Summary
Field experiments with transgenic plants often reveal the functional significance of genetic traits important for plant performance in their natural environments. Until now, only constitutive overexpression, ectopic expression and gene silencing methods have been used to analyze gene-related phenotypes in natural habitats. These methods do not allow sufficient control over gene expression to study ecological interactions in real-time, genetic traits playing essential roles in development, or dose-dependent effects. We applied the sensitive dexamethasone (DEX)-inducible pOp6/LhGR expression system to the ecological model plant Nicotiana attenuata and established a lanolin-based DEX application method to facilitate ectopic gene expression and RNAi mediated gene silencing in the field and under challenging conditions (e.g. high temperature, wind and UV radiation). Fully established field-grown plants were used to silence phytoene desaturase and thereby cause photobleaching only in specific plant sectors, and to activate expression of the cytokinin (CK) biosynthesis gene isopentenyl transferase (ipt). We used ipt expression to analyze the role of CK’s in both the glasshouse and field to understand resistance to the native herbivore Tupiocoris notatus, which attack plants at small spatial scales. By spatially restricting ipt expression and elevating CK levels in single leaves, T. notatus damage increased, demonstrating CK’s role in this plant-herbivore interaction at a small scale. As the arena of most ecological interactions is highly constrained in time and space, these tools will advance the genetic analysis of dynamic traits that matter for plant performance in nature.
Keywords: pOp6, LhGR, dexamethasone, fieldwork, Nicotiana attenuata, Tupiocoris notatus, cytokinin, Manduca sexta, herbivory, pds
Introduction
Experiments with transgenic plants in natural environments are often indispensable for ecological research, because the complex blend of abiotic and biotic factors can reveal plant phenotypes, which might be absent under the coddled conditions of the laboratory and glasshouse (Izawa et al. 2011, Baldwin 2012, Kaur et al. 2012, Dinh et al. 2013).Until now the genetic tools for ecological field studies have been mainly restricted to the use of mutants and constitutive silencing or overexpression technologies. These techniques allow only functional analysis of genes, which do not cause strong developmental defects, since these confound the analysis of traits important for ecological interactions with other organisms. Constitutive techniques also do not allow restricted fine-scale transcriptional regulation in specific plant tissues or developmental stages, which are necessary to address basic questions about the spatial dynamics of herbivore feeding or to study season-specific interactions with herbivores. Additionally they complicate the work with plant traits whose ecological functions are dose dependent or are tightly regulated in time. Expression systems using tissue, stress or developmental specific promotors (Potenza et al. 2004, Moore et al. 2006), like the stress and ontogeny-regulated SARK (Senescence-activated protein kinase) promotor and the stress-inducible HVA22P promotor have been used in the field (Xiao et al. 2009, Qin et al. 2011), but are limited to specific tissues, stresses or developmental stages. Chemically-inducible expression systems provide the flexibility required for studies of ecological interaction, since they allow immediate control over the spatial, temporal and quantitative construct expression. To the best of our knowledge, such expression systems have only been used under controlled laboratory and glasshouse conditions in several plant species, and their use in ecological field research remains untested.
Various chemically-inducible systems, which express the target construct only in the presence of specific compounds, like estradiol, alcohol or dexamethasone (DEX), have been developed (Moore et al. 2006, Corrado and Karali 2009). There are several reports of conditional basal expression (alc system; Salter et al. 1998, Roslan et al. 2001), chimeric patterns (Cre/loxP recombination system; Guo et al. 2003) and side effects for the plant from the chemical elicitors (ethanol; Camargo et al. 2007) or the activated expression system itself (GVG system; Kang et al. 1999). One of the most sensitive expression systems, which allows for the regulation of gene expression with minimal side effects on the plant’s physiology is based on the DEX-inducible pOp6/LhGR system.
The pOp6/LhGR expression system was developed from the pOp/LhG4 system (Moore et al. 1998) by Craft et al. (2005) and Samalova et al. (2005). The system is comprised of a constitutively expressed chimeric transcription factor (LhGR) containing a high affinity DNA binding lac-repressor domain, a Gal4 transcription activator region and the ligand-binding domain of a glucocorticoid receptor. The target construct is under control of a minimal CaMV promotor downstream of an array of lac-operator repeats. In the presence of DEX, the transcription factor dissociates from heat shock proteins (Picard 1993), binds to the lac-operator array and activates the otherwise inactive minimal CaMV promotor, leading to expression of the target construct.
The system was tested for heterologous gene expression, as well as for RNAi mediated gene silencing (Craft et al. 2005, Samalova et al. 2005, Wielopolska et al. 2005). For example, to regulate the expression of the Agrobacterium tumefaciens isopentenyl transferases (ipt) coding gene Tumor morphology root (Tmr), which catalyzes the rate limiting step in the biosynthesis of the cytokinin (CK) trans-zeatin (tZ; Heidekamp et al. 1983, Craft et al. 2005, Samalova et al. 2005, Ueda et al. 2012). Since already minor CK changes affect plant development (Medford et al. 1989, Bohner and Gatz 2001), ipt represents a sensitive visual marker for analyzing dose-dependent induction. Gene-silencing was tested by silencing phytoene desaturase (pds), which is involved in carotenoid biosynthesis, resulting in visually observable photobleaching (Chamovitz et al. 1993, Wielopolska et al. 2005, Zhang et al. 2010). In the traditional application methods, such as spraying or soil drenching (Aoyama and Chua 1997, Samalova et al. 2005), the use of this steroid based system has been restricted to the controlled environment of a laboratory (Moore et al. 2006, Corrado and Karali 2009). The pOp6/LhGR system has found limited use for field studies mostly due to the biological activity of DEX in humans (Walton 1959) and other organisms (Miller et al. 1994), as well as the high risk of contaming the environment.
N. attenuata is intensively used for ecological field experiments (e.g. Kessler et al. 2004, Kessler et al. 2008, Allmann and Baldwin 2010, Long et al. 2010, Weinhold and Baldwin 2011, Meldau et al. 2012a, Schuman et al. 2012). The extensive use of transgenic N. attenuata plants in their native habitat in the analysis of plant-animal and plant-microorganism interactions has made it one of the best-characterized model organisms for understanding genetic traits responsible for ecological performance under natural conditions.
Many ecologically important plant traits, such as defense responses against specific herbivores, are regulated by temporal and tissue-specific changes in plant hormones (Erb et al. 2012). CKs, for example, mediate many developmental and stress-related processes (Werner and Schmülling 2009). Yet, the physiological responses to CKs are highly concentration-dependent and specific for different tissues and developmental stages. Unfettered manipulations of CKs induce severe developmental perturbations (Klee et al. 1987), which may confound the analysis of their role in plant interactions with native herbivores. A field-applicable pOp6/LhGR system, with locally restricted dose-dependent CK manipulations, would allow for rigorous tests of many hypotheses about the roles of CKs in plant ecological interactions (Giron et al. 2013).
Here we describe the application of the DEX-inducible pOp6/LhGR system for precisely controlled overexpression and gene silencing in N. attenuata and present a DEX application approach, which can be used in the field.
Results
Establishment of the pOp6/LhGR system in N. attenuata
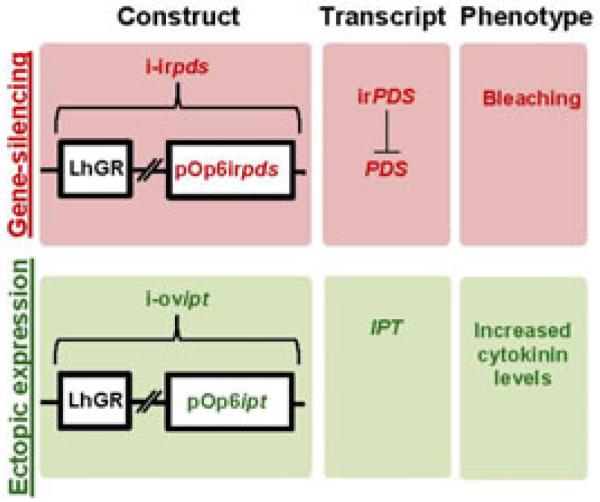
We tested the pOp6/LhGR system for field experiments in N. attenuata using two different pOp6 constructs (Figure 1). The i-irpds expresses a construct for RNAi-mediated gene-silencing (Wesley et al. 2001) of the N. attenuata pds, leading to a visible photobleaching, whereas the i-ovipt line ectopically expresses an ipt from A. tumefaciens (Heidekamp et al. 1983), leading to higher levels of CKs (Figure 1). Both lines contain a specific pOp6 construct (pOp6irpds for i-irpds and pOp6ipt for i-ovipt), as well as the LhGR construct (Figure 1; Figure S1), which were combined by crossing plants homozygous for each of the individual constructs. All pOp6 and LhGR lines were independently screened (Figure S2), before crossings. Separate screening allowed us to combine the most promising lines for both constructs, reuse the screened LhGR line and to combine the pOp6 constructs with inducer lines with tissue or developmental specific expression in the future (Moore et al. 2006). Hemizygosity also reduces possible insertion site effects, by maintaining one wildtype (WT) allele. We modified the screening procedure optimized for N. attenuata (Figure S2; Gase et al. 2011) because the high levels of hptII (Figure S3) silencing, which thwarted efficient hygromycin-based screening. After crossing the pOp6 with homozygous LhGR plants, we visually screened the resulting seedlings on DEX-containing agar plates for photobleaching and CK over-accumulation phenotypes (Figure S4). pOp6 and LhGR lines were selected by choosing the transformed lines that showed highest inducibility. An example of line optimization is shown for LhGR in Figure S5 (line 92 was chosen for final crosses). Photobleaching in pds-silenced plants is only achieved in lines with high silencing efficiency. This allows efficient screening of functional LhGR lines by analyzing crosses with the corresponding i-irpds lines. Lines with insufficient phenotypes in the presence of DEX or with phenotypic changes in the absence of DEX were excluded. The final selected lines exhibit highly DEX-inducible seedling phenotypes, but are phenotypically normal in the absence of DEX (Figure S4).
Figure 1.
pOp6/LhGR system for inducible construct expression.
Constructs used for ectopic expression and gene-silencing, their regulated transcripts and the expected phenotype. The color code (red: gene silencing, green: ectopic expression) is used consistently for all figures. “ir” stands for inverted repeat, “pds” for phytoene desaturase and “ipt” for isopentenyl tranferase.
DEX application procedure
We next established a DEX treatment procedure for mature plants for use under field conditions. We first tested the stability of DEX under conditions relevant for studies in the plant’s natural environment (Table S1; Dinh et al. 2013). To test the effect of elevated temperature, a DEX-containing methanol solution was incubated at 37°C for several days in darkness. Even after six days at 37°C only a small proportion of DEX was degraded (11% degradation, Figure S6a). However, when exposed for six days to the light environment of the glasshouse at 22°C, we found a strong DEX degradation (63% degradation, Figure S6b), suggesting that DEX is sensitive to light. Since our field site in the Great Basin desert (Utah, USA) is characterized by light irradiation (Dinh et al. 2013) that is much higher when compared to glasshouse, DEX stability was considered as limiting factor under these conditions. Therefore DEX should be applied to plant areas protected from direct sunlight and repeatedly applied for long-term treatments. Spray application is not a useful field technique as wind increases the risks of both environment and researcher to DEX exposure. Lanolin is commonly used as matrix for applying lipophilic substances to N. attenuata (Baldwin 1996, Kessler and Baldwin 2001, Meldau et al. 2011, Kallenbach et al. 2012). When the test tubes containing the DEX-methanol solutions were covered with a thin layer of lanolin (~1mm), the DEX degradation in the light environment of the glasshouse was significantly reduced (45% degradation, Figure S6b). Thus lanolin can help to increase the stability of DEX in high-light environments. To dissolve substances in lanolin, it is usually liquefied at 60°C and then mixed with the compound of interest. We tested if heating leads to DEX breakdown. Since we were not able to purify DEX from lanolin, we analyzed its stability when dissolved in MeOH. Our results show that short exposures of DEX to 60°C did not affect DEX stability (Figure S6c). Therefore it is unlikely that DEX degradation occurs during dissolution in lanolin. However, we cannot rule out that DEX has different stabilities in lanolin, when compared to MeOH. To dissolve the DEX in lanolin, we used DMSO as primary solvent. DMSO is expected to improve absorption by the plant tissue (Williams and Barry 2012) and has minimal side effects on a plant’s physiology at low doses, when compared to other solvents, such as ethanol (Samalova et al. 2005, Robison et al. 2006). Since we mainly manipulated the gene expression in leaves, most experiments described here involved applying of a thin layer of DEX-containing lanolin paste to the lower side of the petiole of the targeted leaves (Figure S7).
The pOp6/LhGR system under glasshouse conditions
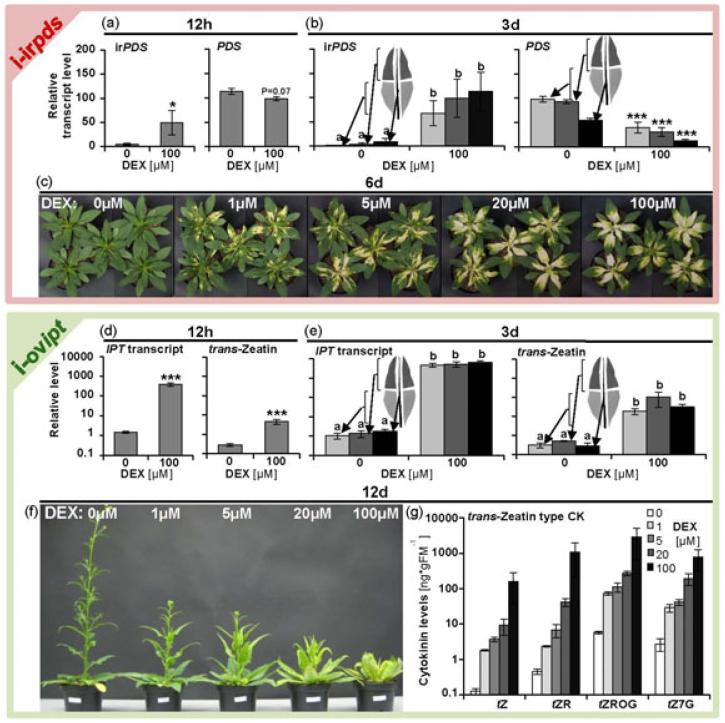
Before performing experiments in the field, we tested the system under glasshouse conditions. To quantify DEX-induced silencing, we measured irPDS and PDS transcript accumulations, while for DEX-induced heterologous expression; we analyzed IPT transcript accumulation and CK levels. i-irpds plants showed 10 fold increases in irPDS transcripts 12h after treatment with 100μM DEX (Figure 2a) and this increased to more than 100 fold one day after DEX applications (Figure S8a). The PDS transcript accumulation was only reduced by approximately 10% at both time points (Figure 2a; Figure S8a). The i-ovipt plants increased IPT transcript abundance 250 fold after 12h, leading to more than 15 fold increases in tZ levels (Figure 2d). After one day, IPT transcripts increased more than 1000 fold and tZ by nearly 35 fold. Three days after a single petiole-treatment with 100μM DEX, the bleaching in i-irpds plants and growth effects due to cytokinin overproduction in i-ovipt plants were clearly visible (Figure S8b,d). The corresponding transcript and metabolite level changes were found to be similar in the basal and apical part and the midvein of the leaves (Figure 2b,e). Three days after DEX treatments, the irPDS transcripts accumulated 10-40 fold in i-irpds plants, when compared to control levels, leading to 60-80% reductions in PDS transcript levels (Figure 2b). Even if the photobleaching was only visible in newly established leaf tissue, completely green leaf parts showed strong pds silencing (apical parts in Figure 2b; compare Ruiz et al. 1998). The IPT transcript abundance increased 4000 fold three days after DEX application, leading to 60-200 fold increases in tZ levels (Figure 2e). The application of different DEX concentrations showed a strong dose-dependent response with a high dynamic range (1-100μM DEX) for gene silencing and for ectopic expression (Figure 2c,f,g). In addition to the clearly observable growth phenotypes (Figure 2c,f), tZ-type CKs increased 5-15, 15-30, 45-100 or 250-2500 fold 12 days after the applications of 1, 5, 20 or 100μM DEX, respectively (Figure 2g). We also observed that treatments of single leaves did not affect adjacent leaves, indicating that the pOp6/LhGR system can be used to manipulate spatially restricted responses in plants (Figure S9). To test if the DEX system can also be used to manipulate responses in roots, plants were grown in hydroponic cultures and DEX was applied directly to the hydroponic solutions. DEX-induction of i-irpds plants by treating roots induced bleaching in the leaves (Figure S10a). i-ovipt plants treated with DEX through the roots reduced root growth and changed growth of the shoots (Figure S10b,c).
Figure 2.
pOp6/LhGR system in Nicotiana attenuata under glasshouse conditions.
(a) Inverted repeat PDS (irPDS) and PDS transcript level in i-irpds plants 12h after application of DEX-containing lanolin paste to the midvein.
(b) irPDS and PDS transcript levels in the basal and apical parts of the leaf lamina, as well as in the midvein of i-irpds plants three days after application of DEX-containing lanolin paste.
(c) i-irpds plants six days after application of DEX-containing lanolin paste.
(d) IPT transcript and trans-zeatin (ng*gFM−1) levels in i-ovipt plants 12h after application of DEX-containing lanolin paste to the midvein.
(e) IPT transcript and trans-zeatin (ng*gFM−1) levels in the basal and apical parts of the leaf lamina, as well as in the midvein of i-ovipt plants three days after application of DEX-containing lanolin paste.
(f) i-ovipt plants 12d after application of DEX-containing lanolin paste.
(g) trans-Zeatin (tZ), trans-zeatin riboside (tZR), trans-zeatin ribosid O-glycoside (tZROG) and trans-zeatin 7-glycoside (tZ7G) levels in i-ovipt plants after 12 days of treatments with different concentrations of DEX-containing lanolin paste.
Lanolin paste was applied every three days. Asterisks indicate significant differences between DEX treated samples and the corresponding control (0μM) (independent samples t test: * P<0.05, *** P<0.001). irPDS, IPT transcript and trans-zeatin data, 12h after DEX treatment were log10 transformed before t-test analysis. Small letters indicate significant differences between samples (one-way ANOVA, Turkey HSD, P<0.05). Error bars show standard errors (a, b: N=6; d: N=5; e, g: N≥3). FM, Fresh mass.
The pOp6/LhGR system under field conditions
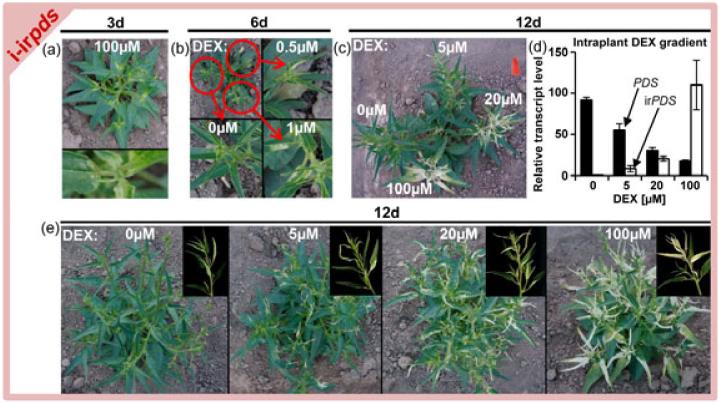
After establishing the pOp6/LhGR system under glasshouse conditions, we tested its utility for fieldwork. When i-irpds plants were treated with 100μM DEX in the field, first signs of photobleaching were visible after three days (Figure 3a), leading to strongly bleached plants after 2 weeks (Figure S11). We tested if gene expression in single branches of pOp6/LhGR lines could be silenced, without affecting the other branches. After decapitation, plants developed several equally-sized side branches, which were treated individually with different DEX concentrations (Figure S12a). DEX applications to side branches of LhGR plants did not influence plant growth (Figure S12b, c), indicating minimal side effects of the DEX treatment under field conditions. Notably, treatments with 0.5μM DEX were sufficient to induce visible photobleaching in the field (Figure 3b). The applications of 0, 5, 20 or 100μM DEX to different side branches of i-irpds plants induced strong concentration-dependent photobleaching (Figure 3c). The irPDS and PDS transcript abundances mirrored the visual photobleaching patterns (Figure 3d). Twelve days of treatment with 100μM DEX induced 2000 fold induction of irPDS transcript abundance and more than 80% pds silencing (Figure 3d). On the control branch, which was directly adjacent to the branch treated with 100μM DEX, no photobleaching was observed (Figure 3c). Also single treatments of complete plants showed reliable, dose-dependent photobleaching phenotypes (Figure 3e). The observed visible bleaching was also correlated with a strong decrease in chlorophyll contents, as expected for pds silencing (Figure S13; Qin et al. 2007). To evaluate the contamination risks for surrounding plants, also adjacent untreated plants and control plants were monitored, but no signs of cross-contaminations were found.
Figure 3.
Inducible gene-silencing in Nicotiana attenuata in its native habitat.
(a) i-irpds plants three days after application of DEX-containing lanolin paste.
(b) i-irpds plants six days after application of DEX-containing lanolin paste on different side branches of the same plant.
(c) i-irpds plant 12 days after application of DEX-containing lanolin paste on different side branches of the same plant.
(d) PDS (solid bars) and inverted repeat PDS (irPDS, open bars) transcript levels in leaves of DEX-containing lanolin paste treated side branches of i-irpds plants.
(e) i-irpds plants 12 days after a single application of DEX-containing lanolin paste.
Lanolin paste was applied every three days. Experiments were performed in the Great Basin Desert, Utah, USA. Error bars show standard errors (N=6).
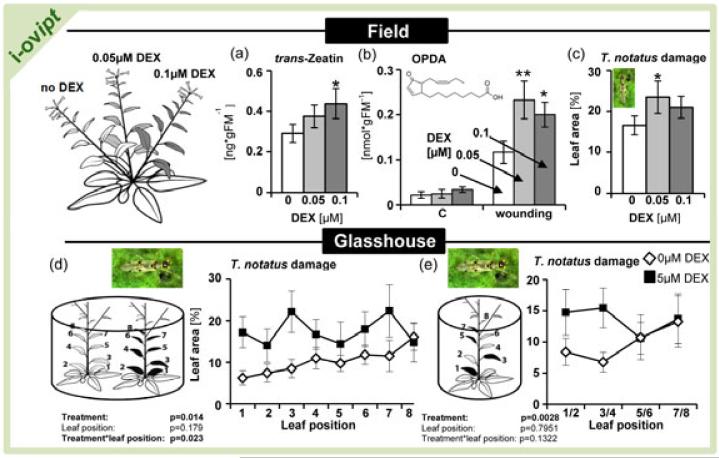
We also analyzed if DEX treatments on different branches of i-ovipt plants lead to branch-specific changes in the levels of CKs or CK-related phenotypes. Different side branches were treated with 0, 0.05 and 0.1μM DEX-containing lanolin paste, which was refreshed every three days. The treatment increased tZ levels only in DEX-treated branches but not after lanolin treatments (Figure 4a). Previous work has shown that external CK-applications to poplar leaves increase wound-induced levels of oxylipins (Dervinis et al. 2010). We found wound-induced levels of the oxylipin 12-oxo-phytodienoic acid (OPDA) to be significantly higher in leaves of branches treated with DEX, compared with the controls (Figure 4b).
Figure 4.
Tissue-specific elevated cytokinin levels increase Tupiocoris notatus damage in Nicotiana attenuata in its native habitat and in the glasshouse.
(a) trans-zeatin levels in leaves of different side branches of i-ovipt plants 15 days after application of different concentrations of DEX-containing lanolin paste.
(b) OPDA levels in untreated control leaves and 60min after wounding. Samples were taken from different side branches of i-ovipt plants 15 days after application different concentrations of DEX-containing lanolin paste.
(c) T. notatus leaf damage on different side branches of i-ovipt plants treated with different concentrations of DEX-containing lanolin paste for 12d.
(d) Between-plants choice assays. T. notatus leaf damage on i-ovipt plants after a single application of either 0 (numbered white leaves) or 5μM (numbered black leaves) DEX-containing lanolin paste. Paired plants were exposed to T. notatus for 14 days.
(e) Within-plant choice assays. T. notatus leaf damage on i-ovipt plants after a single alternating application of either 0 (numbered white leaves) or 5μM (numbered black leaves) DEX-containing lanolin paste. Plants were exposed to T. notatus for 14 days.
Lanolin paste was applied every three days. Experiments were performed under field conditions in the Great Basin Desert, Utah (a-c) or in transparent boxes under glasshouse conditions (d, e). Asterisks indicate significant differences between DEX-treated samples and the corresponding control (0μM) (paired samples t test: * P<0.05, ** P<0.01). T. notatus damage under glasshouse conditions was analyzed by a mixed-effects model. Error bars show standard errors (a: N=8; b: N≥5; c: N≥15; d: N=7; e: N=10). FM, Fresh mass.
Using the pOp6/LhGR system to study plant-herbivore interactions
Since we were able to subtly increase CK levels in specific tissues of plants, we analyzed if this influences the plants’ resistance to native herbivores, as has been suggested (Smigocki et al. 1993, Smigocki et al. 2000, Mujer and Smigocki 2001, Dervinis et al. 2010, Erb et al. 2012, Meldau et al. 2012b, Giron et al. 2013). We quantified herbivore damage on lanolin and DEX-treated branches of i-ovipt plants. We found that one of the most abundant herbivores in the field, the mirid bug Tupiocoris notatus, caused significantly more damage on the DEX treated side branches of i-ovipt plants than on branches treated with lanolin (Figure 4c). The growth of the analyzed i-ovipt plants was not altered by these gentle increases in CK levels (Figure S14), excluding possible developmental perturbations as an explanation for the observed increase in susceptibility to mirids. To examine potential direct effects of DEX on the plant susceptibility, we measured leaf damage levels on LhGR plants treated with high concentrations (up to 20μM) of DEX and found no difference in mirid damage (Figure S15).
We repeated the experiment under glasshouse conditions, using a highly accurate mirid-damage quantification method (Figure S16). We compared the T. notatus-inflicted leaf damage between DEX-treated and control i-ovipt plants in a paired design (Figure 4d). Additionally, a within-plant leaf choice assay was conducted by exposing i-ovipt plants with alternating DEX-treated and control leaves to T. notatus (Figure 4e). In both experiments, the DEX-treated leaves with elevated CK levels were more damaged by T. notatus (Figure 4d,e). Interestingly, the interaction between treatment and leaf position significantly affected the mirid damage in the between-plant choice assays, indicating that the effects of DEX and/or cytokinins on mired damage are leaf position dependent (Figure 4d).
We also tested if the DEX itself influences the performance of the specialist lepidopteran herbivore Manduca sexta and did not find significant effects (Figure S17).
Discussion
Genetic tools for ecological research
In addition to constitutive and stress-, tissue- or developmentally-regulated manipulations, chemically inducible expression technologies are increasingly being used in basic research, as well as agriculture (Corrado and Karali 2009). As pointed out by Corrado and Karali (2009), to study molecular processes in plants, an inducible expression system should show fast, strong and concentration-dependent activity after induction, but have insignificant activity in the absence of the inducer. The inducer should also have no pleotropic effects and be applied in a flexible manner. In contrast, most techniques used in commercial and field based applications have been selected by considerations of cost efficiency and lack of impact on the ecosystem, but lack the necessary precision required for molecular biology research. Here, we developed a system that provides both: the precision needed for surgical manipulation of gene expression and the robustness required for fieldwork.
Adaption of the pOp6/LhGR system for ecological field research
The pOp6/LhGR system is one of the most popular inducible expression systems used in plant molecular biology, but its use has been restricted to the laboratory, since treatments with steroids require controlled environments (Moore et al. 2006, Corrado and Karali 2009). The usual application methods for DEX include incorporation into the growth media and spraying, as well as watering with aqueous DEX solutions (Aoyama and Chua 1997, Samalova et al. 2005). Since DEX is a glucocorticoid known for its anti-inflammatory activities in human (Walton 1959), DEX application strategies should avoid the uncontrolled formation of aerosols, as well as direct skin contact or ingestion. The influence of DEX on other study organisms inhabiting natural environments is also a consideration as DEX can suppress the immune responses of insects like M. sexta, by inhibiting phospholipase A2 and thereby eicosanoid biosynthesis (Miller et al. 1994). Since field experiments are often done under unpredictable conditions with limited technical support, the DEX application method should be simple and work under a variety of environmental conditions, without posing risks for personnel and the environment.
The field site used in this study is located in the Great Basin Desert of Utah, which is a natural habitat for N. attenuata and is characterized by high light irradiation, high temperatures, drought and intense wind (Table S1; Dinh et al. 2013), conditions which make DEX application by spraying or watering untenable. Many studies with N. attenuata have shown that lanolin can be used to apply substances to the plant (Baldwin 1996, Kessler and Baldwin 2001, Steppuhn et al. 2004) in its natural environment. A lanolin-based application method for DEX was also mentioned by Borgi et al. (2010), but no data were reported. We therefore applied DEX-containing lanolin paste to the plants, which prevents aerosol formations, remains locally restricted and is expected to protect DEX from light-mediated degradation (Figure S6b). Lanolin-based applications are also expected to continuously supply DEX to the plant, which ensures stable long-term effects. To reduce the exposure of herbivorous insects to DEX, we only applied a thin layer of DEX-containing lanolin paste to the lower side of the petioles (Figure S7), which represents a shaded position and is less frequently visited by herbivores. This application also allows the DEX to access the leaf vasculature, leading to optimal DEX distribution in the attached leaf. The DEX application also did not affect the performance of T. notatus and M. sexta (Figure S15, Figure S17), however, control experiments should be performed when working with other herbivores. Since the DEX-treated plants are harvested at the end of the field season, DEX contamination of the environment is unlikely. Personal contact with the DEX can be avoided by basic protection measures (protective clothes and gloves).
The efficiency of the pOp6/LhGR system in N. attenuata
We used the silencing of pds (i-irpds) and the heterologous ipt expression (i-ovipt) to evaluate the suitability of the pOp6/LhGR system for field studies. pds is highly expressed in leaf tissues and pds silencing results in bleaching of newly developed above-ground tissues (Figure S8b; Figure S11). Since the consequences of silencing are readily observable in vivo, it represents an ideal marker with which to track the effectiveness of silencing under field conditions. To test the sensitivity of the system, we used ipt-mediated changes in CK levels, because even relatively small increases in ipt expression can lead to changes in plant development (Medford et al. 1989, Bohner and Gatz 2001). As such, ipt expression is a very sensitive marker for “leaky” pOp6 activity. In addition to visual observations and transcript analysis, CK levels of i-ovipt plants were quantified by mass-spectrometry.
In the absence of DEX, no physiological changes were observed in all our selected lines, indicating negligible background activity, while DEX application induced transcript expression within hours (Figure 2a,d). Detailed analysis of different leaf parts revealed that petiole treatments are sufficient to regulate gene expression in the entire leaf (Figure 2b,e). The induced changes could be regulated in a temporal, spatial and quantitative manner and the dynamic range of the system was excellent (Figure 2; Figure S8; Figure S9).
Phenotypes that are obtained in the field should be verified under controlled conditions in the glasshouse and the lab and hence, an inducible expression system should work with similar efficiencies under both conditions. Figure 3 shows this requirements can be fulfilled. One major challenge for experiments with transgenic plants in natural habitats is that only a limited number of plants can be grown. When appropriate, eliciting the growth of equal-sized lateral branches by decapitation, allows for branch-specific transcriptional manipulation (Figure S12a,b) and comparisons between DEX-induced and control side branches from the same plant (Figure 3b,c,d; Figure 4a). In addition to reducing the number of replicate plants required, the use of the same individual plant for treatments and control also helps to compensate for environmental variation between the plants, as well as for insertion side effects of the construct. Clearly, this approach should be used with caution when manipulating systemically transmitted traits.
The pOp6/LhGR system for plant-herbivore interaction studies
CKs are important targets for crop improvement as they influence plant traits such as leaf senescence (Richmond and Lang 1957, Gan and Amasino 1995), drought resistance (Werner et al. 2010, Qin et al. 2011) and resistance against pathogens (Choi et al. 2010, Großkinsky et al. 2011). The influence of CKs on traits important for resistance against insect herbivores in natural environment remains unstudied. Constitutive ipt expression leads to abnormal development, which confounds the analysis of natural plant-herbivore interactions. Here we used the i-ovipt plants to subtly elevate CK levels without affecting plant development and analyzed their role in resistance to natural herbivores in the field. We analyzed leaf damage after elevating CK levels in particular side branches of decapitated plants. As intended, only mild changes in CK levels were measured (Figure 4a). These subtly higher CK levels significantly increased the accumulation of JA precursors after wounding (Figure 4b). Since the JA pathway is known to regulate the levels of defense metabolites involved in N. attenuata’s resistance to herbivores (Halitschke and Baldwin 2003), increased CK levels of leaves were expected to correlate with an enhanced resistant to herbivores. However, the DEX treatment of the i-ovipt plants increased the leaf damage by T. notatus in both the field and the glasshouse (Figure 4c,d,e). Since mirids are only slightly affected by most JA-mediated defense responses, except diterpene glycosides (Dinh et al. 2013), increased OPDA levels might even induce metabolic changes favorable for mirids. OPDA changes were observed after mechanically wounding leaves and it is not clear how far OPDA levels are affected by mirid feeding itself. The within-plant choice assays (Figure 4e) indicate that regulating CK levels are sufficient to shape the patterns of herbivore damage in a plant, which is an important aspect of ecological theories, such as the optimal defense theory (Meldau et al., 2012b). Since CKs regulate source-sink relationships (Kuiper 1993, Ehness and Roitsch 1997, Lara et al. 2004), they likely enhance the nutritional quality of a tissue, which in turn attracts T. notatus feeding, resulting in higher damage levels. Interestingly, Figure 4d indicates that the influence of CK level changes on the mirid damage were leaf position dependent, which might correlate with the inhomogeneous CK distribution between different plant parts (Hewett and Wareing 1973, Ori et al. 1999). Future experiments will reveal the mechanism behind leaf-specific, CK-mediated plant susceptibility to insects and their contributions to herbivore resistance.
Potential future applications of the DEX-inducible pOp6/LhGR system
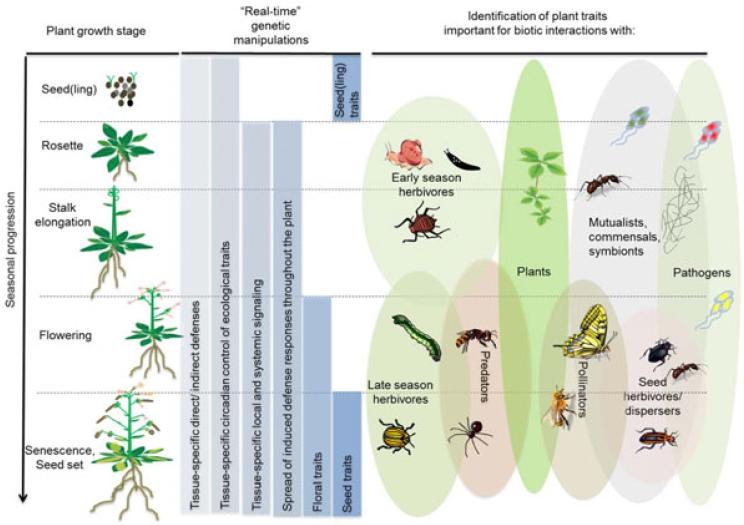
Since plants are the foundation for most food chains on the planet, the timing of their activities profoundly orchestrate most ecological interactions. Not surprisingly, many plant traits are not constitutively expressed throughout a plant’s body, but are restricted to specific tissues or ontogenic stages and at particular times. The expression of these traits likely reflects an evolutionary adaptation to the spatial and temporal activity patterns of interacting organisms (Fig. 5). However, the tools available to ecologists to manipulate these interactions have been too ham-fisted to disentangle these sophisticated environmental interactions. “Real-time” genetic tools, such as the pOp6/LhGR system allow for the manipulation of such conditionally expressed traits in ecologically relevant situations (Fig. 5). Many traits important for interactions with pollinators, such as nectar production and scent emission, are only transiently expressed at particular stages of flower development. Using the DEX system to surgically manipulate these traits can prevent off-target effects in other tissues, which may confound interactions with non-target organisms.
Figure 5.
“Real-time” genetic manipulations for studying plant ecology.
“Real-time” genetic manipulations can be used to study traits involved in biotic interactions of plants in different growth stages during seasonal progression. Black broken lines separate different plant growth stages. Blue boxes illustrate the manipulation of tissue-specific target traits in different growth stages. Differentially colored ovals are examples of season-specific biotic interactions of plants.
The DEX system can also be applied to manipulate the spread of an induced defense, as its elicitation by herbivory. Analyzing the herbivore’s feeding behavior on such plants will shed light on the role of complex spatio-temporal changes that are induced throughout a plant’s body. This is especially valuable for the analysis of defense and signaling components whose constitutive manipulations do not yield viable plants (e.g. Meldau et al. 2011).
The pOp6 lines also offer the possibility for crosses with other activator lines with stress-, tissue- or developmentally-regulated promotors, generating various functional combinations (Moore et al. 2006). This provides the possibility to broaden its application to specific cell types, such as trichomes. Additionally the use of constitutive activators (Moore et al. 1998) might be beneficial for the analysis of tissues, such as roots, which are difficult to treat with DEX under field conditions.
The presented method could also be applied for field experiments with other transformable plant species like petunia (Kessler et al. 2013), peanut (Qin et al. 2011) or rice (Xiao et al. 2009). Next-generation sequencing technologies allow to gain the knowledge on genetic information, which is required for the preparation of species-specific RNAi constructs, from many plant species. This genetic information is not required for DEX-mediated ectopic expressions. We hope that the possibilities offered by the pOp6/LhGR system encourage researchers to develop transformation systems for non-model plant species.
Conclusion and outlook
Flexible control over gene expression is highly desirable for ecological experiments in natural environments. Due to its ease of use and the experimental flexibility it affords in the precise manipulation of gene expression, we predict that the DEX-pOp6/LhGR system described here will allow scientists to revisit hypotheses about the function of traits whose analyses was previously thwarted by the inflexibility of the available technologies.
Experimental procedures
Vector construction
Genomic DNA from transgenic tobacco plants harboring the Agrobacterium tumefaciens tmr gene for IPT under the control of the DEX inducible LhGR/pOp6 promoter system was used as template to PCR amplify the LhGR cassette and the pOp6-ipt cassette with primer pairs described in Table S3. After digestion with SalI the LhGR fragment was cloned in vector pSOL8DC2 cut with SalI and EcoRV, yielding pSOL9LHGRC (GenBank JX185747; Figure S1a). The pOp6-tmr cassette was cloned after digestion with SacI and HindIII in the vector pVKH18 digested with the same enzymes, yielding pPOP6IPT (GenBank JX185749, Figure S1b). The pPOP6IRPDS vector (GeneBank JX185750; Figure S1c) was constructed by replacing the 0.7-kb-SalI-partial-BamHI fragment of pPOP6IPT with a 0.3 kb inverted repeat of a part of the N. attenuata pds gene (GeneBank JX185751), separated by an intron.
The plants were kindly provided by Bretislav Brzobohaty and the pVKH18 vector by Ian Moore.
Plant transformation and growth
Plants from the 30th inbred generation of the inbred ‘UT’ line of N. attenuata (Torr. ex S. Wats.) were transformed with the vectors mentioned above. The transformation with A. tumefaciens (strain LBA 4404), seed germination and growth under glasshouse conditions were done as described by Krügel et al. (2002). Field experiments were conducted under the APHIS permission numbers 11-350-101r, 11-350-101r and 11-350-101r, for the LhGR, i-irpds and i-ovipt plants respectively. For field experiments, plants were grown as described by Schuman et al. (2012). LhGR and i-ovipt plants for herbivore damage analysis were grown in pairs on a field plot located at latitude 37.141 and longitude 114.027 (Figure S11).
DEX application
DEX was dissolved in DMSO and diluted to 100 times the final concentration of the lanolin paste or 2000 times of the GB5 media and hydroponic media, respectively. Aliquots were stored at −20°C. Final DEX concentration for GB5 media was 20μM and for hydroponic solution 1μM. Lanolin was liquefied at 60°C. DEX was added and after thorough mixing, the lanolin paste was taken up by a syringe (1ml, Omnifix), in which the lanolin solidifies after cooling. These syringes can be directly used for plant treatments. As control treatments, the plants were treated with the corresponding amount of DMSO in lanolin without DEX. For phenotypic analyses of seedlings, the seeds were germinated on DEX containing GB5 media. DEX applications to the hydroponic media were performed one week after plants were transferred to the pots. Lanolin treatments were performed earliest three weeks after germination, when plants were already transferred to pots. The time of treatments varied according to the experiment. If not stated otherwise, for glasshouse experiments, plants were treated in early rosette stage growth. For T. notatus experiments and under field conditions, plants were treated during the early flowering stages. Side branches of decapitated plants were treated after exceeding lengths of three to five centimeters. If not stated otherwise, lanolin paste was applied to the lower side of a petiole (Figure S7). Depending on leaf size, between 10 and 30μl lanolin paste were applied per petiole. In contrast, for the short-time experiments (12h and 24h) the entire midveins of leaves were treated.
DEX analysis
For DEX degradation experiments a 50mM DEX stock solution in DMSO was dissolved in pure MeOH to a concentration of 20μM DEX. Aliquots were placed in clear 1.5ml glass vials (Machery Nagel) and were incubated at 37°C in the dark, in a 60°C water bath or under glasshouse conditions with or without a ~1mm lanolin layer covering the vial. Samples were taken at indicated time points. The samples were diluted 1:20 with methanol and stored at −80°C. Before measurement, 200ng of [9,10-2H]dihydro-JA was added per sample as internal standard for relative quantification. Samples were analyzed by LC-MS/MS on a Varian 1200 Triple-Quadrupole-LC-MS system (Varian, Palo Alto, CA, USA). Separation was done on a Kinetex C18 column (50×2.10mm, 2.6μm, 100A, Phenomenex, USA). The mobile phase comprised solvent A (water, 0.05% HCOOH, 0,1% acetonitrile) and solvent B (MeOH) used in a gradient mode: 0-1min, 95%A; 1-8min 5-98%B in A; 8-15.5min 98%B; 15.5-17min, 2-95%A; 17-20min, 95%A with a flow of time/flow (ml/min): 0-0.5min, 0.4 → 0.2ml/min, 0.5-15.5min, 0.2 ml/min, 15.5-16min, 0.2 → 0.4ml/min, 17-20min, 0.4ml/min. For detection, the mass spectrometer was operated in negative mode and a multi-reaction-monitoring (MRM) was performed to monitor analyte parent ion → product ion (Table S4). Varian MS Workstation Version 6.6 software (Varian, Palo Alto, CA, USA) was used for data acquisition and processing.
CK analysis
CK were analyzed by extraction of plant tissue in acidified aqueous methanol followed by two solid-phase extraction (SPE) steps and subsequent measurement with LC-MS/MS. The methodology was adapted according to Dobrev and Kaminek (2002) with the modifications by Kojima et al. (2009).
In brief, 100mg ground frozen plant tissue was extracted twice with 800μl MeOH:H2O:HCOOH (15:4:1) at −20°C. Labelled internal standards were supplemented in the first extraction step. Extraction and SPE were performed in 96 Well BioTubes (1.1ml individual tubes, Arctic White LLC) and Nunc 96-Well Deep Well Plates (Thermo Scientific). The first SPE step was performed on a Multi 96 HR-X column (96 × 25mg) (Macherey-Nagel) conditioned with extraction buffer. The flow through was collected and the MeOH was evaporated under constant nitrogen flow in an Evaporator system (Gals-Col, Terra Haute, USA), at 42°C. After replenishment with 850μl 1M HCOOH, the samples were loaded on a Multi 96 HR-XC column (96 × 25mg) (Macherey Nagel), conditioned with 1M HCOOH. After washing with 1) 1ml 1M HCOOH, 2) 1ml MeOH and 3) 1ml 0.35 M NH4OH, 4) the CK-ribosides, free bases and glucosides were eluted with 1ml 0.35 M NH4OH in 60% MeOH. SPE were performed using a Chromabond Multi 96 vacuum chamber (Macherey-Nagel). After evaporation, samples were reconstituted in 50μl 0.1% acetic acid.
Chromatography was performed on an Agilent 1200 HPLC system (Agilent Technologies, Boeblingen, Germany). For separation a Zorbax Eclipse XDB-C18 column (50×4.6mm, 1.8μm, Agilent Technologies, Germany) was used. The mobile phase comprised of solvent A (water, 0.05% formic acid) and solvent B (acetonitrile) with the elution profile: 0-0.5min, 95%A; 0.5-5min, 5-31.5%B in A; 5.01-6.5min 100% B and 6.51-9min 95% A, with a flow rate of 1.1 ml/min. The column temperature was maintained at 25°C. The liquid chromatography was coupled to an API 5000 tandem mass spectrometer (Applied Biosystems, Darmstadt, Germany) equipped with a Turbospray ion source. For detection the mass spectrometer was operated in positive ionization mode MRM modus to monitor analyte parent ion → product ion (Table S5). Settings were as follows: ionspray voltage, 5500eV; turbo gas temperature, 700°C; nebulizing gas, 70psi; curtain gas, 25psi; heating gas, 60psi and collision gas, 6psi. Both Q1 and Q3 quadrupoles were maintained at unit resolution. Analyst 1.5 software (Applied Biosystems, Darmstadt, Germany) was used for data acquisition and processing. tZ, tZR, tZROG and tZ7G were quantified by using deuterated internal standards (Table S5; Olchemim, Olomouc, Czech Republic).
OPDA analysis
OPDA was extracted and analyzed by LC-MS/MS as described by Kallenbach et al. (2010).
Chemicals
MeOH was purchased by Merck, HCOOH for UPLC by Fisher Scientific, otherwise by Riedel-de Haën, DEX by Enzo Life Science, lanolin, as well as methyl JA and DMSO by Sigma-Aldrich, GB5 by Duchefa, acetic acid by Roth and CK standards by Olchemim. [9,10-2H]dihydro-JA was synthesized by saponification and deuteration of methyl JA.
Transcript analysis
RNA extraction was done with TRIzol (Invitrogene), according to the manufacturer instructions. cDNA was synthesized by reverse transcription using oligo(dT) primer and RevertAid reverse transcriptase (Invitrogen). Quantitative (q)PCR was performed using Actin as standard on a Stratagene Mx3005P qPCR machine using a SYBR Green containing reaction mix (Eurogentec; qPCR Core kit for SYBR Green I No ROX). The primer sequences are summarized in Table S2
Statistics
Data were analyzed with SPSS Statistics 17.0. Either independent samples t-test, paired samples t-test or one-way ANOVA followed by Tukey’s honestly significant difference test were used as indicated. Use of data transformation is indicated. R 3.0.1 was used for statistical analysis of mirid-damage data obtained in the glasshouse experiments (Figure 4d,e). A mixed-effects model was applied with cage and plant as random factors and treatment (DEX application), leaf position and their interaction as fixed factors. The model was simplified by stepwise elimination of fixed factors. To achieve the influence of the different fixed factors, a maximum likelihood ratio test of the different models was done. Mirid damage data were arc sin sqrt transformed.
Tupiocoris notatus damage
T. notatus damage in the field was estimated in percent of the total leaf area. Glasshouse choice assays were carried out on matured N. attenuata plants with removed flowers. Plants were kept in 25 × 25 × 50 cm (l × w × h) sealed glass cages, with 50-100 T. notatus adults and older nymphs at the start of experiment. T. notatus were obtained from an in-house colony. Damage was quantified after 14 days. The inter-plant choice assays were carried out with two plants per cage; one treated with 0 and the other with 5μM DEX (Figure 4d). Damage of the first 8 stem leaves was determined. The intra-plant choice assays were carried out with leaves alternatingly treated with 0 or 5μM DEX. Odd numbered leaves, beginning with the oldest stem leaf were DEX induced, while even numbered leaves served as control (Figure 4e). To determine the rate of damage we took pictures of the leaves and used Adobe Photoshop CS5 for picture analysis. We quantified the area of each leaf and manually marked damaged parts and calculated the percentage of the damaged area. Leaf area was marked as damaged, if the leaf showed clear signs of T. notatus feeding, such as locally bleached chlorotic spots or mirid frass (Figure S16).
Manduca sexta
For M. sexta performance, freshly hatched neonates were placed on early rosette-stage LhGR plants pretreated for one day with 0 or 100μM DEX. Lanolin paste was applied every three days. Caterpillar mass was measured at the indicated time points. M. sexta larvae were obtained from in-house colonies.
Supplementary Material
Acknowledgements
We thank Mario Kallenbach and Meredith Schuman for helpful scientific comments. Mario Kallenbach, Matthias Schöttner, Antje Wissgott, Susanne Kutschbach, Wibke Kröber, and Eva Rothe for technical assistance, Tamara Krügel, Andreas Weber and Andreas Schünzel from the glasshouse team for plant cultivation, Grit Kunert for help with the statistical analysis and the Brigham Young University for the use of their Lytle Preserve field station. Schäfer, Gase, Reichelt and Baldwin are funded by Max-Planck-Society and Meldau and Brütting are funded by Advanced Grant No 293926 of the European Research Council to Baldwin.
Short legends for Supporting Information
Figure S1. Plasmid vector overview.
Figure S2. Screening overview.
Figure S3. Hygromycin resistance silencing.
Figure S4. Seedling phenotype of i-irpds and i-ovipt.
Figure S5. LhGR-efficiency screening.
Figure S6. DEX stability under different temperature and light conditions.
Figure S7. DEX application method.
Figure S8. pOp6/LhGR system in Nicotiana attenuata under glasshouse conditions.
Figure S9. Spatial characteristics of the pOp6/LhGR system after induction with DEX-containing lanolin paste.
Figure S10. pOp6/LhGR system in Nicotiana attenuata - Hydroponic culture.
Figure S11. Inducible gene-silencing in the field.
Figure S12. DEX treatments do not influence plant growth.
Figure S13. Chlorophyll content of leaves with different bleaching grades.
Figure S14. Subtle cytokinin induction in the field does not change plant growth.
Figure S15. DEX treatment does not affect Tupiocoris notatus performance.
Figure S16. Quantification method for Tupiocoris notatus damage.
Figure S17. DEX treatment does not affect Manduca sexta performance.
Table S1. Temperature conditions at the Utah field site at one day within the field season (4pm, 02.06.2012).
Table S2. Sequences of primers used for qPCR.
Table S3. Sequences of primers used for PCR.
Table S4. Multi-reaction-monitoring settings for DEX quantification in negative ionization mode.
Table S5. Multi-reaction-monitoring settings for cytokinin quantification in positive ionization mode.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as an ‘Accepted Article’, doi: 10.1111/tpj.12301
References
- Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Chua N-H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- Baldwin I. Methyl jasmonate-induced nicotine production in Nicotiana attenuata: inducing defenses in the field without wounding. In: Städler E, Rowell-Rahier M, Bauer R, editors. Proceedings of the 9th International Symposium on Insect-Plant Relationships. Springer; Netherlands: 1996. pp. 213–220. [Google Scholar]
- Baldwin IT. Training a new generation of biologists: the genome-enabled field biologists. Proc. Am. Philos. Soc. 2012;156:205–214. [Google Scholar]
- Bohner S, Gatz C. Characterisation of novel target promoters for the dexamethasone-inducible/tetracycline-repressible regulator TGV using luciferase and isopentenyl transferase as sensitive reporter genes. Mol. Gen. Genet. 2001;264:860–870. doi: 10.1007/s004380000376. [DOI] [PubMed] [Google Scholar]
- Borghi L. Inducible gene expression systems for plants. In: Hennig L, Köhler C, editors. Plant developmental biology. Humana Press; 2010. pp. 65–75. [DOI] [PubMed] [Google Scholar]
- Camargo SR, Cancado GM, Ulian EC, Menossi M. Identification of genes responsive to the application of ethanol on sugarcane leaves. Plant Cell Rep. 2007;26:2119–2128. doi: 10.1007/s00299-007-0430-8. [DOI] [PubMed] [Google Scholar]
- Chamovitz D, Sandmann G, Hirschberg J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993;268:17348–17353. [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev. Cell. 2010;19:284–295. doi: 10.1016/j.devcel.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Corrado G, Karali M. Inducible gene expression systems and plant biotechnology. Biotechnol. Adv. 2009;27:733–743. doi: 10.1016/j.biotechadv.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Craft J, Samalova M, Baroux C, Townley H, Martinez A, Jepson I, Tsiantis M, Moore I. New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J. 2005;41:899–918. doi: 10.1111/j.1365-313X.2005.02342.x. [DOI] [PubMed] [Google Scholar]
- Dervinis C, Frost CJ, Lawrence SD, Novak NG, Davis JM. Cytokinin primes plant responses to wounding and reduces insect performance. J. Plant Growth Regul. 2010;29:289–296. [Google Scholar]
- Dinh TS, Galis I, Baldwin IT. UVB radiation and 17-hydroxygeranyllinalool diterpene glycosides provide durable resistance against mirid (Tupiocoris notatus) attack in field-grown Nicotiana attenuata plants. Plant, Cell & Environment. 2013;36:590–606. doi: 10.1111/j.1365-3040.2012.02598.x. [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kamínek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A. 2002;950:21–29. doi: 10.1016/s0021-9673(02)00024-9. [DOI] [PubMed] [Google Scholar]
- Ehness R, Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–548. doi: 10.1046/j.1365-313x.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SS, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Gase K, Weinhold A, Bozorov T, Schuck S, Baldwin IT. Efficient screening of transgenic plant lines for ecological research. Molecular Ecology Resources. 2011;11:890–902. doi: 10.1111/j.1755-0998.2011.03017.x. [DOI] [PubMed] [Google Scholar]
- Giron D, Frago E, Glevarec G, Pieterse CMJ, Dicke M. Cytokinins as key regulators in plant–microbe–insect interactions: connecting plant growth and defence. Funct. Ecol. 2013 [Google Scholar]
- Großkinsky DK, Naseem M, Abdelmohsen UR, Plickert N, Engelke T, Griebel T, Zeier J, Novák O, Strnad M, Pfeifhofer H, van der Graaff E, Simon U, Roitsch T. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011;157:815–830. doi: 10.1104/pp.111.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HS, Fei JF, Xie Q, Chua NH. A chemical-regulated inducible RNAi system in plants. Plant J. 2003;34:383–392. doi: 10.1046/j.1365-313x.2003.01723.x. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003;36:794–807. doi: 10.1046/j.1365-313x.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Heidekamp F, Dirkse WG, Hille J, van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983;11:6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett EW, Wareing PF. Cytokinins in Populus×robusta: qualitative changes during development. Physiol. Plant. 1973;29:386–389. [Google Scholar]
- Izawa T, Mihara M, Suzuki Y, Gupta M, Itoh H, Nagano AJ, Motoyama R, Sawada Y, Yano M, Hirai MY, Makino A, Nagamura Y. Os-GIGANTEA confers robust diurnal rhythms on the global transcriptome of rice in the field. Plant Cell. 2011;23:1741–1755. doi: 10.1105/tpc.111.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiol. 2010;152:96–106. doi: 10.1104/pp.109.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach M, Bonaventure G, Gilardoni PA, Wissgott A, Baldwin IT. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proc. Natl. Acad. Sci. USA. 2012;109:E1548–E1557. doi: 10.1073/pnas.1200363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-G, Fang Y, Singh KB. A glucocorticoid-inducible transcription system causes severe growth defects in Arabidopsis and induces defense-related genes. Plant J. 1999;20:127–133. doi: 10.1046/j.1365-313x.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Kaur H, Shaker K, Heinzel N, Ralph J, Gális I, Baldwin IT. Environmental stresses of field growth allow cinnamyl alcohol dehydrogenase-deficient Nicotiana attenuata plants to compensate for their structural deficiencies. Plant Physiol. 2012;159:1545–1570. doi: 10.1104/pp.112.196717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305:665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. Field experiments with transformed plants reveal the sense of floral scents. Science. 2008;321:1200–1202. doi: 10.1126/science.1160072. [DOI] [PubMed] [Google Scholar]
- Kessler D, Diezel C, Clark DG, Colquhoun TA, Baldwin IT. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecol. Lett. 2013;16:299–306. doi: 10.1111/ele.12038. [DOI] [PubMed] [Google Scholar]
- Klee H, Horsch R, Rogers S. Agrobacterium-mediated plant transformation and its further applications to plant biology. Annual Review of Plant Physiology. 1987;38:467–486. [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, Mizutani M, Ashikari M, Ueguchi-Tanaka M, Matsuoka M, Suzuki K, Sakakibara H. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12:177–183. [Google Scholar]
- Kuiper D. Sink strength: Established and regulated by plant growth regulators. Plant, Cell & Environment. 1993;16:1025–1026. [Google Scholar]
- Lara MEB, Garcia MCG, Fatima T, Ehness R, Lee TK, Proels R, Tanner W, Roitsch T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–1287. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HH, Sonntag DG, Schmidt DD, Baldwin IT. The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol. 2010;185:554–567. doi: 10.1111/j.1469-8137.2009.03079.x. [DOI] [PubMed] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau DG, Long HH, Baldwin IT. A native plant growth promoting bacterium, Bacillus sp. B55, rescues growth performance of an ethylene insensitive plant genotype in nature. Front. Plant Sci. 2012a;3 doi: 10.3389/fpls.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Baldwin IT, Wu J. SGT1 regulates wounding- and herbivory-induced jasmonic acid accumulation and Nicotiana attenuata’s resistance to the specialist lepidopteran herbivore Manduca sexta. New Phytol. 2011;189:1143–1156. doi: 10.1111/j.1469-8137.2010.03558.x. [DOI] [PubMed] [Google Scholar]
- Meldau S, Erb M, Baldwin IT. Defence on demand: mechanisms behind optimal defence patterns. Ann. Bot. 2012b;110:1503–1514. doi: 10.1093/aob/mcs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Nguyen T, Stanley-Samuelson DW. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. USA. 1994;91:12418–12422. doi: 10.1073/pnas.91.26.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore I, Galweiler L, Grosskopf D, Schell J, Palme K. A transcription activation system for regulated gene expression in transgenic plants. Proc. Natl. Acad. Sci. USA. 1998;95:376–381. doi: 10.1073/pnas.95.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore I, Samalova M, Kurup S. Transactivated and chemically inducible gene expression in plants. Plant J. 2006;45:651–683. doi: 10.1111/j.1365-313X.2006.02660.x. [DOI] [PubMed] [Google Scholar]
- Mujer CV, Smigocki AC. Cytokinin- and wound-inducible cytochrome P450 from Nicotiana plumbaginifolia. Physiol. Plant. 2001;111:172–181. [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Picard D. Steroid-binding domains for regulating the functions of heterologous proteins in cis. Trends Cell Biol. 1993;3:278–280. doi: 10.1016/0962-8924(93)90057-8. [DOI] [PubMed] [Google Scholar]
- Potenza C, Aleman L, Sengupta-Gopalan C. Targeting transgene expression in research, agricultural, and environmental applications: Promoters used in plant transformation. In Vitro Cell.Dev.Biol.-Plant. 2004;40:1–22. [Google Scholar]
- Qin G, Gu H, Ma L, Peng Y, Deng XW, Chen Z, Qu LJ. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007;17:471–482. doi: 10.1038/cr.2007.40. [DOI] [PubMed] [Google Scholar]
- Qin H, Gu Q, Zhang J, Sun L, Kuppu S, Zhang Y, Burow M, Payton P, Blumwald E, Zhang H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011 doi: 10.1093/pcp/pcr125. [DOI] [PubMed] [Google Scholar]
- Richmond AE, Lang A. Effect of kinetin on protein content and survival of detached Xanthium leaves. Science. 1957;125:650–651. [Google Scholar]
- Robison MM, Smid MPL, Wolyn DJ. Organic solvents for the glucocorticoid inducer dexamethasone: are they toxic and unnecessary in hydroponic systems? Can. J. Bot. 2006;84:1013–1018. [Google Scholar]
- Roslan HA, Salter MG, Wood CD, White MR, Croft KP, Robson F, Coupland G, Doonan J, Laufs P, Tomsett AB, Caddick MX. Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana. Plant J. 2001;28:225–235. doi: 10.1046/j.1365-313x.2001.01146.x. [DOI] [PubMed] [Google Scholar]
- Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937–946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Paine JA, Riddell KV, Jepson I, Greenland AJ, Caddick MX, Tomsett AB. Characterisation of the ethanol-inducible alc gene expression system for transgenic plants. Plant J. 1998;16:127–132. [Google Scholar]
- Samalova M, Brzobohaty B, Moore I. pOp6/LhGR: a stringently regulated and highly responsive dexamethasone-inducible gene expression system for tobacco. Plant J. 2005;41:919–935. doi: 10.1111/j.1365-313X.2005.02341.x. [DOI] [PubMed] [Google Scholar]
- Schuman MC, Barthel K, Baldwin IT. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife. 2012;1:e00007. doi: 10.7554/eLife.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigocki A, Heu S, Buta G. Analysis of insecticidal activity in transgenic plants carrying the ipt plant growth hormone gene. Acta Physiol. Plant. 2000;22:295–299. [Google Scholar]
- Smigocki A, Neal JW, Jr., McCanna I, Douglass L. Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene. Plant Mol. Biol. 1993;23:325–335. doi: 10.1007/BF00029008. [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS Biol. 2004;2:e217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Kojima M, Suzuki K, Sakakibara H. Agrobacterium tumefaciens tumor morphology root plastid localization and preferential usage of hydroxylated prenyl donor is important for efficient gall formation. Plant Physiol. 2012;159:1064–1072. doi: 10.1104/pp.112.198572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CH. Clinical experience with dexamethasone. Can. Med. Assoc. J. 1959;81:724–726. [PMC free article] [PubMed] [Google Scholar]
- Weinhold A, Baldwin IT. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. USA. 2011;108(13):7855–7859. doi: 10.1073/pnas.1101306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Schmulling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 2001;27:581–590. doi: 10.1046/j.1365-313x.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- Wielopolska A, Townley H, Moore I, Waterhouse P, Helliwell C. A high-throughput inducible RNAi vector for plants. Plant Biotechnol. J. 2005;3:583–590. doi: 10.1111/j.1467-7652.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Williams AC, Barry BW. Penetration enhancers. Adv. Drug Delivery. Rev. 2012;64(Supplement):128–137. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Xiao B-Z, Chen X, Xiang C-B, Tang N, Zhang Q-F, Xiong L-Z. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol. Plant. 2009;2:73–83. doi: 10.1093/mp/ssn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gase K, Baldwin I, Galis I. Enhanced fluorescence imaging in chlorophyll-suppressed tobacco tissues using virus-induced gene silencing of the phytoene desaturase gene. Biotechniques. 2010;48:125–133. doi: 10.2144/000113345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.