SE. The sorption of water vapor by high polymers. J Am Chem Soc. 1947;69(3):646–651. doi: 10.1021/ja01195a053. [DOI] [PubMed] [Google Scholar]
- Togrul H, Arslan N. Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosys Eng. 2006;95(2):181–195. doi: 10.1016/j.biosystemseng.2006.06.011. [DOI] [Google Scholar]
- Wang N, Brennan J. Moisture sorption isotherm characteristics of potatoes at four temperatures. J Food Eng. 1991;14(4):269–287. doi: 10.1016/0260-8774(91)90018-N. [DOI] [Google Scholar]
- Yan Z, Sousa-Gallagher MJ, Oliveira FAR. Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. J Food Eng. 2008;86(3):342–348. doi: 10.1016/j.jfoodeng.2007.10.009. [DOI] [Google Scholar]
- Yazdani M, Sazandehchi P, Azizi M, Ghobadi P. Moisture sorption isotherms and isosteric heat for pistachio. Eur Food Res Technol. 2006;223(5):577–584. doi: 10.1007/s00217-006-0256-6. [DOI] [Google Scholar]