Abstract
Immunology-based therapy is rapidly developing into an effective treatment option for a surprising range of cancers. We have learned over the last decade that powerful immunologic effector cells may be blocked by inhibitory regulatory pathways controlled by specific molecules often called “immune checkpoints.” These checkpoints serve to control or turn off the immune response when it is no longer needed to prevent tissue injury and autoimmunity. Cancer cells have learned or evolved to use these mechanisms to evade immune control and elimination. The development of a new therapeutic class of drugs that inhibit these inhibitory pathways has recently emerged as a potent strategy in oncology. Three sets of agents have emerged in clinical trials exploiting this strategy. These agents are antibody-based therapies targeting cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). These inhibitors of immune inhibition have demonstrated extensive activity as single agents and in combinations. Clinical responses have been seen in melanoma, renal cell carcinoma, non-small cell lung cancer, and several other tumor types. Despite the autoimmune or inflammatory immune-mediated adverse effects which have been seen, the responses and overall survival benefits exhibited thus far warrant further clinical development.
Keywords: Immune checkpoints, PD-1, PD-L1, CTLA4, immunotherapy, immune modulators
Immune checkpoints refer to regulatory pathways in the immunome that inhibit a portion of an active immune response against a specific target or set of targets[1]. Immune checkpoints are therefore seen as a normal part of the immune system's regulatory cascade and necessary to modulate and maintain immune homeostasis. These regulatory pathways are redundant, multi-faceted, and complex (Figure 1). Their presence and evolutionary development are thought to reflect the fact that a normal immune response can also be potentially deadly to a host if not properly regulated, as evidenced by autoimmune disease.
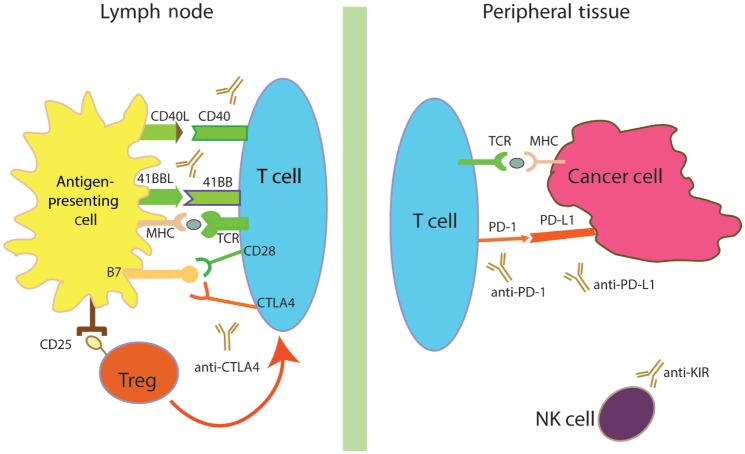
Figure 1. Regulatory pathways in immuno-oncology.
The immune system has multiple levels of co-stimulatory (shown in green) and co-inhibitory (shown in red) pathways, helping to maintain immune homeostasis in the midst of responding to antigenic stimulation. Antibodies blocking cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) have shown remarkable clinical activity and further development is underway. Agonist antibodies, which directly interact with co-stimulatory pathways such as 41BB and CD40, are also in clinical development. Other means of affecting the immune responses are being explored, including direct actionon regulatory T (Treg) cells and natural killer (NK) cells. MHC, major histocompatibility complex; TCR, T-cell receptor.
As immunotherapy has gained a foothold in the anticancer armamentarium, there is a commonly held belief among oncologists that cancers thwart these regulatory mechanisms to evade immune detection and continue their growth and spread. However, the whole picture is more complex. In many chronic infections, immune checkpoints are exploited by parasitic and viral pathogens as evidenced by T-cell exhaustion in these conditions[2]. Cancer, however, arises from within the host. While some immune checkpoints are engaged to avoid immune-mediated eradication, others are merely a part of the normal immune response to stimuli. We are only beginning to understand these pathways.
As a therapeutic class, drugs that inhibit these co-inhibitory signaling pathways, also known as immune checkpoint inhibitors, have emerged as a mainstay in melanoma therapy, with potential roles in the treatment of renal cell carcinoma (RCC), non–small cell lung cancer (NSCLC), urothelial cancer, head and neck cancer, ovarian cancer, and various lymphomas[3]–[11]. In fact, ClinicalTrials.gov now lists dozens of trials with immune checkpoint inhibitors in a broad range of indications. As the targets of therapy are components of the immune system, there is currently no theoretical reason to exclude any potential histologies or tumor types from clinical evaluation. However, from a drug development perspective, this can be a challenge, as the resources are constantly constrained. It will be important to optimize the development of strategies to efficiently explore the therapeutic potentials.
While a variety of agents could be deemed to interact with immune checkpoint inhibitors, the focus of this review are limited to the most advanced agents in clinical trials, those targeting the cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) pathway. The early trials involving CTLA4- and PD-1/PD-L1-targeting agents have shown how the addition of just one blocking antibody can reveal a hidden immune response, with potentially massive therapeutic benefit for patients. Immune modulators targeting other mechanisms (Table 1), such as indoleamine 2,3-dioxigenase (IDO)[12],[13], lymphocyte-activation gene 3 (LAG3)[14], T-cell membrane protein 3 (TIM3)[15], signal transducer and activator of transcription 3 (STAT3)[16], glucocorticoid-induced tumor necrosis factor receptor (TNFR) family-related protein (GITR)[17], and agents against the human inhibitory killer IgG-like receptor (anti-KIR) targeting natural killer (NK) cells have also entered the clinical trials arena[18]–[20].
Table 1. Other immunotherapeutic agents in development.
| Target | Name | Indication(s) | Company | Phase | Clinical Trials.gov identifier (selected trials) |
| B7.1 | Galiximab | Lymphoma | Biogen Idec | Phase II | NCT00516217 |
| B7H3 | MGA271 | Solid tumors | Macrogenics | Phase I | NCT01391143 |
| LAG3 | IMP321 | Solid tumors | Immuntep | Phase I/II | NCT00349934 |
| BMS-986016 | Solid tumors | Bristol-Myers Squibb | Phase I | NCT01968109 | |
| CD137 | BMS-663513 | Solid tumors | Bristol-Myers Squibb | Phase I/II | NCT00309023 |
| PF-05082566 | Lymphoma | Pfizer | Phase I | NCT01307267 | |
| KIR | IPH2101 | Myeloma, AML | Innate Pharma (BMS) | Phase II | NCT01248455 |
| NCT01256073 | |||||
| CCR4 | KW-0761 | ATL, CTCL | Kyowa Kirin | Phase I/II | NCT00920790 |
| CD27 | CDX-1127 | Solid tumors & Heme | CellDex Therapeutics | Phase I | NCT01460134 |
| Ox40 | MEDI-6469 | Solid tumors | MedImmune/AZ | Phase I | NCT02205333 |
| CD40 | CP-870,893 | Pancreatic | Genentech | Phase I | NCT01456585 |
Heme, hematologic tumors; ATL, acute T-cell leukemia; CTCL, cutaneous T-cell lymphoma; AML, acute myeloid leukemia.
CTLA4 Inhibition and Human Cancers
CTLA4 background
CTLA4 is one of several co-inhibitory molecules that aid in modifying the T-cell response to antigen activation. The primary role of CTLA4 is to modify T-lymphocyte response to stimuli. CTLA4 regulates the clonal burst size during priming and secondary expansion, which is thought to be proportional to the activation strength of the complex formed by the T-cell receptor-major histocompatibility complex (TCR-MHC complex), both in terms of binding affinity and co-stimulation by accessory signals. CTLA4, also known as cluster of differentiation (CD) 152, is an inhibitory molecule found on T cells, and its counterpart is CD28. CD28 is a co-stimulatory signal, but it is important to note that CTLA4 has higher avidity for its ligands than CD28, which suggests a dominance of inhibitory signals in immune activation. The ligands for both CD28 and CTLA4 are known as B7 proteins, which are found on antigen- presenting cells (APCs). There are two types of B7 proteins: B7-1 (also known as CD80) and B7-2 (also known as CD86)[21],[22].
In normal circumstances, T lymphocytes are thought to express CTLA4 on their surface immediately upon response to antigenic stimulation of TCR. Because a blocking antibody would attenuate the inhibitory signal, an anti-CTLA4 antibody would appear to have a clear role in enhancing antitumor immunity, as the interaction of B7 with CD28 would thereby be enhanced. Indeed, in preclinical models, this is exactly what was seen[23]. However, B7 is rarely present in the tumor microenvironment, leading to a second hypothesis for how anti-CTLA4 antibodies may enhance immune-mediated tumor rejection[22]. CTLA4 is differentially expressed among separate T-lymphocyte subsets. Indeed, higher levels of surface expression of CTLA4 are seen in regulatory T cells (Treg cells) as compared with effector T cells (Teff cells). Researchers have established that higher Teff/Treg ratios in both CD4-positive and CD8-positive tumor-infiltrating lymphocytes are associated with enhanced tumor eradication when CTLA4 blockade is employed in tumor models[24],[25].
Other mechanisms of action involving Treg cells have been hypothesized. For example, Treg cells are known to reduce B7 expression on both human and animal APCs[26]. While B7 is thought to directly signal CTLA4, leading to its co-inhibitory effect, the reverse, or CTLA4 signaling to APCs via B7, also occurs. CTLA4 reverse signaling via B7 can lead to increased expression of IDO. IDO leads to a reduced level of local tryptophan stores in the microenvironment, which inhibits T-lymphocyte activation and proliferation[27]. Lastly, recently discovered alternatively spliced mRNA molecules encode soluble CTLA4 molecules lacking transmembrane domains. These soluble inhibitors could also mediate immune suppression, either by down-regulating B7 expression as noted above or by blocking the potential interaction of B7 and the co-stimulatory molecule CD28[28].
Ipilimumab
The first anti-CTLA4 agent in clinical development, ipilimumab, was approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of metastatic melanoma following research showing improved survival[29]. Ipilimumab is a fully humanized immunoglobulin (Ig) G1 kappa monoclonal antibody that antagonizes CTLA4 and prevents ligand binding[30].
A phase III combination study of ipilimumab at 3 mg/kg with a glycoprotein 100 (gp100) peptide vaccine was conducted with 676 patients with stage III or IV unresectable melanoma, randomized in a 3:1:1 ratio. The results showed the median overall survival (OS) in the ipilimumab plus gp100 group was 10.0 months [95% confidence interval (CI), 8.5 to 11.5 months], as compared with 6.4 months (95% CI, 5.5 to 8.7 months) in the gp100 alone group [hazard ratio (HR) for death, 0.68; P < 0.001]. The median OS in the ipilimumab alone group was 10.1 months (95% CI, 8.0 to 13.8 months; HR for death with ipilimumab alone as compared with gp100 alone, 0.66; P = 0.003). Grade 3 or 4 immune-related adverse events occurred in 10% to 15% of patients treated with ipilimumab and in 3% of patients treated with gp100 alone. The investigators concluded that ipilimumab, with or without a gp100 peptide vaccine, as compared with gp100 alone, improved OS in patients with previously treated metastatic melanoma. Adverse events can be severe, long-lasting, or both, but most are reversible with appropriate treatment, particularly corticosteroids[29].
Another supportive study in metastatic melanoma involved 502 patients in a 1:1 randomized trial of ipilimumab at a dose of 10 mg/kg plus dacarbazine at a dose of 850 mg/m2 of body surface area versus dacarbazine and placebo. The OS was longer in the group receiving ipilimumab-dacarbazine therapy than in the dacarbazine-placebo arm (11.2 months vs. 9.1 months). Survival rates were also higher for the ipilimumab-dacarbazine arm at 1 year (47.3% vs. 36.3%), 2 years (28.5% vs. 17.9%), and 3 years (20.8% vs. 12.2%), with an HR for death at 0.72 (P < 0.001). Grade 3 or 4 adverse events were recorded in 56.3% of patients in the experimental arm and only 27.5% in the control arm[31]. These findings further support the use of ipilimumab in metastatic melanoma.
The preliminary results of the first adjuvant trial were reported at the 2014 Annual Meeting of the American Society of Clinical Oncology (ASCO). The European Organization for Research and Treatment of Cancer (EORTC) conducted a trial with 951 patients with stage III melanoma following complete resection (EORTC 18071, NCT00636168). Patients were randomized to receive ipilimumab at a dose of 10 mg/kg versus placebo every 3 weeks for 4 doses. The median relapse-free survival was 26.1 months for the ipilimumab arm and 17.1 months for the placebo arm, with an HR of 0.75 (95% CI, 0.64 to 0.90; P = 0.001). The adverse event profile was thought to be generally consistent with that seen in advanced melanoma, but a higher incidence of endocrinopathies were reported[32].
While the results were largely seen as positive, there are still unresolved issues that may limit the ability of regulatory agencies to approve ipilimumab in the adjuvant setting. In particular, patients with stage III melanoma have the option of high-dose interferon, which is the standard of care in the United States[33]. In addition, as noted above, the dose chosen in EORTC 18071 trial was 10 mg/kg of ipilimumab, higher than the dose used in the metastatic setting. Another large adjuvant trial, E1609 (NCT01274338), addresses the questions of the optimal dose of ipilumumab and its comparative effectiveness to high-dose interferon. In this trial, conducted by the Eastern Cooperative Oncology Group (ECOG) under the sponsorship of the United States National Cancer Institute (NCI), 1,500 patients with stage III or IV melanoma that has been fully resected are randomized to three arms: high-dose interferon, ipilimumab at 3 mg/kg, and ipilimumab at 10 mg/kg[30]. The trial is close to completing accrual and the outcome data are eagerly awaited.
Other indications with ipilimumab are still under clinical development. On ClinicalTrials.Gov, a search with the term “ipilimumab” returns 191 trials, over 100 of which remain open. Much of the attention in ipilimumab clinical development has moved to combination therapy, particularly with the anti-PD-1 agent nivolumab.
The development of ipilumumab brought up interesting ques-tions about the best endpoints of efficacy assessment for this class of agents. In the phase III melanoma trials with ipilimumab described above, improvements in OS seen in the metastatic setting were not accompanied by significant radiographic responses to therapy. This has complicated efficient drug development, as response rate is less likely to serve as a readout of efficacy, at least when ipilimumab is used as a monotherapy. Monotherapy is still being explored in various settings, however. In one example, in a single institution case series, 5 patients with recurrent glioblastoma multiforme (GBM) who were treated with ipilimumab were followed. Patients had time to progression ranging from 1 to 6 months, and 1 patient remains recurrence-free 19 months after therapy, suggesting penetration of this drug across the blood-brain barrier[34].
Currently, there are no biomarkers to predict which patients or tumor types are more likely to respond to anti-CTLA4 therapies. Some analysis suggested that a high mutational load may be associated with a clinical benefit from ipilimumab. In particular, one group of researchers has focused on identifying a “neoantigen signature” that correlates with benefit. Findings are preliminary, but this suggests that tumor genetics might explain the divergent outcomes among patients treated with ipilimumab[35].
Tremelimumab
Tremelimumab (formerly CP-675,206) is a human IgG2 monoclonal antibody specific for CTLA4. In a large, single-arm phase II trial, 241 response-evaluable patients with advanced refractory or relapsed melanoma were treated at a dose of 15 mg/kg intravenously every 3 months. Objective responses were observed in 6.6% of those patients, which led to a phase III trial in a similar patient population[36]. In the phase III trial in advanced melanoma, 655 patients were enrolled and randomly assigned to treatment with tremelimumab or chemotherapy. Median OS by intent to treat was 12.6 months (95% CI, 10.8 to 14.3 months) for tremelimumab and 10.7 months (95% CI, 9.36 to 11.96 months) for chemotherapy (HR, 0.88; P = 0.127). Of note, while objective response rates were similar in the two arms (10.7% in the tremelimumab arm and 9.8% in the chemotherapy arm), duration of response was significantly longer among patients treated with tremelimumab (35.8 months vs. 13.7 months, P = 0.0011)[37]. Regardless, the study failed to demonstrate a statistically significant survival advantage of treatment with the investigational drug over standard-of-care chemotherapy.
While the results were disappointing, there was a possibility that the dosing schedule of tremelimumab (once every 3 months) was insufficient to achieve results similar to that of ipilimumab. As a result, ongoing efforts with tremelimumab as a monotherapy have used more frequent dosing. The preferred dose and schedule for tremelimumab for ongoing trials has generally been 10 mg/kg intravenously, every 4 weeks for 6 months. A phase II, single-arm study of tremelimumab as a second-line treatment was conducted with 29 patients with malignant mesothelioma. Results showed a partial response in 4 patients (13.8%) and stable disease in 11 patients (37.9%), with OS of 11.5 months[38]. As a result, a phase III, double-blinded, randomized trial is currently underway using tremelimumab in mesothelioma[39]. Tremelimumab is also being tested in various combinations in a variety of other tumor types.
PD-1/PD-L1 Inhibition and Human Cancers
PD-1/PD-L1 background
Compared to the limited development with CTLA4, there is an immense level of interest and investment by pharmaceutical companies in the PD-1/PD-L1 pathway (Table 2). Seven major companies have entered the fray, with multiple phase III registration trials already underway in a variety of malignancies, most notably melanoma, RCC, and NSCLC (Table 3). This enthusiasm is justified, as clinical trial results thus far confirm that targeting the PD-1/PD-L1 pathway represents the most exciting opportunity for advancing cancer immunotherapy to date.
Table 2. Agents targeting PD-1/PD-L1 in clinical development.
| Company | Agent targeting PD-1 | Agent targeting PD-L1 |
| Bristol-Myers Squibb | BMS-936558/MDX-1106 Nivolumab (fully human IgG4 mAb) | BMS-936559/MDX-1105 (fully human IgG4 mAb) |
| CureTech | CT-011Pidilizumab (humanized IgG1 mAb) | N/A |
| Genentech | N/A | MPDL3280A (IgG1 mAb, Fc modified) |
| MedImmune/AZ | AMP-514 | MEDI4736 (fully human mAb) |
| Merck | MK-3475Pembrolizumab (humanized IgG4 mAb) | N/A |
| EMD Serono | N/A | MSB0010718C |
| Aurigene and Pierre Fabre | AUNP 12 (peptide) | N/A |
PD-1, programmed death 1 receptor; PD-L1, programmed cell death ligand 1; IgG4, immunoglobulin G4; mAb, monoclonal antibody; N/A, not available.
Table 3. Selected ongoing PD-1/PD-L1 trials in melanoma, renal cell cancer (RCC), and non–small cell lung cancer (NSCLC).
| Sponsor | Tumor | Setting | Phase | Comparison | Primary endpoint | Sample size | Primary completion date | Clinical trials.gov identifier |
| Bristol-Myers Squibb | Melanoma | 1st-line | II | Ipilimumab +/– nivolumab | ORR | 150 | Jul 2014 | NCT01927419 |
| Bristol-Myers Squibb | NSCLC | 3rd-line squamous cell | II | Nivolumab vs. docetaxel | OS | 264 | Jan 2016 | NCT01642004 |
| Bristol-Myers Squibb | NSCLC | 2nd-/3rd-line non-squamous cell | III | Nivolumab vs. docetaxel | OS | 582 | May 2015 | NCT01673867 |
| Merck | Melanoma | 1st-/2nd-line (ipi-naïve) | III | Pembrolizumab vs. ipilimumab | OS & PFS | 645 | Feb 2015 | NCT01866319 |
| Merck | Melanoma | 2nd-line | II | Pembrolizumab vs. chemotherapy | OS & PFS | 510 | Mar 2015 | NCT01704287 |
| Bristol-Myers Squibb | Melanoma | 2nd-line post-ipi | III | Nivolumab vs. chemotherapy | OS | 405 | May 2015 | NCT01721746 |
| Merck | NSCLC | 2nd-line | II/III | Pembrolizumab vs. docetaxel | OS & PFS | 920 | Sep 2015 | NCT01905657 |
| Bristol-Myers Squibb | Melanoma | 1st-/2nd-line | III | Nivolumab vs. chemotherapy | OS | 410 | Sept 2015 | NCT01721772 |
| Bristol-Myers Squibb | RCC | 2nd- to 4th-line | III | Nivolumab vs. everolimus | OS | 822 | Feb 2016 | NCT01668784 |
| Genentech | NSCLC | 2nd-line | II | MPDL3280A vs. docetaxel | OS | 287 | Mar 2016 | NCT01903993 |
| Bristol-Myers Squibb | Melanoma | 1st-line | III | Ipilimumab +/– nivolumab | OS | 915 | Oct 2016 | NCT01844505 |
| Genentech | NSCLC | 2nd-line | III | MPDL3280A vs. docetaxel | OS | 850 | Jun 2017 | NCT02008227 |
| AstraZeneca | NSCLC (stage III) | 1st-line after concurrent chemoradiation | III | MEDI4736 vs. placebo | OS & PFS | 702 | May 2017 | NCT02125461 |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; ipi, ipilumumab. Other footnotes as in Table 2.
Whereas CTLA4 is involved in central tolerance and control, the PD-1 receptor is critical in peripheral tolerance. PD-1 is expressed on T lymphocytes during thymic development. Similar to CTLA4, PD-1 becomes expressed on CD4- and CD8-positive T lymphocytes during antigenic stimulation, serving as a co-inhibitory signal. In addition, PD-1 is expressed on numerous other immune cells, including natural killer T cells, B cells, monocytes, and certain dendritic cell subsets[40],[41]. PD-1 has two main ligands, namely PD-L1 (also known as B7-H1) and PD-L2 (also known as B7-DC). While PD-L2 has a much higher affinity for PD-1, it is expressed chiefly on activated dendritic cells, macrophages, certain B-cell subsets, and other immune cells. PD-L1 is more widely expressed on hematologic and non-hematologic tissues[1].
As a co-inhibitory signal, PD-1 engagement results in reduced cytokine production, cytolytic activity, and lymphocyte proliferation[41]. PD-1 is up-regulated in T lymphocytes following viral infections and down-regulated following viral clearance. In chronic viral infections, however, CD8-positive T lymphocytes express PD-1 constitutively, likely through gene demethylation, contributing to what has been termed a T-cell exhaustion phenotype. In human immunodeficiency virus (HIV) infection, for example, high levels of PD-1 expression allow for persistent interaction between PD-L1 expressed by APCs and subsequent T-cell dysfunction. T-lymphocyte activation is essentially blocked in this setting. In an experiment using B cells from HIV-infected individuals, researchers were able to show increased responses to HIV antigens in the presence of PD-1 blocking antibodies in vitro[42]. This suggests that an active immune response can be induced if effective blockade of the PD-1/PD-L1 pathway can be implemented. A clinical trial sponsored by the US National Institutes of Health is currently planned to investigate that possibility using the anti-PD-L1 antibody, BMS-936559 (NCT02028403).
Similarly, tumors use the same pathway, meant to induce peripheral immune tolerance, to evade T-lymphocyte-mediated immune eradication. Tumors evading an active immune response are thought to express PD-L1 following T lymphocyte infiltration and expression of interferon-gamma. Expression of PD-L1 has been associated with a poor prognosis in a wide variety of human tumors[11],[43]–[51]. Interestingly, in one analysis of glioma cases, a better prognosis was seen in patients whose tumor-adjacent brain tissue expressed PD-L1 and whose tumors did not express PD-L1. This suggests a general role for PD-L1 in protecting normal tissue from immune attack, though it would be important to see these findings replicated elsewhere and in other tumor types[52].
In general, the results of PD-1/PD-L1 blockade have been encouraging. Higher response rates and durable responses were seen with PD-1/PD-L1 blocking antibodies than with CTLA4 blockade in melanoma, RCC, and unexpectedly, NSCLC. In addition, although similar immune-mediated adverse events were seen with both CTLA4 and PD-1/PD-L1 agents, the severity of adverse events has generally been lower, with the caveat that fatal pneumonitis was seen in 1% of the patients on the nivolumab phase I trial described below[53].
PD-1-targeting agents
Nivolumab
Nivolumab (BMS-936558) is a fully human IgG4 monoclonal antibody targeting PD-1. A phase I/II study was reported in 2012 in the New England Journal of Medicine in parallel with a plenary session at the 2012 ASCO Annual Meeting[53]. Patients on the trial had advanced melanoma, NSCLC, castration-resistant prostate cancer, RCC, or colorectal cancer (CRC). Patients received nivolumab at doses of 0.1 to 10.0 mg/kg of body weight every 2 weeks for up to 12 cycles until disease progression or a complete response occurred. Of the 296 patients enrolled, only 14% experienced grade 3 or 4 drug-related adverse events, but there were 3 deaths from pulmonary toxicity. No maximum tolerated dose (MTD) was defined. Cumulative response rates (all doses) were 18.4% (14/76) among patients with NSCLC, 27.6% (26/94) among patients with melanoma, and 27.3% (9/33) among patients with RCC[53]. A subsequent analysis of the 104 patients in that trial with metastatic melanoma was performed. The investigators reported that the median OS in nivolumab-treated metastatic melanoma patients was 16.8 months, and 1- and 2-year survival rates were 62% and 43%, respectively. Among the 33 patients (30.8%) with objective tumor regressions, the Kaplan-Meier estimated median response duration was 2 years. Of note, responses were not observed in prostate cancer and colon cancer patients on the trial[54].
While preliminary results from the phase I/II trial suggested that PD-L1 could be an appropriate biomarker for patient selection, subsequent analyses have shown numerous PD-L1-negative patients had responded to treatment with nivolumab, although lower rates are seen. Given the variability and limitation of the available PD-L1 assays, a true absence of PD-L1 expression in those patients cannot be confirmed. At this time it remains to be determined whether PD-L1 is a predictive marker of response for PD-1 pathway inhibitors.
At the 2014 ASCO Annual Meeting, preliminary results of other nivolumab clinical trials were reported. In a small dose-escalation trial in Japan, 3 of 13 patients with platinum-resistant ovarian cancer had objective responses to nivolumab. The initial 10 patients were treated at a dose of 1 mg/kg, and 2 responders were from that cohort. The subsequent cohort that received 3 mg/kg had only 3 patients, but 1 was a responder. These results, though preliminary, were promising[3]. In addition, investigators reported early results using nivolumab at a dose of 3 mg/kg every 2 weeks in patients with treatment-naïve advanced NSCLC. The initial results on 20 patients revealed an objective response rate of 30%. Two patients had a greater than 80% target lesion reduction at 18 weeks. Of the 15 evaluable tumor samples, 9 were PD-L1-positive (defined as greater than 5% PD-L1 expression using a Dako kit), and the response rate was 67% in PD-L1-positive patients; whereas no responses were observed in the 6 PD-L1-negative patients[55]. Combinations of nivolumab with conventional chemotherapy or epidermal growth factor receptor (EGFR) inhibitors were also reported, but results are difficult to interpret in the absence of randomized comparisons[56],[57].
Pembrolizumab
Pembrolizumab (MK-3475, formerly lembrolizumab) is a humanized IgG4 monoclonal antibody targeting PD-1. Pembro-lizumab has been very successful in treating melanoma and NSCLC, similar to nivolumab. Significant differences cannot be assessed in the absence of a randomized trial comparing the two agents. However, binding affinities of the agents are different. Nivolumab is a fully human IgG4, and pembrolizumab is humanized. In phase I trials, neither agent has been found to have a maximally tolerated dose. That said, more time and energy has been spent on searching for an appropriate dose for pembrolizumab.
In the first major publication involving pembrolizumab, Hamid et al.[58] reported that patients with advanced melanoma were analyzed after being treated with three separate dosing strategies: 10 mg/kg of body weight every 2 or 3 weeks or 2 mg/kg every 3 weeks. Ultimately, 135 patients with advanced melanoma were treated. Adverse events were similar to those found in patients treated with nivolumab, including fatigue, rash, pruritus, and diarrhea. Response rates across all dose levels were 38% (95% CI, 25% to 44%). Investigators found no difference among those with and without prior ipilimumab therapy. Responses were durable, and the median progression-free survival (PFS) among the 135 patients was longer than 7 months[58].
A subsequent prospective, randomized analysis was performed using both the 2 mg/kg and the 10 mg/kg doses, given every 3 weeks to patients with ipilimumab-refractory advanced melanoma. There were 89 patients in the 2 mg/kg cohort and 84 patients in the 10 mg/kg cohort. The response rate was 26% at both doses. Safety profiles were similar and there were no deaths reported[59]. Other attempts to analyze patients with melanoma have been reported, including an analysis of 411 melanoma patients treated across multiple dose levels and multiple trials, which was reported at the 2014 ASCO Annual Meeting. Median OS data was not available, but 1-year OS rate over all dose cohorts was 71%. Response rates were encouraging as well, ranging from 26% to 57%, varying based on ipilimumab prior treatment, dose, and schedule[60].
Investigators also reported preliminary results of a phase I trial of previously treated patients with locally advanced or metastatic NSCLC. Enrolled patients with PD-L1 detected in their tumors by a preliminary immunohistochemical assay were randomized to pembrolizumab at a dose of 10 mg/kg every 2 weeks or every 3 weeks. In addition, some patients with tumors without PD-L1 expression who had received at least two prior lines of therapy were treated at a dose of 10 mg/kg every 2 weeks. Ultimately, 102 patients comprised the every-2-week cohort (including 43 whose tumors did not express PD-L1), and 119 patients comprised the every-3-week cohort. Investigators reported that 48% of patients experienced drug-related adverse events, with 6% experiencing grade 3/4 adverse events. As in the nivolumab trial reported above, pneumonitis was a concern, with 3 cases of drug-related grade 3/4 pneumonitis. The Response Evaluation Criteria in Solid Tumors (RECIST) response rate in all patients was 21%. The response rate was slightly higher (24%) for patients with PD-L1-positive tumors (more specifically, 31% on the every-2-week cohort and 22% on the every-3-week cohort). RECIST response rates were 8% for patients without PD-L1 expression[61].
Pidilizumab
Pidilizumab is a humanized IgG1 antibody targeting PD-1. The agent was initially evaluated in a phase I trial targeting hematologic malignancies. Presently, there are a number of clinical trials underway in both hematologic and solid tumors[62].
The results of two pidilizumab clinical trials were recently published in peer-reviewed journals. In a single-center, single-arm, phase II trial, 32 patients with relapsed follicular lymphoma received pidilizumab at a dose of 3 mg/kg every 4 weeks for 4 infusions with up to 8 additional infusions administered. In addition, rituximab was given at a dose of 375 mg/m2 of body surface area every week for 4 weeks. Investigators reported that 19 of 29 evaluable patients achieved an objective response, with complete responses in 15 patients (51.7%)[63].
An additional phase II trial involved patients with diffuse large B-cell lymphoma (DLBCL) following autologous hematologic stem cell transplantation (AHSCT). Sixty-six patients were treated with 3 doses of pidilizumab in the first 1 to 3 months after AHSCT. The PFS rate was 72% at 6 months after AHSCT (90% CI, 60% to 82%), meeting the primary endpoint. Thirty-five patients had measurable disease following AHSCT, and the response rate in those patients was 51%[7].
Investigators also presented results on the use of pidilizumab in metastatic melanoma at the 2014 ASCO Annual Meeting. In this trial, 103 patients were randomized in a 1:1 ratio to receive either 1.5 mg/kg or 6 mg/kg every 2 weeks for 27 doses. Response rates were lower than those observed in patients treated with other PD-1-targeting agents, but the OS rate at 12 months was 64.5% (90% CI, 55.6% to 72.0%). No significant differences were seen between different strata or dose groups[64].
PD-L1-targeting agents
MPDL3280A
MPDL3280A is an engineered human IgG1 monoclonal antibody that targets PD-L1. Of note, MPDL3280A was engineered to eliminate antibody-dependent cell-mediated cytotoxicity (ADCC) effector function. As a result, MPDL3280A, unlike some other anti-PD-L1 antibodies, does not deplete cells expressing PD-L1.
Phase I results and data from multiple expansion cohorts in a variety of histologies were presented at the 2013 ASCO Annual Meeting. Pharmacokinetic data supported dosing at 15 mg/kg every 3 weeks or a fixed dose equivalent, as no maximally tolerated dose was defined. Patients were treated for up to 1 year[65]. In an expansion cohort for previously treated patients with metastatic NSCLC, 52 subjects were enrolled and treated at a dose of 15 mg/kg. Of note, 62% of those patients had heavily pretreated NSCLC with more than 3 prior treatments. The response rate was 22%[66]. Similar results were seen in a RCC cohort, in which the response rate was 13% (6/47)[67]. In patients with metastatic melanoma treated in the phase I portion of the trial, the response rate was 26% (9 of 35 patients)[68]. In all of the above cohorts, patients were analyzed for PD-L1 status, and a correlation between PD-L1 status and efficacy was observed. Early results from patients with other tumor histologies were also considered promising[69].
At the 2014 ASCO Annual Meeting, investigators presented preliminary data from a clinical trial involving patients with metastatic bladder cancer. Patients were divided into PD-L1-positive and PD-L1-negative cohorts, based on an analysis of the PD-L1 expression of immune-infiltrating cells within tumor specimens. In the PD-L1-positive cohort, 20 patients were enrolled, and of those, 10 responded to therapy (9 partial responses and 1 complete response). Response rates for patients with PD-L1-negative tumors have yet to be published[5].
MEDI4736
MEDI4736 is a human IgG1 monoclonal antibody recognizing human PD-L1. It is similar to MPDL3280A, as the molecule also has mutations in the Fc receptor, which therefore eliminates complement-mediated cytotoxicity and ADCC[70]. Results from early clinical trials are still pending. A phase I dose of 10 mg/kg every 2 weeks is currently being evaluated in several histologies in an expansion phase[71]. Of note, at the 2014 ASCO Annual Meeting, investigators reported the preliminary results of 13 NSCLC patients treated with MEDI4736, with 3 partial responses already observed[72]. Several large combination trials in lung cancers are ongoing.
MSB0010718C
MSB0010718C is a fully human IgG1 monoclonal antibody targeting PD-L1. As opposed to the PD-L1-targcting agents from Genentech and MedImmune/AstraZeneca, MSB0010718C has a native Fc receptor, allowing for ADCC. Results from a phase I trial with the agent were reported at the 2014 ASCO Annual Meeting[66]. Twenty-seven patients with relapsed cancers were treated. No maximally tolerated dose was determined, and the investigators demonstrated that MSB0010718C could be safely administered in doses up to 20 mg/kg every 2 weeks. Further clinical trials are planned in a variety of tumors.
Combinations of Immunotherapeutic Agents
In spite of the promising single agent indications mentioned above, a majority of patients will not respond to immune checkpoint inhibition in the histologies tested thus far. The need for newer combination strategies has become obvious.
Early in the development of anti-CTLA4 antibodies, there was an effort to combine these therapies with high-dose interferon in order to take advantage of the immunomodulatory effects already noted in advanced melanoma. In one such trial, tremelimumab was administered at a dose of 15 mg/kg every 12 weeks while high-dose interferon was administered concurrently in standard doses, involving 20×106 U/m2 of body surface area for 5 days a week, followed by maintenance interferon at a dose of 10 × 106 U/m2 of body surface area subcutaneously 3 times a week for 8 weeks. Thirty-six patients were ultimately enrolled. Of the 33 evaluable patients, there were 3 complete responses and 7 partial responses. The median OS was 15.9 months[36]. A similar trial with ipilimumab and high-dose interferon is currently enrolling patients (NCT01708941).
A randomized phase II trial of ipilumumab investigated the potential synergy of adding granulocyte macrophage colony-stimulating factor (GM-CSF, or sargramostim) with ipilumumab[73]. In this trial, patients with metastatic melanoma were randomized to either ipilumumab alone at a dose of 10 mg/kg every 3 weeks for 4 cycles followed by maintenance of ipilimumab every 12 weeks or to the same dose and schedule of ipilumumab in addition to GM-CSF at 250 µg subcutaneously on days 1-14 of 21-day cycles for 4 cycles. Toxicity and response rates did not differ significantly between the two arms. However, the median OS in the combination arm was 17.5 months versus 12.7 months in the ipilimumab alone arm, which was shown to translate to a 36% reduction in mortality risk (P = 0.014). Of note, there were 2 grade 5 adverse events in the combination arm and 7 grade 5 events in the ipilumumab alone arm, suggesting the possibility that GM-CSF improved the tolerability of ipilimumab therapy.
One area of remarkable success involved the use of nivolumab and ipilimumab in patients with metastatic melanoma. In the first trial combining these two immune checkpoint inhibitors, 53 patients received concurrent therapy with nivolumab and ipilimumab, and 33 received sequenced treatment. Among all patients enrolled who received concurrent treatment, the response rate was 40%. In the concurrent arm using 3 mg of ipilimumab and 1 mg of nivolumab every 3 weeks, 53% of patients had an objective response, and all of those responding in that arm showed a tumor reduction of 80% or more. Grade 3 or 4 adverse events related to therapy occurred in 53% of patients in the concurrent treatment cohort. In the sequenced cohort, 18% of patients had grade 3 or 4 adverse events and a response rate of 20% was reported[74].
The combination of ipilimumab and nivolumab has subsequently been evaluated in a variety of malignancies. In RCC, two variations of the combination were tested. In the arm with nivolumab at a dose of 3 mg/kg and ipilimumab at a dose of 1 mg/kg, 21 patients were treated, and there were 6 objective responses and 7 patients with stable disease. In the arm with nivolumab at a dose of 1 mg/kg and ipilimumab at a dose of 3 mg/kg, 23 patients were treated, and there were 9 objective responses and 9 patients with stable disease[75].
The same regimen was evaluated in NSCLC, with disappointing results. Fewer responses were reported, in part due to early treatment discontinuation caused by adverse events. Further safety evaluations are now underway at lower doses (1 mg/kg of ipilimumab and 1 mg/kg of nivolumab)[76]. This stresses the need for investigators to carefully tailor regimens to specific patient populations. It is clear that the number and severity of co-morbidities, average age of patients, and number and intensity of prior treatments may alter the suitability of a given immunotherapy.
A number of other combinations among immunotherapeutic agents are currently underway or planned. Time will tell if the remarkable successes of the combinations noted above will be replicated when other immunotherapeutic agents are used in combination.
Conclusions
Immunotherapy for cancer was declared to be the breakthrough of the year in 2013 as a result of the significant advances seen and described above[77]. While much has been accomplished, more still needs to be done. A wide variety of important clinical and scientific questions must be addressed efficiently to significantly benefit patients and to improve understanding of the interactions of tumor biology and human immunology. If translational scientists and clinical investigators work together to solve these problems, what is now thought to be the breakthrough of the year can soon be thought of as the medical breakthrough of the century.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Ikeda T, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2014;(32 suppl):abstr 5511. [Google Scholar]
- 4.Seiwert TY, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. J Clin Oncol. 2014;(32 suppl):abstr 6011. [Google Scholar]
- 5.Powles T, Vogelzang NJ, Fine GD, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) J Clin Oncol. 2014;(32 suppl):abstr 5011. [Google Scholar]
- 6.Bachy E, Coiffier B. Anti-PD1 antibody: a new approach to treatment of lymphomas. Lancet Oncol. 2014;15:7–8. doi: 10.1016/S1470-2045(13)70587-4. [DOI] [PubMed] [Google Scholar]
- 7.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malas S, Harrasser M, Lacy KE, et al. Antibody therapies for melanoma: new and emerging opportunities to activate immunity. Oncol Rep. 2014 doi: 10.3892/or.2014.3275. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava N, McDermott D. Update on benefit of immunotherapy and targeted therapy in melanoma: the changing landscape. Cancer Manage Res. 2014;6:279–289. doi: 10.2147/CMAR.S64979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer. 2014;85:101–109. doi: 10.1016/j.lungcan.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunturi A, McDermott DF. Potential of new therapies like anti-PD1 in kidney cancer. Curr Treat Options Oncol. 2014;15:137–146. doi: 10.1007/s11864-013-0268-y. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy E, Rossi GR, Vahanian NN, et al. Phase 1/2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus ipilimumab for the treatment of unresectable stage 3 or 4 melanoma. J Clin Oncol. 2014;(32 suppl):abstr TPS9117. [Google Scholar]
- 13.Khleif S, Munn D, Nyak-Kapoor A, et al. First-in-human phase 1 study of the novel indoleamine-2,3-dioxygenase (IDO) inhibitor NLG-919. J Clin Oncol. 2014;(32 suppl):abstr TPS3121. [Google Scholar]
- 14.Wang-Gillam A, Plambeck-Suess S, Goedegebuure P, et al. A phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinoma. Invest New Drugs. 2013;31:707–713. doi: 10.1007/s10637-012-9866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193:1525–1530. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ok CY, Chen J, Xu-Monette Z, et al. Clinical implications of phosphorylated STAT3 expression in de novo diffuse large B-cell lymphoma. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0683. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Xu X, Zhang B, et al. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. doi: 10.1186/1479-5876-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson DM, Jr, Hofmeister CC, Padmanabhan S, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vey N, Bourhis JH, Boissel N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 20.Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–686. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linsley PS, Brady W, Grosmaire L, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2014 doi: 10.1093/intimm/dxu076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 24.Quezada SA, Peggs KS, Curran MA, et al. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 28.Magistrelli G, Jeannin P, Herbault N, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarhini AA, Thalanayar PM. Melanoma adjuvant therapy. Hematol Oncol Clin North Am. 2014;28:471–489. doi: 10.1016/j.hoc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Ipilimumab versus placebo after complete resection of stage III melanoma: initial efficacy and safety results from the EORTC 18071 phase III trial. J Clin Oncol. 2014;(32 suppl):abstr LBA9008. [Google Scholar]
- 33.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 34.Hu JL, Sharma P, Yu J, et al. Ipilimumab for recurrent glioblastoma: a single-institution case series. J Clin Oncol. 2014;(32 suppl):abstr e13010. [Google Scholar]
- 35.Snyder Charen A, Makarov V, Merghoub T, et al. The neoantigen landscape underlying clinical response to ipilimumab. J Clin Oncol. 2014;(32 suppl):abstr 3003. [Google Scholar]
- 36.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 37.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabro L, Morra A, Fonsatti E, et al. A phase 2 single-arm study with tremelimumab at an optimized dosing schedule in second-line mesothelioma patients. J Clin Oncol. 2014;(32 suppl):abstr 7531. [Google Scholar]
- 39.Maio M, Scherpereel A, Di Pietro A, et al. Randomized, double-blind, placebo-controlled study of tremelimumab for second- and third-line treatment of unresectable pleural or peritoneal mesothelioma. J Clin Oncol. 2014;(32 suppl):abstr TPS7609. [Google Scholar]
- 40.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedoeem A, Azoulay-Alfaguter I, Strazza M, et al. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153:145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Nicholas KJ, Zern EK, Barnett L, et al. B cell responses to HIV antigen are a potent correlate of viremia in HIV-1 infection and improve with PD-1 blockade. PloS One. 2013;8:e84185. doi: 10.1371/journal.pone.0084185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JR, Moon YJ, Kwon KS, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PloS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maine CJ, Aziz NH, Chatterjee J, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oba J, Nakahara T, Abe T, et al. Expression of programmed death receptor ligand 1 in melanoma may indicate tumor progression and poor patient survival. J Am Acad Dermatol. 2014;70:954–956. doi: 10.1016/j.jaad.2014.01.880. [DOI] [PubMed] [Google Scholar]
- 47.Shi SJ, Wang LJ, Wang GD, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PloS One. 2013;8:e76012. doi: 10.1371/journal.pone.0076012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamai K, Nakamura M, Mizuma M, et al. Suppressive expression of CD274 increases tumorigenesis and cancer stem cell phenotypes in cholangiocarcinoma. Cancer Sci. 2014;105:667–674. doi: 10.1111/cas.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xylinas E, Robinson BD, Kluth LA, et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014;40:121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–573. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Carlsson R, Ambjorn M, et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013;33:14231–14245. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 2014;(32 suppl):abstr 8024. [Google Scholar]
- 56.Antonia SJ, Brahmer JR, Gettinger SN, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;(32 suppl):abstr 8113. [Google Scholar]
- 57.Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol. 2014;(32 suppl):abstr 8022. [Google Scholar]
- 58.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60958-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) J Clin Oncol. 2014;(32 suppl):abstr LBA9000. [Google Scholar]
- 61.Garon EB, Leighl NB, Rizvi NA, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) J Clin Oncol. 2014;(32 suppl):abstr 8020. [Google Scholar]
- 62.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–376. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 63.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. J Clin Oncol. 2014;(32 suppl):abstr 9001. [Google Scholar]
- 65.Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;(31 suppl):abstr 3000. [Google Scholar]
- 66.Spigel DR, Gettinger SN, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) J Clin Oncol. 2013;(31 suppl):abstr 8008. [Google Scholar]
- 67.Cho DC, Sosman JA, Sznol M, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;(31 suppl):abstr 4505. [Google Scholar]
- 68.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) J Clin Oncol. 2013;(31 suppl):abstr 9010. [Google Scholar]
- 69.Tabernero J, Powderly JD, Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic CRC, gastric cancer (GC), SCCHN, or other tumors. J Clin Oncol. 2013;(31 suppl):abstr 3622. [Google Scholar]
- 70.Khleif S, Lutzky J, Segal N, et al. MEDI4736, an anti-PD-L1 antibody with modified Fc domain: preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors. Eur J Cancer. 2013:S161–S161. [Google Scholar]
- 71.Fairman D, Narwal R, Liang M, et al. Pharmacokinetics of MEDI4736, a fully human anti-PDL1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;(32 suppl):abstr 2602. [Google Scholar]
- 72.Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol. 2014;(32 suppl):abstr 8021. [Google Scholar]
- 73.Hodi FS, Lee SJ, McDermott DF, et al. Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (IPI) versus IPI alone in metastatic melanoma: E1608. J Clin Oncol. 2013;(31 suppl):abstr CRA9007. [Google Scholar]
- 74.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammers HJ, Plimack ER, Infante JR, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2014;(32 suppl):abstr 4504. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antonia SJ, Gettinger SN, Chow LQM, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: interim phase I results. J Clin Oncol. 2014;(32 suppl):abstr 8023. [Google Scholar]
- 77.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]