Abstract
Rain-splash dispersal of Phyllosticta citricarpa (syn. Guignardia citricarpa) conidia (pycnidiospores) from infected oranges was studied in still air and combined with wind. High power microscopy demonstrated the presence of conidia in splash droplets from diseased oranges, which exuded conidia for over one hour during repeated wetting. The largest (5 mm) incident drops produced the highest splashes (up to 41.0 cm). A linear-by-quadratic surface model predicted highest splashes to be 41.91 cm at a horizontal distance of 25.97 cm from the target orange. Large splash droplets contained most conidia (4–5.5 mm splashes averaged 308 conidia), but were splashed <30 cm horizontal distance. Most (80–90%) splashes were <1 mm diameter but carried only 0–4 conidia per droplet. In multiple splash experiments, splashes combined to reach higher maxima (up to 61.7 cm; linear-by-quadratic surface model prediction, 62.1 cm) than in the single splash experiments. In combination with wind, higher wind speeds carried an increasing proportion of splashes downwind travelling horizontally at least 8 m at the highest wind speed tested (7 m/s), due to a small proportion of droplets (<1 mm) being aerosolised. These experiments suggest that P. citricarpa conidia can be dispersed from infected oranges by splashes of water in rainfall events.
Phyllosticta citricarpa (McAlpine) van der Aa1 (previously Guignardia citricarpa Kiely2) is a fungal pathogen of citrus plants such as orange and lemon, causing the disease citrus black spot (CBS). The pathogen is absent from Europe and the suitability of weather conditions for it to complete its life cycle in southern parts of Europe where susceptible citrus trees are grown, including commercial citrus plantations, is debated3,4,5,6,7,8,9. Wind dispersed ascospores of Phyllosticta citricarpa are produced from infected leaf debris and are actively discharged into the air after wetting30. This active dispersal is typical of many ascomycetes and other fungi and promotes dispersal of spores by escaping the layer of relatively still air close to surfaces31. However asexually produced conidia (pycnidiospores) are not actively dispersed in air but are dispersed in water by splashing. This type of spore is produced in pycnidia on fruit, twigs and also leaf litter10. Although the role of conidia in CBS epidemics was discussed in early work in Australia and Zimbabwe2,11 and their importance in disease epidemiology has been recently studied in Brazil in field conditions12,13,14, the mechanism of their rain-splash dispersal from infected citrus fruit has never been investigated. A better understanding of the dispersal mechanism of P. citricarpa conidia by rain-splash from infected fruit would help to clarify the potential role of infected fruit in the introduction and spread of this pathogen. This study investigated the splash dispersal characteristics of conidia of P. citricarpa from the surfaces of artificially infected oranges, using a purpose-built rain-tower and wind-tunnel facility15 at Rothamsted Research, UK.
The objectives were to use established methods to collect experimental data, produced under replicated conditions, on the splash dispersal of P. citricarpa conidia from infected citrus fruit, particularly on the dispersal distance of the droplets containing the fungal conidia splashed upwards from the citrus fruits by incident drops. Splash dispersal (trajectory of splashed droplets and concentrations of conidia per droplet) of various pathogens such as Parastagonospora nodorum, Rhynchosporium commune and Pyrenopeziza brassicae has been investigated previously at Rothamsted using the rain-tower and combined wind-tunnel facility15. However, it was shown that the height of splash droplets varied according to the properties of the surface they were splashed from16. The approach used in the present study was to simulate rain-splash events using distilled water drops of various sizes onto infected oranges in both still air and combined with a wind current allowing the combination of wind and rain to be investigated. Splashed droplets were collected after individual and multiple incident rain drops, of known diameter, fell on infected oranges. The horizontal and vertical location of deposited droplets was assessed and their frequency and trajectories determined. A subset of splashed droplets was assessed for numbers of conidia present and data analysis was carried out to determine effects of rain-splash on P. citricarpa spore dispersal from rain-splash events.
Results
Simulated release of spores from an infected orange during a rain shower
Infected oranges released conidia into the water film on their surface for up to 1 hour. On a time lapse basis (minutes) the numbers of conidia per ml in the surface water film after selected periods of misting were on average (mean of two samples) estimated to be in the region of: 343,500 (1 min), 462,500 (10 mins), 343,750 (20 mins), 337,500 (30 mins), 78,000 (40 mins), 31,250 (50 mins) and 200,000 (60 mins). During an hour the average concentration of conidia in the liquid on the orange surface was estimated to be approximately 258,125 per ml (mean SE 190,000).
Frequency of conidia in splashed droplets on spore suspension and melinex tape
The size of the splashed droplets varied from 0.1 mm to 5.5 mm in diameter. The smaller droplets were more numerous. Observations under a high power microscope showed the mean numbers of conidia by droplet size (n = 10) were estimated to be: 1.7 (range 0–4) in 0–1 mm droplets; 21.5 (range 3–39) in 1–1.99 mm droplets; 58.5 (range 29–99) in 2–2.99 mm droplets; 141.5 (range 70–233) in 3–3.99 mm droplets; 308.6 (range 158–473) in 4–5.5 mm droplets. The maximum number of conidia was 473 in a droplet measuring 5.1 mm. The smallest droplets (0–1 mm), which were the most numerous, only contained 0–4 conidia. Thus, larger drops contain more conidia and P. citricarpa conidia were clearly visible within the droplets (Fig. 1a and b).
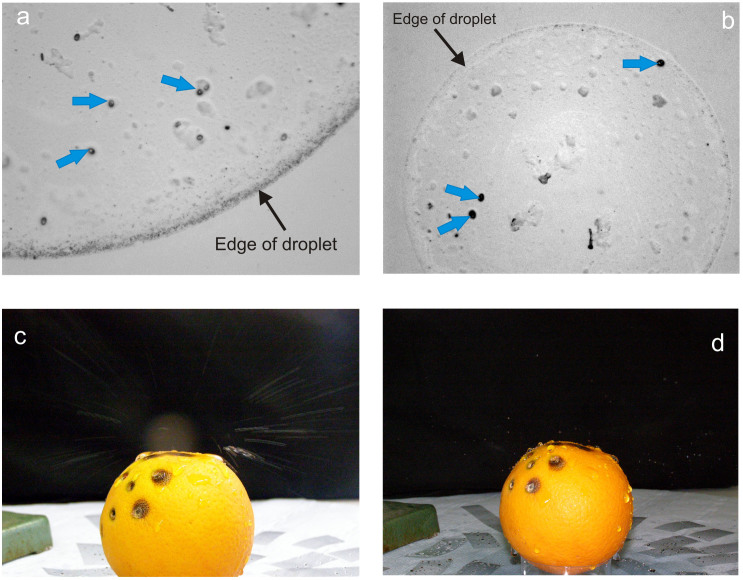
Figure 1.
(a). Conidia (blue bold arrows) in a 4 mm splashed droplet (edge indicated by fine arrow) observed under high power microscopy; (b). Three conidia (blue bold arrows) in a 1 mm droplet (edge indicated by fine arrow); (c). Splash emanating from infected orange (long exposure to show splash trajectories); (d). Splash droplets mid-flight (flash, short-term exposure).
Presence of conidia in splashed droplets from infected oranges
In a sterile flow cabinet, the infected orange had been misted with sterile water and checked to confirm that conidia were present in the film/beads of water on the surface. Observation of some of the slides showed that conidia were present in the splashed droplets which came from the infected orange – present in 5 cm distance slides. The splashes were 5–6 mm in diameter. It showed that conidia were produced from the lesions into the surface water and were able to be splashed away from the orange. However, not all splashes were found to contain conidia as drops hitting uninfected parts of the orange did not pick up conidia. In the rain-tower, the experiment confirmed that conidia were present in splashes dispersed from the infected orange, up to 30 cm away (greater distances were not measured). Microscopic observation at high power showed there to be numerous conidia in the splashed droplets. Photography captured the pattern of splash trajectory and dispersal of drops from the infected orange (Fig. 1c and d).
Rain-tower Experiments
Effect of single incident drops on splash height, distance and trajectory in still air
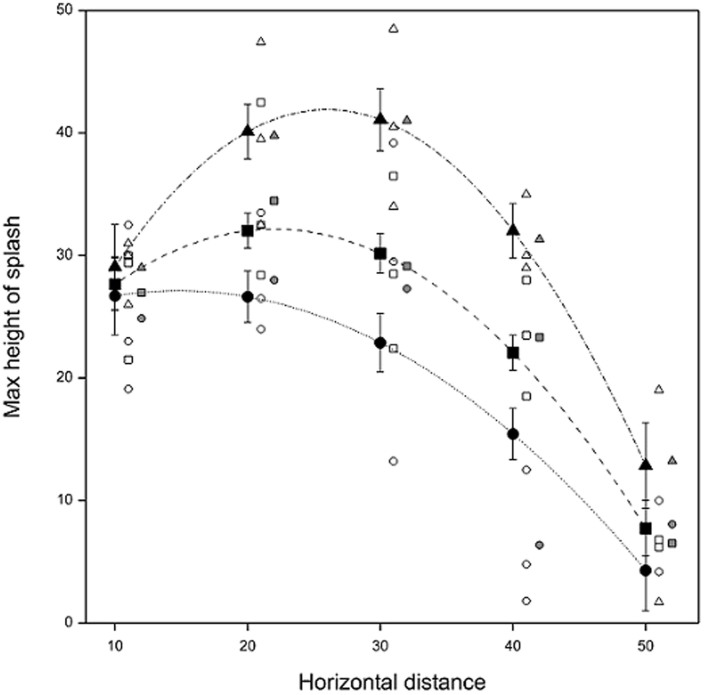
Splash height varied with distance from the orange (Fig. 2). The greatest individual height (48.5 cm) was recorded at 30 cm from the orange for the largest incident drop size (5 mm). Average observed maximum splash height was also greatest (41.0 cm) for this drop size and distance. For the other two incident drop sizes, the greatest average maximum splash height was recorded 20 cm from the orange (34.5 cm and 28.0 cm for 3.5 mm and 2.5 mm drops, respectively). A linear (drop size) by quadratic (horizontal distance) surface model predicted average splash heights of 41.10 cm at 30 cm distance (5 mm drop, SE 2.552), and 32.03 cm (3.5 mm drop, SE 1.426) and 26.63 cm (2.5 mm drop, SE 2.088) at 20 cm distance. The fitted linear-by-quadratic surface model had the form given in equation (1)
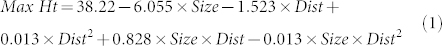 |
(where Size is size of incident drop in cm and Dist is horizontal distance from orange in cm) and accounted for approximately 69% of the variation in the splash heights, with no statistical lack of fit evident (P>0.05). This model predicts maximum heights of 41.91 cm (at 25.97 cm horizontal distance, SE 2.478), 32.15 cm (at 22.02 cm horizontal distance, SE 1.472) and 27.13 cm (at 14.81 cm horizontal distance, SE 2.259) for 5.0, 3.5 and 2.5 mm incident drops falling at their terminal velocity, respectively.
Figure 2. Maximum vertical splash height achieved in still air in Rothamsted rain-tower against horizontal distance from infected orange for single incident drops of three sizes: 2.5 (circle), 3.5 (square) and 5 (triangle) mm.
Symbols: individual observations (open), observed means (solid grey), predictions from linear-by-quadratic surface model at observed distances (solid black). Observations and observed means are shifted right by one and two units of distance, respectively, for clarity. Vertical bars represent ± SE.
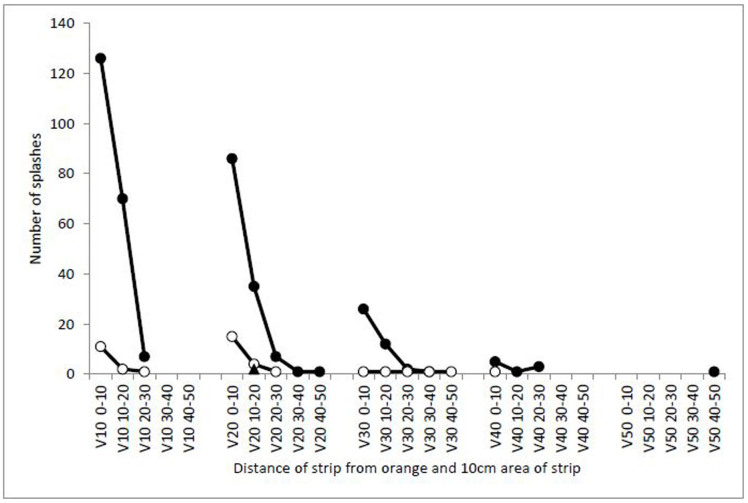
The numbers of splash droplets of different sizes reduced with distance as they radiated out from the orange; most splashes falling on the vertical strips (32%) were less than 1 mm in diameter and fell within 10 cm of the orange. 24% of splashed droplets were less than 1 mm and hit a maximum height at 20 cm from the orange (Fig. 3). The variation and frequency of different sized splashes landing on vertical strips was large; most splashes (90%) were less than 1 mm in diameter, 9.4% of splashes were 1–2 mm and only 0.5% of the splashes were over 2 mm. The largest splash landing on the vertical strips was 2–3 mm in diameter and fell on the strip 20 cm from the orange at a height of 10–20 cm. Splash-droplets collected on melinex tapes were observed to contain conidia, whose numbers decreased with increasing height and distance.
Figure 3. Numbers of splash droplets of different sizes falling on vertical strips (V) of water-sensitive paper at increasing heights (0–10, 10–20, 20–30, 30–40, 40–50 cm high) with increasing horizontal distance (10, 20, 30, 40, 50 cm) from an infected orange and relative percentages of the total numbers of droplets (5 mm incident drop).
Data were also collected using 2.5 mm incident drops, producing a similar pattern (data not shown). Symbols: <1 mm (solid circles), 1–2 mm (open circles), 2–3 mm (solid triangles).
Observations of the horizontal strips showed the smallest splash-droplets, less than 1 mm in diameter, were the most frequent (81%) size of splash (Table 1). Most (76%) of splashes fell within 10 cm of the orange and 90% fell within 20 cm. The smallest splashes as well as being the most numerous occasionally splashed the furthest away – only splashes less than 2 mm travelled further than 50 cm from the orange. The largest splashes (4–5 mm) did not travel so far and all fell within 30 cm of the orange, half of these (52.3%) were within 5 cm of the orange (96.8% within 10 cm of the orange). Although most splash droplets were <1 mm, the ones that travelled furthest were 1–2 mm. Droplets collected on melinex tape showed conidia to be contained within the splashes and numbers of conidia decreased with increasing distance as the splashes also decreased in size.
Table 1. Frequency of splash droplets of different sizes on water sensitive paper strips placed horizontally at different directions (NW = North West; NE = North East; SW = South West; SE = South East) and at increasing distances from the orange (horizontal distances in cm).
| Mean values of NW, NE, SW, SE | ||||||
|---|---|---|---|---|---|---|
| Distance (cm) from orange | <1 mm | 1–2 mm | 2–3 mm | 4–5 mm | Total | % |
| 5 | 313.3 | 31.7 | 11.25 | 8.25 | 364.5 | 35.9 |
| 10 | 323 | 63.25 | 10 | 7 | 403.3 | 39.7 |
| 20 | 128.75 | 18 | 1.25 | 0.25 | 148.3 | 14.6 |
| 30 | 55 | 12 | 1.25 | 0.25 | 68.5 | 6.8 |
| 40 | 3.5 | 12.5 | 0.5 | 16.5 | 1.6 | |
| 50 | 2 | 3.75 | 5.8 | 0.6 | ||
| 60 | 1 | 5 | 6.0 | 0.6 | ||
| 70 | 1 | 1 | 2.0 | 0.2 | ||
| Total | 827.6 | 147.2 | 24.3 | 15.8 | ||
| % | 81.6 | 14.5 | 2.4 | 1.6 | ||
Simulated rain shower event – multiple splashes in still air
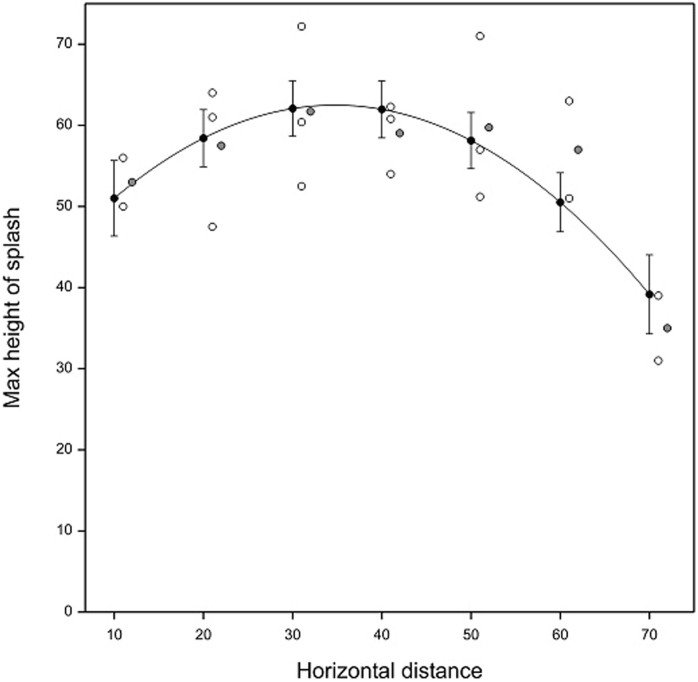
The maximum individual height of splash droplets in the simulated rain shower was 72.2 cm, which was reached at 30 cm from the oranges and the average observed maximum height was 61.7 observed at the same distance (Fig. 4), suggesting that splashes combined and resulted in erratic splashes that went much higher than in the single drop experiments. This is thought to occur due to combination of adjacent splashes, which alters the splash trajectory to force some of the splashed droplets to go higher than from a single splash event. A quadratic model of the form given in equation (2) (where Dist = horizontal distance from orange in cm) predicts a maximum splash height of 62.50 cm (SE 3.486) at a horizontal distance of 34.74 cm away from the target oranges and accounted for approximately 45% of the variation in the splash heights, with no statistical lack of fit evident (P = 0.403).
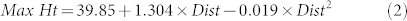 |
Figure 4. Maximum vertical splash height achieved in still air in Rothamsted rain-tower against horizontal distance from an infected orange for multiple drops of size 5 mm.
Symbols: individual observations (open), observed means (solid grey) and predictions from quadratic surface model at observed distances (solid black). Observations and observed means are shifted right by one and two units of distance, respectively, for clarity.
Effect of wind using single incident drops on splash height, distance and trajectory
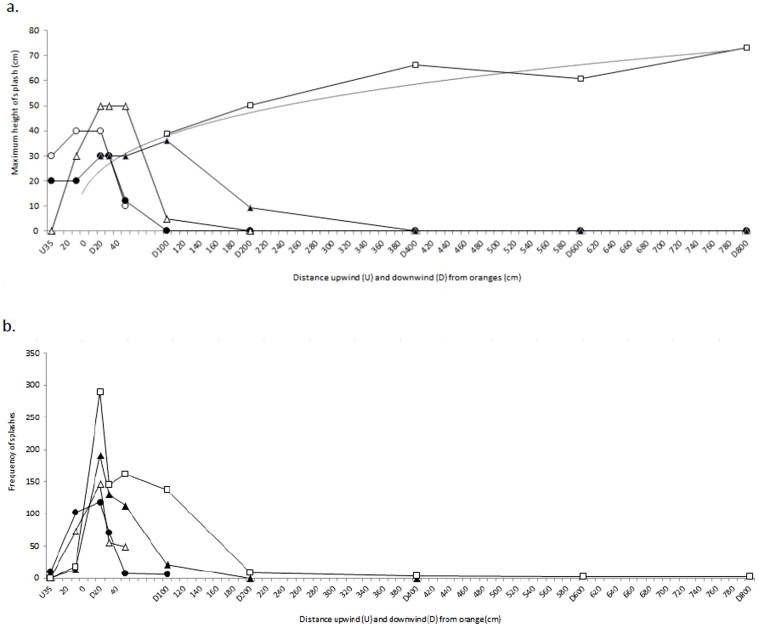
Higher wind speeds dispersed the splashes from the oranges further downwind than in still air or in low wind speeds (Fig. 5a). Some ‘ballistic splashes’ went upwind, especially at the lower wind speeds. The 1 m wind speed produced a similar pattern of splash trajectories to that of still air. The 4 m/s wind speed carried splashes to 2 m downwind and the 7 m/s wind speed carried some splashes further, up to 8 m away. Numbers of splashes dispersing downwind increased with wind speed as increasing numbers were influenced by the wind to be blown downwind rather than being dispersed more radially (Fig. 5b). At the highest wind speed (7 m/s), a component of the smallest splash droplets (<1 mm in diameter) became aerosolized and entrained into the airflow, staying at their original splash height or even dispersing higher, up to 73.2 cm at 8 m, with distance downwind. This occurred in the three separate repeat runs at this wind speed. The highest droplets at 7 m/s wind speed followed a trajectory reasonably described by an exponential equation (R2 = 0.916) of the form given in equation (3) (where Dist = horizontal distance from orange in cm).
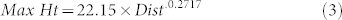 |
At this highest speed (7 m/s) the droplets were dispersing within the air rather than following ballistic trajectories. The proportion of droplets travelling to different distances both up- and down-wind at various heights suggests that ballistic drops are dispersed up to 2 m at wind speed of 7 m/s, but an increase in number of droplets reaching 60 cm height at over 2 m away suggests some droplets at this wind speed were aerosolized and were dispersed at least 8 m away.
Figure 5.
(a). Maximum mean height of splashes in wind speed experiments with distance upwind and downwind from the source and 7 m/s regression line (Max Ht = 22.15 × Dist 0.2717, R2 = 0.916), (b). Frequency of splashes at differing wind speeds with distance upwind and downwind from the source. Symbols: still air (open circles), 1 m/s (solid circles), 2 m/s (open triangles), 4 m/s (solid triangles), 7 m/s (open squares) and 7 m/s regression line (solid line no markers) (mean of two repeats, except for 7 m/s which has three repeats).
Discussion
Simulated rain-splash experiments using a rain-tower and wind tunnel were used to determine the potential for dispersal of P. citricarpa conidia (pycnidiospores) from infected oranges. Conidia exuded continually for over an hour from wetted pycnidia in CBS lesions, which means that the film of water on the infected positions will contain a suspension of conidia throughout a rain event and these conidia will be available to be splash-dispersed. It is known that rain and irrigation splashes remove conidia in water films from plant surfaces by incorporating them into splash droplets, most of which travel only a few centimetres and some over 1 m18. High power microscopy demonstrated presence of conidia of P. citricarpa in splash droplets. The surface texture, angle of orientation, flexibility and plasticity of a surface is known to affect the characteristics of splashes from the surface. To our knowledge, splash dispersal of conidia from oranges has not been studied previously.
In still air, incident drops falling at their terminal velocity caused splash-droplets to be splashed highest by the largest (5 mm) incident drops, reaching 41.0 cm high (mean maximum recorded heights), between 20 and 30 cm horizontal distance. Larger splashed droplets contained the most conidia but get splashed <30 cm, while most (80–90%) splashes were <1 mm diameter but carried on average only one spore. The droplets that were splashed the greatest horizontal distance were 1–1.99 mm in diameter, which reached up to 70 cm horizontal distance in still air and contained an average of 21 conidia. Results of this study therefore fit well with previous studies on other splash-dispersed pathogens, which have demonstrated splash dispersal of conidia of Colletotrichum acutatum to distances up to 80 cm from plastic sheeting, 60 cm from soil and 50 cm from straw16 and modelled theoretical maxima of ballistic splashed droplets 1 mm in diameter as 75 cm height and 120 cm horizontal distance in still air19. The largest rain drop size possible is reported to be 6 mm, though this is extreme20. Large incident rain drops are known to remove more conidia and to splash them further due to their increased kinetic energy compared to small rain drops21,22,23. As in other splash-dispersed fungal pathogens16,24, ground characteristics such as paved floor in open-air fruit waste facilities, bare soil or weed cover in citrus orchards25,26, might influence the splash dispersal potential of P. citricarpa conidia from CBS-affected fruit.
In multiple splash experiments, in which a 15 second simulated shower of rain fell onto infected oranges, mean maximum splash heights in still air reached 61.7 cm, compared to 41.0 cm with single splash experiments. This is likely to be due to a vastly increased number of splash events, i.e. greater technical replication since the multiple splash experiment was estimated to have comprised about 2,000 splash events, compared to 25 individual incident drops per run with single splashes. However, it was also observed in the multiple splash experiments that adjacent splashes combined together and the altered trajectory appeared to be forced higher than with individual incident drops. Model predictions by others similarly predicted splash heights of 60 cm being reached by single incident drops as affected by the product of their velocity and diameter27. Our finding of 61.6 cm maximum height suggests a small additive effect of multiple adjacent splashes simultaneously.
In addition to ballistic splashed droplets, which describe parabolic trajectories and are relatively unaffected by wind, smaller splash droplets, are affected by wind, and particularly for the smallest droplets, can become aerosolized and able to be dispersed much longer distances. In experiments combining wind speed with rain-splash, progressively higher wind speeds caused an increasing proportion of splashes that would have travelled upwind to be turned downwind and generally splashes travelled increasingly further downwind. At wind speeds up to 4 m/s, splash droplets described an arc that was skewed due to the wind but travelled less than 4 m horizontal distance downwind. However, at wind speeds of 7 m/s, splash droplets were found to disperse at least 8 m and a small proportion of droplets (<1 mm) were found to be dispersed higher than originally splashed (up to 75 cm) suggesting that they remain aerosolized and were affected by turbulence rather than behaving as ballistic droplets. These fine droplets, despite carrying an average of only one spore, are very numerous. As the experiments were conducted in relatively cool and humid conditions (15°C; RH 70–80%), effects of evaporation on the small droplets in moving air within the 1.14 s flight time (at 7 m/s) were considered to be negligible. In any case, evaporation of fine droplets to dryness would leave an aerosolized dry spore, which could be deposited onto leaves and be available to infect the new host if infection conditions occurred subsequently.
This study shows that conidia of P. citricarpa are able to be dispersed from pycnidia in CBS-affected orange fruit in splashes of rain and when combined with moderate wind speeds (7 m/s equates to 25.2 Km/h, which is not unusual during wind-driven rain events), the pathogen can be dispersed at least 8 m and to heights of at least 75 cm. Rain events are often combined with strong winds and although modelling of splash dispersal during rain events is complex due to secondary splash, loss of conidia due to wash-out and depletion of the spore source over time, diffusion models23 and random jump models28 have been used. Studies of dispersal from citrus trees to the nearest newly infected tree in Florida suggest that rain-splash dispersed pathogens (i.e. the citrus canker bacterium Xanthomonas campestris pv. citri) can travel up to 3.5 km, most probably in a tropical storm event29, which would be at greater wind-speeds than the present study. Clearly the risk of spore dispersal from CBS-affected fruit depends on the incidence and severity of infection. This study, using artificially inoculated fruit, demonstrates that conidia are able to be dispersed from discarded oranges at ground level to heights and distances that would allow deposition onto (susceptible) leaves of citrus trees growing in close proximity.
Methods
Fungal cultures and inoculum production
Two isolates of P. citricarpa were used in this study; one designated as IVIA-GC072 (GenBank Accession No. KF709953) and the second (IVIA-GC092, Genbank ID: 1701230) supplied by A. Vicent (IVIA, Spain) and which were originally obtained from sweet orange fruits from South Africa. The isolates were sub-cultured at Rothamsted onto PDA agar plates and kept at 20°C under UV light. After several days, dark masses of mycelium were observed, colonising the plates (Fig. 6a). Some of these were used to produce a spore suspension to inoculate the oranges for use in the experiments below. When masses of conidia were observed on mycelium, plates were flooded with sterile distilled water in a flow cabinet and agitated with a sterile L-shaped glass rod and the resulting suspension poured into a tube. A small amount was placed on a glass cavity slide and observed microscopically to ensure presence of conidia (Fig. 6b).
Figure 6.
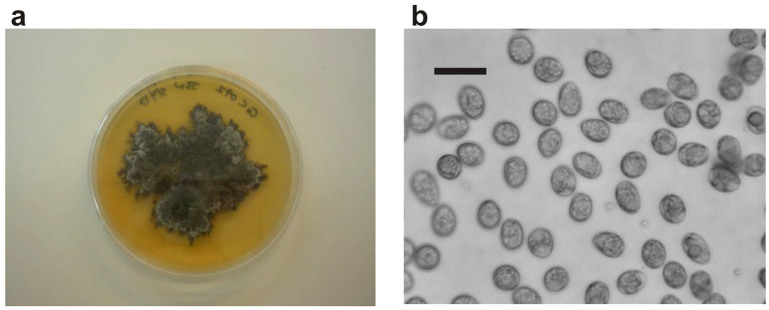
(a). Phyllostica citricarpa colony on a PDA agar plate; (b). P. citricarpa conidia in aqueous suspension (bar represents 10 µm).
Rothamsted holds a permit from the UK plant health authorities to keep fungal cultures for research (FERA UK plant health permit amended 101941/201284/1) and a risk assessment was made. All experiments were done within a single building in contained conditions with all surfaces disinfected with 70% alcohol and waste materials autoclaved. No air was vented directly to outdoors. Additionally, no citrus or other Rutaceae plants are known to be grown outside near Rothamsted Research.
Microscopy and Imaging
High-power microscopy (Fig. 1a, 1b & 6b) in this study used an Olympus S-BH 2 microscope, with images captured using a Hamamatsu C8484-05G01 digital CCD camera and associated software (2007 version).
Fruit inoculation
The experiments required fruit with lesions containing mature pycnidia that were able to produce conidia. Mature fruits of sweet orange (Citrus sinensis Osbeck), cultivar Navel-Late (from Spain and South Africa, depending on the season) and free of any kind of blemishes were purchased commercially. Fruits were washed and surface disinfected using 70% ethanol. Two methods were used to inoculate the fruits with single isolates in a sterile flow cabinet. Oranges were inoculated in batches with one or other of the isolates, they were never used mixed. A) A suspension of conidia (in sterile distilled water) was injected, 100 µl at a time, into a dozen different locations on the top surface of each orange, using a hypodermic needle. It was carefully inserted into the albedo of the orange (the white pith area just below the peel). B) Additionally, some fruit were inoculated with mycelia. Growing edges of a fungal colony were collected from the margin using a fine scalpel. A small incision was made in a dozen parts of the upper surface of the oranges. The mycelia were inserted into the incisions being careful to ensure the material reached the white albedo beneath the peel. The inoculated oranges were incubated in sterile plastic boxes at 20°C under a lighting rig providing a 12 hour photoperiod. After a few weeks, typical ‘hard spot’ symptoms were observed in the infection points, with development of lesions and subsequently pycnidia after some 4–6 weeks. Some oranges (approx. 20%) developed Penicillium rot and were discarded. Eight batches of nine oranges were inoculated over several months to ensure a continuous supply of infected oranges for use in experiments. Oranges were misted with sterile distilled water and a drop of surface film water collected and observed for spore production by microscopy. Statistical analysis (not shown) of experimental data found neither significant trends caused by either the source of the oranges (from Spain or South Africa) nor the two isolates used in the study.
Simulated release of spore production by an infected fruit during rain shower
An infected orange with distinct disease lesions was misted, in a flow cabinet, with sterile distilled water three times in the minutes preceding the experiment. This was to encourage conidia to exude from the disease lesions. The film of water rapidly coagulated into large drops of water on the orange surface, most of which then ran off the orange. However, drops of water remained in the hollows associated with lesions. After set periods of time up to one hour, water was drawn off the orange surface and placed on a hemocytometer for microscopic observation to determine presence and numbers of P. citricarpa conidia. The orange was repeatedly misted throughout, as in simulation of a continuous light rain shower. This was repeated twice with different infected oranges. The aim of this was to determine how long conidia were produced for during a light rain shower.
Frequency of conidia in splashed droplets
This experiment was done to determine numbers of conidia that were carried in splashed droplets of different sizes. A spore suspension was produced by flooding a fungal colony with sterile distilled water. The surface was then scraped, the content poured off and filtered through sterile muslin. The resulting spore concentration was quantified by hemocytometer slide and was adjusted to 70,000 per ml. On the platform at the base of the rain-tower, strips of melinex tape were placecd horizontally around a small glass Petri dish containing a 1 mm layer of the above spore suspension. Fifty drops of sterile distilled water were released in the rain-tower onto the dish containing the spore suspension and drop splashes caught on the surrounding melinex strips. These were then carefully lifted and left in a sterile flow cabinet to dry. The number of conidia in ten drops each of various size categories was recorded using high power microscopy.
Presence of conidia in splashed droplets from infected oranges
This experiment determined whether conidia were carried within the splashed-droplets from an infected orange. In a sterile flow hood, an infected orange was misted with sterile distilled water. After 10 minutes, 0.1 ml of the liquid-film on the surface of the infected orange was drawn-up using a hypodermic syringe and placed on a slide. High power microscopic observation showed numerous conidia of P. citricarpa were present and thus able to be potentially splashed off the orange. A series of slides were placed radially around the orange inside the flow cabinet at 5 cm and 10 cm distances. A 1 ml syringe filled with distilled water was held in place 40 cm above the orange. Individual drops of water were forced down out of the syringe onto the orange to produce splashes. The slides were assessed under high power microscope for presence of conidia and these were counted.
This experiment was repeated in the rain-tower using sections of transparent melinex tape as droplet collection surfaces radiating out from an infected orange. To confirm the tape was an appropriate surface to use, a spore suspension of differing size drops (concentration 50,000 per ml) was dripped onto melinex strips and measured whilst wet. They were left to dry in a flow hood to determine firstly whether the position of the dried drop was still visible and secondly whether conidia could be observed and counted accurately once dried out. The presence of conidia in the liquid-film on the surface of a sterile water misted infected orange was checked by placing a drop onto a microscope slide at the start of the experiment. This confirmed that there were indeed P. citricarpa conidia present. Incident drops (100, 5 mm size) were dropped on to an infected orange in a rain-tower experiment. Sections of tape were observed under high power microscopy to determine presence of conidia in splashes dispersed from the infected orange.
Rain-tower experiments
Rain-splash experiments were conducted in the Rothamsted rain-tower15. This is an 11 m tower, 1.2 m × 1.2 m wide. The top is open with a framework for attaching syringes for creating drops of water and the height of the tower allows the drops to reach terminal velocity. The bottom is also open and is at the leading end of a wind tunnel. The base is enclosed with transparent Perspex doors. At the base there is a flat platform on which a target (bulls-eye ring) was overlain with 10 cm increasing circles, up to a maximum of 70 cm from the central point. Vertical retort stands were placed at various locations on this platform onto which collection tapes were placed to catch splashed droplets. Having monitored presence of conidia by microscopy in splash-droplets collected on transparent melinex tape, a simpler method was employed for the majority of further experiments to quantify splash dispersal by counting splashed water-droplets collected on water sensitive paper. Digital and video photography (Fig. 1c & 1d) used an Olympus SP-51ouz camera to record the pattern of splash from the oranges. The average concentration of conidia in the film around the misted orange was assessed by microscopy using a haemocytometer slide at the start of a rain simulation event. To take account of both random (design structure) and fixed (treatment) effects, the data were analysed as a linear mixed model using restricted maximum likelihood (REML) in GenStat Version 1617.
Effect of single incident drops on splash height, distance and trajectory in still air
An orange, with pycnidia visible in infection lesions, was placed in the target-centre at the base of the rain-tower and was misted with sterile distilled water to encourage a film (including individual drops) of water on the surface in which conidia became suspended. Individual drops of water of pre-determined diameter (5 mm, 3.5 mm and 2.5 mm) were dropped onto the orange (25 drops per experiment) from 1 ml syringe (5 mm drops) or 1 ml syringe plus two different hypodermic needles (for 2.5 and 3.5 mm drops). The resulting splashed droplets emanating from the orange surfaces were collected on water sensitive paper strips (70 mm × 20 mm) and (50 cm × 20 mm) which were placed at differing heights and distances from the orange either sides of the narrow column of falling simulated rain (a) horizontally at increasing distance from the target orange (5, 10, 20, 30, 40, 50, 60, 70 cm) and (b) mounted vertically on retort stands at different heights (10, 20, 30, 40, 50 cm), located at different distances around the target orange (10, 20, 30, 40, 50 cm horizontal distance).
Simulated rain shower event – multiple splashes in still air
This experiment involved releasing a 15 second timed shower on to a collection of infected oranges placed on a wire mesh cage at the base of the rain-tower. The mesh cage platform was surrounded by paper tissue to soak up any drops that had either missed the oranges or would be secondarily splashed from the solid platform to interfere with those splashes directly from the oranges. Water sensitive strips were placed vertically on rods on clamp stands radiating out from 10 cm to 70 cm away from the infected oranges. The rods were also raised to the same base-height as the oranges. The oranges were misted to induce spore production and then a rain shower was simulated for 15 seconds and is estimated to have comprised approximately 2,000 incident drops of diameter 5 mm.
Effect of wind using single incident drops on splash height, distance and trajectory
This experiment investigated the effect of different wind speeds on the splash pattern resulting from 5 mm drops onto infected oranges. It was conducted using the rain-tower and integral wind tunnel which was shut at both ends and included a filtration system on the circulating air. The wind speeds investigated were 1, 2, 4 and 7 m/s. Instead of being placed in a radial layout as in still air experiments, the retort stands containing vertical arrangements of water sensitive paper strips were placed in a slightly offset linear pattern up-wind and down-wind of the infected orange at the base of the rain-tower. The reason to offset collection positions was to avoid shielding subsequent positions. The stands were placed at different distances up to a maximum of 8 m downwind from the orange.
Author Contributions
J.S.W. designed and supervised the project, J.S.W. and S.A.M.P. conducted the experiments, S.J.C. conducted statistical analyses, S.A.M.P. wrote the manuscript, which was edited by J.S.W., S.J.C. and S.A.M.P.
Acknowledgments
This study was funded by the European Food Safety Authority; project EFSA-Q-2013-00011. We thank Antonio Vicent (IVIA, Valencia, Spain) for proof-reading the article and provision of isolates of Phyllosticta citricarpa IVIA-GS072 (GenBank Accession No. KF709953) and IVIA-GC092 (Genbank ID: 1701230). Isolates were used under UK PHA license 101941/201284/1. Rothamsted Research receives grant-aided support from the BBSRC. The present paper is published under the sole responsibility of the authors and may not be considered as a scientific output by the European Food Safety Authority (EFSA). The position and opinions presented in this publication are those of the authors alone and do not necessarily represent the views of EFSA.
References
- Aa van der H. A. Studies in Phyllosticta I. Stud Mycol 5, 1–110 (1973). [Google Scholar]
- Kiely T. B. Preliminary studies on Guignardia citricarpa, n. sp.: The ascigenous stage of Phoma citricarpa McAlp. and its relation to black spot of citrus. Proc Linnean Soc N S Wales 68, 249–292 (1948). [Google Scholar]
- Paul I., van Jaarsveld A. S., Korsten L. & Hattingh V. The potential global geographical distribution of Citrus Black Spot caused by Guignardia citricarpa Kiely: The likelihood of disease establishment in the European Union. Crop Prot 24, 297–308 (2005). [Google Scholar]
- E F. S. A. Scientific opinion of the panel on plant health on a request from the European Commission on Guignardia citricarpa Kiely. EFSA J 925, 1–108 (2008). [Google Scholar]
- E F. S. A. Scientific opinion on the risk of Phyllosticta citricarpa (Guignardia citricarpa) for the EU territory with identification and evaluation of risk reduction options. EFSA J 12 (2), 3557 [243 pp.]. 10.2903/j.efsa.2014.3557 (2014). [Google Scholar]
- Vicent A. & García-Jiménez J. Risk of establishment of nonindigenous diseases of citrus fruit and foliage in Spain: An approach using meteorological databases and tree canopy climate data. Phytoparasitica 36, 7–19 (2008). [Google Scholar]
- Yonow T., Hattingh V. & de Villiers M. CLIMEX modelling of the potential global distribution of the citrus black spot disease caused by Guignardia citricarpa and the risk posed to Europe. Crop Prot 44, 18–28 (2013). [Google Scholar]
- Fourie P. H., Schutte G. C., Serfontein S. & Swart S. H. Modelling the effect of temperature and wetness on Guignardia pseudothecium maturation and ascospore release in citrus orchards. Phytopathology 103, 281–292 (2013). [DOI] [PubMed] [Google Scholar]
- Graham J. H. et al. Response to “Potential distribution of citrus black spot in the United States based on climatic conditions”, Er et al. 2013. Eur J Plant Pathol 139, 231–234 (2014). [Google Scholar]
- Timmer L. W. Diseases of fruit and foliage Citrus Health Management. Timmer, L. W. & Duncan, L. W. (eds.) 107–115 (APS, St. Paul, MN., USA, 1999).
- Whiteside J. O. Sources of inoculum of the black spot fungus, Guignardia citricarpa, in infected Rhodesian citrus orchards. Rhod Zam Mal J Agr Res 5, 171–177 (1967). [Google Scholar]
- Sposito M. B., Amorim L., Ribeiro P. J., Bassanezi R. B. & Krainski E. T. Spatial pattern of trees affected by black spot in citrus groves in Brazil. Plant Dis 91, 36–40 (2007). [DOI] [PubMed] [Google Scholar]
- Sposito M. B., Amorim L., Bassanezi R. B., Filho A. B. & Hau B. Spatial pattern of black spot incidence within citrus trees related to disease severity and pathogen dispersal. Plant Pathol 57, 103–108 (2008). [Google Scholar]
- Spósito M. B. et al. Relative importance of inoculum sources of Guignardia citricarpa on the citrus black spot epidemic in Brazil. Crop Prot 30, 1546–1552 (2011). [Google Scholar]
- Fitt B. D. L. et al. A rain-tower and wind-tunnel for studying the dispersal of plant pathogens by wind and rain. Ann Appl Biol 109, 661–71 (1986). [Google Scholar]
- Yang X., Madden L. V., Wilson L. L. & Ellis M. A. Effects of surface-topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology 80, 1115–1120 (1990). [Google Scholar]
- VSN International. The Guide to the GenStat® Command Language (Release 16), Part 2: Statistics Payne, R. W. (ed.). (VSN International, Hemel Hempstead, UK, 2013). [Google Scholar]
- Fitt B. D. L., McCartney H. A. & Walklate P. J. Role of rain in the dispersal of pathogen inoculum. Annu Rev Phytopathol 27, 241–270 (1989). [Google Scholar]
- MacDonald O. C. & McCartney H. A. Calculation of splash droplet trajectories. Agr Forest Meteorol 39, 95–110 (1987). [Google Scholar]
- Villermaux E. & Bossa B. Single-drop fragmentation determines size distribution of raindrops. Nat Phys 5, 697–702 (2009). [Google Scholar]
- McCartney H. A. & Fitt B. D. L. Construction of dispersal models. Mathematical modelling of crop disease, Advances in Plant Pathology 3 Gilligan, C. A. (ed.) 107–143 (Academic Press, London 1985). [Google Scholar]
- Fitt B. D. L. et al. Dispersal of Rhyncosporium secalis conidia from infected barley leaves or straw by simulated rain. Ann Appl Biol 112, 49–59 (1988). [Google Scholar]
- Yang X. et al. Motion analysis of drop impaction on a strawberry surface. Agr For Meteorol 56, 67–92 (1991). [Google Scholar]
- Ntahimpera N., Hacker J. K., Wilson L. L., Hall F. R. & Madden L. W. Characterisation of splash droplets from different surfaces with a phase doppler particle analyzer. Agr For Meteorol 97, 9–19 (1999). [Google Scholar]
- Caparra P., Foti F., Scerra M., Sinatra M. C. & Scerra V. Solar-dried citrus pulp as an alternative energy source in lamb diets: Effects on growth and carcass and meat quality. Small Ruminant Res 68, 303–311 (2007). [Google Scholar]
- Agustí M. Citricultura 422 pp (Ediciones Mundi-Prensa, Madrid, 2012). [Google Scholar]
- Walklate P. J., McCartney H. A. & Fitt B. D. L. Vertical dispersal of plant pathogens by splashing. Part 11: Experimental study of the relationship between raindrop size and the maximum splash height. Plant Pathol 38, 64–70 (1989). [Google Scholar]
- Pielaat A. & van den Bosch F. A model for dispersal of plant pathogens by rain-splash. IMA J Math Appl Med Biol 15, 117–134 (1998). [Google Scholar]
- Gottwald T. R. et al. Geo-referenced spatiotemporal analysis of the urban citrus canker epidemic in Florida. Phytopathology 92, 361–377 (2002). [DOI] [PubMed] [Google Scholar]
- Truter M. et al. A sampler to determine available Guignardia citricarpa inoculum on citrus leaf litter. Biosyst. Eng. 89, 515–519 (2004). [Google Scholar]
- West J. S. Plant Pathogen Dispersal. eLS (John Wiley & Sons Ltd., Chichester, UK, 2014). [Google Scholar]