Abstract
A non-invasive method to characterize human mesenchymal stromal cells during adipogenic differentiation was developed for the first time. Seven fatty acid methyl esters (FAMEs), including methyl laurate, methyl myristate, methyl palmitate, methyl linoleate, methyl oleate, methyl elaidate and methyl stearate, were used for characterizing adipogenic differentiation using headspace solid-phase microextraction (HS-SPME) which is a very simple and non-invasive method for the extraction of volatile compounds. Glassware was used for culturing mesenchymal stromal cells rather than the common plasticware to minimize contamination by volatile impurities. The optimal SPME fiber was selected by comparing diverse fibers containing two pure liquid polymers (PDMS and PA) and two porous solids (PDMS/DVB and CAR/PDMS). Using optimized procedures, we discovered that seven FAMEs were only detected in adipogenic differentiated mesenchymal stromal cells and not in the mesenchymal stromal cells before differentiation. These data could support the quality control of clinical mesenchymal stromal cell culture in the pharmaceutical industry in addition to the development of many clinical applications using mesenchymal stromal cells.
One of the current primary research themes in biomedicine is stem cell biology, which encompasses both regenerative medicine and cell therapy. Two broad types of stem cells, embryonic stem cells and adult stem cells, and several subcategories of adult stem cells, bone marrow-, adipose- and blood-derived stem cells, have been demonstrated to date. Among them, human mesenchymal stromal cells, which are derived from bone marrow, have been extensively investigated because of their low immunogenicity when used for clinical remedies, physiological self-renewal and immunomodulation or immunosuppression1,2. Compared to embryonic stem cells, there are few ethical problems, which allows for the development of clinical applications3,4,5.
The usefulness of mesenchymal stromal cells in many therapies resides in the regeneration capacity to differentiate into the targeted tissue or organ6,7. To date, a myriad of studies have demonstrated the regenerative potencies of mesenchymal stromal cells, which were distributed into many organs or tissues (liver, heart, neuron and blood vessels, etc.) by various routes (intravenous, intracoronary and intramuscular infusion)8,9,10,11,12. Meanwhile, the most essential constituent among the characteristics of clinical mesenchymal stromal cells is to maintain the stemness and, hence, multipotency before transplanting into the target location13. In other words, grafting the mesenchymal stromal cells should prevent unwanted differentiation. This would be paramount step if clinical mesenchymal stromal cells could maintain their stemness, as it would allow for industrial production of stem cells for pharmaceutical applications.
Quality assurance (QA) would be a significant factor for mass production of mesenchymal stromal cells, as is the case with other pharmaceutical drugs. However, unlike small molecule drugs, protein drugs and other biomedicines do not have an acceptable standard quality control (QC) procedure, given that the preparations of final product using the same procedures result in heterogeneous molecules because of unintended modifications14. Process analytical technology (PAT), an application for addressing difficulties in QC, was first introduced by the U.S. Food and Drug Administration in 200415. PAT is a risk-based QA framework that manages the risk associated with the process of manufacturing and that can produce trust in the quality of final products while removing any unexpected factors. Thus, it should also include procedures to demonstrate the stemness of clinical stem cells during the manufacturing process because this is the most important aspect for the QA of stem cell products.
PAT basically recommends the at-line, on-line and in-line measurement of process analyzers. In other words, the process analyzers should not disrupt the manufacturing streamline when quality is checked15. Common experimental molecular and cell biology techniques have been used to assess the stemness of mesenchymal stromal cells, but most of these methods are too invasive and strenuous to be applied to process analyzers. For example, RNA purification is performed after whole cell lysis to study gene expression, and signal transduction studies involve the use of immunoblot analysis, which also requires detergent-mediated cell lysis.
In this study, we attempted to combine headspace solid-phase microextraction/gas chromatography-mass spectrometry (HS-SPME/GC-MS) with metabolome analyses to identify volatile organic compound (VOC) markers of adipogenic differentiation. HS-SPME is a simple, rapid, non-invasive and solvent-free sample preparation technique that was developed by Pawliszyn and co-workers16,17,18. In regard to process analyzers for PAT, near infrared (NIR) and ultraviolet (UV) spectrometry are the most commonly used, and mass spectrometry (MS) has also been suggested19. From among these process analyzers, we chose HS-SPME as an extraction method because it corresponds well with the purpose of PAT. This method can extract, pre-concentrate and analyze the volatile compounds using a non-invasive in-line measurement within the manufacturing process. HS-SPME has already been utilized for the quality assessment of many products with volatile markers20,21,22. Furthermore, in scientific studies, HS-SPME is considered a promising technique for sampling living organisms because of its unique characteristics, such as portability and simple extraction methods23,24,25.
The main purpose of this study was to identify new volatile biomarkers of adipogenic differentiation of mesenchymal stromal cells using HS-SPME followed by quantitative GC-MS analysis. For the first time, we detected fatty acid methyl esters (FAMEs), as VOC markers, using the HS-SPME/GC-MS method during adipogenic differentiation in human bone marrow-derived mesenchymal stromal cells.
Results and Discussion
Verifying the stemness of mesenchymal stromal cells in this experiment
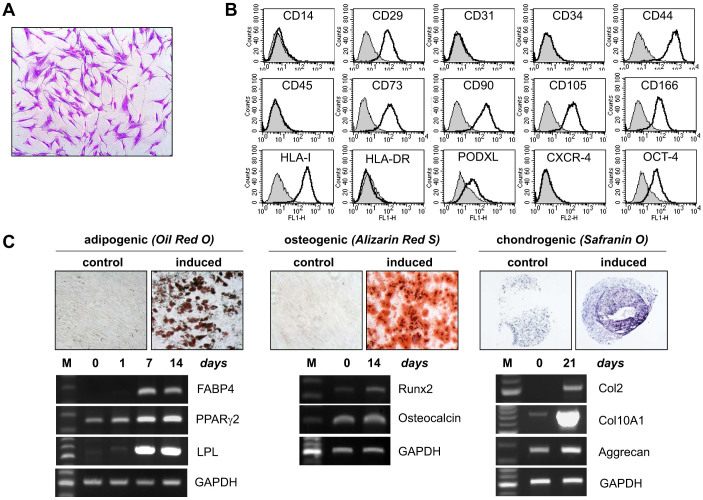
We isolated bone marrow stromal cells from a healthy donor and characterized the mesenchymal stromal cell properties. The cells exhibited a fibroblast-like shape when cultured on plastic culture plates (Figure 1A). Well-known mesenchymal stromal cell surface markers, including CD29, CD44, CD73, CD90, CD105, CD166 and PODXL, were highly expressed; however, hematopoietic and endothelial markers, including CD14, CD31, CD34, CD45, CXCR4 and HLA-DR, were negative (Figure 1B). Oct-4, an embryonic stem cell marker, was also expressed on the cell surface (Figure 1B). To evaluate the stem cell property of mesenchymal stromal cells, the multipotency to differentiate along the adipogenic, osteogenic and chondrogenic lineages was assayed. When the stem cells were cultured in adipogenic, osteogenic and chondrogenic media, they successfully differentiated into adipocytes (visualized by Oil Red O), osteoblasts (visualized by Alizarin Red S) and chondrocytes (visualized by toluidine blue), respectively (Figure 1C). Consistent with the cell type-specific cytochemical staining results, the appropriate cell type-specific marker genes were up-regulated at the transcriptional level during each differentiation (Figure 1C). During adipogenic differentiation, FABP4, PPARγ2 and LPL were highly induced, and when mesenchymal stromal cells were grown in osteogenic medium, runx2 and OCN were up-regulated. In addition, the mRNA expression levels of type II collagen, type X collagen and aggrecan were greatly increased during chondrogenic differentiation. Thus, these results eliminated the possibility that the cells used in this study were pre-adipocytes or adipocyte precursor cells, and furthermore, these results confirmed the identity of the mesenchymal stromal cells that were used in this study.
Figure 1. Characterization of the bone marrow-derived mesenchymal stromal cells.
(A) Cultured mesenchymal stromal cells exhibited a typical fibroblast morphology in two-dimensional cell culture (visualized with crystal violet staining). (B) The marker expression of the mesenchymal stromal cells was analyzed by flow cytometry. The mesenchymal stromal cells expressed well-known mesenchymal stromal cell markers but did not express hematopoietic/endothelial markers. (C) The multipotency of the mesenchymal stromal cells was evaluated by an in vitro differentiation assay. Upon appropriate induction, mesenchymal stromal cells successfully differentiated into adipocytes (stained red with Oil Red O), osteocytes (stained red with Alizarin Red S) and chondrocytes (stained purple with Toluidine Blue). The mRNA expression of the molecular markers for each cell type is also shown.
Choice of the cultureware to optimize the extraction of FAMEs
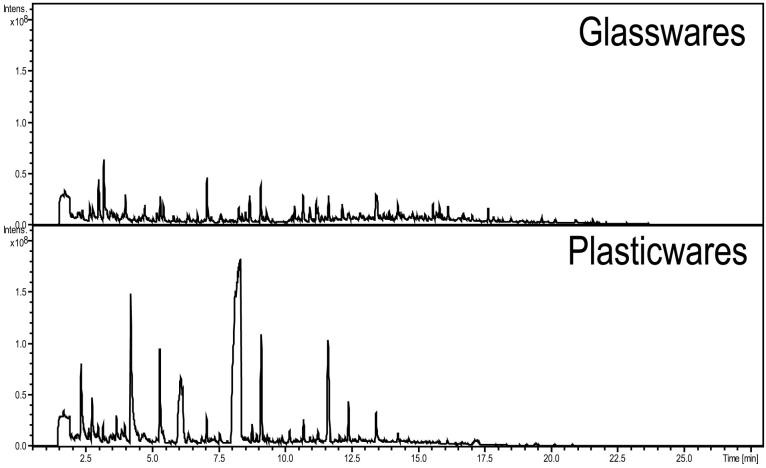
The culture flask that is commonly used for mesenchymal stromal cells is not the proper flask to be used with the HS-SPME procedure because unidentified impurities may leach from the plasticware, which could deteriorate the detection limit or linearity range of the targeted VOC due to interferences of plasticizer-related impurities, followed by a decrease in the relative response to the fiber26,27. Moreover, regarding investigational new drugs (IND), massive production of stem cells should apply for approval, which requires an evaluation of the all processes and materials that were used. During the evaluation, all materials that come into contact with the pharmaceutical compounds or cells should be investigated. Unlike glass, plastics have the possibility of containing plasticizers or polymers that can be released during the manufacturing of plastics, and these impurities should be excluded from the final product because they may be harmful. IND approval requires that detailed documentation of the results be submitted. Regarding PAT, many plasticizer-related impurities could diffuse into the clinical materials, and this possible problem could be caught by QA procedures. Thus, there are advantages of glassware culture systems, although glass could reduce cell proliferation. In this study, we compared the cell culture use of plasticware and glassware using the same extraction method used in the main experiment. As shown in the chromatogram in Figure 2, the plasticware culture flask emitted more impurities compared to glassware, and there was an especially high level of compounds detected at 4.2, 6.1 and 8.3 min. These results conclude that based on the HS-SPME measurement of VOCs, glassware is preferred over plasticware.
Figure 2. Observations of volatile impurities originating from glassware and plasticware (common culture flask) with SPME fiber extraction.
Included are chromatograms of blank samples with glassware (above) and plasticware (below).
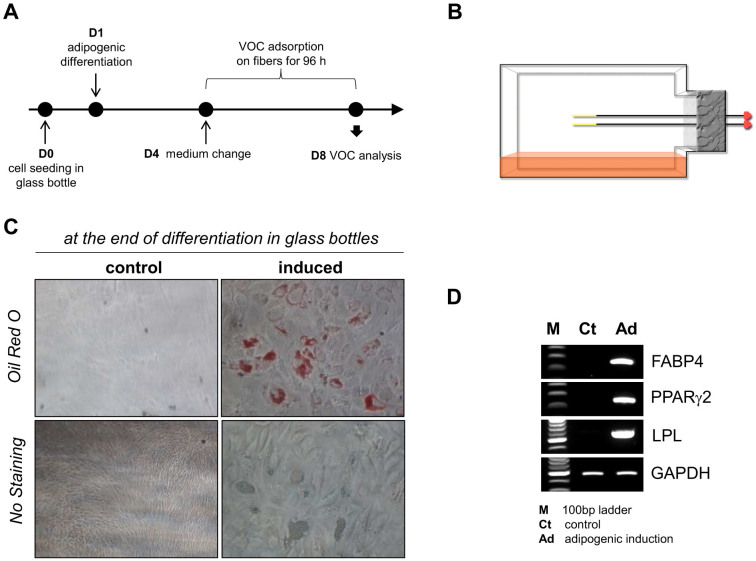
To determine whether the mesenchymal stromal cells could attach to, spread on and proliferate on glass bottles rather than plastic tissue culture plates, the cell adhesion and proliferation abilities were tested by culturing the cells on plastic tissue culture plates and glass bottles. Although the plastic tissue culture plates were better for cell proliferation, the cells exhibited normal fibroblast-like morphology, adhesion and proliferation in the glass bottles (data not shown), indicating that glass bottles can be used for mesenchymal stromal cell culture. Mesenchymal stromal cells were seeded in square glass bottles (100 mL, SCHOTT-DURAN, Germany) at a density of 1 × 106 cells/bottle. The next day, the growth medium was replaced with the adipogenic medium to induce differentiation, and control cells were re-fed with fresh growth medium. After 3 days, the adipogenic and growth media were replaced with fresh media, followed immediately by the placement of SPME fibers inside the bottles, which were then loosely sealed to allow enough air flow for cell growth (Figure 3B). The cells were incubated for an additional 96 h without a medium change in a 5% humidified CO2 incubator (Figure 3A), after which the remaining cells were then subjected to neutral lipid-specific staining and RT-PCR. Lipid droplets in differentiated cells were normally stained with Oil Red O, verifying our differentiation protocol in glass bottles instead of culture plasticware (Figure 3C). Adipogenic differentiation was also confirmed by lineage-specific markers, including FABP4, PPARγ2 and LPL (Figure 3D).
Figure 3.
(A) The process of VOC extraction. (B) A schematic representation of headspace solid-phase microextraction devices placed inside a bottle. (C) After the SPME fibers were removed at the end of the differentiation process, the remaining cells were stained with Oil Red O to verify the effective adipogenic differentiation of the mesenchymal stromal cells. (D) At the end of the differentiation process, RNA was isolated from the remaining cells, and RT-PCR was performed to confirm the adipogenic differentiation of mesenchymal stromal cells. The mRNA expression levels of adipocyte markers, including FABP4, PPARγ2 and LPL, were highly up-regulated during adipogenic differentiation.
Optimizing the SPME conditions for characterizing adipogenic differentiation
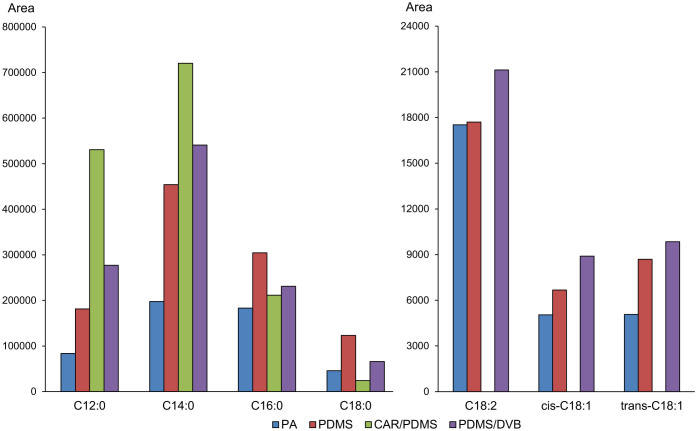
First, VOCs that were detected in mesenchymal stromal cells and adipogenic-differentiated mesenchymal stromal cells using scan mode were assigned to determine potential candidates for differentiation indicators for in-line assessment. The VOCs that were released from adipogenic medium and mesenchymal stromal cell growth medium were assigned (Supplementary Table S1). In the cells, before and after the differentiation, a few VOCs increased quantitatively, indicating the release of VOCs from cells, as shown in Supplementary Tables S2 and S3. There were seven FAMEs that could distinguish well between adipogenic-differentiated and non-differentiated mesenchymal stromal cells. Using these seven FAMEs, optimized conditions for the characterization of adipogenic differentiation were developed. To lower the variation in the quality of the products, we optimized all conditions for stem cell culturing. Non-invasive extraction for in-line measurement during the manufacturing process requires a limited temperature range to maintain product quality, and therefore, the best cell culture temperature of 36°C was adopted. In addition, the performance of the SPME fibers to extract FAMEs was tested, including many fibers that were composed of coating materials with two pure liquid polymers (polydimethylsiloxane (PDMS) and polyacrylate (PA)) and two porous solids (polydimethylsiloxane/divinylbenzene (PDMS/DVB) and carboxen/polydimethylsiloxane (CAR/PDMS)). It is known that PDMS extracts non-polar analytes, that PA and PDMS/DVB absorb polar analytes and that CAR/PDMS absorbs low molar mass analytes28. Four SPME fibers were exposed to the FAMEs standard solution at a known concentration. After the separation of the FAMEs in the scan mode was verified, the analysis was changed to selected ion monitoring mode (SIM mode) to allow for better quantification results (Supplementary Figure S1). The results showed that PA had poor extraction performance for all FAMEs and that CAR/PDMS could not extract the unsaturated FAMEs. Between PDMS and PDMS/DVB, PDMS had better extraction performance for methyl palmitate and methyl stearate. However, the other five FAMEs were adsorbed well onto PDMS/DVB, and thus, it was selected as an optimized fiber to extract the seven FAMEs (Figure 4). Next, the extraction time for FAME saturation on the PDMS/DVB fiber was optimized (Supplementary Figure S2). The non-exhaustive extraction kinetics of SPME can obtain quantitative results when adsorption reaches the saturation of the fiber. Thus, the saturation time of PDMS/DVB should be determined. As shown in Supplementary Figure S2, the saturation was almost complete at 24 h, which was similar to the saturation at 96 h. However, C12:0, C14:0 and C16:0 had a slight increase in the detection limit, and consequently, 96 h was adopted as the extraction time for confirming FAME alterations.
Figure 4. Selection of SPME fibers out of two pure liquid polymers (PDMS and PA) and two porous solids (PDMS/DVB and CAR/PDMS), which could be utilized to detect the seven FAMEs.
Validation of the GC-MS analysis
The linearity, slope, LOD and LOQ values from GC-MS quantification are shown in Table 1, and the accuracy and precision are shown in Table 2. The linearity is presented as the correlation coefficient (R2), ranging from 0.9922 to 0.9989. The accuracy was calculated by analyzing the VOCs of the chromatographic standards in triplicate at 0.1, 0.5 and 1 μg/mL within the calibration range. The intra- and inter-day accuracy values at the three concentration levels were within the range of 61 ~ 138%. The precision was evaluated as the relative standard deviation (RSD), and the intra-day and inter-day precision varied from 1% and 31%, respectively. The LOD values ranged from 0.001 to 0.005 μg/mL, and the LOQ values ranged from 0.005 to 0.01 μg/mL.
Table 1. The linear range, slope (log-log scale), correlation coefficient (R2), LODs and LOQs of the FAMEs.
| Linear range (μg/mL) | Correlation coefficient (R2) | Slope (log-log scale) | LOD (μg/mL) | LOQ (μg/mL) | |
|---|---|---|---|---|---|
| Methyl laurate | 0.005–1 | 0.9989 | 0.997 | 0.005 | 0.005 |
| Methyl myristate | 0.005–1 | 0.9964 | 0.8889 | 0.001 | 0.005 |
| Methyl palmitate | 0.01–1 | 0.9971 | 0.9828 | 0.001 | 0.01 |
| Methyl linoleate | 0.01–1 | 0.9981 | 1.0258 | 0.005 | 0.01 |
| Methyl oleate | 0.01–1 | 0.9922 | 1.0369 | 0.005 | 0.01 |
| Methyl elaidate | 0.01–1 | 0.9922 | 1.0211 | 0.005 | 0.01 |
| Methyl stearate | 0.01–5 | 0.9932 | 0.8732 | 0.001 | 0.01 |
Table 2. Precision and accuracy of the seven FAMEs. Values were measured using three different concentrations (0.1, 0.5 and 1 μg/mL) of each FAME.
| 0.1 μg/mL | 0.5 μg/mL | 1 μg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | Intra-day | Inter-day | |||||||
| Unit: % | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision |
| Methyl laurate | 90–106 | 6 | 67–125 | 25 | 78–91 | 6 | 95–104 | 3 | 99–127 | 11 | 86–138 | 19 |
| Methyl myristate | 95–127 | 10 | 74–116 | 15 | 92–114 | 9 | 102–138 | 10 | 81–105 | 9 | 73–77 | 2 |
| Methyl palmitate | 63–101 | 18 | 84–104 | 13 | 94–127 | 12 | 98–134 | 13 | 84–111 | 12 | 74–130 | 24 |
| Methyl linoleate | 65–104 | 19 | 66–129 | 28 | 97–113 | 7 | 88–99 | 5 | 83–110 | 13 | 65–128 | 31 |
| Methyl oleate | 74–96 | 13 | 85–99 | 7 | 102–105 | 1 | 86–104 | 8 | 81–110 | 16 | 71–129 | 28 |
| Methyl elaidate | 61–83 | 13 | 64–87 | 13 | 93–111 | 8 | 79–108 | 13 | 74–114 | 19 | 76–114 | 19 |
| Methyl stearate | 74–94 | 8 | 63–112 | 22 | 113–129 | 5 | 78–130 | 18 | 92–113 | 7 | 77–125 | 20 |
Alteration of the FAME levels during adipogenic differentiation of mesenchymal stromal cells
The amounts of FAMEs produced during adipogenic differentiation were calculated by subtracting the peak areas of the FAMEs in adipogenic medium alone from those of the FAMEs that were produced from adipogenic-differentiated mesenchymal stromal cells. Seven FAMEs (methyl laurate, C12:0; methyl myristate, C14:0; methyl palmitate, C16:0; methyl linoleate, C18:2; methyl oleate, cis- C18:1; methyl elaidate, trans- C18:1; and methyl stearate, C18:0) were detected in four samples (two were stem cells and the other two were blank media), and they are tabulated as the chromatographic area of the precursor ion for the seven FAMEs (Table 3). As shown in the results, both adipogenic cell and adipogenic media contained FAMEs. The percentage of adipocyte/adipogenic medium varied from 513 to 4,884%, which indicated that there was a substantial release of FAMEs from the adipogenic-differentiated mesenchymal stromal cells. Comparatively, non-differentiated mesenchymal stromal cells displayed ratios from 0 to 76%, showing the reduced quantity of FAMEs. In other words, FAMEs were taken up by the mesenchymal stromal cells, not released. Although two different media contained FAMEs, the fact that differentiated and non-differentiated mesenchymal stromal cells showed reciprocal release-absorption patterns indicates that regardless of the quantities of FAMEs in either medium, FAMEs were only released in differentiated mesenchymal stromal cells. Additionally, although FAMEs were detected in all samples, the levels of FAMEs were below the LOQ for the mesenchymal stromal cell and growth medium control samples. These results imply that FAMEs do not exist in non-differentiated mesenchymal stromal cells at high levels. This result indicates that FAMEs can be considered as volatile biomarker candidates for the differentiation of mesenchymal stromal cells into adipogenic cells.
Table 3. Quantitative results of FAME in each cell type (ADI, adipogenic cell; MSC, mesenchymal stromal cell) and blank medium (DMC, differentiation medium control for ADI; GMC, growth medium control for MSC). The calculated ratio of cell/medium for the two cell types and the calculated concentration of the seven FAMEs are shown.
| Average (n = 4) | Methyl laurate | Methyl myristate | Methyl palmitate | Methyl linoleate | Methyl oleate | Methyl elaidate | Methyl stearate |
|---|---|---|---|---|---|---|---|
| ADIa | 1,150,729 | 5,587,207 | 21,254,867 | 345,702 | 764,046 | 93,147 | 4,821,435 |
| DMCa | 23,558b | 277,589 | 3,458,594 | 53,752 | 148,931 | 3,846b | 711,969 |
| ADI/DMC (%) | 4,885 | 2,013 | 615 | 643 | 513 | 2,422 | 677 |
| Release from cell (μg/mL) | 110.64 | 69.20 | 789.52 | 171.23 | 409.51 | 66.11 | 978.83 |
| MSCa | 2,526b | 2,404b | 4,273b | 0 | 0 | 0 | 0 |
| GMCa | 3,294b | 8,047b | 36,114b | 5,856b | 29,158b | 2,397b | 30,958b |
| MSC/GMC (%) | 77 | 30 | 12 | 0 | 0 | 0 | 0 |
| Release from cell (μg/mL) | −0.07c | −0.03c | −1.26c | −3.79c | −21.64c | −1.91c | −3.63c |
achromatographic area with SIM mode of each precursor ion.
barea under the LOQ.
cnegative release, same as the absorption into the cell.
As mentioned above, mesenchymal stromal cell products require the maintenance of stemness or homogeneity, and the properties must remain constant to meet the necessary conditions to treat disease. However, in the case of the large-scale production of stem cells, spontaneous differentiation into other cell types during ex vivo expansion disrupts the desired homogeneity in the cell population, and therefore, there is an increasing demand for assays to detect the existence of differentiated cells for quality control. Current assays to characterize mesenchymal stromal cells can also be adopted. However, the method proposed herein does not require any chemicals for the extraction of markers, so there will not be the potential problem of biological product alteration. Furthermore, with our method, in-line measurement with non-invasive sampling, as suggested by the PAT guidelines, is possible, which is a major advantage of our method over previous methods. Because mesenchymal stromal cells have been characterized only by traditional and invasive methods to date, this non-invasive quality control method based on HS-SPME/GC-MS can help to produce stable and homogeneous cell therapy products with greater efficiency.
Possibilities for FAME production during the adipogenic differentiation of mesenchymal stromal cells
This is the first study to show that FAMEs were detected only in adipogenic-differentiated mesenchymal stromal cells. Meanwhile, according to some previous studies, FAMEs could also be artifacts due to the methanol used in the experimental procedure29,30 or the enzymatic esterification of free fatty acids31. However, the production of FAMEs requires a much higher quantity of methanol, and in this study, we did not use any methanol during the extraction procedure, which eliminates the possibility of FAME introduction into samples as an artifact32.
The primary substrates, lipids (such as free fatty acids), were undoubtedly present in the fetal bovine serum, and thus, there was no deficiency of substrates in either the mesenchymal cells or adipogenic-differentiated cells33. Accordingly, lipid uptake, enzymatic regulation and changes in the biochemical equilibria that are related to the process of FAME production can explain the production of FAMEs only by adipogenic differentiation. Fatty acids, one class of substrates, are agonists of the peroxisome proliferator-activated receptors (PPARs), especially PPARα and γ, which regulate adipogenesis of mesenchymal stromal cells34,35,36. Thus, FAME production, in which fatty acids are used as substrates, can be a regulation step in the adipogenic differentiation of mesenchymal stromal cells. Further studies on this biological phenomenon may be needed to define the FAME-related metabolism of stem cells.
Methods
Mesenchymal stromal cell isolation and flow cytometry
Bone marrow aspirates were collected from the iliac crest of a healthy male donor after obtaining informed consent (approved by Inha University Medical School Institutional Review Board). Isolation of the mesenchymal stromal cells and subsequent culturing was performed as previously described37. The established mesenchymal stromal cell line was then analyzed for several stem cell markers using flow cytometry. The antibodies used for the analysis were as follows: anti-CD14, anti-CD29, anti-CD31, anti-CD34, anti-CD44, anti-CD73, anti-CD90, anti-CD105, anti-CD106, anti-CD166, anti-CXCR-4, anti-HLA-DR, anti-PODXL and anti-Oct-4 antibodies (BD Biosciences Pharmingen, San Diego, CA, USA). For the Oct-4 analysis, the cells were permeabilized with Triton X-100, and the cells were then analyzed by a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA). Isotype-matched control antibodies were used as controls.
Verifying the multipotency of the mesenchymal stromal cells in vitro
Normal mesenchymal stromal cells have credible multipotency and can differentiate into many cell types. Therefore, we investigated the potency with adipogenic, chondrogenic, and osteogenic differentiation. Mesenchymal stromal cells were plated in a four-well plate at a density of 6 × 104 cells/well. The following day, the sub-confluent cells were incubated in adipogenic medium composed of DMEM with high glucose, 10% calf serum, 10−7 M dexamethasone (DEX; Sigma-Aldrich, St. Louis, MO, USA), 10 μg/mL insulin, 0.5 mM 1-methyl-3-isobutylxanthine (IBMX; Sigma-Aldrich, St. Louis, MO, USA) and 50 μg/mL indomethacin (Sigma-Aldrich, St. Louis, MO, USA). The cells were allowed to differentiate into adipocytes over 4–5 days. The cells were then fixed with 4% formaldehyde and stained with Oil Red O for 30 minutes, followed by counterstaining with hematoxylin for 10 min. A pellet culture system was used for chondrogenic differentiation. A total of 2.0 × 105 mesenchymal stromal cells were placed in a 15 mL conical tube and were pelleted by centrifugation. The pellet was cultured in 500 μL of serum-free chondrogenic medium [α-MEM supplemented with 10 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN, USA), 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN, USA) and 1% insulin-transferrin-selenous acid premix (BD Biosciences, Minneapolis, MN, USA)]. The chondrogenic medium was changed every 3 days for 3 weeks. The cell pellet was then embedded in OCT compound (Sakura Finetek, Torrance, CA, USA), frozen, sectioned into 8-mm slices and then stained with Toluidine Blue. For osteogenic induction, mesenchymal stromal cells that were seeded in a four-well plate at a density of 6 × 104 cells/well were cultured in an osteogenic medium (α-MEM containing 10% FBS, 50 μg/mL ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA), 10−8 M DEX and 10 mM β-glycerophosphate). The osteogenic medium was changed every 3 days for 3 weeks. The cells were then fixed with 4% paraformaldehyde and subjected to Alizarin Red S staining. For reverse transcription-polymerase chain reaction (RT-PCR), total RNA was extracted from either control cells or differentiated mesenchymal stromal cells using the EasyBlue RNA isolation reagent (Intron, Sungnam, Korea). cDNA was synthesized from 1 μg of total RNA using the AccuPower cDNA synthesis kit (Bioneer, Daejeon, Korea), and semi-quantitative RT-PCR was performed using the AccuPower PCR premix (Bioneer, Daejeon, Korea). The amplified PCR products were electrophoresed on 1% agarose gels containing SyberSafe (Invitrogen, Carlsbad, CA, USA) and analyzed using a fluorescence image analyzer (LAS4000 mini; Fuji PhotoFilm, Tokyo, Japan). The PCR primer sequences were as follows: FABP4 forward (f) 5′-CATCAGTGTGAATGGGGATG-3′, reverse (r) 5′-GTGGAAGTGACGCCTTTCAT-3′; PPARγ2 (f) 5′-GACCACTCCCACTCCTTTGA-3′, (r) 5′-CGACATTCAATTGCCATGAG-3′; LPL (f) 5′-TACAGGGCGGCCACAAGTTTT-3′, (r) 5′-ATGGAGAGCAAAGCCCTGCTC-3′; Runx2 (f) 5′-TATGAAAAACCAAGTAGCAAGGTTC-3′, (r) 5′-GTAATCTGACTCTGTCCTTGTGGAT-3′; osteocalcin (f) 5′-GTGCAGAGTCCAGCAAAGGT-3′, (r) 5′-CTAGCCAACTCGTCACAGTC-3′; Col2 (f) 5′-TTTCCCAGGTCAAGATGGTC-3′, (r) 5′-TCACCTGGTTTTCCACCTTC-3′; Col10A1 (f) 5′-GCCCAAGAGGTGCCCCTGGAATAC-3′, (r) 5′-CCTGAGAAAGAGGAGTGGACATAC-3′; aggrecan (f) 5′-GCTACACCCTAAAGCCACTGCT-3′, (r) 5′-CGTAGTGCTCCTCATGGTCATC-3′; and GAPDH (f) 5′-AACGGATTTGGTCGTATTGG-3′, (r) 5′-TGTGGTCATGAGTCCTTCCA-3′.
HS-SPME/GC-MS analysis
The SPME holder and the fibers used for this study were purchased from Supelco (Bellefonte, PA, USA). Four types of fiber coatings that are primarily used for the adsorption of FAMEs were tested: polyacrylate (PA) 85 μm, polydimethylsiloxane (PDMS) 100 μm, carboxen/polydimethylsiloxane (CAR/PDMS) 75 μm and polydimethylsiloxane/divinylbenzene (PDMS/DVB) 65 μm38,39,40. Before sampling, all of the fibers were activated at 250°C for 20 min and then exposed to germicidal UV radiation (tube type, 15–30 W) for 15 min. After the FAMEs had been extracted, each SPME fiber was then inserted into the GC inlet to desorb the extracts. The GC-MS analysis was performed using a 7890A GC system (Agilent Technologies, Wilmington, DE, USA) equipped with a 5975C inert XL mass spectrometer with a triple-axis detector (Agilent Technologies, Wilmington, DE, USA). We used a DB-5 capillary column (length 30 m, I.D. 0.25 mm, film thickness 0.25 μm; Agilent Technologies), and helium was used as the carrier gas at a constant flow rate of 1 mL/min. The gas chromatography system was operated in splitless mode with the split/splitless injector maintained at 250°C. The temperature programs of the column were as follows: initial temperature of 40°C, hold for 3 min, increase at a rate of 5°C/min to 300°C, hold for 5 min for scan mode, and initial temperature of 100°C, increase at a rate of 5°C/min to 215°C, hold for 2 min, increase at the rate of 5°C/min to 230°C for SIM mode. The temperatures of the ion source and the transfer line were 250°C and 240°C, respectively. The MSD system was operated in the electron impact (EI) mode at an ionization energy of 70 eV, and the mass analyzer was a quadrupole operated at a temperature of 150°C. Scan mode detection range was from 25 to 550 m/z, and SIM mode for the quantification of seven FAMEs adopted the molecular weight of each FAME (C12:0, 214.3; C14:0, 242.4; C16:0, 270.4; C18:2, 294.5; cis and trans C18:1, 296.5; C18:0, 298.5) with the same analytical parameters. The volatile products were identified by comparing the chromatographic retention times and the mass spectra with those of the standards. MSD ChemStation (Agilent Technologies, Wilmington, DE, USA) was used for signal acquisition and data processing.
Quantification and method validation
Standards for the FAMEs (i.e., methyl laurate, methyl myristate, methyl palmitate, methyl linoleate, methyl oleate, methyl elaidate and methyl stearate; Sigma-Aldrich, St. Louis, MO, USA) were used for both intra- and inter-day validation of FAMEs that were found to be up-regulated during the adipogenic differentiation of the mesenchymal stromal cells. The standard solutions were dissolved in methylene chloride, after which they were reconstituted in buffered blank media. Headspace, sample and container volume and all the other protocols for the quantitative validation were the same as those described above. The linearity was calculated using regression analysis between the analyte concentration and the peak area. The intra-day variation was measured in triplicate over a single day, and the inter-day variation was assessed over three separate days. The accuracy was evaluated using three separate FAMEs measurements of the standards, and the accuracy was then calculated using the following formula: percentage recovery (%) = CF × 100/CA, where CF is the experimental amount of the FAME and CA is the spiked amount of the FAME. The precision was estimated using the relative standard deviation (RSD) of the triplicate calculation: RSD (%) = CV% = σ × 100/X (X = average, CV = coefficient of variation, σ = standard deviation). The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the international conference on harmonization (ICH) guidelines41.
Author Contributions
Conceived and designed the experiments: D.K.L., K.E.P., S.U.S. and S.W.K. Performed the experiments: D.K.L., T.G.Y., K.E.P., H.J.L., Y.K.C., M.Y.L., S.U.S. and S.W.K. Analyzed the data: D.K.L., T.G.Y., K.E.P., S.J.L., S.U.S. and S.W.K. Contributed reagents/materials/analysis tools: J.L., J.H.P. and M.Y.L. Wrote the manuscript: D.K.L., T.G.Y., K.E.P., J.L. and S.W.K.
Supplementary Material
Supplementary data
Acknowledgments
This work was supported by the Bio-Synergy Research Project of the Ministry of Science, ICT and Future Planning through the National Research Foundation (NRF-2012M3A9C4048796), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2009-0083533) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0023057).
References
- Bianco P. et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19, 35–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi U. & Giordano A. The Gap Between the Physiological and Therapeutic Roles of Mesenchymal Stem Cells. Med Res Rev (2014). [DOI] [PubMed] [Google Scholar]
- Uccelli A., Moretta L. & Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8, 726–736 (2008). [DOI] [PubMed] [Google Scholar]
- Kolf C. M., Cho E. & Tuan R. S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9, 204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R. C. & Melton D. A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002). [DOI] [PubMed] [Google Scholar]
- Yi T. & Song S. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Archives of Pharmacal Research 35, 213–221 (2012). [DOI] [PubMed] [Google Scholar]
- Pittenger M. F. et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 284, 143–147 (1999). [DOI] [PubMed] [Google Scholar]
- Sato Y. et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106, 756–763 (2005). [DOI] [PubMed] [Google Scholar]
- Fu Y.-S. et al. Conversion of Human Umbilical Cord Mesenchymal Stem Cells in Wharton's Jelly to Dopaminergic Neurons In Vitro: Potential Therapeutic Application for Parkinsonism. STEM CELLS 24, 115–124 (2006). [DOI] [PubMed] [Google Scholar]
- Segers V. F. & Lee R. T. Stem-cell therapy for cardiac disease. Nature 451, 937–42 (2008). [DOI] [PubMed] [Google Scholar]
- Sykova E., Jendelova P., Urdzikova L., Lesny P. & Hejcl A. Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cell Mol Neurobiol 26, 1113–29 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillard L. et al. Infusion of allogeneic-related HLA mismatched mesenchymal stem cells for the treatment of incomplete engraftment following autologous haematopoietic stem cell transplantation. Leukemia 21, 568–570 (2007). [DOI] [PubMed] [Google Scholar]
- Otte A., Bucan V., Reimers K. & Hass R. Mesenchymal stem cells maintain long-term in vitro stemness during explant culture. Tissue Eng Part C Methods 19, 937–48 (2013). [DOI] [PubMed] [Google Scholar]
- Hinz D. C. Process analytical technologies in the pharmaceutical industry: the FDA's PAT initiative. Anal Bioanal Chem 384, 1036–42 (2006). [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research, C.f.V.M., Office of Regulatory Affairs. Guidance for Industry; PAT - A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. (ed. Services, H. A. H.) 1–16 (Food and Drug Administration, 2004).
- Pawliszyn J. Sampling and sample preparation for field and laboratory: fundamentals and new directions in sample preparation, (Elsevier, 2002). [Google Scholar]
- Arthur C. L. & Pawliszyn J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Analytical Chemistry 62, 2145–2148 (1990). [Google Scholar]
- Pawliszyn J. Solid phase microextraction: Theory and practice, (Wiley-VCH New York, 1997). [Google Scholar]
- Bakeev K. A. Process Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical Industries, (Wiley, 2010). [Google Scholar]
- Vichi S. et al. HS-SPME coupled to GC/MS for quality control of Juniperus communis L. berries used for gin aromatization. Food Chemistry 105, 1748–1754 (2007). [Google Scholar]
- Ezquerro Ó., Pons B. & Tena M. A. T. Headspace solid-phase microextraction–gas chromatography–mass spectrometry applied to quality control in multilayer-packaging manufacture. Journal of Chromatography A 1008, 123–128 (2003). [DOI] [PubMed] [Google Scholar]
- Hettinga K. A., van Valenberg H. J. F. & van Hooijdonk A. C. M. Quality control of raw cows' milk by headspace analysis. International Dairy Journal 18, 506–513 (2008). [Google Scholar]
- Rudnicka J., Kowalkowski T., Ligor T. & Buszewski B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J Chromatogr B Analyt Technol Biomed Life Sci 879, 3360–6 (2011). [DOI] [PubMed] [Google Scholar]
- Miekisch W., Fuchs P., Kamysek S., Neumann C. & Schubert J. K. Assessment of propofol concentrations in human breath and blood by means of HS-SPME-GC-MS. Clin Chim Acta 395, 32–7 (2008). [DOI] [PubMed] [Google Scholar]
- Musteata F. M. & Pawliszyn J. In vivo sampling with solid phase microextraction. J Biochem Biophys Methods 70, 181–93 (2007). [DOI] [PubMed] [Google Scholar]
- Rocha S., Ramalheira V., Barros A., Delgadillo I. & Coimbra M. A. Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J Agric Food Chem 49, 5142–51 (2001). [DOI] [PubMed] [Google Scholar]
- Kalua C. M., Bedgood Jr D. R. & Prenzler P. D. Development of a headspace solid phase microextraction-gas chromatography method for monitoring volatile compounds in extended time–course experiments of olive oil. Analytica Chimica Acta 556, 407–414 (2006). [Google Scholar]
- Augusto F. & Luiz Pires Valente A. Applications of solid-phase microextraction to chemical analysis of live biological samples. TrAC Trends in Analytical Chemistry 21, 428–438 (2002). [Google Scholar]
- Lough A. K., Felinski L. & Garton G. A. The production of methyl esters of fatty acids as artifacts during the extraction or storage of tissue lipids in the presence of methanol. Journal of Lipid Research 3, 478–480 (1962). [Google Scholar]
- Fischer G. A., Sauk J. J. & Kabara J. J. The occurrence of natural and artificial methyl esters in lipid extracts. Microchemical Journal 11, 461–468 (1966). [Google Scholar]
- Yurkowski M. & Walker B. L. Formation of methyl esters by rat mucosa in methanol. FEBS Letters 2, 265–266 (1969). [DOI] [PubMed] [Google Scholar]
- Lough A. K. & Garton G. A. The lipids of human pancreas with special reference to the presence of fatty acid methyl esters. Lipids 3, 321–323 (1968). [DOI] [PubMed] [Google Scholar]
- Rodrigues A. F., Amaral A. I., Carmo M., Alves P. & Coroadinha A. The Role of Culture Medium Lipids and Lipid Metabolism in Retroviral Vector Production Under Serum Deprivation. in Proceedings of the 21st Annual Meeting of the European Society for Animal Cell Technology (ESACT), Dublin, Ireland, June 7–10, 2009, Vol. 5 (eds. Jenkins, N., Barron, N. & Alves, P.) 705–712 (Springer Netherlands, 2012). [Google Scholar]
- Brun R. P. et al. Differential activation of adipogenesis by multiple PPAR isoforms. Genes & Development 10, 974–984 (1996). [DOI] [PubMed] [Google Scholar]
- Kliewer S. A. et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences 91, 7355–7359 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E. & Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994). [DOI] [PubMed] [Google Scholar]
- Song S. U. et al. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev 17, 451–61 (2008). [DOI] [PubMed] [Google Scholar]
- Llompart M. A., Lourido M., Landín P., García-Jares C. & Cela R. Optimization of a derivatization–solid-phase microextraction method for the analysis of thirty phenolic pollutants in water samples. Journal of Chromatography A 963, 137–148 (2002). [DOI] [PubMed] [Google Scholar]
- Vergnais L., Masson F., Montel M. C., Berdagué J. L. & Talon R. Evaluation of Solid-Phase Microextraction for Analysis of Volatile Metabolites Produced by Staphylococci. Journal of Agricultural and Food Chemistry 46, 228–234 (1998). [DOI] [PubMed] [Google Scholar]
- Lord H. L. & Pawliszyn J. Method Optimization for the Analysis of Amphetamines in Urine by Solid-Phase Microextraction. Analytical Chemistry 69, 3899–3906 (1997). [DOI] [PubMed] [Google Scholar]
- Validation of Analytical Procedures: Methodology Q2B., <www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html>. (1996) Date of access: 12/10/2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data