Abstract
At present, only 0.9% of PDB-deposited structures are of membrane proteins in spite of the fact that membrane proteins constitute approximately 30% of total proteins in most genomes from bacteria to humans. Here we address some of the major bottlenecks in the structural studies of membrane proteins and discuss the ability of the new technology, the Single-Protein Production (SPP) system, to help solve these bottlenecks.
Keywords: SPP, membrane protein, NMR
Introduction
Although membrane proteins play crucial roles in living cells, including the transport of materials, signal transduction, cell-cell communication, and many other essential cellular functions, the determination of their three-dimensional structures has been a major challenge in molecular biology. The analysis of genomes from bacteria to humans reveals that as much as 30% of the total proteins are, indeed, membrane proteins (Wallin and von Heijne, 1998). However, in spite of the fact that as many as 58,886 three-dimensional structures have been deposited in the PDB to date (July, 2009), only 197 of these structures are from membrane proteins (approximately 0.9% of the total three-dimensional structures).
There are two major reasons for this surprising lack of structural studies of membrane proteins. First, membrane proteins are highly hydrophobic, which makes it extremely difficult to purify them to the homogeneity required for both X-ray and NMR structural studies. When membrane proteins that are solubilized by detergents are purified by conventional purification methods such as ion exchange column chromatography, affinity column chromatography and gel filtration, they do not behave in a manner similar to that of the soluble proteins, resulting in low purity and yield even after tedious purification steps. Second, the expression of membrane proteins is generally very poor, and cell-free synthesis of membrane proteins is problematic, as they cannot easily assemble in the membrane.
If there were a way to obviate the need to purify a protein before undertaking structural studies, the major bottleneck for membrane structural biology would be solved. This could be achieved if one could isotopically label only a single membrane protein of interest without labeling any other cellular (soluble and membrane) proteins in living cells. In this manner, the NMR structural study of membrane proteins could potentially be carried out without purification. Even if one choses to purify the targeted membrane protein, for crystallization or NMR experiments, the ability to characterize its structural features prior to purification and to compare its NMR spectrum before and after purification/reconstitution would help ensure the biological significance of structural studies of the purified protein. For these reasons, the Single-Protein Production (SPP) system can potentially revolutionize the structural biology of membrane proteins and significantly increase our understanding of the structure and function of membrane proteins.
Creation of the SPP system
E. coli has an operon on its chromosome encoding a toxin, MazF, and its cognate antitoxin, MazE (See Figure 1). MazF has been shown to be a sequence-specific endoribonuclease, cleaving cellular mRNAs at ACA (Zhang et al., 2003). Since the discovery of MazF, a number of sequence-specific endoribonucleases encoded by bacterial genomes have also been discovered, some of which are more specific than E. coli MazF and recognize five-base sequences (Yoshizumi et al., 2009; Zhu et al., 2006). These enzymes are now termed mRNA interferases and appear to be involved in the regulation of cell growth under various stress conditions (Yamaguchi and Inouye, 2009), bacterial pathogenicity (Yoshizumi et al., 2009; Zhu et al., 2006), as well as in the programmed cell death of a developmental bacterium, Myxococcus xanthus (Nariya and Inouye, 2008).
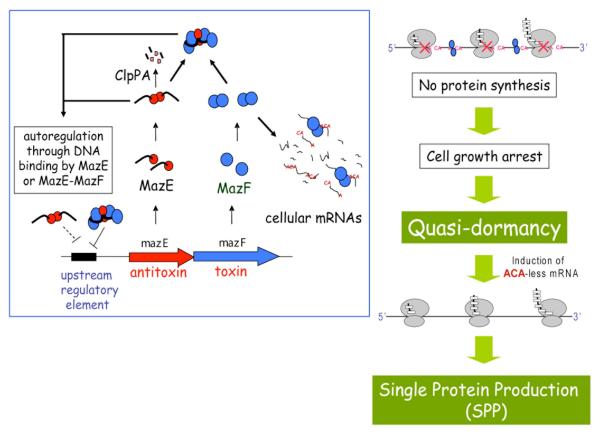
Figure 1. A schematic model for the SPP system in E. coli.
In the left panel, the regulatory mechanism for the MazE-MazF toxin-antitoxin (TA) system is shown. The genes for MazE (upstream) and MazF (downstream) are co-transcribed from a single promoter upstream of the mazE gene. Both MazE and MazF from form a dimer, and one MazE dimer forms a complex with two MazF dimmers so that the toxicity of MazF is suppressed. The MazE dimer and the MazE-MazF complex also bind to a palindromic sequence in the promoter region for the mazE-mazF operon and therefore, MazE and MazF production is negatively auto-regulated by either MazE or the MazE-MazF complex. MazE is much more unstable than MazF as it is specifically degraded by ClpPA, a stress-induced serine protease. Under stress conditions such as amino acid starvation and the exposure to antibiotics, free MazF is released in the cell to cleave mRNAs at ACA sequences. As a result, cell growth is arrested converting cells into the quasi-dormant state as shown in the right panel. The quasi-dormant cells are still fully metabolically active, and therefore if an ACA- less mRNA is induced, cells are capable of producing a protein from that mRNA. Thus, that is the only protein produced in the cell resulting in the single-protein-production (SPP) system.
Since almost all E. coli mRNAs contain ACA sequences, induction of MazF in E. coli leads to complete inhibition of cellular protein synthesis, resulting in total cell growth arrest (Zhang et al., 2003). However, when a specific mRNA was engineered to avoid all ACA sequences by altering them to non-MazF-cleavable sequences without changing the amino acid sequence of the protein encoded by the mRNA, the cells were found to be capable of producing the protein in the total absence of any other cellular proteins (Figure 2A). This indicates that although the MazF-induced cells are completely incapable of growth and thus are in a dormant state, they still maintain the full capacity to produce proteins. This dormant state is termed quasi-dormancy (Suzuki et al., 2005). Importantly, cells under the quasi-dormancy are fully capable for ATP production, amino acid and nucleotide biosynthesis, and RNA and protein synthesis.
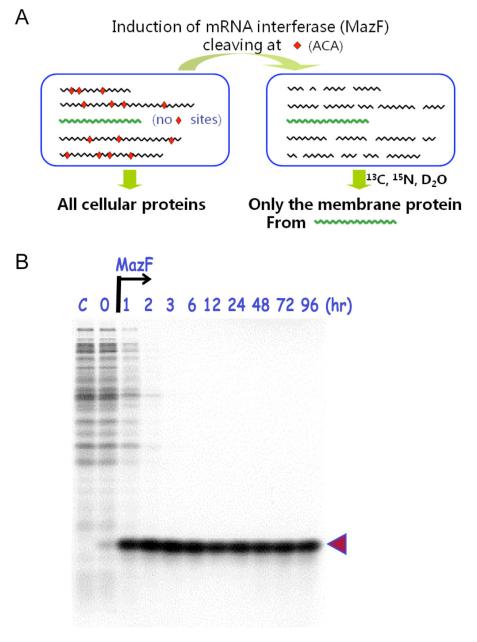
Figure 2. Exclusive production of human eotaxin in the SPP system.
(A), the single protein production (SPP) system in E. coli. (B), the gene for eotaxin was synthesized without ACA-sequences and inserted in pColdI vector. At the times indicated after MazF induction, cells were pulse-labeled with [35S]-methionine (Suzuki et al., 2005).
In the experiment shown in Figure 2B (Suzuki et al., 2005), the mazF gene was cloned under a lactose inducible promoter, and a synthetic ACA-less human eotaxin gene, encoding a 74-residue cytokine was cloned into a high expression, cold-shock induced vector, pCold. Eotaxin was indeed exclusively produced in the cells when MazF was co-expressed. After 2 hr of induction, background cellular protein synthesis was almost completely inhibited and at 3 hr, 35S-methionine was incorporated only into eotaxin and most impressively, the rate of the eotaxin production did not diminish for at least 96 hr. A number of other proteins were also independently expressed in the same manner to a level of 20 to 30% of total cellular proteins as demonstrated by Coommassie Brilliant Blue staining. In this manner, the SPP system in E. coli was created. We found that the E. coli SPP system is universally applicable to any protein including membrane proteins, provided that its mRNA is engineered to eliminate all ACA sequences. Notably, any ACA sequence can be altered to MazF-uncleavable sequence without changing the amino acid sequence of the protein of interest irrespective of their location in a gene.
The potential of the SPP system goes beyond currently available conventional methods. In particular, this system offers an ideal technology for membrane proteins, as it potentially eliminates the need for their purification for NMR structure studies. In addition, it was found that the culture for the SPP system can be condensed from 10 to 100 times depending on the construct, without affecting the final protein yield. Using this condensed SPP (cSPP) system, a one-liter culture can be condensed to a mere 25 or 10 ml, resulting in 95% or 99% cost saving for isotope labeling (Suzuki et al., 2007; Suzuki et al., 2006). We have already successfully expressed a number of membrane proteins from both prokaryotes and eukaryotes by using the cSPP system.
Since there are currently no general methods to circumvent the problems uniquely associated with the purification of membrane proteins, the use of the cSPP system for structural studies of membrane proteins will open a completely new avenue for both solution and solid-state NMR. NMR structural studies of membrane proteins can proceed without extensive purification or with samples prepared with a very simple purification procedure to eliminate the major contaminants that may disturb the NMR spectra. The cSPP technology also allows screening of detergents optimal for the NMR structural study of individual membrane proteins, even prior to developing a purification protocol for the target protein (Mao et al., 2009).
Approaches
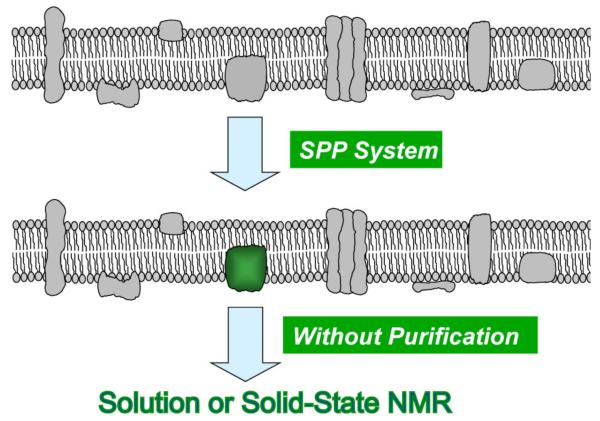
In the SPP method, isotopes (15N, 13C and 2H in the form of NH4Cl, glucose and deuterium oxide, respectively) or isotope-labeled amino acids in the culture medium are incorporated only into the target membrane protein in E. coli cells. The membrane fraction isolated from such a culture, therefore, contains only the target protein isotope-labeled, which can be detected by NMR spectroscopy. Although the membrane fraction from such a culture contains hundreds of other membrane proteins, none of them are isotope-labeled and, therefore, they are not detected in the NMR spectra (Figure 3). Importantly, this technology may be able to significantly improve three other aspects in the structural biology of membrane proteins. First, as mentioned earlier, the cost for the use of isotopes and isotope-labeled compounds may be substantially reduced over the conventional methods currently used, since the culture for isotope labeling may be condensed 10- to 100-fold, reducing the cost to as little as 1% of that incurred using conventional methods. Second, the “purification yield” of the membrane protein of interest for the structural studies will be 100% or nearly 100%, since no purification steps will be needed except for isolation of the membrane fraction. Another important advantage of the proposed technology is the significant time-saving, as one does not have to spend time for the tedious purification of membrane proteins. Third, toxic compounds such as 2H2O and various amino acid analogues are essential for preparing samples for structural studies. However, their toxicity reduces the yield of protein production. As these compounds are incorporated only into the target protein in the SPP system, and not into any other cellular proteins, they are not found to be detrimental to target protein production. Recently we have demonstrated very effective incorporation of 2H into multiple proteins independently using the cSPP system (Schneider et al., 2009).
Figure 3.
Production and isotope-enrichment of membrane proteins in the SPP system.
Conclusion
In summary, the condensed single protein production system (cSPP) system will likely revolutionize the structural determination of membrane proteins not only by NMR but also by X-ray crystallography given the high yields of membrane proteins. It is expected this will make major contributions to our understanding of the structure and function of membrane proteins. Notably, the cSPP system may be valuable for solid-state NMR studies of the structures of membrane proteins, since the membrane fraction can directly be used for structure study (Figure 3). Therefore, the cSPP technology has the potential to provide a major leap in the structural biology of membrane proteins. In order to further improve the cSPP system, there are several new other approaches which we will explore such as (a) how to prevent the incorporation of 13C into lipids from 13C-glucose, which is important for solid-state NMR, (b) how to overcome the inhibitory effect of some membrane proteins on the membrane potential, (c) how to develop the best inducible system for membrane proteins to maximize the incorporation of isotopes, (d) how to optimize the best condition for triple labeling of membrane proteins with 2H, 15N and 13C, (e) how to increase the capacity on the membrane insertion site in the cell, (f) how to streamline membrane protein structure genomics by the SPP system and (g) how to create a system to study the interaction of a membrane protein with other proteins using the SPP system.
Acknowledgements
This work was supported by the National Institutes of Health Grants U54 GM074958 (G.T.M. and M. I.), U54 GM75026 (G.T.M. and M. I.), and 1R01 GM085449 (M.I.), and partially by a research fund from Takara-Bio., Inc.
References
- Mao L, Tang Y, Vaiphei ST, Shimazu T, Kim S, Mani R, Fakhoury E, White E, Montelione GT, Inouye M. Production of membrane proteins for NMR Studies using the condensed single protein production (cSPP) system. Journal of Structural and Functional Genomics. 2009 doi: 10.1007/s10969-009-9072-0. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariya H, Inouye M. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell. 2008;132:55–66. doi: 10.1016/j.cell.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Schneider WM, Tang Y, Vaiphei ST, Mao L, Shen Y, Raman S, Baker D, Inouye M, Roth MJ, Montelione GT. Efficient production of perdeuterated soluble and membrane proteins for NMR studies. Nature Methods. 2009 Submitted. [Google Scholar]
- Suzuki M, Mao L, Inouye M. Single protein production (SPP) system in Escherichia coli. Nat Protoc. 2007;2:1802–1810. doi: 10.1038/nprot.2007.252. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Roy R, Zheng H, Woychik N, Inouye M. Bacterial bioreactors for high yield production of recombinant protein. J Biol Chem. 2006;281:37559–37565. doi: 10.1074/jbc.M608806200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Zhang J, Liu M, Woychik NA, Inouye M. Single protein production in living cells facilitated by an mRNA interferase. Mol Cell. 2005;18:253–261. doi: 10.1016/j.molcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Inouye M. mRNA interferases, sequence-specific endoribonucleases from the toxin-antitoxin systems. Prog Mol Biol Transl Sci. 2009;85:467–500. doi: 10.1016/S0079-6603(08)00812-X. [DOI] [PubMed] [Google Scholar]
- Yoshizumi S, Zhang Y, Yamaguchi Y, Chen L, Kreiswirth BN, Inouye M. Staphylococcus aureus YoeB homologues inhibit translation initiation. J Bacteriol. 2009 doi: 10.1128/JB.00623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12:913–923. doi: 10.1016/s1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Teh JS, Zhang J, Connell N, Rubin H, Inouye M. Characterization of mRNA interferases from Mycobacterium tuberculosis. J Biol Chem. 2006;281:18638–18643. doi: 10.1074/jbc.M512693200. [DOI] [PubMed] [Google Scholar]