Abstract
Dried residues from four different vegetables, viz. pea pod (pp), cauliflower waste (CW), potato peel (PP) and tomato peel (TP) were extracted using four solvents i.e., hexane, chloroform, ethyl acetate and methanol. Among the four solvents, methanolic extracts showed the highest total phenolic content (TPC) for all the four vegetable residues. Methanolic extracts were evaluated for antioxidant activities using diphenylpicryl-hydrazyl (DPPH) and reducing power assay. Tomato peel extract showed highest phenolic content of 21.0 mg GAE/g-dw and 80.8 % DPPH free radical scavenging ability, whereas potato peel extract had a low phenolic content, and it also showed the least antioxidant activity among the residues examined in this study. Total phenolic content and DPPH free radical scavenging activity in pea pods and cauliflower waste were 13.6 mg GAE/g-dw and 72 % and 9.2 mg GAE/g-dw and 70.7 %, respectively. The coefficient of determination (r2) for correlation between TPC and reducing power, DPPH and TPC, DPPH and reducing power for all extracts was 0.85, 0.91and 0.87, respectively, suggesting an important role of phenolics in imparting antioxidant ability. Extracts from vegetables residues therefore represent a significant source of phenolic antioxidants for use as nutraceuticals or biopreservatives.
Keywords: Antioxidant activity, Correlation, DPPH, Reducing power, Total phenolic content vegetable residues
Introduction
Lipid peroxidation is one of the principal causes of food quality deterioration. Lipid peroxidation results in the formation of reactive oxygen species and free radicals, which are associated with carcinogenesis, mutagenesis, inflammation, DNA changes, aging and cardiovascular diseases. Antioxidants are the substances that are able to prevent or inhibit oxidation processes in human body and deterioration in food products. It is hypothesized that phytochemicals in plant foods exert health beneficial effects because they combat oxidative stress in body by maintaining a balance between oxidants and antioxidants. Butylatedhydroxyanisole (BHA), Butylatedhydroxytoluene (BHT), and tert-Butylhydroquinone (TBHQ) are commonly used as synthetic antioxidants. Because of the possible role of synthetic antioxidants as promoters of carcinogenesis (Ito et al. 1986) and liver swelling (Martin and Gilbert 1968), the search for endogenous protective ingredients in foods has been intensified.
Antioxidants such as flavonoids, tannins, coumarins, curcumanoids, phenolics are found in various plant parts (e.g. fruits, leaves, seeds and oils) and have been reported to have multiple biological effects including antioxidant activity. For this reason, there is growing interest in separating these plant antioxidants and using them as natural antioxidant. Agriculture and industrial residues are attractive sources of natural antioxidants. The waste is obtained mainly in the form of seeds, peels, cuttings and trimmings. Large quantities of solid wastes are being left out and disposal of these waste materials usually represent a problem that is further aggravated by legal restrictions. Thus new aspects concerning the use of these wastes as by-products for further exploitation in the production of food additives or supplements is environmental friendly and economically attractive. Khiari and Makris (2012) reported that the non-edible portions of onion bulbs, which are considered as wastes contained phenolics with peculiar structures that are not found in the edible part.
Phenolics are broadly distributed in the plant kingdom and are the most abundant secondary metabolites found in plants. These phenolic substances or polyphenols include many classes of compounds ranging from phenolic acids, coloured anthocyanins, simple flavonoids and complex flavonoids (Spanos and Wrolstad 1992). All phenolic compounds possess an aromatic ring bearing one or more hydroxyl groups. The antioxidant compounds from agricultural residues may not only increase the stability of foods by preventing lipid peroxidation, but may also protect cell organelles from oxidative damage. Antioxidants are able to prevent the radical chain reactions of oxidation by cutting in the initiation and propagation step, which leads to the termination of the reaction and a delay in degradation reactions. The ability of compounds to act as antioxidants is based on the fact that they are able to form delocalized unpaired electrons, stabilizing the formed phenoxyl radical after reaction with lipid radicals (Gordon 1990). These properties allow the molecule to act as reducing agent, hydrogen donator and singlet oxygen quencher.
Vegetable is generally classified according to the edible plant parts, such as leaf, stem, flower or fruit. In this study, natural compounds with antioxidant property were isolated from common vegetable by products viz. Tomato peel (TP), cauliflower waste (CW), potato peel (PP) and pea pod (pp). These selected vegetables are cultivated across the globe and generate substantial amounts of biowaste in the form of peels, pods and inedible portions. Tomato (Lycopersicon esculentum) is a versatile vegetable that is consumed fresh as well as in the form of processed products. Tomato processing industries generate large amounts of waste in the form of seeds and peels. Tomato peel is a rich source of lycopene which has potential to be used in anti-cancer medicine (Wenli et al. 2001). Carotenoids like lycopene, β-carotene, lutein, zeaxanthin are known to be the most efficient singlet oxygen quencher in the biological systems without the production of any oxidizing products (Das et al. 2010). Cauliflower (Brassica oleracae L) is an important vegetable grown all over the world. Cauliflower has the highest waste index i.e. ratio of edible portion to non-edible portion and thus enormous amount of organic waste is generated which is not put to any commercial use (Oberoi et al. 2007). Similarly, pea pod (Pisum sativum) waste is also generated in large quantities which has only limited application in the form of cattle feed. Peels are a major by-product of potato processing and a potential source of functional compounds. Antioxidant activity of potato (Solanum tuberosum) peel has been studied but scarce information is available on the total phenolic content (TPC) and antioxidant activity for TP and CW. We have not yet come across any published report or literature describing the nutraceutical potential of pea pods. Thus, for the present study, we have focused on tomato, potato, peas and cauliflower which have a high waste index and residues of such vegetables are not put to any significant use.
Several methods were developed recently for measuring the total antioxidant capacity of food and beverages (Pellegrini et al. 2000). These assays differ in their chemistry generation of different radicals/or target molecules and in the way the end products are measured (Pellegrini et al. 2000). Because different antioxidant compounds may act in vivo through different mechanisms, no single method can fully evaluate the total antioxidant capacity of foods. With a hypothesis that the antioxidant constituents of these different vegetable wastes could be efficiently extracted using solvents with different polarities, this comparative study was aimed (i) to evaluate the efficiency of four different solvents in recovery of phenolics from the four residues (ii) to determine the antioxidant activities and reducing activities of the different extracts obtained using the evaluated solvent (iii) to analyze the possible correlation between the antioxidant activity, reducing activity and TPC.
Materials and methods
Materials
The vegetables were procured from local market and other sources, such as vegetable farm of Punjab Agricultural University, Ludhiana, India. The non-edible portions such as peels and cuttings were separated manually from the edible portions. 1, 1 diphenyl-2-picryl hydrazyl (DPPH), gallic acid, and butylated hydroxy toluene (BHT) were procured from Sigma-Aldrich (St. Louis, MO, USA). Analytical grade n-hexane, chloroform, ethyl acetate and methanol were procured from Fisher Scientific (Mumbai, India).
Sample preparation and extraction
Extraction of bioactive compounds was done according to the method of Velioglu et al. (1998). Potato peel samples were steamed at 100 °C for 15 min to prevent browning of flesh, cooled and dried in a hot-air oven. The samples from the other three residues were cut into small pieces and directly dried in a hot-air oven at 60 °C until complete drying. The dried samples were subsequently ground in a laboratory blender (Rumboa et al. 2009). Steam blanching for potato peel samples was employed primarily to inactivate degradative enzymes while minimizing losses of phenolic substances due to leaching (Rumboa et al. 2009). The samples (50 g each) were extracted with each of the four extraction solvents at 80 % concentration separately (n-hexane, chloroform, ethyl acetate and methanol) through refluxing at 60 °C. The four solvents used in the present study have been extensively tried by many researchers for efficient extraction of phenolic compounds (Ignat et al. 2011). The extracts were filtered using Buchner funnel lined with Whatman no.1 filter paper. The process was repeated twice for each extract and the final volume was made to 100 ml with deionized water. Samples were concentrated using rotary evaporator (Hahn Shin, Korea) at 40 °C for removal of the solvents (Babbar et al. 2011). The extracts were stored in a refrigerator and analyzed for TPC, reducing power and antioxidant activity. In the present study, water was not used for extraction of phenolic compounds because previous studies suggested that water was not very efficient in extraction of phenolic compounds (Negi et al. 2003; Prasad et al. 2009). It is also felt that only a few phenolic compounds, such as anthocyanins and proanthocyanadins present abundantly in extracts of litchi pericarp and grape seeds are water soluble (Babbar et al. 2011). We feel that the most of the phenolic compounds present in the four residues examined in the present study are composed of phenolics, such as flavanols, phenolic acids, sinapic acid derivatives and hydroxycinnamic acid derivatives, which cannot be effectively extracted using water (Ignat et al. 2011), thereby necessitating the use of organic solvents for their extraction.
Determination of total phenolic content (TPC) in vegetable residues
Total phenolic content of the methanolic extracts was determined with Folin-Ciocalteu colorimetric method (Velioglu et al. 1998). Briefly, 0.5 ml extract was mixed with 0.5 ml Folin-Ciocalteu reagent. The contents were mixed by manual shaking for 15–20 s. After 3 min, 0.50 ml of saturated sodium carbonate solution was added and the solution diluted to 5 ml with deionized water. The reaction mixture was incubated in dark at room temperature for 2 h and its absorbance was measured at 765 nm against deionized water using a dual beam UV–vis spectrophotometer (T-60, PG Instruments, UK). The total phenolic content was determined using a calibration curve prepared with gallic acid standard (0.01–0.1 %) as a reference. The values were reported as mg of gallic acid equivalent (GAE) by reference to gallic acid standard curve and the results were expressed as milligrams of GAE per gram dry weight (g-dw) of residues.
Reducing power
Reducing power of methanolic extracts was determined according to the method of Oyaizu (1986). 0.5 ml extract made to 1.0 ml with distilled water was mixed with 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 mL, 1 % potassium ferricyanide [K3 Fe (CN) 6]. The mixture was incubated at 50 °C for 20 min and centrifuged at 5000 g after addition of 2.5 ml of 10 % trichloroacetic acid. A 2.5 ml aliquot of upper layer (supernatant) was collected and mixed with 0.5 ml, 0.1 % FeCl3. The absorbance was measured at 700 nm against a blank using UV–vis spectrophotometer. Increase in absorbance was directly correlated to increase in reducing power.
Radical scavenging activity towards DPPH
The DPPH assay of extracts of residues and standard (BHT) was performed according to the method of Yamaguchi et al. (1998). 500 μl extract was mixed with 500 μl Tris- HCl buffer (50 mM, pH 7.4). 1 ml, 0.1 mM DPPH prepared in methanol was added to the reaction mixture. The tubes containing reaction mixture were incubated at ambient temperature for 20 min in dark. The DPPH absorption values at 517 nm were recorded every 5 min until 35 min. Different concentrations of BHT in 80 % methanol were used as standard. Antioxidant activity as scavenging activity (SA) was calculated as percent inhibition relative to control using following equation and expressed as % inhibition
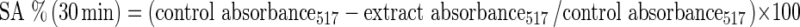 |
Statistical analysis
All the experiments were conducted in triplicate and the mean and standard deviation were calculated using MS Excel software and the treatment means were statistically analyzed using JMP software (SAS Inc., Cary, NC, USA).
Results and discussion
Total phenolic content
The polarity of extracting solvent and the solubility of chemical constituents in the extracting solvent might influence the TPC of the extracts. Therefore, in this study, the samples were extracted using four different solvents in order to determine the recovery of TPC using such solvents. The results revealed that methanol and ethyl acetate were better than the other two solvents in extraction of phenolic compounds (Table 1). This could probably be because of their higher polarity and better solubility for phenolic components present in plant materials (Zhao et al. 2006). The data in Table 1 shows that the extraction efficiency with methanol was highest for extracting phenolic compounds from all the four residues. Pryzbylski et al. (1998) reported that the antioxidant activity of buck-wheat extracts varied with polarity of the solvent with methanolic extract showing relatively higher antioxidant ability. The diverse chemical structures of the phenolic compounds ranging from simple to polymerized forms might consequently change their solubility behaviors. Methanol is often used for extraction of medium polar and polar phenolic compounds such as flavonoid glycosides and phenolic acids (Harborne 1998). The methanol extract and its fractions from brown seaweed exhibited higher 2,2′-azinobis(3-thylbenzothizoline-6-sulfonic acid) radical scavenging activity with more than 90 % scavenging in butanol and ethyl acetate fractions (Sachindra et al. 2010). The highest phenolic content was found in TP followed by pp, CW and PP. Quercetin is the only identified phenolic compounds present in tomato peel till date (Zeyada et al. 2008). Our results are in line with the results of George et al. (2004) who reported TPC of tomato waste in the range of 12.15–49.61 mg GAE/g dry residue. Phenolic compounds are the major antioxidants present in brassica vegetables due to their high content and high antioxidant activity (Podsedek 2007). The TPC in CW has been reported to range from 3.4 to 3.8 mM galllic acid/g (Valentina et al. 2008). The dominating phenolic acids in Brassica vegetables are sinapic acid derivatives (Winter and Herrmann 1986). The total phenolic content in potato samples analyzed in a study by Rumboa et al. (2009) ranged from 34.4 to 50.0 mg GAE/100 g dry sample, whereas our results show a much higher TPC for PP residues (Table 1). In a study Mohdaly et al. (2010) dried the potato peels at 40 °C in a hot-air oven and ground the material to a fine powder before extraction of phenolic compounds, however, the total phenolics in methanolic extract of potato peels reported by them was only 2.91 mg GAE/g which is significantly lower than the TPC analyzed in the present study. This suggests that steam blanching is important to preserve phenolics which are otherwise susceptible to degradation by peroxidases and catalases. However, it is important to optimize the steaming conditions as extended steaming may result in losses of phenolic compounds due to their susceptibility to leaching from the plant tissues and degradation of heat sensitive phenolic substances (Kalt 2005). Potato peels contain about 50 % of the total phenolics present in potato (Friedman 1997) and thus, it is important to ensure that such phenolics are not degraded before estimation. Sample treatment and extraction methods including homogenization and heating result in higher yields as demonstrated in literature (Huang et al. 2005). Studies have shown that chlorogenic acid, caffeic acid and quercitin, a flavanol are the major phenolic compounds in potatoes (Pratt and Watts 1964). Yalcin et al. (2011) reported that incorporation of potato peel, orange peel and apple pomace extracts prevented the oxidation in sunflower oil during storage for 45 days at 40 °C. The literature on phenolic compounds and antioxidant activity of pea pod is not available. The results mentioned above confirmed the important role of extracting solvent in altering the recovery of TPC in different vegetable wastes. On the basis of the above results, it is clear that methanolic extracts of all the four residues examined in this study showed comparable or higher TPC reported in published literature. However, more studies are needed to characterize the specific phenolic compounds present in the methanolic extracts of CW and pp and identify the role of those compounds in imparting the antioxidant ability.
Table 1.
Effect of different solvents on extraction of total phenols from different vegetable residues
| Vegetable residue | Total phenols (mg GAE/g-dw) | |||
|---|---|---|---|---|
| Methanol extract | Ethyl acetate extract | Chloroform extract | n-hexane extract | |
| Tomato peel | 21.0a ± 0.40 | 19.1a ± 0.89 | 18.9a ± 0.56 | 17.6a ± 0.67 |
| Pea pod | 13.6b ± 0.20 | 12.2b ± 0.78 | 8.0b ± 0.34 | 8.6b ± 0.34 |
| Cauliflower waste | 9.2c ± 0.60 | 9.1c ± 0.67 | 6.0c ± 0.34 | 4.3c ± 012 |
| Potato peel | 5.4d ± 0.40 | 5.2d ± 0.67 | 2.0d ± 0.45 | 2.1d ± 0.23 |
| LSD (p < 0.05) | 0.79 | 0.78 | 0.88 | 0.87 |
Values are mean ± standard deviation, n = 3
GAE gallic acid equivalent
dw dry weight
Reducing power
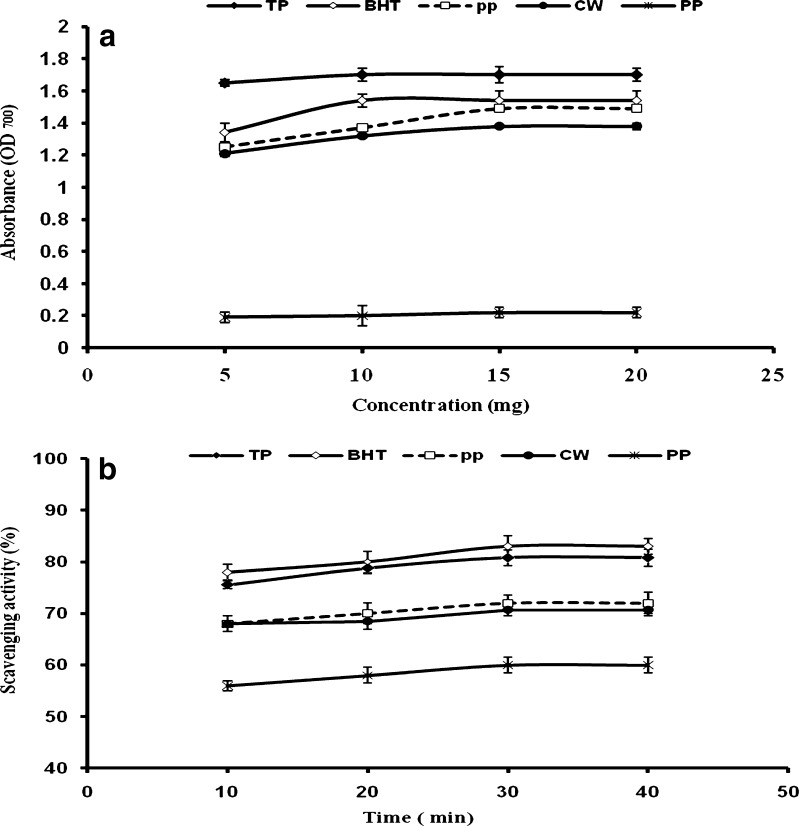
The reducing power of a compound is related to the electron transfer ability of that compound. In reducing power assay, the presence of antioxidant in an extract may lead to the reduction of Fe (III)/ferric cyanide complex to the ferrous form [Fe (II)] by donating an electron. Therefore, the reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. Methanolic extracts of four vegetable residues clearly display a dose dependent reducing power. Reducing power threshold value recorded at around 10 mg/ml for TP extracts was significantly higher than those of other vegetable residues (Fig. 1a), whereas the reducing power for extracts of the remaining residues levelled off at a concentration around 15 mg/ml (Fig. 1a). Reducing power for BHT which was used as a standard for comparison levelled off at a concentration of 10 mg/ml. The absorbance at 700 nm ranged from 0.22 to 1.65 for the extracts obtained from vegetable residues examined in the present study. We have not yet come across any published report describing reducing power for the vegetable residues examined in the present study.
Fig. 1.
Reducing power and Antioxidant activity of methanolic extracts obtained from vegetable residues and BHT. a LSD (p < 0.05) values for TP, BHT, pp, CW and PP were 0.07, 0.10, 0.02, 0.02 and 0.08, respectively. Values depicted are mean ± SD for n = 3. TP: Tomato peel, BHT: Butylated hydroxytoluene, CW: cauliflower waste, pp: Pea pods, PP: Potato peel. b LSD (p < 0.05) values for TP, BHT, pp, CW and PP were 2.4, 3.4, 3.3, 2.5 and 2.6, respectively. Values depicted are mean ± SD for n = 3. TP: Tomato peel, BHT: Butylated hydroxytoluene, CW: cauliflower waste, pp: Pea pods, PP: Potato peel
Among the vegetable residues investigated in this study, TP showed reducing power comparable to that of BHT. High reducing power at low concentration indicates high antioxidant potential. Previous studies have reported that the reducing power of bioactive compounds was associated with the antioxidant activity (Siddhuraju et al. 2002; Yen et al. 1993). Thus, it is necessary to determine the reducing power of phenolic compounds to elucidate the relationship between antioxidant effect of selected vegetable residues and their reducing power. In absence of available literature, it is not possible to discuss the results obtained for reducing power in methanolic extracts of the four residues. However, this study forms the basis for future work in this direction especially for characterization of residues like CW and pp.
Radical scavenging activity towards DPPH free-radical
This assay allows comparison of the reactivities of powerful antioxidants such as, BHT with those present in the vegetable extracts. DPPH possesses a proton free radical with a characteristic absorption which decreases significantly on exposure to proton radical scavengers (Yamaguchi et al. 1998). Further it is well accepted that the DPPH free-radical scavenging by antioxidants is due to their hydrogen donating ability (Chen and Ho 1995). Additionally, DPPH has the advantage of being unaffected by certain side reactions of polyphenols, such as metal ion chelation and enzyme inhibition. Figure 1(b) depicts the kinetics of DPPH bleaching by samples of vegetable extracts in comparison to BHT at a concentration of 5 mg/ml. All methanolic extracts showed considerable difference in antioxidant activity or scavenging activity method used in this study (Table 2). Clearly, the highest rate of DPPH decay occurs within the first 10 min of reaction. SA% was calculated at 30 min, as all the extracts showed maximum activity at 30 min. The efficiency of radical decomposition by vegetable extracts at 5 mg/ml was 80.8 % for TP followed by pp (72 %), CW (70.7 %) and PP (60 %) while that of BHT was 83 % (Table 2). It is clear from Fig. 1(b) that TP and BHT exhibited comparable DPPH radical scavenging ability. Antioxidants such as lycopene by virtue of their ability to interact with reactive oxygen species can mitigate the damaging effects of oxidants and may play a significant role in the prevention of chronic diseases (Rao and Rao 2007). The data obtained in this study revealed that the extracts obtained from TP were free radical scavengers which reacted with DPPH radical by their electron-donating ability. Wenli et al. (2001) concluded that lycopene is effective in scavenging such reactive oxygen species as superoxide anion, hydroxyl radical, singlet oxygen and lipid free radical. As was the case with reducing power of methanolic extracts, potato peel showed the least DPPH activity among the residues analyzed in the present study. In addition to phenolic compounds (as mentioned in the previous section) patain, the tuber storage protein of potato is associated with the antioxidant activity of potato (Liu et al. 2003). Previous studies have reported SA of 30.7 to 66.8 % for waste obtained from different cultivars of cauliflower (Scalzo 2005). The low antioxidant activity in PP in comparison with the reported literature may be a consequence of steam blanching prior to drying. Dao and Friedman (1992) studied the effect of boiling, microwave-baking and oven baking on whole potato tubers. They found that boiling destroyed 65 %, microwave baking 45 % and oven baking 100 % of the original amount of the chlorogenic acid. Phenolic compounds and vitamin C are the major antioxidants of brassica vegetables, due to their high content and high antioxidant activity. Lipid-soluble antioxidants (carotenoids and vitamin E) are responsible for up to 20 % of the brassica total antioxidant activity (Podsedek 2007).
Table 2.
Antioxidant activity of methanolic extracts obtained from vegetable residues
| Sample | SA (%)a |
|---|---|
| Tomato peel | 80.8b ± 0.10 |
| Pea pod | 72.0c ± 0.30 |
| Cauliflower waste | 70.7d ± 0.20 |
| Potato peel | 60.0e ± 0.50 |
| BHT | 83.0a ± 0.20 |
| LSD (p < 0.05) | 0.53 |
Values are mean ± standard deviation, n = 3
a SA Scavenging activity at 30 min = % inhibition relative to a control
BHT butylated hydroxytoluene
Relationship between antioxidant activity and total phenolic content
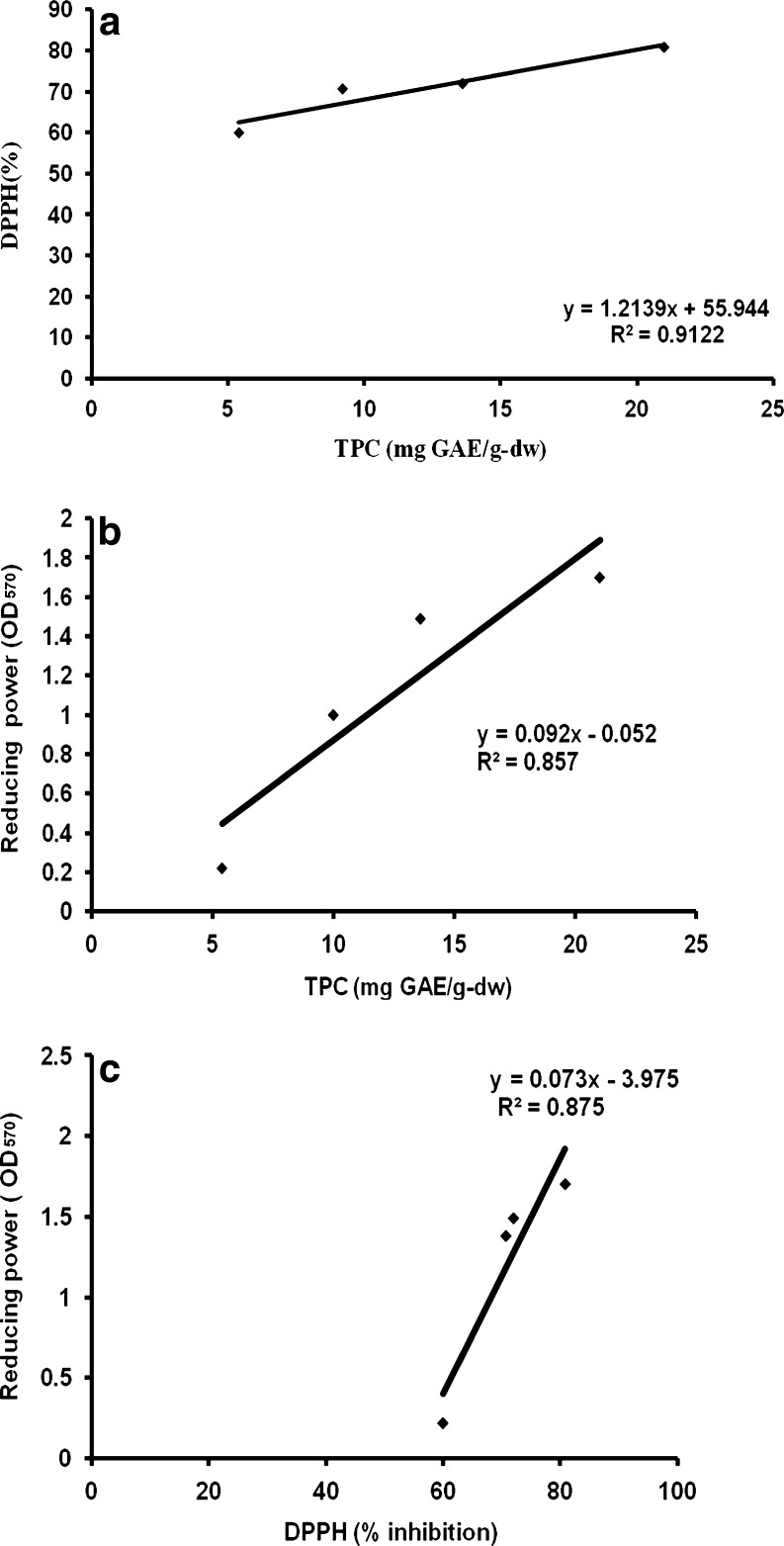
In order to establish the contributing effect of the total phenolic content (TPC) to the total antioxidant activities of vegetable residue extracts, linear correlation studies were performed between DPPH and TPC, DPPH and reducing activity, TPC and reducing activity. Among the extracts analyzed in this study, TPC was highest for tomato peel extract followed by pp and CW. Cauliflower waste and pp also showed a considerable antioxidant activity for DPPH scavenging abilities as mentioned previously. Potato peel extract had a lower concentration of phenolics and also showed a lower antioxidant activity. The correlation between DPPH scavenging ability and TPC was demonstrated by linear regression analysis. The r2 values determined on the basis of correlation between TPC and DPPH for all extracts was 0.91 (Fig. 2a). This shows that there is a high degree of correlation between TPC and DPPH. The r2 values determined between TPC and reducing power for all extracts was 0.85 (Fig. 2b) and r2 value between DPPH and reducing power was 0.87 (Fig. 2c). This study showed that TP, pp and CW can serve as good substrates for extraction of bioactive compounds which hold great promise for use in the food industry. Methanolic extract of tomato peel extract presented more promising results as an antioxidant, when evaluated for DPPH, reducing power and TPC. The phenolic concentration and antioxidant potential in extracts obtained from potato peel were relatively low and thus, such residues could be exploited for other applications like use as a dietary fibre in bakery. Previous studies demonstrated a linear correlation between phenolic content and antioxidant ability in fruits and vegetables (Kaur and Kapoor 2000). However, in one of our previous studies we showed a low degree of correlation between TPC and antioxidant activity for fruit residues (Babbar et al. 2011). High correlation coefficients between phenolic content and antioxidant activities have been reported for various food commodities such as sorghum (r2 = 0.971) and cactus pear (r2 = 0.97) (Rabah et al. 2004). The antioxidant activity of phenolic compounds is due to their ability to scavenge free radicals, donate hydrogen atoms or electrons, or chelate metal cations (Amarowicz 2004). The structure of phenolic compounds is a key determinant of their radical scavenging and metal chelating activity. The antioxidant activity of phenolic acids increases with increasing degree of hydroxylation. However, substitution of the hydroxyl groups at 3 and 5 position with methoxyl groups as in syringic acid reduces the activity (Rice-Evans 1996). We are now trying to exploit the potential use of such extracts as biopreservatives in foods and also for enhancing the shelf life of perishables. In addition, their use as effective nutraceuticals can also be exploited.
Fig. 2.
Relationship between total phenolic content, reducing power and DPPH radical scavenging activity for vegetable residues (a) total phenolic content and DPPH radical scavenging activity (b) total phenolic content and reducing power (c) total reducing power and DPPH
Conclusions
In the present study, four vegetable residues, viz., tomato peel, cauliflower waste, potato peel and pea pods were evaluated for their total phenolic content and antioxidant ability. Out of the four solvents used for extraction of phenolic compounds from the vegetable residues, methanolic extraction showed higher phenolic content for all the four vegetable residues. Tomato peel had the highest total phenolic content, reducing power and DPPH radical scavenging ability among the vegetable residues examined in the present study. Pea pods and cauliflower waste also showed good phenolic content and antioxidant ability. High degree of correlation between total phenolic content and DPPH radical scavenging activity indicates that phenolic compounds are responsible for antiradical activity in the methanolic extracts of the vegetable residues examined in this study. This study has set a platform for more research to characterize the specific phenolic compounds imparting the antioxidant characteristic to the four vegetable residues. Cauliflower waste and pea pods which account for a significant percentage of the total vegetable waste on fresh weight basis need to be exploited extensively for nature and concentration of phenolic constituents. Due to the low cost of vegetable residues, which otherwise would be discharged as waste in the environment, they should be regarded as potential nutraceutic resource, capable of offering low cost, dietary supplement.
Acknowledgments
Authors thankfully acknowledge the financial assistance received from Indian Council of Agricultural Research (ICAR), under AMAAS project of ICAR.
References
- Amarowicz Free- radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Chen CW, Ho CW. Antioxidant properties of polyphenols extracted from green and black tea. J Food Lipids. 1995;2:35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- Dao L, Friedman M. Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. J Agric Food Chem. 1992;40:2152–2156. doi: 10.1021/jf00023a022. [DOI] [Google Scholar]
- Das L, Bhaumik E, Raychaudhari U, Chakraborty R (2010) Role of nutraceuticals in human health. J Food Sci Technol. doi:10.1007/s13917-011-0269-4 [DOI] [PMC free article] [PubMed]
- Friedman M. Chemistry, biochemistry and dietary role of potato polyphenols- A review. J Agric Food Chem. 1997;45:1523–1540. doi: 10.1021/jf960900s. [DOI] [Google Scholar]
- George B, Kaur C, Khurdiya DS, Kapoor HC (2004) Antioxidants in tomato (Lycopersicum esculentum) as a function of genotype. Food Chem 84:45–51
- Gordon MH. The mechanism of antioxidant in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elseiver Applied Science; 1990. pp. 12–18. [Google Scholar]
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. 3. London, England: Chapman and Hall; 1998. [Google Scholar]
- Huang YC, Chang YH, Shao YY. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2005;98:529–538. doi: 10.1016/j.foodchem.2005.05.083. [DOI] [Google Scholar]
- Ignat I, Volf I, Popa VI. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Ito N, Hirose M, Fukishima S, Isuada H, Shirai T, Tatematsu M. Studies on antioxidants: their anticarcinogenic and modifying effects on chemical carcinogenesis. Food Chem Toxicol. 1986;24:1099–1102. doi: 10.1016/0278-6915(86)90291-7. [DOI] [PubMed] [Google Scholar]
- Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:11–19. doi: 10.1111/j.1365-2621.2005.tb09053.x. [DOI] [Google Scholar]
- Kaur C, Kapoor HC. Antioxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2000;37:153–161. doi: 10.1046/j.1365-2621.2002.00552.x. [DOI] [Google Scholar]
- Khiari Z, Makris DP (2012) Stability and transformation of major flavonols in onion (Allium cepa) solid wastes. J Food Sci Technol 49:489–494 [DOI] [PMC free article] [PubMed]
- Liu YW, Han CH, Lee MH, Hsu FL, Hou WC. Patain, the tuber storage protein of potato, exhibits antioxidant activity in vitro. J Agric Food Chem. 2003;51:4389–4393. doi: 10.1021/jf030016j. [DOI] [PubMed] [Google Scholar]
- Martin AD, Gilbert D. Enzyme changes accompanying liver enlargement in rats treated with 3-tert-butyl-4-hydroxyanisole. J Biochem. 1968;106:22–23. [Google Scholar]
- Mohdaly AAA, Sarhan MA, Smetanska I, Mahmoud A. Antioxidant properties of various solvent extracts of potato peels, sugar beet pulp and sesame cake. J Sci Food Agric. 2010;90:218–226. doi: 10.1002/jsfa.3796. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK, Jena BS. Antioxidant and antimutagenic activities of pomegranate peel extract. Food Chem. 2003;80:393–397. doi: 10.1016/S0308-8146(02)00279-0. [DOI] [Google Scholar]
- Oberoi HS, Kalra KL, Uppal DS, Tyagi SK. Effects of different drying methods of cauliflower waste on drying time, colour retentionand glucoamylase production by Aspergillus niger NCIM 1054. Int J Food Sci Technol. 2007;42:228–234. doi: 10.1111/j.1365-2621.2006.01331.x. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pellegrini N, Simonetti P, Gardana C, Brenna O, Brighenti F, Pietta PG. Polyphenol content and total antioxidant activity of Vini novella (young red wines) J Agric Food Chem. 2000;48:732–735. doi: 10.1021/jf990251v. [DOI] [PubMed] [Google Scholar]
- Podsedek A. Natural antioxidant capacity of brassica vegetables: a review. LWT- Food Sci Technol. 2007;40:1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Prasad KN, Yang B, Yang S, Chen Y, Zhao M, Ashraf M, Jiang Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009;116:1–7. doi: 10.1016/j.foodchem.2009.01.079. [DOI] [Google Scholar]
- Pratt DE, Watts BM. The antioxidant ctivity of vegetable extracts. J Food Sci. 1964;29:27–33. doi: 10.1111/j.1365-2621.1964.tb01689.x. [DOI] [Google Scholar]
- Pryzbylski R, Lee YC, Eskin NAM. Antioxidant and radical scavenging activities of buckwheat seed components. J Am Oil Chem. 1998;75:1595–1601. doi: 10.1007/s11746-998-0099-3. [DOI] [Google Scholar]
- Rabah IO, Hou DX, Komine SI, Fujii M. Potential chemopreventive properties of extract from baked sweet potato. J Agric Food Chem. 2004;23:7152–7157. doi: 10.1021/jf049368w. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Rice-Evans Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Rumboa RGO, Cornago DF, Geronimo IM. Phenolic content and antioxidant capacity of Philippine potato tubers. J Food Compos Anal. 2009;22:546–550. doi: 10.1016/j.jfca.2008.11.004. [DOI] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47:94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo J. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–213. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Mohn PS, Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia Fistula L): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002;79:61–69. doi: 10.1016/S0308-8146(02)00179-6. [DOI] [Google Scholar]
- Spanos GA, Wrolstad RE. Phenolics of apple, pear and white grape juice and their changes with processing and storage- a review. J Agric Food Chem. 1992;40:1478–1487. doi: 10.1021/jf00021a002. [DOI] [Google Scholar]
- Valentina S, Paul A, Andrew P, Esra I, Senol I. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready to eat expanded snacks. J Food Eng. 2008;87:554–563. doi: 10.1016/j.jfoodeng.2008.01.009. [DOI] [Google Scholar]
- Velioglu YS, Mazza G, Gho L, Onmah BD. Antioxidant activity and total phenolics in selected fruits and vegetables and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Wenli Y, Yaping Z, Zhen X, Hui J, Dapu W. The antioxidant properties of lycopene concentrate extracted from tomato paste. JOAC. 2001;78:697–701. doi: 10.1007/s11746-001-0328-6. [DOI] [Google Scholar]
- Winter M, Hermann K. Esters and glucosides of hydroxycinnamic acids in vegetables. J Agric Food Chem. 1986;34:616–620. doi: 10.1021/jf00070a007. [DOI] [Google Scholar]
- Yalcin H, Karaman S, Ozturk I. Evaluation of antioxidant efficiency of potato and orange peel and apple pomace extract in sunflower oil. Italian J Food Sci. 2011;23:55–61. [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical scavenging activity of foods by using 1,1-diphenyl-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–1220. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- Yen GC, Duh PD, Tsai C. Relationships between antioxidant activity and maturity of peanut hulls. J Agric Food Chem. 1993;41:67–70. doi: 10.1021/jf00025a015. [DOI] [Google Scholar]
- Zeyada NN, Zeitoum MAM, Barbary OM. Utilization of some vegetables and fruit waste as natural antioxidants. Alex J Food Sci Technol. 2008;5:1–11. [Google Scholar]
- Zhao M, Yang B, Wang J, Li B, Jiang Y. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 2006;98:539–544. doi: 10.1016/j.foodchem.2005.06.028. [DOI] [Google Scholar]