Abstract
Transforming growth factor-β (TGF-β) signaling regulates diverse cellular processes, including cell proliferation, differentiation, apoptosis, cell plasticity, and migration. TGF-β signaling can be mediated by Smad proteins or other signaling proteins such as MAP kinases and Akt. TGF-β signaling is tightly regulated at different levels along the pathways to ensure its proper physiological functions in different cells and tissues. Deregulation of TGF-β signaling has been associated with various kinds of diseases, such as cancer and tissue fibrosis. This paper focuses on our recent work on regulation of TGF-β signaling.
1. Introduction
Transforming growth factor-β (TGF-β) family is a group of structurally related growth factors, which includes TGF-β, activin, nodal, bone morphogenetic proteins (BMPs), and others. These growth factors play critical roles in regulating a wide range of biological processes during embryonic development and adult tissue homeostasis, and deregulation of the signal transduction has been associated with many human diseases, including cancer and tissue fibrosis [1–3]. TGF-β signaling is initiated by the binding of TGF-β to its serine and threonine kinase receptors, the type II and type I receptors on the cell membrane. Ligand binding triggers the formation of the receptor heterocomplex, in which type II receptor phosphorylates type I receptor at the threonine and serine residues in its TTSGSGSG motif, leading to the activation of type I receptor [1, 4, 5]. The activated type I receptor recruits and phosphorylates the R-Smad proteins, which then form a heterocomplex with the co-Smad Smad4. The Smad complexes are then accumulated in the nucleus and regulate transcription of the target genes by cooperating with other cofactors [6, 7].
For each member of the TGF-β family, they have their own combination of type I and type II receptors and R-Smads. For TGF-β signaling, the type I receptor TβRI/ALK5 and the type II receptor TβRII are employed to activate Smad2/3. For BMP signaling, ALK1/2/3/6 can activate Smad1/5/8 with type II receptor BMPRII, ActRII, and ActRIIB [8, 9]. ALK4/7 can activate Smad2/3 with ActRII and ActRIIB to mediate activin/nodal signaling [10, 11]. In addition, TGF-β can also activate mitogen-activating protein kinases (MAPKs) including ERK, p38 and JNK, phosphatidylinositol 3 kinase (PI3K)/Akt, and small GTPases [12–14]. In this review, we mainly summarize our work on the regulation of the activity and stability of TGF-β receptors and Smads, highlighting the current understanding and perspectives of TGF-β signaling modulation.
2. Membrane Trafficking Regulates the Activity and Stability of TGF-β Receptors
Cell surface receptors are internalized through two major endocytic pathways: clathrin-mediated endocytosis and lipid raft/caveolae-mediated endocytosis [15–17]. Clathrin-mediated endocytosis is the best characterized pathway, which is employed by many cell surface receptors such as G protein-coupled receptors, tyrosine kinase receptors, low-density lipoprotein receptor, and transferring receptor [18]. The receptors are first concentrated on the clathrin-coated pits, which are assembled on the cytoplasmic face of the plasma membrane by the recruitment of the adaptor complex AP2, clathrin, and other accessory proteins such as Eps15, epsin, disabled-2, synaptotagmin, and amphiphysin [19–21]. These pits undergo invagination and then pinch off from the plasma membrane in a dynamin GTPase-dependent manner [22]. After uncoating with dissociation of adaptors and clathrin, the vesicle is fused with early endosomes.
Besides clathrin-coated pits, cholesterol-enriched, and specialized detergent-insoluble lipid rafts can also be found in the plasma membrane, which can serve as signaling centers for nitric oxide, calcium, G protein-coupled receptors, and protein tyrosine kinases, or as virus entrance [23, 24]. Some of these membrane microdomains are specialized as caveolae in the presence of caveolin. Caveolae mediates the internalization of various proteins such as choleratoxin, glycosylphosphatidylinositol (GPI)-anchored proteins, endothelin receptor, and growth hormone receptor [25, 26]. The internalized cargos are transported to not well-characterized caveosomes and eventually to later endosomes or lysosomes.
TGF-β receptors are partitioned between the lipid rafts and nonraft areas on the plasma membrane [27–32]. Ligand binding to its receptor at the cell surface not only initiates signaling events but also triggers internalization of both ligand and receptors. We and others have demonstrated that TGF-β receptors can be endocytosed via clathrin-coated vesicles as TGF-β endocytosis can be blocked by potassium depletion and the GTPase deficient dynamin K44A mutant [33–35]. Internalization of TGF-β receptors through clathrin-dependent endocytosis to EEA1-positive endosomes is more likely to promote signaling as the FYVE domain-containing protein SARA are enriched in EEA1-positive endosomes and can facilitate R-Smads activation [36–38]. To support this idea, we found that endofin, which share a homology with SARA, can interact with TGF-β receptors and Smad4 and promote TGF-β-induced Smad complex formation [39]. The internalized receptors can be recycled to the membrane in a Rab11-dependent manner [40]. TGF-β receptors located in lipid raft regions enter cells via lipid raft/caveolae and are found in caveolin-positive vesicles [36]. Lipid raft/caveolae is indicated to facilitate the degradation of TGF-β receptors and therefore turnoff of TGF-β signaling (Figure 1).
Figure 1.

Membrane trafficking regulates the activity and stability of TGF-β receptors. Internalization of TGF-β receptors through clathrin-dependent endocytosis enhances TGF-β-Smad signaling, whereas caveolin-mediated endocytosis promotes the ubiquitination and degradation of the receptors and thus the turnoff of signaling. c-Cbl neddylates TβRII and facilitates its clathrin-dependent endocytosis, while PICK1 promotes lipid raft/caveolae localization and caveolin-mediated endocytosis of TβRI. Dapper2 locates in late endosomes and accelerates the lysosomal degradation of TβRI. The lipid raft localization of TGF-β receptors is critical for TGF-β-mediated MAPK activation.
The partitioning and internalization of TGF-β receptors are regulated processes [41]. One of the major regulators we identified is Casitas B-lineage lymphoma (c-Cbl), a protooncogene with widespread mutations in hematopoietic malignancies [42]. Unlike its classic role as a ubiquitin E3 ligase mediating receptor tyrosine kinases (RTKs) ubiquitination and degradation, c-Cbl interacts with TβRII and conjugates neural precursor cell-expressed, developmentally downregulated 8 (NEDD8), a ubiquitin-like protein, to TβRII at Lys556 and Lys567 [43]. Neddylation has been reported to regulate substrate protein activity, stability, and subcellular localization [44]. In the case of TβRII, we demonstrated that c-Cbl-mediated neddylation could target TβRII into EEA1-positive early endosomes and prevent its endocytosis to caveolin-positive compartments. Consequently, c-Cbl stabilizes TβRII by attenuating its ubiquitination and degradation and thereby enhances cellular TGF-β responsiveness.
It has been well established that c-Cbl mutations contribute to leukemia by negatively regulating the activity and stability of receptor tyrosine kinases [45–47]. Besides, disruption of TGF-β signaling, which is a major antiproliferation and prodifferentiation signal for hematopoietic stem/progenitor cells [48], greatly promotes lymphoblastic and myeloid leukemia in mouse models [49, 50]. We demonstrated that c-Cbl overexpression stabilizes TβRII and sensitizes leukemia cells to TGF-β-induced growth inhibition. We also identified a neddylation-activity-defective c-Cbl mutation from leukemia patients, implying that c-Cbl inactivation contributes to leukemia development not only by amplifying the mitogenic signals from RTKs, but also by releasing the antiproliferative effects of TGF-β.
We demonstrated that PICK1 (protein that interacts with C kinase 1), opposite to c-Cbl, promotes lipid raft/caveolae localization and caveolin-mediated endocytosis of TGF-β receptors [51]. As an adaptor protein, PICK1 has been shown to interact with a number of membrane proteins and regulate their subcellular trafficking, such as AMPAR [52–55], acid-sensing ion channel [56], and ErbB2/Her-2 [57]. Our biochemical analyses reveal that PICK1 directly interacts with the C-terminus of TβRI via its PDZ domain and acts as a scaffold protein to enhance the interaction between TβRI and caveolin-1, leading to increased lipid raft/caveolae localization [51]. Therefore, PICK1 increases caveolin-mediated endocytosis, ubiquitination, and degradation of TβRI and suppresses TGF-β signaling.
Previous studies associated the deviant expression of PICK1 in brain with mental disorders such as schizophrenia [58–60]. However, PICK1 is ubiquitously expressed in many organs outside the nervous system, and its physiological functions have not been fully investigated. By modulating the signaling, PICK1 may participate in TGF-β-related processes. Indeed, we observed a significant negative correlation between PICK1 expression and TβRI or phospho-Smad2 levels in human breast tumors, indicating that PICK1 may be involved in breast cancer development through inhibition of TGF-β signaling [51]. This idea is also supported by other reports suggesting that PICK1 is associated with human cancer development [57, 61–63].
In fact, distribution of TGF-β receptors in lipid rafts does not simply promote receptor degradation. We showed that localization of TGF-β receptors in the lipid raft regions is required for TGF-β-mediated MAPK activation. Disturbance of distribution of TGF-β receptors in lipid rafts by cholesterol depletion blocks TGF-β-induced MAPK activation and epithelial-mesenchymal transition (EMT) [64]. Consistent with this, specific targeting of the intracellular domain of TβRI to lipid rafts directly activates ERK and triggers EMT. This suggests a distinct role of lipid rafts in controlling the canonical TGF-β/Smad signaling and the TGF-β/noncanonical MAPK signaling.
We have also identified another regulator of TGF-β receptors trafficking and turnover, Dapper2. Interacting with Dishevelled with its C-terminal PDZ-binding motif, Dapper1 was first identified as a Wnt signaling antagonist in Xenopus [65]. Then, the inhibitory effect of Dapper2 on TGF-β/nodal signaling was demonstrated in zebrafish mesoderm induction [66], and its function is later found to be conserved in mammalian cells [67]. Dapper2 preferentially interacts with TβRI/ALK5 and activin receptor ActRIB/ALK4 in the Rab7-positive late endosomes and accelerates their lysosomal degradation, suggesting that Dapper2 facilitates the transport of endocytosed receptors from late endosomes to lysosomes. However, its detailed mechanism is unclear.
3. Regulation of TGF-β Receptor Ubiquitination and Stability
TGF-β receptors localized in lipid raft/caveolae and caveolin-1-positive vesicles undergo ubiquitination-mediated degradation [36, 68, 69]. Recruitment of the WW-HECT-type E3 ubiquitin ligases Smurf1, Smurf2, NEDD4-2 and WWP1 to TβRI is essential for its ubiquitination, in which process Smad7 acts as a critical adaptor [70]. Smad7 can bind to TβRI and HECT domain-containing E3 ligases and thus facilitate the assembly of the TβRI-Smad7-E3 complex, in which both TβRI and Smad7 are ubiquitinated and degradated [71–75] (Figure 2(a)).
Figure 2.
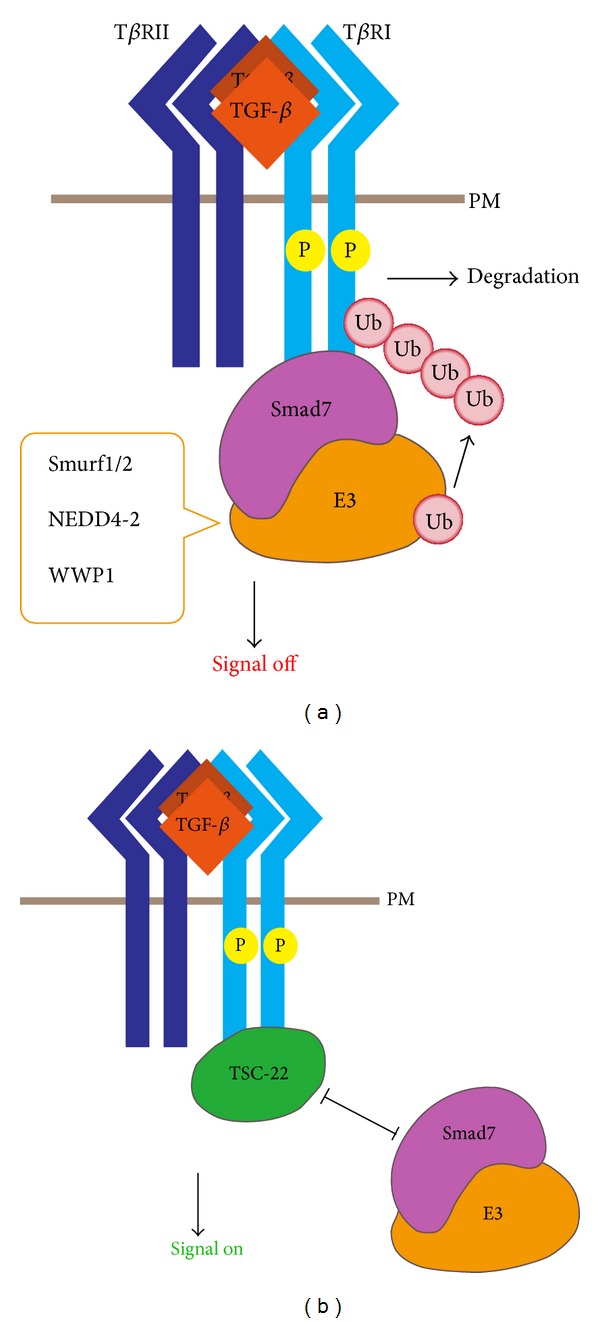
Regulation of TGF-β receptor degradation and expression. (a) TGF-β receptors localized in lipid raft/caveolae and caveolin-1-positive vesicles undergo ubiquitination. Smad7 recruits HECT domain-containing E3 ligases to mediate ubiquitination and degradation of TβRI. (b) TSC-22 competes with Smad7/Smurfs for TβRI binding and therefore decreases the ubiquitination and degradation of the receptor, leading to enhanced TGF-β signaling. PM: plasma membrane.
TβRI ubiquitination is finely controlled by multiple proteins, one of which we found is TGF-β-stimulated clone 22 (TSC-22). TSC-22, which was first reported as a TGF-β-upregulated gene in MC3T3E1 mouse osteoblastic cells, contains a leucine zipper-like structure and a nuclear export signal [76]. Accumulated evidence indicates that TSC-22 has an antiproliferative activity and is downregulated in several types of tumor cells [77–82]. We identified TSC-22 as a TβRI-binding partner using a yeast two-hybrid screen [83]. As a TGF-β target, TSC-22 can disrupt the binding of Smad7/Smurfs with TβRI and therefore decrease the ubiquitination and degradation of the receptor, leading to enhanced TGF-β signaling [83] (Figure 2(b)). This positive-feedback loop may be involved in myocardial fibrosis as an elevated TSC-22 level was correlated with TGF-β signaling activation and enhanced expression of fibrotic genes in the isoproterenol-induced heart fibrosis model. However, it is unclear whether TSC-22 prevents Smad7-induced receptor ubiquitination/degradation in lipid rafts or in nonraft regions.
4. Regulation of TGF-β Receptor Expression
Although modulation of receptor activities is a critical step for TGF-β signaling regulation, the regulation of TGF-β receptor expression is also important. Histone acetylation has been indicated to regulate TGF-β receptor expression [84–87]. Other mechanisms may be also employed to control their transcription. In search for miRNAs interfering type I receptor expression, we found that microRNA miR-24 reduces the mRNA and protein levels of human activin type I receptor ALK4 (ALK4) by targeting the 3′-untranslated region of ALK4 mRNA and inhibits activin signaling [88]. Consequently, miR-24 represses the activin-mediated erythroid differentiation of K562 cells, erythroid colony formation, and maturation of human CD34+ hematopoietic progenitor cells. TβRII expression is also repressed by mir-106b [89].
5. Modulation of Smad Activation
Upon being phosphorylated by TβRII, the activated TβRI recruits and phosphorylates Smad2/3 at the C-terminal (Figure 3(a)). Various proteins associated with the receptors complex have been reported to regulate R-Smad recruitment [90], such as SARA and endofin as mentioned above. BMP and activin membrane-bound inhibitor (BAMBI) has been reported as a general antagonist of TGF-β family members. Acting as a pseudoreceptor, BAMBI interferes with the interaction between type I and type II receptors of the TGF-β family [91]. In addition to blocking the heterocomplex formation of TGF-β receptors, our recent work showed that BAMBI cooperates with Smad7 to inhibit TGF-β signaling [92]. BAMBI can form a ternary complex with Smad7 and TβRI and inhibit the interaction between TβRI and Smad3, which impairs Smad3 activation (Figure 3(b)). Besides, we also found that p21-activated kinase 2 (PAK2) can directly phosphorylate Smad2 at Ser417, which interferes with the TβRI-Smad2 association and thus blocks TGF-β-induced Smad2 activation and signaling [93].
Figure 3.
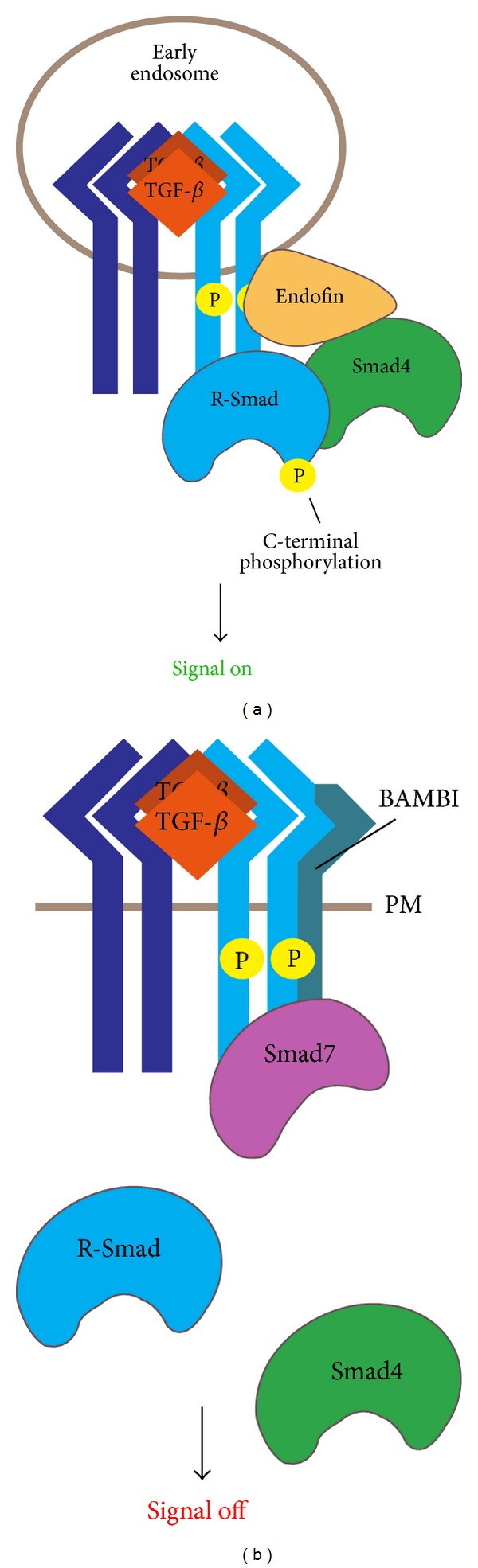
Modulation of Smad activation. (a) Activated TβRI recruits and phosphorylates Smad2/3 at the C-terminal, and then the phosphorylated Smad2/3 binds Smad4 to form a Smad heterocomplex to mediate signal transduction. Endofin recruits Smad4 to the receptor complex in early endosomes and facilitates the association of receptor-activated Smad2/3 with Smad4. (b) BAMBI forms a ternary complex with receptors and Smad7 and inhibits the interaction between TβRI and Smad3, impairing Smad3 activation. PM: plasma membrane.
Phosphorylated Smad2/3 binds Smad4 to form a Smad heterocomplex, which mediates downstream signal transduction. We have reported that the FYVE domain-containing protein endofin can interact with both TβRI and Smad4 [39]. As a scaffold protein, endofin recruits Smad4 to TβRI in early endosomes and facilitates the association of receptor-activated Smad2 with Smad4 (Figure 3(a)).
6. Regulation of Smad Activity
Smad4 is the common Smad critical for both TGF-β/activin and BMP signaling. However, several studies have also revealed Smad4-independent R-Smad signaling [94–96]. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein (SARS-CoV N protein) is a 46 kDa viral RNA-binding protein that shares little homology with the N proteins of other known coronaviruses [97]. We found that SARS-CoV N interacts with Smad3 and enhances Smad3-p300 interaction, which specifically potentiates the Smad3-mediated transcriptional responses of TGF-β such as the expression of plasminogen activator inhibitor-1 (PAI-1) [98]. At the same time, the SARS-CoV N interferes with the complex formation between Smad3 and Smad4 and inhibits TGF-β-induced Smad4-mediated proapoptotic genes expression and cell apoptosis (Figure 4(a)).
Figure 4.

Regulation of Smad activity in the nucleus. (a) SARS-CoV N protein interacts with Smad3 and enhances the Smad3-p300 interaction, potentiating the Smad3-mediated transcription of fibrotic genes. SARS-CoV N protein can also interfere with the complex formation between Smad3 and Smad4, thereby inhibiting Smad4-mediated expression of apoptotic genes. (b) Smad7 directly binds to DNA and represses TGF-β signaling by interfering with the functional R-Smad/Smad4-DNA complex on target gene promoters. (c) YY1 can cooperate with Smad7 to inhibit TGF-β signaling in the nucleus via recruiting HDAC1.
In addition, we reported that in some cell lines, including Hep3B, HeLa, L17 cells (a mutant mink lung epithelial Mv1Lu cell line lacking TβRI) and human normal lung epithelial HPL-1 cells, Smad7 is predominantly localized in the nucleus and can inhibit the transcriptional activity of the functional R-Smad-Smad4 complex, independently, of inhibition of the type I receptors [99]. Unlike R-Smads and Smad4, which bind to DNA through their MH1 domains, biotinylated oligonucleotide pull-down assays and single-molecule force spectroscopy studies showed that Smad7 binds to DNA through its MH2 domain and thus represses TGF-β signaling by interfering with the functional R-Smads/Smad4-DNA complex formation on the target gene promoters [99, 100] (Figure 4(b)). These results suggest that Smad7 can inhibit TGF-β signaling in the nucleus by a novel mechanism.
Furthermore, we identified Yin Yang 1 (YY1), a ubiquitously expressed transcription repressor, as a critical cooperator of Smad7 in the nucleus [101]. Although it has been reported that YY1 can attenuate TGF-β/Smad signaling independently of its DNA binding ability [102], we found that YY1 and Smad7 could interact with each other and synergistically suppress TGF-β-induced transcription in the nucleus. Mechanistically, Smad7 enhances the interaction of YY1 with the histone deacetylase HDAC1 (Figure 4(c)). These studies reveal the important function of Smad7 to attenuate TGF-β signaling in the nucleus. This notion is supported by a recent report showing that nuclear Smad7 can promote myogenesis independent of TGF-β/Smad3 signaling [103].
7. Conclusions and Perspectives
Modulating the activity and stability of TGF-β receptors is a critical step for regulation of TGF-β signaling. Although much effort has been made to understand the regulatory mechanisms of TGF-β receptors, many important questions still remain unsolved. For instance, although degradation of TGF-β receptors is sensitive to the inhibitors of lysosome and proteasome, it is unclear how these two degradation pathways cooperate to achieve full degradation of TGF-β receptors. In addition to the caveosome pathway, TGF-β receptors can be transported to lysosomes via early endosomes and later endosomes. How is the intracellular sorting of TGF-β receptors regulated? Ubiquitination is known to promote TGF-β receptors degradation. However, its role in mediating TGF-β receptors partition and internalization is unclear. In addition, how the receptors in lipid rafts activate MAPK is another important subject of future investigation.
For Smad regulation, many questions await to be addressed too. It is well documented that the TGF-β receptor-mediated C-terminal phosphorylation of Smad2/3 is the key event for Smad activation. TGF-β receptors can also induce the Smad2/3 phosphorylation in the linker region [104, 105]. The linker phosphorylation has been shown to inhibit Smad activity or induce Smad degradation [6]. How the inhibitory linker phosphorylation and the activating C-terminal phosphorylation are coordinated is unknown. In the nucleus, Smad7 can bind to DNA via its MH2 domain and inhibit TGF-β-driven transcription by interfering with the R-Smad/Smad4-DNA association. It will be interesting to investigate whether Smad7 has other function independent of inhibition of TGF-β signaling.
Regulation of TGF-β signaling has been extensively investigated. However, as TGF-β signaling controls a wide range of biological responses and distinct regulatory mechanism is employed by different tissue at different time, exploration of the molecular mechanisms of how the TGF-β signaling is modulated in specific pathological or physiological processes will be an exciting field.
Acknowledgments
The authors are grateful to the past and current members in the Chen Lab, who have contributed to the works discussed in this paper. The current research in the Chen lab is supported by the Grants from NSFC (31330049, 31221064), and the 973 Program (2011CB943803, 2011CBA01104, 2010CB833706), and Bing Zhao is supported by postdoctoral fellowships from Amgen China and from Tsinghua-Peking Center for Life Sciences. Ye-Guang Chen is the Bayer Chair Professor.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 2.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nature Reviews Molecular Cell Biology. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 3.Massagué J. TGFβ in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massagué J, Chen Y-G. Controlling TGF-β signaling. Genes and Development. 2000;14(6):627–644. [PubMed] [Google Scholar]
- 5.Huang F, Chen Y-G. Regulation of TGF-β receptor activity. Cell and Bioscience. 2012;2(1, article 9) doi: 10.1186/2045-3701-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes and Development. 2005;19(23):2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 7.Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through smads. Annual Review of Cell and Developmental Biology. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 8.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine and Growth Factor Reviews. 2009;20(5-6):343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews. 2005;16(3):251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Walton KL, Makanji Y, Harrison CA. New insights into the mechanisms of activin action and inhibition. Molecular and Cellular Endocrinology. 2012;359(1-2):2–12. doi: 10.1016/j.mce.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y-G, Wang Q, Lin S-L, Chang CD, Chung J, Ying S-Y. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Experimental Biology and Medicine. 2006;231(5):534–544. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 12.Moustakas A, Heldin C-H. Non-Smad TGF-β signals. Journal of Cell Science. 2005;118(16):3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 13.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Research. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77(3):759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 17.Brown DA, London E. Functions of lipid rafts in biological membranes. Annual Review of Cell and Developmental Biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annual Review of Biochemistry. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 19.Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends in Cell Biology. 2001;11(9):385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 20.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nature Reviews Molecular Cell Biology. 2003;4(5):409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annual Review of Biochemistry. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 22.Hinshaw JE. Dynamin and its role in membrane fission. Annual Review of Cell and Developmental Biology. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RGW, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 24.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 25.van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends in Cell Biology. 2003;13(2):92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 26.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. Journal of Cell Biology. 2003;161(4):673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Wang Q, Jiang Y, Xiao Z, Fang X, Chen Y-G. Lateral diffusion of TGF-β type I receptor studied by single-molecule imaging. Biochemical and Biophysical Research Communications. 2007;356(1):67–71. doi: 10.1016/j.bbrc.2007.02.080. [DOI] [PubMed] [Google Scholar]
- 28.Luga V, McLean S, Le Roy C, O'Connor-McCourt M, Wrana JL, Di Guglielmo GM. The extracellular domain of the TGFβ type II receptor regulates membrane raft partitioning. Biochemical Journal. 2009;421(1):119–131. doi: 10.1042/BJ20081131. [DOI] [PubMed] [Google Scholar]
- 29.Xiao LZ, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-β receptor compartmentalization and turnover enhances TGF-β1 signaling. Journal of Biological Chemistry. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 30.Atfi A, Dumont E, Colland F, et al. The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. Journal of Cell Biology. 2007;178(2):201–208. doi: 10.1083/jcb.200612046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C-L, Shuan SH, Huang JS. Cellular heparan sulfate negatively modulates transforming growth factor-β1 (TGF-β1) responsiveness in epithelial cells. The Journal of Biological Chemistry. 2006;281(17):11506–11514. doi: 10.1074/jbc.M512821200. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. The Journal of Biological Chemistry. 2004;279(24):25326–25332. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- 33.Lu Z, Murray JT, Luo W, et al. Transforming growth factor β activates Smad2 in the absence of receptor endocytosis. Journal of Biological Chemistry. 2002;277(33):29363–29368. doi: 10.1074/jbc.M203495200. [DOI] [PubMed] [Google Scholar]
- 34.Penheiter SG, Mitchell H, Garamszegi N, Edens M, Doré JJE, Jr., Leof EB. Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Molecular and Cellular Biology. 2002;22(13):4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes S, Chawla A, Corvera S. TGFβ receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. Journal of Cell Biology. 2002;158(7):1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nature Cell Biology. 2003;5(5):410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Chuang J-Z, Xu K, McGraw TG, Sung C-H. SARA, a FYVE domain protein affects RAb5-mediated endocytosis. Journal of Cell Science. 2002;115(24):4755–4763. doi: 10.1242/jcs.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panopoulou E, Gillooly DJ, Wrana JL, et al. Early endosomal regulation of Smad-dependent signaling in endothelial cells. Journal of Biological Chemistry. 2002;277(20):18046–18052. doi: 10.1074/jbc.M107983200. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y-G, Wang Z, Ma J, Zhang L, Lu Z. Endofin, a FYVE domain protein, interacts with smad4 and facilitates transforming growth factor-β signaling. The Journal of Biological Chemistry. 2007;282(13):9688–9695. doi: 10.1074/jbc.M611704200. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and -independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and rab11. Molecular Biology of the Cell. 2004;15(9):4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y-G. Endocytic regulation of TGF-β signaling. Cell Research. 2009;19(1):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 42.Naramura M, Nadeau S, Mohapatra B, et al. Mutant Cbl proteins as oncogenic drivers in myeloproliferative disorders. Oncotarget. 2011;2(3):245–250. doi: 10.18632/oncotarget.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo W, Huang F, Chiang YJ, et al. C-Cbl-mediated neddylation antagonizes ubiquitination and degradation of the TGF-beta type II receptor. Molecular Cell. 2013;49(3):499–510. doi: 10.1016/j.molcel.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell. 2011;19(2):168–176. doi: 10.1016/j.ccr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Kales SC, Ryan PE, Nau MM, Lipkowitz S. Cbl and human myeloid neoplasms: the Cbl oncogene comes of age. Cancer Research. 2010;70(12):4789–4794. doi: 10.1158/0008-5472.CAN-10-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nature Reviews Cancer. 2008;8(5):341–350. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- 47.Thien CBF, Langdon WY. CBL: many adaptations to regulate protein tyrosine kinases. Nature Reviews Molecular Cell Biology. 2001;2(4):294–305. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2853. [PubMed] [Google Scholar]
- 49.Quere R, Karlsson G, Hertwig F, et al. Smad4 binds Hoxa9 in the cytoplasm and protects primitive hematopoietic cells against nuclear activation by Hoxa9 and leukemia transformation. Blood. 2011;117(22):5918–5930. doi: 10.1182/blood-2010-08-301879. [DOI] [PubMed] [Google Scholar]
- 50.Wolfraim LA, Fernandez TM, Mamura M, et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. The New England Journal of Medicine. 2004;351(6):552–559. doi: 10.1056/NEJMoa031197. [DOI] [PubMed] [Google Scholar]
- 51.Zhao B, Wang Q, Du J, Luo S, Xia J, Chen Y-G. PICK1 promotes caveolin-dependent degradation of TGF-β type I receptor. Cell Research. 2012;22(10):1467–1478. doi: 10.1038/cr.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PD domain-containing protein PICK1. Neuron. 1999;22(1):179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 53.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nature Cell Biology. 2008;10(3):259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47(3):407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. The EMBO Journal. 2005;24(18):3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. The Journal of Biological Chemistry. 2002;277(52):50463–50468. doi: 10.1074/jbc.M208848200. [DOI] [PubMed] [Google Scholar]
- 57.Jaulin-Bastard F, Saito H, Le Bivic A, et al. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. Journal of Biological Chemistry. 2001;276(18):15256–15263. doi: 10.1074/jbc.M010032200. [DOI] [PubMed] [Google Scholar]
- 58.Hong C-J, Liao D-L, Shih H-L, Tsai S-J. Association study of PICK1 rs3952 polymorphism and schizophrenia. NeuroReport. 2004;15(12):1965–1967. doi: 10.1097/00001756-200408260-00026. [DOI] [PubMed] [Google Scholar]
- 59.Dev KK, Henley JM. The schizophrenic faces of PICK1. Trends in Pharmacological Sciences. 2006;27(11):574–579. doi: 10.1016/j.tips.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hikida T, Mustafa AK, Maeda K, et al. Modulation of D-serine levels in brains of mice lacking PICK1. Biological Psychiatry. 2008;63(10):997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin W-J, Chang Y-F, Wang W-L, Huang C-YF. Mitogen-stimulated TIS21 protein interacts with a protein-kinase-Ca-binding protein rPICK1. Biochemical Journal. 2001;354(3):635–643. doi: 10.1042/0264-6021:3540635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashbourne Excoffon KJD, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. Journal of Cell Science. 2004;117(19):4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- 63.Zhang B, Cao W, Zhang F, et al. Protein interacting with C α kinase 1 (PICK1) is involved in promoting tumor growth and correlates with poor prognosis of human breast cancer. Cancer Science. 2010;101(6):1536–1542. doi: 10.1111/j.1349-7006.2010.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo W, Chen Y-G. Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Molecular Biology of the Cell. 2009;20(3):1020–1029. doi: 10.1091/mbc.E08-09-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheyette BNR, Waxman JS, Miller JR, et al. Dapper, a Dishevelled-associated antagonist of β-catenin and JNK signaling, is required for notochord formation. Developmental Cell. 2002;2(4):449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Zhou H, Su Y, et al. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science. 2004;306(5693):114–117. doi: 10.1126/science.1100569. [DOI] [PubMed] [Google Scholar]
- 67.Su Y, Zhang L, Gao X, et al. The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. The FASEB Journal. 2007;21(3):682–690. doi: 10.1096/fj.06-6246com. [DOI] [PubMed] [Google Scholar]
- 68.Itoh S, ten Dijke P. Negative regulation of TGF-β receptor/Smad signal transduction. Current Opinion in Cell Biology. 2007;19(2):176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Lönn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGFβ receptors and Smads. Cell Research. 2009;19(1):21–35. doi: 10.1038/cr.2008.308. [DOI] [PubMed] [Google Scholar]
- 70.Yan X, Chen Y-G. Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochemical Journal. 2011;434(1):1–10. doi: 10.1042/BJ20101827. [DOI] [PubMed] [Google Scholar]
- 71.Ebisawa T, Fukuchi M, Murakami G, et al. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. Journal of Biological Chemistry. 2001;276(16):12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89(7):1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 73.Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Molecular Cell. 2000;6(6):1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 74.Kuratomi G, Komuro A, Goto K, et al. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-β (transforming growth factor-β) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-β type I receptor. Biochemical Journal. 2005;386(3):461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komuro A, Imamura T, Saitoh M, et al. Negative regulation of transforming growth factor-β (TGF-β) signaling by WW domain-containing protein 1 (WWP1) Oncogene. 2004;23(41):6914–6923. doi: 10.1038/sj.onc.1207885. [DOI] [PubMed] [Google Scholar]
- 76.Shibanuma M, Kuroki T, Nose K. Isolation of a gene encoding a putative leucine zipper structure that is induced by transforming growth factor β1 and other growth factors. Journal of Biological Chemistry. 1992;267(15):10219–10224. [PubMed] [Google Scholar]
- 77.Iida M, Anna CH, Gaskin ND, Walker NJ, Devereux TR. The putative tumor suppressor Tsc-22 is downregulated early in chemically induced hepatocarcinogenesis and may be a suppressor of Gadd45b. Toxicological Sciences. 2007;99(1):43–50. doi: 10.1093/toxsci/kfm138. [DOI] [PubMed] [Google Scholar]
- 78.Nakashiro KI, Kawamata H, Hino S, et al. Down-regulation of TSC-22 (transforming growth factor β-stimulated clone 22) markedly enhances the growth of a human salivary gland cancer cell line in vitro and in vivo. Cancer Research. 1998;58(3):549–555. [PubMed] [Google Scholar]
- 79.Uchida D, Kawamata H, Omotehara F, et al. Over-expression of TSC-22 (TGF-β stimulated clone-22) markedly enhances 5-fluorouracil-induced apoptosis in a human salivary gland cancer cell line. Laboratory Investigation. 2000;80(6):955–963. doi: 10.1038/labinvest.3780098. [DOI] [PubMed] [Google Scholar]
- 80.Shostak KO, Dmitrenko VV, Garifulin OM, et al. Downregulation of putative tumor suppressor gene TSC-22 in human brain tumors. Journal of Surgical Oncology. 2003;82(1):57–64. doi: 10.1002/jso.10180. [DOI] [PubMed] [Google Scholar]
- 81.Xu Y, Iyengar S, Roberts RL, Shappell SB, Peehl DM. Primary culture model of peroxisome proliferator-activated receptor γ activity in prostate cancer cells. Journal of Cellular Physiology. 2003;196(1):131–143. doi: 10.1002/jcp.10281. [DOI] [PubMed] [Google Scholar]
- 82.Yu J, Ershler M, Yu L, et al. TSC-22 contributes to hematopoietic precursor cell proliferation and repopulation and is epigenetically silenced in large granular lymphocyte leukemia. Blood. 2009;113(22):5558–5567. doi: 10.1182/blood-2009-02-205732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan X, Zhang J, Pan L, et al. TSC-22 promotes transforming growth factor β-mediated cardiac myofibroblast differentiation by antagonizing Smad7 activity. Molecular and Cellular Biology. 2011;31(18):3700–3709. doi: 10.1128/MCB.05448-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee BI, Park SH, Kim JW, et al. MS-275, a histone deacetylase inhibitor, selectively induces transforming growth factor β type II receptor expression in human breast cancer cells. Cancer Research. 2001;61(3):931–934. [PubMed] [Google Scholar]
- 85.Ammanamanchi S, Brattain MG. Restoration of transforming growth factor-β signaling through receptor RI induction by histone deacetylase activity inhibition in breast cancer cells. The Journal of Biological Chemistry. 2004;279(31):32620–32625. doi: 10.1074/jbc.M402691200. [DOI] [PubMed] [Google Scholar]
- 86.Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. Trichostatin A induces transforming growth factor β type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1·NF-Y complex. Journal of Biological Chemistry. 2005;280(11):10047–10054. doi: 10.1074/jbc.M408680200. [DOI] [PubMed] [Google Scholar]
- 87.Osada H, Tatematsu Y, Sugito N, Horio Y, Takahashi T. Histone modification in the TGFβRII gene promoter and its significance for responsiveness to HDAC inhibitor in lung cancer cell lines. Molecular Carcinogenesis. 2005;44(4):233–241. doi: 10.1002/mc.20135. [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, Huang Z, Xue H, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008;111(2):588–595. doi: 10.1182/blood-2007-05-092718. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Liu J, Zong Y, et al. MiR-106b aberrantly expressed in a double transgenic mouse model for Alzheimer's disease targets TGF-β type II receptor. Brain Research. 2010;1357:166–174. doi: 10.1016/j.brainres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 90.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends in Cell Biology. 2009;19(8):385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Onichtchouk D, Chen Y-G, Dosch R, et al. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature. 1999;401(6752):480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 92.Yan X, Lin Z, Chen F, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. Journal of Biological Chemistry. 2009;284(44):30097–30104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan X, Zhang J, Sun Q, et al. p21-activated kinase 2 (PAK2) inhibits TGF-β signaling in Madin-Darby Canine Kidney (MDCK) epithelial cells by interfering with the receptor-Smad interaction. The Journal of Biological Chemistry. 2012;287(17):13705–13712. doi: 10.1074/jbc.M112.346221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MAS, Massagué J. Hematopoiesis Controlled by Distinct TIF1γ and Smad4 Branches of the TGFβ Pathway. Cell. 2006;125(5):929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 95.Hirota M, Watanabe K, Hamada S, et al. Smad2 functions as a co-activator of canonical Wnt/β-catenin signaling pathway independent of Smad4 through histone acetyltransferase activity of p300. Cellular Signalling. 2008;20(9):1632–1641. doi: 10.1016/j.cellsig.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ijichi H, Otsuka M, Tateishi K, et al. Smad4-independent regulation of p21/WAF1 by transforming growth factor-β . Oncogene. 2004;23(5):1043–1051. doi: 10.1038/sj.onc.1207222. [DOI] [PubMed] [Google Scholar]
- 97.Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 98.Zhao X, Nicholls JM, Chen Y-G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-β signaling. Journal of Biological Chemistry. 2008;283(6):3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang S, Fei T, Zhang L, et al. Smad7 antagonizes transforming growth factor β signaling in the nucleus by interfering with functional Smad-DNA complex formation. Molecular and Cellular Biology. 2007;27(12):4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi X, Chen F, Yu J, et al. Study of interaction between Smad7 and DNA by single-molecule force spectroscopy. Biochemical and Biophysical Research Communications. 2008;377(4):1284–1287. doi: 10.1016/j.bbrc.2008.10.145. [DOI] [PubMed] [Google Scholar]
- 101.Yan X, Pan J, Xiong W, et al. Yin Yang 1 (YY1) synergizes with Smad7 to inhibit TGF-β signaling in the nucleus. Science China Life Sciences. 2014;57(1):128–136. doi: 10.1007/s11427-013-4581-2. [DOI] [PubMed] [Google Scholar]
- 102.Kurisaki K, Kurisaki A, Valcourt U, et al. Nuclear factor YY1 inhibits transforming growth factor β- and bone morphogenetic protein-induced cell differentiation. Molecular & Cellular Biology. 2003;23(13):4494–4510. doi: 10.1128/MCB.23.13.4494-4510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyake T, Alli NS, McDermott JC. Nuclear function of Smad7 promotes myogenesis. Molecular and Cellular Biology. 2010;30(3):722–735. doi: 10.1128/MCB.01005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang G, Matsuura I, He D, Liu F. Transforming growth factor-β-inducible phosphorylation of Smad3. Journal of Biological Chemistry. 2009;284(15):9663–9673. doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao S, Alarcón C, Sapkota G, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-β signaling. Molecular Cell. 2009;36(3):457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


