Abstract
Background
Distal pancreatectomy (DP) is a surgical procedure performed to remove the pancreatic tail jointly with a variable part of the pancreatic body and including a spleen resection in the case of conventional distal pancreatectomy or not in the spleen-preserving distal pancreatectomy.
Methods
In this article, we describe a standardized operative technique for fully robotic distal pancreatectomy.
Results
In the last decade, the use of robotic systems has become increasingly common as an approach for benign and malignant pancreatic disease treatment. Robotic Distal Pancreatectomy (RDP) is an emerging technology for which sufficient data to draw definitive conclusions in surgical oncology are still not available because the follow-up period after surgery is too short (less than 2 years).
Conclusions
RDP is an emerging technology for which sufficient data to draw definitive conclusions of value in surgical oncology are still not available, however this techniques is safe and reproducible by surgeons that possess adequate skills.
Keywords: pancreatic surgery, robotic surgery
Background
Distal pancreatectomy (DP) is a surgical procedure performed to remove the pancreatic tail jointly with a variable part of the pancreatic body and including a spleen resection in the case of conventional distal pancreatectomy or not in the spleen-preserving distal pancreatectomy. In this article we describe a technical note on RDP.
Methods
Operative technique
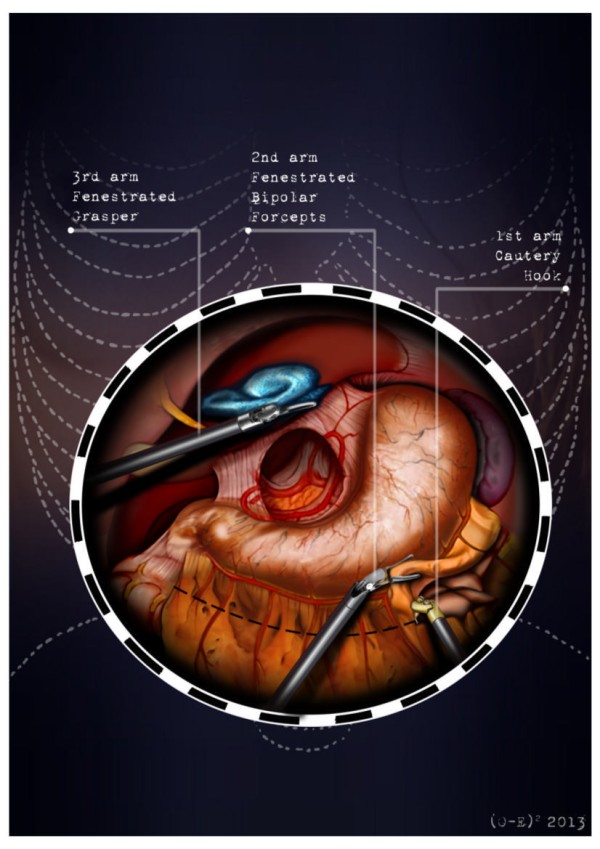
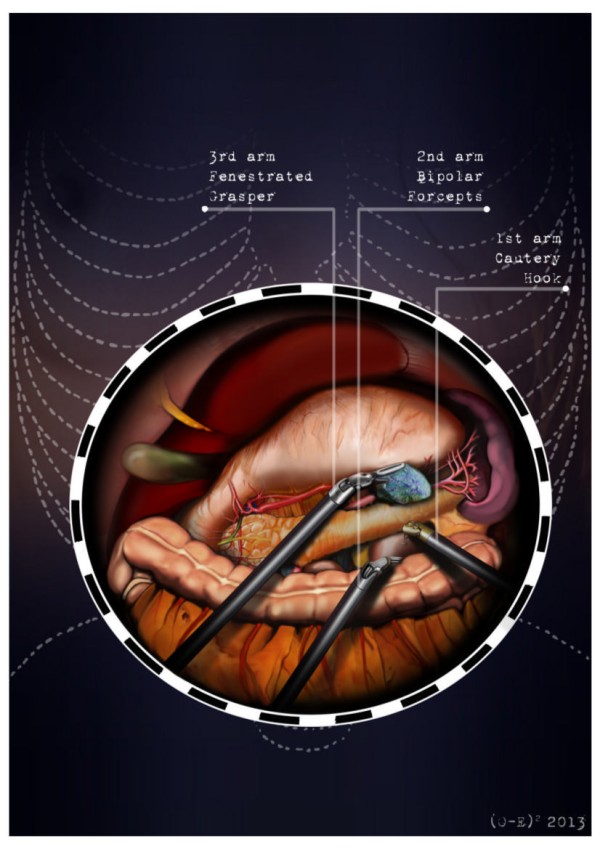
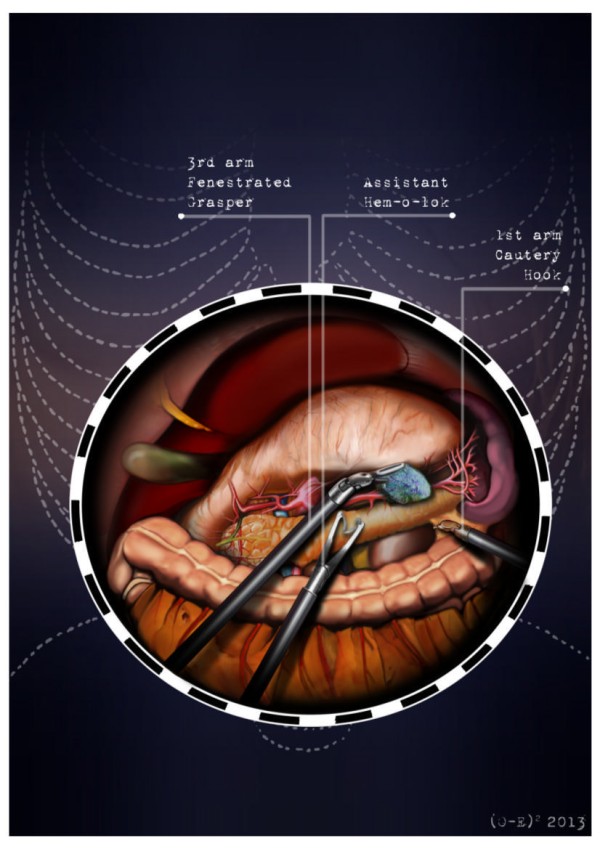
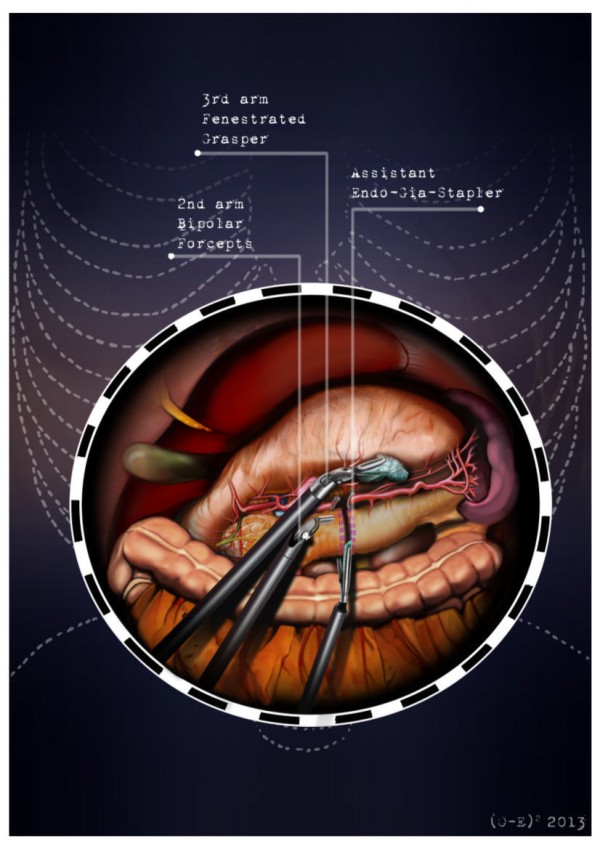
After the induction of general anesthesia, the patient’s arms are abducted and his legs are spread apart in order to allow the placement of the assistant surgeon. A nasogastric tube and urinary catheter are also applied. After preparation of the skin with povidone-iodine is completed, the abdomen is insufflated with CO2 using a veress needle through a one millimeter diameter periumbilical incision. The ınsufflator is set to a constant pressure of 12 mmHg. The trocars are placed following a concave and arcuate line (Figure 1). Usually, the optical trocar is inserted just above and to the left of the umbilicus. In practice, however, its position could vary in relation to the patient’s anatomy and pancreatic lesion localization, which is why a preliminary introduction of an assistant 12-mm extra port on the transverse umbilical line in between the xifopubic and left middle axillary line could be useful in order to check the internal anatomy and evaluate the optimal position of the optical trocar. The first robotic trocar is positioned at the intersection of the left middle axillary line and the transverse umbilical line, the second robotic trocar at the intersection of the right anterior axillary line and the transverse umbilical line, and the third robotic trocar in the right hypochondrium. The assistant surgeon in the various surgical phases will be able to introduce an aspirator, a pair of forceps, a mechanical stapler or a suture thread through the assistant port. The robotic cart is placed between the patient’s head and left shoulder after rotating the operation table to the right and consequently docking the robotic system. The robotic camera is inserted through the periumbilical trocar port, the cautery hook is placed on arm number 1, the fenestrated bipolar forceps is placed on arm number 2, and the double fenestrated grasper on arm number 3. The gastrocolic ligament is cut from the right to the left side with the help of a cautery hook, until complete exposure of the pancreatic isthmus is obtained and the gastrolienal ligament is reached (Figure 2). Subsequently, the short gastric vessels are meticulously identified and dissected by ultrasound dissector or bipolar forceps; when necessary clips and Hem-o-loks could also be applied. The stomach is lifted upward by the third robotic arm, and the transverse colon is moved downwards (Figure 3). In this manner a passage that leads to the lesser sac is obtained, helping us to distinguish and dissect the splenic artery at the superior pancreatic edge. The artery is ligated distally using Hem-o-loks and sectioned (Figures 4 and 5). The colosplenic ligament is sectioned so that the spleen is completely mobilized. The inferior spleen pole is pulled to the right with the help of a pair of fenestrated bipolar forceps, thus allowing the complete section of the splenorenal ligament by the cautery hook (Figure 6). During this procedure, attention must be paid to avoid injury to the left adrenal gland. This moment is particularly important as it identifies the precise level for the forthcoming dissection. Dissection of the lower edge of the pancreas should be performed following a retropancreatic avascular plane of dissection until visualization of the splenic vein on the posterior surface of the gland. Before ligature, the splenic vein should be isolated from the fibrotic lamina surrounding it. The splenic vein could be sectioned using proximal and distal ligatures with a Hem-o-lok or stapler. Two suspension sutures are placed at the lower edge of the pancreas at the expected level of gland resection. The pancreatic section is performed with robotic Ultracision, placed on the arm number 1, gradually reaching the duct of Wirsung, which must be tied before it is sectioned (Figures 7 and 8). Alternatively, this step can be performed using a mechanical stapler. The pancreas is finally isolated from the posterior abdominal wall by dissecting along the soft avascular tissue behind the retropancreatic band and the splenic hilum, until complete mobilization of both the organs (Figure 9). After checking the correct detachment of the surgical specimen, it is extracted with an Endocath through a McBurney or Pfannenstiel abdominal incision (Figure 10). After checking the hemostasis, a Jackson-Pratt drain is placed close to the site of the pancreatic section and incisions are sutured.
Figure 1.
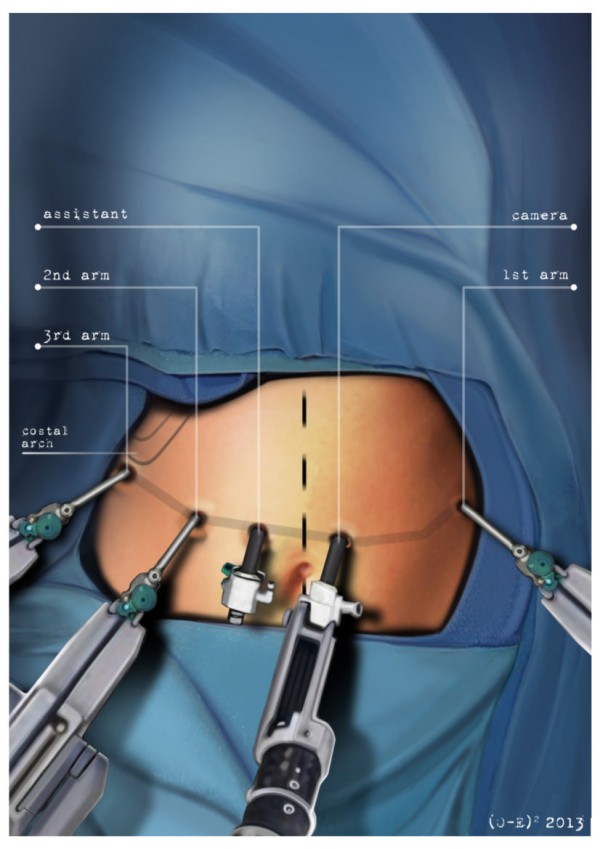
The trocars are placed following a concave and arcuated line.
Figure 2.
The gastrocolic ligament is cut from the right to the left side with the help of a cautery hook, until complete exposure of the pancreatic isthmus is obtained and the gastrolienal ligament is reached.
Figure 3.
The stomach is lifted upward by the third robotic arm and the transverse colon is moved downwards.
Figure 4.
Splenic artery dissection.
Figure 5.
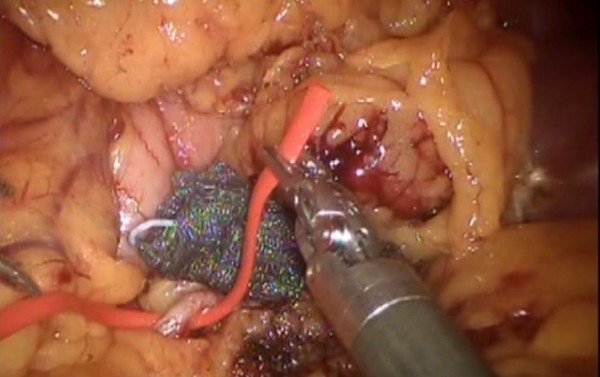
Splenic artery ligature with Hem-o-loks and sectioning.
Figure 6.
Section of the splenorenal ligament by the cautery hook.
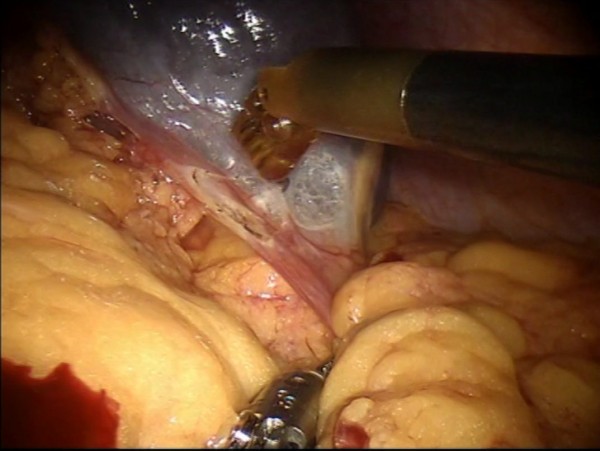
Figure 7.
Pancreatic section.
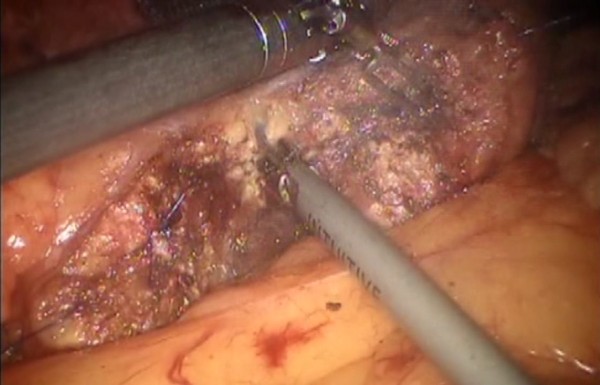
Figure 8.
The pancreatic section is performed with robotic Ultracision, placed on the arm no 1, gradually reaching Wirsung’s duct.
Figure 9.
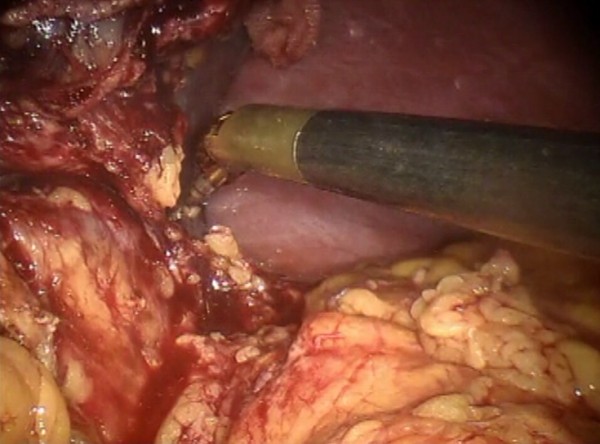
Pancreas is isolated from the posterior abdominal wall by dissecting along the soft avascular tissue behind the retropancreatic band and the splenic ilum.
Figure 10.
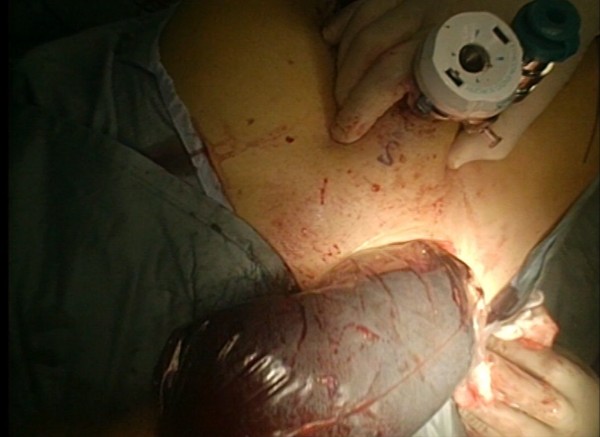
Surgical specimen is extracted with an Endocath through a McBurney or Pfannenstiel abdominal incision.
Results and discussion
In 1913, Mayo standardized the surgical procedure for DP [1], after the first described DP was performed by Trendelemburg in a case of pancreatic sarcoma [2]. Currently, there are reports that describe safely performing a spleen preserving pancreatectomy in cases of trauma, benign lesions of the body and tail of the pancreas next to the duct of Wirsung, or chronic pancreatitis. Spleen preservation allows many well-demonstrated advantages in terms of morbidity and mortality, preventing the development of infections and facilitating a faster postoperative recovery [3]. However this type of surgical intervention is rarely performed due to the need to select patients, technical difficulties, and the dependence of these procedures on the experience of the surgeon. Mallet-Guy standardized the technique of DP with spleen preservation in chronic pancreatitis: the splenic vessels are identified and dissected from the posterior portion of the gland, followed by the resection of the body/tail of the pancreas [4]. Quenu and Leger point out a collateral blood circulation that can be used to preserve the spleen through the short gastric vessels and the gastroepiploic vessels. Their technique may also be used in the case of interruption of the blood flow of the splenic vessels caused by their iatrogenic rupture or section. Some authors, Leger among others, underline the risk of developing a segmental portal hypertension and suggest performing splenectomy when it is not possible to preserve the splenic vein [5]. In 1988, Warshaw revised the spleen-preserving DP and showed that the use of the short gastric vessels is not only useful to preserve the spleen in the case of damage to the splenic vessels but can also be exploited as a technique of choice in selected cases [6, 7]. The advent of laparoscopy has led to evaluation of the feasibility of a minimally invasive approach for DP. In 1994 Cuschieri performed the first laparoscopic distal pancreatectomy (LDP) [8], followed by Gagner et al., who presented their experience on this topic [9]. Thereafter, a large number of studies reported results; nevertheless, all of them are limited by a small sample size [10–13]. LDP is a procedure considered technically demanding due to the known limitations of the traditional laparoscopic approach. In the last decade, the use of robotic systems has become increasingly common as an approach for benign and malignant pancreatic disease treatment. The robotic system adds precision to the movements and greatly increases the comfort of the surgeon dealing with a delicate minimally invasive dissection phase. Robotic surgical system instrumentation allows the use of a magnified and three-dimensional viewing field [14, 15], a steady traction, tremor suppression [16], flexibility of the instruments [17], and thus, safe suturing. A recent literature review of robotic distal pancreatectomy (RDP) shows that RDP is an emergent technology, for which there is, as yet, insufficient data to draw definitive benefit with respect to conventional or laparoscopic surgery. The mean duration of RDP is longer with the Da Vinci robot, but the hospital stay is shorter even if influenced by different hospital protocols [18]. However, we cannot reach a precise conclusion on the indications for the different approaches because the number of patients treated with the robot is low, studies presented in the literature present a small number of patients, and randomized trials are absent. In this article we describe a technical note on RDP.
Conclusions
RDP is an emerging technology for which sufficient data to draw definitive conclusions of value in surgical oncology are still not available and for which the follow-up period after surgery is too short (less than 2 years) [18]; however this techniques is safe and reproducible by experienced surgeons. We performed an update of the literature review from January 2003 to February 2014; we found 31 studies, whose characteristics are reported in Table 1. None of the studies was a randomized clinical trial. The definition of the robotic approach was heterogeneous: the technique was defined as fully robotic, robotic, robotic-assisted, robot-assisted laparoscopic and hybrid robotic [19–47]. The dissection and resection were also heterogeneous, sequentially combining different approaches: laparoscopic/robotic and only robotic. In this article we have presented a standardized operative technique for fully robotic distal pancreatectomy.
Table 1.
Review of the literature
| Study (Author/year/type) | Duration (year) | Setting City Nation | Patients | Author’s definition of Robotic DP | Type of dissection and resection |
|---|---|---|---|---|---|
| Han [ [19]] 2014 Case report | 2013 | Seoul South Korea | 1 | Robotic RAMPS | Robotic |
| Hanna [ [20]] 2013 CCT | 2006-2012 | Charlotte, NC, USA | 39 | Robotic-assisted laparoscopic distal pancreatectomy | Robotic-laparoscopic |
| Zhang [ [21]] 2013 Review | Beijing, China | Robotic-assisted distal pancreatectomy | |||
| Milone [ [22]] 2013 Review | Chicago, IL, USA | Robotic distal pancreatectomy | |||
| Benizri [ [23]] 2013 CCT | 2004-2011 | Vandoeuvre-les-Nancy, France | 11 | Robot-assisted distal pancreatectomy | Robotic |
| Fernandes [ [24]] 2013 Review | Chicago, IL, USA | RADP | Robotic | ||
| Chen [ [25]] 2013 Review | Shanghai China | Robot-assisted distal pancreatectomy | |||
| Lai [ [26]] 2013 Review | 2013 | Hong Kong China | Robot-assisted laparoscopic distal pancreatectomy | ||
| Wayne [ [27]] 2013 Case series | 2011-2012 | New York, NY, USA | 12 | Robotic pancreatic distal resection | NR |
| Jung [ [28]] 2013 Review | Geneva, Switzerland | Robotic distal pancreatectomy | |||
| Strijker [ [29]] 2012 Review | Utrecht Netherlands | Robot-assisted distal pancreatectomy distal pancreatectomy | |||
| Winer [ [30]] 2012 Review | Pittsburgh, PA, USA | Minimally Invasive RADP | Robotic-laparoscopic | ||
| Hwang [ [31]] 2012 CCT | 2007- 2011 | Seoul South Korea | 22 | Robot-assisted spleen-preserving DP | Robotic |
| Daouadi [ [32]] 2012 CCT | 2004- 2011 | Pittsburgh, PA, USA | 30 | Minimally Invasive RADP | Robotic- laparoscopic |
| Suman [ [33]] 2012 CCT | 2006- 2010 | Ridgewood, NJ, USA | 40 | Robot spleen-preserving DP | NR |
| Buturrini [ [34]] 2012 CCT | NR | Verona Italy | 5 | Hybrid Robotic DP | Robotic-laparoscopic |
| Fully Robotic DP | Robotic | ||||
| Choi [ [35]] 2012 Case series | NR | Seoul South Korea | 4 | Robotic RAMPS | Robotic |
| Kang [ [36]] 2011 CCT | 2006- 2010 | Seoul South Korea | 20 | RADP | NR |
| Ntourakis [ [37] 2011 Case report | 2010 | Strasbourg France | 1 | Robotic Left Pancreatectomy | Robotic |
| Chan [ [38]] 2011 Case series | 2009- 2010 | Hong Kong China | 2 | Robotic spleen preserving DP | Robotic |
| Kim [ [39]] 2011 Case report | 2009 | Seoul South Korea | 1 | Robot Assisted spleen-preserving laparoscopic DP | Robotic |
| Yiengpruksawan [ [40] 2011 Technical note | 2010 | Ridgewood, NJ, USA | NR | RADP | Robotic-laparoscopic |
| Ntourakis [ [41] 2010 Case series | NR | Strasbourg France | 2 | Robotic Distal Splenopancreatectomy | Robotic |
| Waters [ [42]] 2010 CCT | 2008- 2009 | Indianapolis, IN, USA | 17 | Robotic DP | Robotic |
| Giulianotti [ [43] 2010 Case series | 2000- 2007 | Chicago, IL, and Grosseto, Italy | 46 | RADP | Robotic |
| Vasilescu [ [44] 2009 Case report | 2008 | Bucharest Romania | 1 | Robotic spleen-preserving DP | Robotic |
| Machado [ [45] 2009 Case report | NR | Sao Paulo Brazil | 1 | Robotic resection | Robotic-laparoscopic |
| D’Annibale [ [46] 2006 Case series | 2001- 2004 | Padova Italy | 2 | Robotic resection | Robotic |
| Melvin [ [47]] 2003 Case report | NR | Ohio OH, USA | 1 | Robotic resection | Robotic |
DP, distal pancreatectomy; NR, not reported; RADP, robot-assisted distal pancreatectomy; Robotic RAMPS, robotic radical antegrade modular pancreatico-splenectomy.
Consent
Written informed consent was obtained from the patient for the publication of this report and any accompanying images.
Acknowledgements
The authors gratefully acknowledge Konstantinos G. Economou for the preparation of the illustrations and Dr. Suzanne K. Polmar for her editorial review of the manuscript.
Abbreviations
- DP
distal pancreatectomy
- LDP
laparoscopic distal pancreatectomy
- NR
not reported
- RADP
robot-assisted distal pancreatectomy
- RAMPS
radical antegrade modular pancreatico-splenectomy
- RDP
robotic distal pancreatectomy.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed equally to the manuscript. All authors edited, read and approved the final manuscript.
Contributor Information
Amilcare Parisi, Email: amilcareparisi@virgilio.it.
Francesco Coratti, Email: corattif@gmail.com.
Roberto Cirocchi, Email: cirocchiroberto@yahoo.it.
Veronica Grassi, Email: veronicagrassi@hotmail.it.
Jacopo Desiderio, Email: veronicagrassi@hotmail.it.
Federico Farinacci, Email: federico.farinacci@gmail.com.
Francesco Ricci, Email: f.fricci@libero.it.
Olga Adamenko, Email: adamenko@powergee.com.
Anastasia Iliana Economou, Email: ilianags500@gmail.com.
Alban Cacurri, Email: alban_ac@yahoo.it.
Stefano Trastulli, Email: stefano.trastulli@hotmail.it.
Claudio Renzi, Email: renzicla@virgilio.it.
Elisa Castellani, Email: elisa.ecv@gmail.coml.
Giorgio Di Rocco, Email: giorgiodirocco@virgilio.it.
Adriano Redler, Email: adriano.redler@uniroma1.it.
Alberto Santoro, Email: albert.santoro@tiscali.it.
Andrea Coratti, Email: corattian@gmail.com.
References
- 1.Mayo WJ. I. The surgery of the pancreas: I. Injuries to the pancreas in the course of operations on the stomach. II. Injuries to the pancreas in the course of operations on the spleen. III. Resection of half the pancreas for tumor. Ann Surg. 1913;58:145–150. doi: 10.1097/00000658-191308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sulkowski U, Meyer J, Reers B, Pinger P, Waldner M. The historical development of resection surgery in pancreatic carcinoma. Zentralbl Chir. 1991;116:1325–1332. [PubMed] [Google Scholar]
- 3.Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164–168. doi: 10.1001/archsurg.137.2.164. [DOI] [PubMed] [Google Scholar]
- 4.Mallet-Guy P, Vachon A. Pancreatites Chroniques Gauches. Paris: Masson & Cie; 1943. [Google Scholar]
- 5.Leger L, Bréhant J. Chirurgie du Pancréas. Paris: Masson et Cie; 1956. [Google Scholar]
- 6.Warshaw AL. Distal pancreatectomy with preservation of the spleen. J Hepatobiliary Pancreat Sci. 2010;17:808–812. doi: 10.1007/s00534-009-0226-z. [DOI] [PubMed] [Google Scholar]
- 7.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 8.Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb. 1994;39:178–184. [PubMed] [Google Scholar]
- 9.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051–1054. doi: 10.1016/S0039-6060(96)80054-7. [DOI] [PubMed] [Google Scholar]
- 10.Jin T, Altaf K, Xiong JJ, Huang W, Javed MA, Mai G, Liu XB, Hu WM, Xia Q. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford) 2012;14:711–724. doi: 10.1111/j.1477-2574.2012.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui CJ, Li B, Yang JM, Wang SJ, Zhou YM. Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg. 2012;35:1–8. doi: 10.1016/j.asjsur.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 13.Xie K, Zhu YP, Xu XW, Chen K, Yan JF, Mou YP. Laparoscopic distal pancreatectomy is as safe and feasible as open procedure: a meta-analysis. World J Gastroenterol. 2012;18:1959–1967. doi: 10.3748/wjg.v18.i16.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad SM, Maniar HS, Chu C, Schuessler RB, Damiano RJ. Surgical robotics: impact of motion scaling on task performance. J Am Coll Surg. 2004;199:863–868. doi: 10.1016/j.jamcollsurg.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Byrn JC, Schluender S, Divino CM, Conrad J, Gurland B, Shlasko E, Szold A. Three-dimensional imaging improves surgical performance for both novice and experienced operators using the da Vinci Robot System. Am J Surg. 2007;193:519–522. doi: 10.1016/j.amjsurg.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Veluvolu KC, Ang WT. Estimation and filtering of physiological tremor for real-time compensation in surgical robotics applications. Int J Med Robot. 2010;6:334–342. doi: 10.1002/rcs.340. [DOI] [PubMed] [Google Scholar]
- 17.Chitwood WR, Jr, Nifong LW, Chapman WH, Felger JE, Bailey BM, Ballint T, Mendleson KG, Kim VB, Young JA, Albrecht RA. Robotic surgical training in an academic institution. Ann Surg. 2001;234:475–484. doi: 10.1097/00000658-200110000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirocchi R, Partelli S, Coratti A, Desiderio J, Parisi A, Falconi M. Current status of robotic distal pancreatectomy: a systematic review. Surg Oncol. 2013;22:201–207. doi: 10.1016/j.suronc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Han DH, Kang CM, Lee WJ, Chi HS. A five-year survivor without recurrence following robotic anterior radical antegrade modular pancreatosplenectomy for a well-selected left-sided pancreatic cancer. Yonsei Med J. 2014;55:276–279. doi: 10.3349/ymj.2014.55.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna EM, Rozario N, Rupp C, Sindram D, Iannitti DA, Martinie JB. Robotic hepatobiliary and pancreatic surgery: lessons learned and predictors for conversion. Int J Med Robot. 2013;9:152–159. doi: 10.1002/rcs.1492. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wu WM, You L, Zhao YP. Robotic versus open pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1774–1780. doi: 10.1245/s10434-012-2823-3. [DOI] [PubMed] [Google Scholar]
- 22.Milone L, Daskalaki D, Wang X, Giulianotti PC. State of the art of robotic pancreatic surgery. World J Surg. 2013;37:2761–2770. doi: 10.1007/s00268-013-2275-3. [DOI] [PubMed] [Google Scholar]
- 23.Benizri EI, Germain A, Ayav A, Bernard JL, Zarnegar R, Benchimol D, Bresler L, Brunaud L. Short-term perioperative outcomes after robot-assisted and laparoscopic distal pancreatectomy. J Robot Surg. 2014;8(2):125–132. doi: 10.1007/s11701-013-0438-8. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes E, Giulianotti PC. J Hepatobiliary Pancreat Sci. 2013. Robotic-assisted pancreatic surgery. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Yan J, Yuan Z, Yu S, Wang Z, Zheng Q. A meta-analysis of robotic-assisted pancreatectomy versus laparoscopic and open pancreatectomy. Saudi Med J. 2013;34:1229–1236. [PubMed] [Google Scholar]
- 26.Lai EC, Tang CN. Current status of robot-assisted laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a comprehensive review. Asian J Endosc Surg. 2013;6:158–164. doi: 10.1111/ases.12040. [DOI] [PubMed] [Google Scholar]
- 27.Wayne M, Steele J, Iskandar M, Cooperman A. Robotic pancreatic surgery – no substitute for experience and clinical judgment: an initial experience and literature review. World J Surg Oncol. 2013;11:160. doi: 10.1186/1477-7819-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung MK, Buchs NC, Azagury DE, Hagen ME, Morel P. Robotic distal pancreatectomy: a valid option? Minerva Chir. 2013;68:489–497. [PubMed] [Google Scholar]
- 29.Strijker M, van Santvoort HC, Besselink MG, van Hillegersberg R, Borel Rinkes IH, Vriens MR, Molenaar IQ. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB (Oxford) 2013;15:1–10. doi: 10.1111/j.1477-2574.2012.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winer J, Can MF, Bartlett DL, Zeh HJ, Zureikat AH. The current state of robotic-assisted pancreatic surgery. Nat Rev Gastroenterol Hepatol. 2012;9:468–476. doi: 10.1038/nrgastro.2012.120. [DOI] [PubMed] [Google Scholar]
- 31.Hwang HK, Kang CM, Chung YE, Kim KA, Choi SH, Lee WJ. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc. 2013;27:774–781. doi: 10.1007/s00464-012-2551-6. [DOI] [PubMed] [Google Scholar]
- 32.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Ann Surg. 2013. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. [DOI] [PubMed] [Google Scholar]
- 33.Suman P, Rutledge J, Yiengpruksawan A. Robotic spleen preserving distal pancreatectomy is safe and feasible. Gastroenterology. 2012;142:S1060–S1061. doi: 10.1053/j.gastro.2012.03.012. [DOI] [Google Scholar]
- 34.Butturini G, Damoli I, Esposito A, Daskalaki D, Marchegiani G, Salvia R, Bassi C. Robotic distal pancreatectomy: is hybrid operation a viable approach? J Pancreas (Online) 2012;13(Suppl):592. [Google Scholar]
- 35.Choi SH, Kang CM, Hwang HK, Lee WJ, Chi HS. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg. 2012;16:868–869. doi: 10.1007/s11605-012-1825-6. [DOI] [PubMed] [Google Scholar]
- 36.Kang CM, Kim DH, Lee WJ, Chi HS. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc. 2011;25:2004–2009. doi: 10.1007/s00464-010-1504-1. [DOI] [PubMed] [Google Scholar]
- 37.Ntourakis D, Marzano E, De Blasi V, Oussoultzoglou E, Jaeck D, Pessaux P. Robotic left pancreatectomy for pancreatic solid pseudopapillary tumor. Ann Surg Oncol. 2011;18:642–643. doi: 10.1245/s10434-010-1376-6. [DOI] [PubMed] [Google Scholar]
- 38.Chan OC, Tang CN, Lai EC, Yang GP, Li MK. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18:471–480. doi: 10.1007/s00534-011-0389-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim DH, Kang CM, Lee WJ, Chi HS. The first experience of robot assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J. 2011;52:539–542. doi: 10.3349/ymj.2011.52.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yiengpruksawan A. Technique for laparobotic distal pancreatectomy with preservation of spleen. J Robotic Surg. 2011;5:11–15. doi: 10.1007/s11701-010-0218-7. [DOI] [PubMed] [Google Scholar]
- 41.Ntourakis D, Marzano E, Lopez Penza PA, Bachellier P, Jaeck D, Pessaux P. Robotic distal splenopancreatectomy: bridging the gap between pancreatic and minimal access surgery. J Gastrointest Surg. 2010;14:1326–1330. doi: 10.1007/s11605-010-1214-y. [DOI] [PubMed] [Google Scholar]
- 42.Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, Ball CG, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD, Schmidt CM. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, Caravaglios G, Coratti A. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–1657. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 44.Vasilescu C, Sgarbura O, Tudor S, Herlea V, Popescu I. Robotic spleen-preserving distal pancreatectomy. A case report. Acta Chir Belg. 2009;109:396–399. doi: 10.1080/00015458.2009.11680446. [DOI] [PubMed] [Google Scholar]
- 45.Machado MA, Makdissi FF, Surjan RC, Abdalla RZ. Robotic resection of intraductal neoplasm of the pancreas. J Laparoendosc Adv Surg Tech A. 2009;19:771–775. doi: 10.1089/lap.2009.0164. [DOI] [PubMed] [Google Scholar]
- 46.D'Annibale A, Orsini C, Morpurgo E, Sovernigo GL. chirurgia robotica. Considerazioni dopo 250 interventi. Chir Ital. 2006;58:5–14. [PubMed] [Google Scholar]
- 47.Melvin WS, Needleman BJ, Krause KR, Ellison EC. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A. 2003;13:33–36. doi: 10.1089/109264203321235449. [DOI] [PubMed] [Google Scholar]


