Abstract
Lignin modification has been a breeding target for the improvements of forage digestibility and energy yields in forage and bioenergy crops, but decreased lignin levels are often accompanied by reduced lodging resistance. The rice mutant gold hull and internode2 (gh2) has been identified to be lignin deficient. GH2 has been mapped to the short arm of chromosome 2 and encodes cinnamyl-alcohol dehydrogenase (CAD). We developed a long-culm variety, ‘Leaf Star’, with superior lodging resistance and a gh phenotype similar to one of its parents, ‘Chugoku 117’. The gh loci in Leaf Star and Chugoku 117 were localized to the same region of chromosome 2 as the gh2 mutant. Leaf Star had culms with low lignin concentrations due to a natural mutation in OsCAD2 that was not present in Chugoku 117. However, this variety had high culm strength due to its strong, thick culms. Additionally, this variety had a thick layer of cortical fiber tissue with well-developed secondary cell walls. Our results suggest that rice can be improved for forage and bioenergy production by combining superior lodging resistance, which can be obtained by introducing thick and stiff culm traits, with low lignin concentrations, which can be obtained using the gh2 variety.
When developing new rice varieties for feed and biofuel, the main goals are to enhance biomass production and lodging resistance as well as to improve feed digestibility and lignocellulose saccharification for biofuel production. Cell wall composition affects digestibility and saccharification efficiency. As the lignin concentration in the shoot increases, efficiency decreases1,2. Therefore, lignin modification in feed and bioenergy crops has been the main objective when breeding new plants for improved energy yields1.
The brown midrib phenotype has been used to identify lignin synthesis mutants in grasses. Brown midrib (Bmr) mutants have been discovered in maize3, which is a model for studying forage digestibility in livestock. Bmr mutants have been used to breed varieties with high forage digestibility4,5, and commercial Bmr varieties of sorghum have been bred in many countries for foraging.
In rice, gold hull and internode 2 (gh2) was identified as a lignin-deficient mutant6. gh2 exhibits a reddish-brown pigmentation in the hull and the internode, is located on the short arm of chromosome 2 and codes for cinnamyl-alcohol dehydrogenase (CAD). CAD catalyzes the final step in monolignol biosynthesis, reducing hydroxycinnamaldehydes to hydroxycinnamyl-alcohol monomers, such as p-coumaryl, coniferyl and syringyl alcohols. In the gh2 mutant, coniferyl alcohol dehydrogenase activity is reduced and sinapyl alcohol dehydrogenase activity is not detectable. GH2 is a multifunctional enzyme that has both activities. The rice genome contains 12 CAD-like genes7. OsCAD2 was cloned using the gh2 mutant by map-based cloning6. The lignin concentration of the OsCAD2 null mutant, gh2, was reduced in comparison to the wild type (WT). OsCAD7 is responsible for the flexible culm 1 (fc1) phenotype, which shows reduced mechanical strength8. Hirano et al.9 suggested that OsCAD2 is largely responsible for monolignol biosynthesis. Previous studies have not investigated whether OsCAD2 affects mechanical strength.
Reduced lignin concentrations are often associated with reduced lodging resistance8,10,11,12. Therefore, reduced lignin concentrations must be accompanied by strong culms for commercial use. In Japan, rice cultivation for animal feed and biofuel has been promoted on abandoned cultivated land. Recently, new high-biomass, long-culm rice varieties have been developed for whole-crop silage (WCS). To fully utilize WCS varieties, it is important to determine which traits are associated with superior lodging resistance and to introduce those traits into new WCS varieties by crossing them with superior lodging-resistant varieties.
We developed a new high-biomass, long-culm WCS variety and named it ‘Leaf Star’. Among the improved varieties, Leaf Star has the highest bending moment at the basal culm at breaking because of its large section modulus, which is an indicator of culm thickness and higher bending stress, and thus, culm stiffness13. Thus, Leaf Star has superior resistance to lodging because of its combination of culm thickness and culm stiffness. This variety also has the same reddish-brown pigmentation of the hull and the internode as that observed in its parent ‘Chugoku 117’ and in the gh mutants13. In spite of its reduced lignin concentration, Leaf Star experiences high bending stress13. This is a very important characteristic that contributes to the lodging resistance of rice and other gramineous crops that use rice germplasms with low lignin concentrations.
In this study, we sought to investigate the relationships between the physiological and genetic factors of gh and the low lignin concentrations in Leaf Star and its parent Chugoku 117. Specifically, we examined enzyme activity, substrate specificity and the expression of OsCAD2. In addition, we examined the effects of a natural CAD mutation on culm strength using Kinmaze, which has been widely cultivated as a semi-dwarf variety in the southern part of Japan and from which many mutant resources have been developed, and a gh2 null mutant with a Kinmaze background14. Finally, to investigate which traits are responsible for strong culm strength in Leaf Star, we focused on cell wall components, such as cellulose, hemicellulose and lignin and on the morphological characteristics of the cortical fiber tissue in the culm.
Results
Genetic factors responsible for the low levels of lignin in Leaf Star
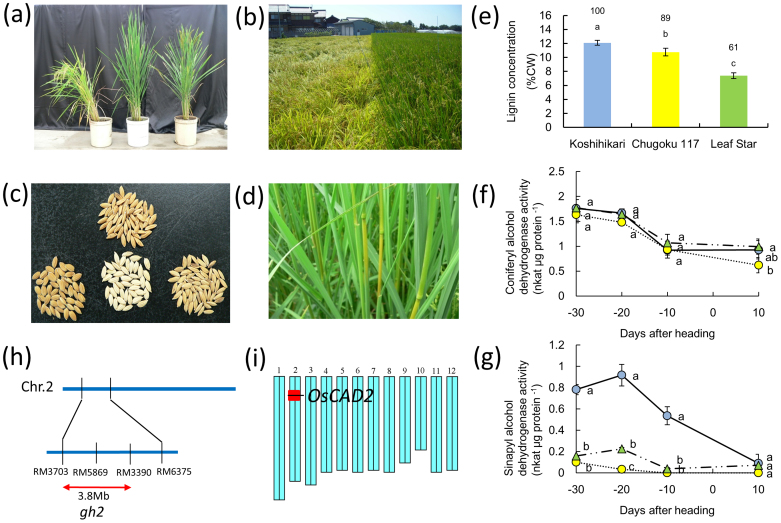
The new long-culm variety Leaf Star showed superior lodging resistance. Leaf Star did not lodge until harvest, while severe lodging occurred with Koshihikari (Fig. 1a, b). Leaf Star had a gold hull and internode phenotype, similar to Chugoku 117 and the Kinmaze-gh2 mutant (Fig. 1c, d). To determine the effects of gh on the lignin concentrations in the leaves and stems of Leaf Star, the lignin concentrations of the fifth internode cell walls of Leaf Star and its parents, Chugoku 117 (gh line) and Koshihikari (non-gh variety), were compared at 20 days after heading (Fig. 1e). The lignin levels in the Chugoku 117 cell walls were 11% lower than those in Koshihikari, and the lignin levels in Leaf Star were 39% lower than those in Koshihikari.
Figure 1. The natural gh2 mutation in Chugoku 117 and Leaf Star.
(a) Plant appearance. Koshihikari (left); Leaf Star (center); Chugoku 117 (right). (b) Lodging during grain ripening in a paddy field immediately after a strong typhoon hit. Koshihikari (left); Leaf Star (right). (c) Gold hull phenotype. Kinmaze-gh2 mutant (left); Koshihikari (lower center); Chugoku 117 (upper center); Leaf Star (right). (d) Gold internode phenotype in Leaf Star. (e) Lignin concentrations of fifth internodes 20 days after heading. Blue bar: Koshihikari; yellow bar: Chugoku 117; green bar: Leaf Star. Each column represents the mean ± s.d. (three replicates). The means followed by different letters are significantly different (5% confidence level) according to Tukey's test. The values in parentheses are the percentages relative to Koshihikari. (f, g) Coniferyl alcohol dehydrogenase activity (f) and sinapyl alcohol dehydrogenase activity (g) in fifth internodes from 30 days before heading to 10 days after heading. Blue circles: Koshihikari; yellow circles: Chugoku 117; green triangles: Leaf Star. The means followed by different letters are significantly different (5% confidence level) according to Tukey's test. (h) Location of gh2 estimated by RILs and introgression lines between Chugoku 117 and Koshihikari. The location of gh2 was predicted to be between markers RM3703 and RM6375 on chromosome 2. The putative gh2 region was narrowed down to the 3.8 Mb region between RM3703 and RM3390 by mapping. (i) Graphical genotype of NIL-gh2 on rice chromosomes. The red bar indicates the chromosome segment of gh2 including OsCAD2. Photographs (a)–(d) were taken by Taiichiro Ookawa.
To identify the factors responsible for the low lignin levels in Leaf Star, the enzyme activity and substrate specificity of CAD in the basal internodes of Leaf Star and its parents were investigated (Fig. 1f, g). CAD activity peaked at 30 days before heading and then declined until 10 days before heading. There were no significant differences in CAD activity among the varieties investigated. Sinapyl alcohol dehydrogenase activity in the non-gh variety Koshihikari peaked at 20 days before heading and then declined until 10 days after heading. In the gh varieties Leaf Star and Chugoku 117, sinapyl alcohol dehydrogenase activity was markedly lower than in Koshihikari and was not detectable at 10 days before heading.
Based on quantitative trait locus (QTL) analyses using backcross inbred lines (BILs) derived from a cross between Chugoku 117 and Koshihikari and recombinant inbred lines (RILs) derived from a cross between Leaf Star and Koshihikari, the gh locus was found to be located in the same region as gh2 on chromosome 2 (Supplementary Table S1 and Supplementary Table S2). In the BILs derived from Chugoku 117 and Koshihikari, one QTL mapped to the short arm of chromosome 2 (nearest the simple sequence repeat (SSR) marker RM3703, LOD 18.6, R2 value 0.40) (Supplementary Table S1). Using backcrossed recombinant homozygous lines (BC4F4), the location of gh was narrowed down to a 3.83-Mb region between RM3703 and RM3390, which included OsCAD2 (Fig. 1h). To identify the QTL, we bred a near-isogenic line (NIL) of gh with the Koshihikari genetic background. We confirmed that this NIL showed the gh phenotype (Fig. 1i). For the RILs derived from Leaf Star and Koshihikari, only one QTL also mapped to the short arm of chromosome 2 (nearest the SNP marker aa02000707, LOD 38.3, R2 value 0.78) (Supplementary Table S2).
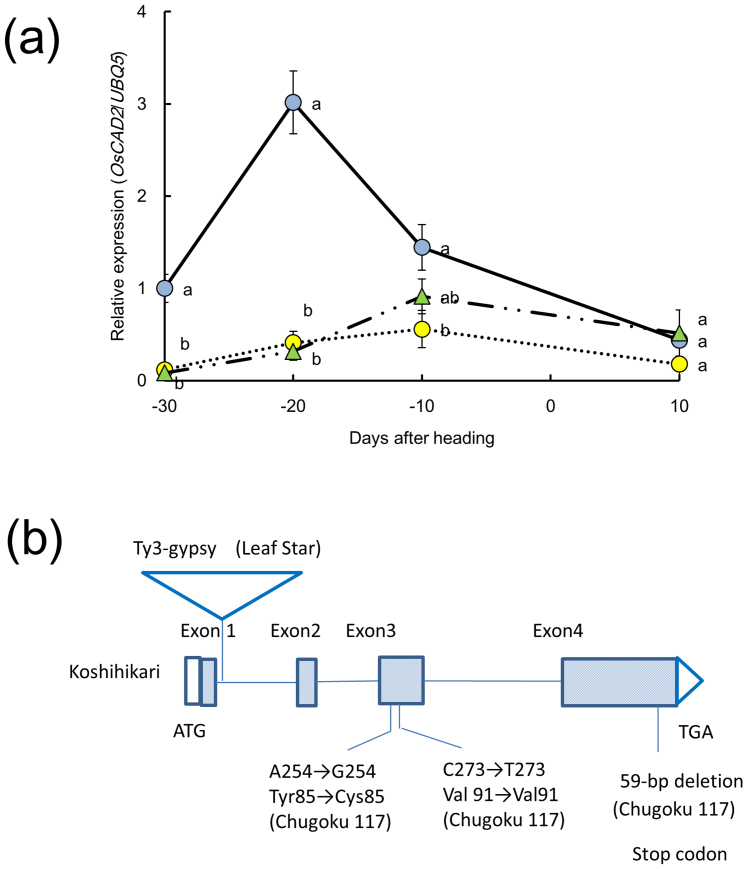
OsCAD2 expression in the fifth internode of Leaf Star and its parents was compared at 30 days before heading up to 10 days after heading (Fig. 2a). In Koshihikari, OsCAD2 expression peaked at 20 days before heading and then decreased rapidly. In contrast, in Leaf Star and Chugoku 117, no OsCAD2 expression was detected at 30 days before heading, and peak expression was delayed until 10 days before heading. In addition, the expression levels in Leaf Star and Chugoku 117 were markedly lower than those in Koshihikari during the fifth internode elongation.
Figure 2. Properties of OsCAD2 in the gh2 varieties Leaf Star and Chugoku 117.
(a) Expression of OsCAD2 relative to UBQ5 in the fifth internode from 30 days before heading to 10 days after heading. Blue circles: Koshihikari; yellow circles: Chugoku 117; green triangles: Leaf Star. The means followed by different letters are significantly different (5% confidence level) according to Tukey's test. (b) OsCAD2 gene structure and mutation sites in Leaf Star. There were no differences in the exon sequences of Leaf Star and Koshihikari, but a 506-bp region of a retrotransposon is inserted in the first intron in Leaf Star.
To determine the cause of the reduced OsCAD2 gene expression, the amino acid sequences of OsCAD2 were compared between Leaf Star and its parents (Supplementary Fig. S1). The amino acid sequence of OsCAD2 in Chugoku 117 differed from those in Koshihikari and Leaf Star. In Chugoku 117, Tyr85 underwent a point mutation to Cys85 at nucleotide 254 (A → G) in exon 3. Amino acids 342–359 in Chugoku 117 also differed from those in Koshihikari and Leaf Star due to a 59-bp deletion in exon 4 (Fig. 2b). This deletion created a premature stop codon in the Chugoku 117 allele.
The OsCAD2 gene structure and mutation sites in Leaf Star and Koshihikari are shown in Fig. 2b. There were no sequence differences in the exons of Leaf Star and Koshihikari, but a 506-bp Ty3-gypsy retrotransposon was inserted into the first intron of the Leaf Star allele. Thus, Leaf Star had a natural OsCAD2 mutation that caused it to be distinct from the Chugoku 117 allele.
Because it has been reported that two gene families contain the primary CAD synthesis genes8, the expression of OsCAD7 in the fifth internode was also compared in Leaf Star and its parents at 20 days before heading. No differences in expression were observed (Supplementary Fig. S2).
Effects of gh2 on culm stiffness
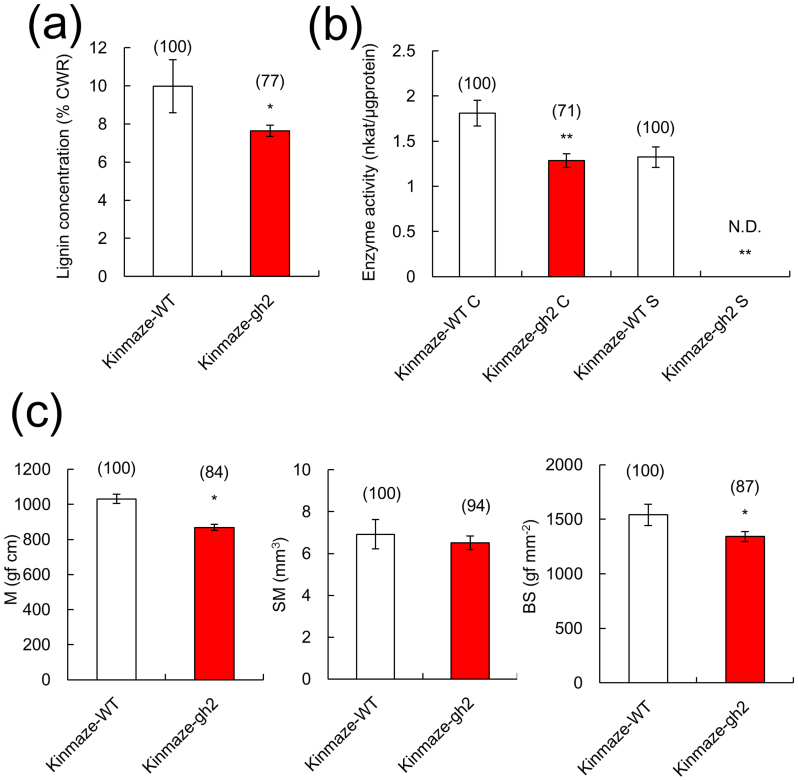
The gh2 mutant with the Kinmaze background was more susceptible to lodging compared to the WT for both years. To determine the effects of the gh2 mutation on culm stiffness, the culm strength, the lignin concentrations of the cell walls and the CAD enzyme activity in the fifth internode in the Kinmaze-gh2 mutant were compared with those of the Kinmaze-WT at 20 days after heading (Fig. 3).
Figure 3. Lignin properties and culm strength in a gh2 mutant.
(a) Lignin concentration in the fifth internode 20 days after heading. (b) Coniferyl alcohol dehydrogenase activity (C) and sinapyl alcohol dehydrogenase activity (S) in the fifth internode 20 days before heading. (c) M, SM and MS of the fifth internode 20 days after heading. Open bars: Kinmaze-WT; red bars: Kinmaze-gh2. Each column represents the mean ± s.d. (three replicates). The means followed by different letters are significantly different (5% confidence level) according to Tukey's test. The values in parentheses are the percentages relative to Kinmaze-WT.
The lignin concentration in Kinmaze-gh2 was 23% lower than that in Kinmaze-WT (Fig. 3a). Coniferyl alcohol dehydrogenase activity in Kinmaze-gh2 was 29% lower than that in Kinmaze-WT. Sinapyl alcohol dehydrogenase activity was high in Kinmaze-WT, but it was not detectable in Kinmaze-gh2, indicating that it was involved in lignin synthesis (Fig. 3b).
To investigate the effects of reduced lignin levels on the traits associated with culm strength, the bending moment at breaking (M), section modulus (SM) and bending stress (BS) were measured in Kinmaze-gh2 and Kinmaze-WT (Fig. 3c). The M value in Kinmaze-gh2 was 16% lower than that in Kinmaze WT. This difference resulted from a lower BS in Kinmaze-gh2.
Based on these results, the OsCAD2 mutation reduced lignin concentrations and affected culm stiffness.
Factors responsible for the stiff culm of Leaf Star
In our previous study13, we have found that the M value of Leaf Star is higher than that of its parents because it has a larger SM and a higher BS. In this study, the same differences in M, SM and BS were observed among these varieties (Supplementary Fig. S3). Thus, Leaf Star, unlike Kinmaze-gh2, has a stiff culm despite its low lignin concentration.
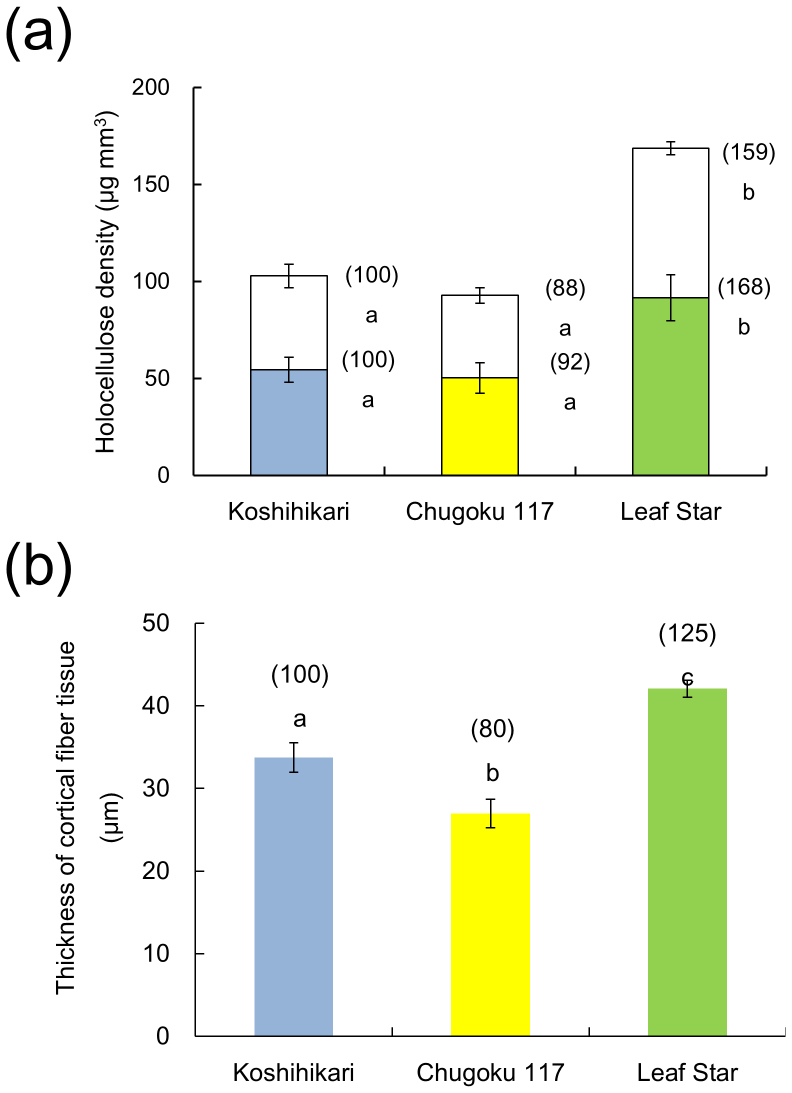
To investigate whether the cell wall densities in Leaf Star are responsible for its stiff culm, the densities of cellulose and hemicellulose in the fifth internode were measured in Leaf Star and its parents at 20 days after heading (Fig. 4a). The densities of both cellulose and hemicellulose in Leaf Star were higher than those in Chugoku 117.
Figure 4. Accumulation of cell wall components and thickness of cortical fiber tissue in culms.
(a) The densities of cellulose (shaded) and hemicellulose (unshaded). (b) Thickness of cortical fiber tissue. Blue bars: Koshihikari; yellow bars: Chugoku 117; green bars: Leaf Star. Each column represents the mean ± s.d. (three replicates). The means followed by different letters are significantly different (5% confidence level) according to Tukey's test. The values in parentheses are the percentages relative to Koshihikari.
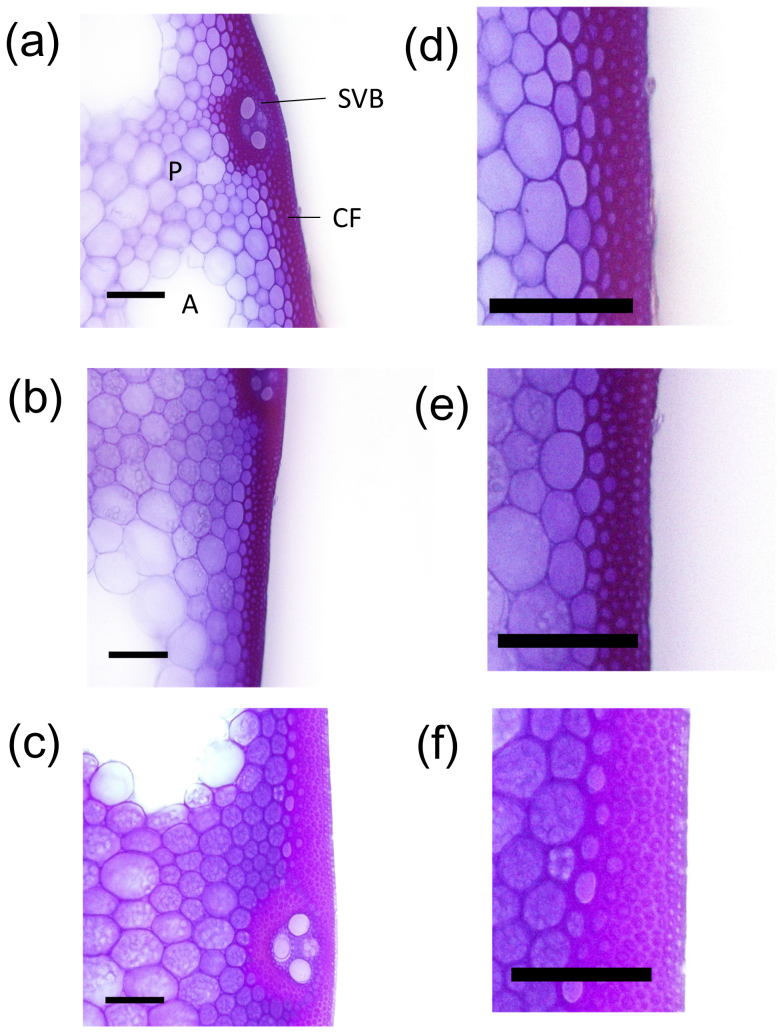
To analyze the morphological characteristics of the culm tissues in the stiff culm of Leaf Star, cross-sections of the basal internodes were stained with crystal violet to visualize the lignified cell walls. Violet staining was observed in the cortical fiber tissues and in the small vascular bundles (Fig. 5).
Figure 5. Morphological characteristics of transverse sections of cortical fiber tissues in culms.
(a, d) Koshihikari, (b, e) Chugoku 117, (c, f) Leaf Star. Scale bar: 100 μm. Transverse sections of fifth internodes 20 days after heading were stained with crystal violet. CF: cortical fiber tissue; SVB: small vascular bundle; P: parenchyma; A: aerenchyma.
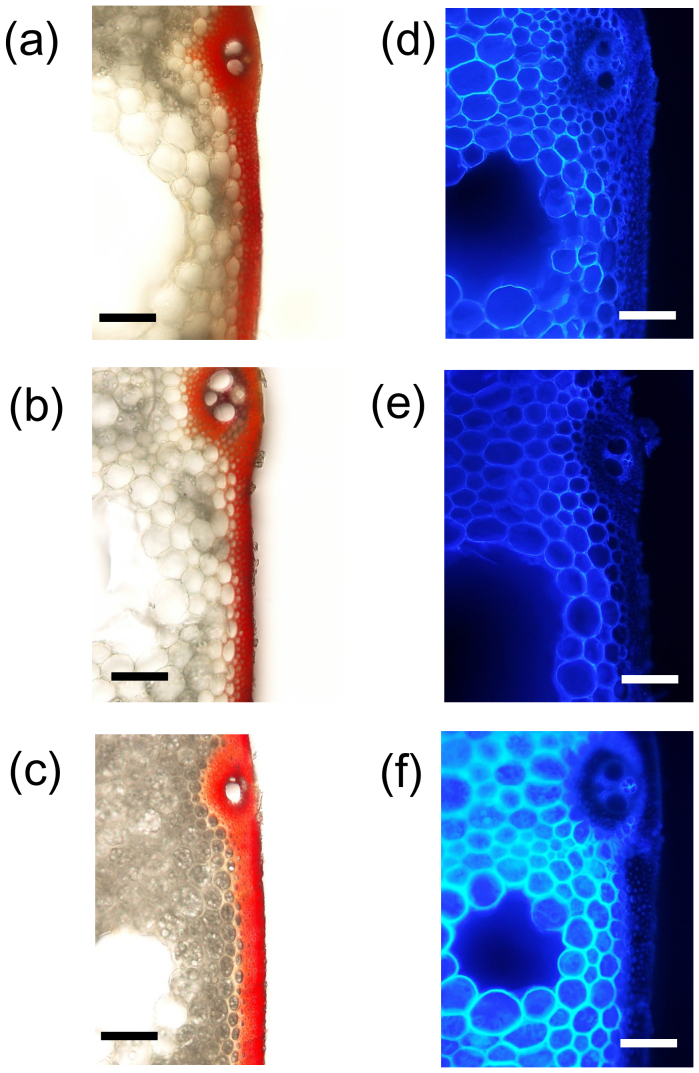
Next, cross-sections of the basal internodes were treated with phloroglucinol or with calcofluor to detect lignin and glucans. Red phloroglucinol staining was observed in the cortical fiber tissues and small vascular bundles of Leaf Star, confirming that it had a well-developed secondary cell wall (Fig. 6). The blue-white calcofluor staining was stronger in the thick cell walls of the parenchyma cells in Leaf Star than in those of both parents (Fig. 6). This was due to the high densities of cellulose and hemicellulose in the basal culm of Leaf Star.
Figure 6. Accumulation of cell wall components in basal culms of Leaf Star and both parents.
Transverse sections of fifth internodes 20 days after heading were stained with phloroglucinol (left) or calcofluor (right). Phloroglucinol staining (red color) was observed in cortical fiber tissues and in small vascular bundles. Calcofluor staining was stronger in the parenchyma cells of Leaf Star than in the parenchyma cells of both parents. (a,d) Koshihikari, (b,e) Chugoku 117, (c,f) Leaf Star. Scale bar: 100 μm.
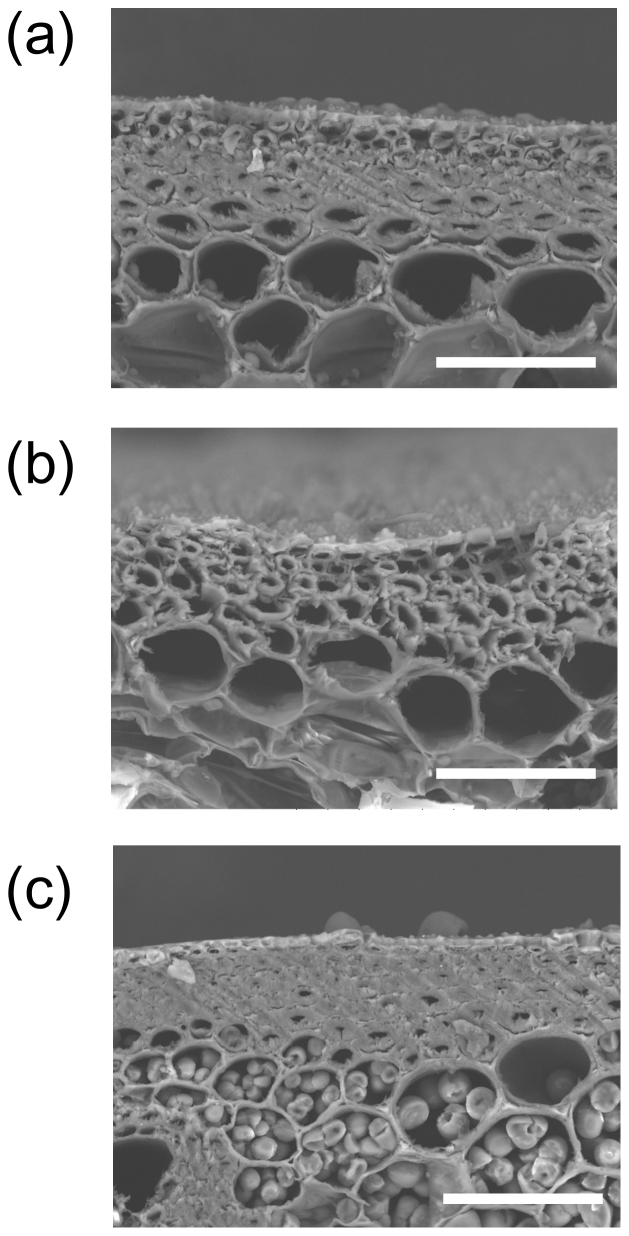
The cortical fiber tissue in the fifth internode was approximately 40% thicker in Leaf Star than in Chugoku 117 (Fig. 4b and Fig. 5). The ratio of cortical fiber tissue thickness to culm wall thickness was higher in Leaf Star than in Chugoku 117. Koshihikari had a higher percentage of cortical tissue than Chugoku 117. The secondary walls of the cortical fiber cells of Leaf Star and Koshihikari were well-developed, whereas many hollow cortical fiber cells were observed in Chugoku 117 (Fig. 7). Thus, Leaf Star inherited extremely thick cortical fiber tissues with well-developed secondary walls from Koshihikari.
Figure 7. Development of cortical fiber cells in culms.
(a) Koshihikari, (b) Chugoku 117, (c) Leaf Star. Scale bar: 50 μm. Transverse sections of fifth internodes 20 days after heading were observed using a scanning electron microscope.
Discussion
Reduction of lignin concentration and mechanical strength in an OsCAD2 mutant
OsCAD2 is the major CAD gene responsible for lignin biosynthesis in rice culms9. A deficiency in OsCAD2 causes the bm phenotype, which is observed in grains, such as maize and sorghum, and the gh phenotype15. However, it is not known whether OsCAD2 affects the mechanical strength of rice culms. The lignin concentration in the OsCAD2-null mutant Kinmaze-gh2 was 23% lower than that in the WT (Fig. 3a), and Kinmaze-gh2 had low coniferyl alcohol dehydrogenase activity and no detectable sinapyl alcohol dehydrogenase activity (Fig. 3b). The loss of sinapyl alcohol dehydrogenase activity has also been observed in other gh2 mutants6.
Regarding the characteristics associated with culm strength, the mechanical strength of the Kinmaze-gh2 culms was 42% lower than that of the WT culms, and severe lodging occurred in this mutant as a result of a weak BS (Fig. 3c). This is the first study demonstrating that OsCAD2 affects the mechanical strength of the basal internode involved in lodging. A T-DNA-tagged OsCAD7 mutant, fc1, also shows 13.4% less lignin and a 34% reduction in mechanical strength at the uppermost internode8. Because weak culms cause lodging problems, these mutants cannot be used in rice breeding for the production of livestock feed or biofuel.
A natural mutation in OsCAD2 reduces lignin concentrations in some rice varieties
Leaf Star and Chugoku 117 exhibited natural gh2 mutations in chromosome 2 (Fig. 1h, Supplementary Table S1 and Supplementary Table S2). For Leaf Star, the lignin concentrations of the culms were significantly reduced compared with those of the two parents, Koshihikari and Chugoku 117 (Fig. 1e). The two varieties with the gh2 mutations had different amounts of lignin. The sinapyl alcohol dehydrogenase activities of both of the gh2 varieties were significantly lower than those in Koshihikari (Fig. 1g). OsCAD2 expression was repressed in the developing internodes of Leaf Star and Chugoku 117. No differences in OsCAD7 expression were observed in Leaf Star and its parents (Supplementary Table S2). The reason for the smaller reduction in lignin levels in Chugoku 117 compared to that in Leaf Star is unclear. In some studies, no differences in the lignin concentrations of the gh2 mutants and WT have been found. Because the lignin biosynthetic pathway is very complex, the activities of other lignin biosynthetic enzymes may be higher in Chugoku 117; this possibility needs further investigation.
Leaf Star and Chugoku 117 have different natural OsCAD2 mutations. There were large differences in the nucleotide and amino acid sequences of OsCAD2 in both varieties (Fig. 2b and Supplementary Fig. S1). The mutations in the Chugoku 117 allele included a Tyr85-to-Cys85 point mutation in exon 3 and a 59-bp deletion in exon 4. Furthermore, the deletion in exon 4 created a premature stop codon in the Chugoku 117 allele. It has been reported that the premature stop codon not only produces a truncated and inactive protein product but also destabilizes the mRNA and leads to its degradation16. This mutation may be responsible for the weak expression of OsCAD2 and the low activity of CAD2 in Chugoku 117.
Although there were no differences in the exon sequences of Leaf Star and Koshihikari, the Ty3-gypsy retrotransposon was inserted into the first intron of the Leaf Star allele (Fig. 2b). It has been reported that the insertion of a transposon into intron 1 of maize CAD2 in the maize strain bm1 is responsible for the nonsense or the frame shift mutations that create the truncated CAD2 protein17. Additionally, in bm1, cad2 mRNA expression was reduced and the lignin concentration was low18.
This natural mutation accidently occurred in the Koshihikari allele during the individual or the line selection in the breeding of Leaf Star, which was derived from a cross between Chugoku 117 and Koshihikari. During these selections, a retrotransposon may have inserted in the first intron region of the Koshihikari allele. This new OsCAD2 allele in Leaf Star can be utilized in future breeding projects to further reduce lignin concentrations for feed and bioenergy production.
Key factors associated with strong culms in Leaf Star
Leaf Star experienced high bending stress, and thus, possessed a high mechanical strength despite its low lignin concentration conferred by its natural OsCAD2 mutation (Fig. 1e). The densities of cellulose and hemicellulose were higher in Leaf Star than in Chugoku 117 (Fig. 4a). The densities of cell wall components are correlated with culm stiffness13,19,20. Cellulose and hemicellulose usually accumulate in the secondary cell wall. In the basal culms of Leaf Star, they accumulated in the secondary cell walls of the cortical fiber cells (Fig. 4b and Fig. 5). In rice plants that highly express cell wall biosynthetic genes, reduced lignin concentrations have been reported, which lead to compensatory increases in cellulose and hemicellulose21. Transgenic aspen trees with the suppression of the lignin biosynthetic gene Pt4CL1 exhibit a reduction in lignin, which is compensated for by an increase in cellulose22. This compensatory regulation may be one of the causal factors for the high accumulation of cellulose and hemicellulose in Leaf Star. Further investigations are needed to clarify the regulatory mechanisms underlying the accumulation of cell wall materials in mechanical tissues.
Leaf Star has been utilized as a commercial rice variety for whole-crop silage and used as a model variety for studying the efficiency of saccharification to produce biofuel from rice straw in Japan13,23. Rice varieties with high cellulose and hemicellulose levels and low lignin concentrations, such as Leaf Star, may improve lodging resistance and the efficient utilization of lignocellulose for feed and biofuel production.
Furthermore, calcofluor staining showed that Leaf Star has well-developed parenchymal cells with thick cell walls (Fig. 6). These structural properties may be responsible for the stiff culm of Leaf Star. It has been reported that cortical fiber tissues are very important to bending stress24. Leaf Star had well-developed and thick cortical fiber tissues, which were inherited from the japonica variety Koshihikari, compared with the tropical japonica line Chugoku 117 (Fig. 7). In our previous study19, the bending stresses in the typical japonica fine-culm varieties in Japan were shown to be higher than those in the typical tropical japonica and indica thick-culm varieties. Further investigation is needed to determine the genetic basis for the structural traits associated with cortical fiber tissues.
Physiological factors associated with differentiation and development of the cortical fiber tissues in rice internodes are unknown. The duration of periclinal division in developing internodes has been found to be associated with cortical fiber tissue development25. It has been reported that in brittle culm (bc) mutants, the accumulation of cellulose, hemicellulose and lignin and the thickness of the secondary walls in the sclerenchyma tissues are reduced; additionally, BC genes have been found to regulate the development of secondary cell walls26,27. Further studies are needed to investigate the physiological and genetic aspects of morphogenesis, such as cell division and cell elongation, that are associated with the formation of the thick cortex fiber tissues and with the thickening of the secondary walls in the cortical fiber cells.
This study demonstrated that superior lodging resistance could be introduced into lignin-deficient varieties by incorporating rice genes that confer thick, stiff culms, such as the natural OsCAD2 mutation found in Leaf Star. To successfully develop long-culm and lignin-deficient varieties with superior lodging resistance and high biomass using marker-assisted selection, it is important to determine the causes of the thick and stiff culms and to identify the QTLs associated with the strong culms. We have identified some QTLs responsible for strong culms and culm thickness, such as SCM228 and SCM3. Further genetic studies are needed to investigate the molecular regulation of culm thickness and stiffness. These studies will provide new strategies for improving lodging resistance in rice, wheat and other gramineous crops during the next green revolution.
Methods
Cultivation
The rice (Oryza sativa L.) cultivar Leaf Star and its parents, Koshihikari and Chugoku 117, were cultivated in 2010 and 2011. The gh2 mutant with the Kinmaze background (Kinmaze-gh2) was generated by the ethyl-methanesulfonate (EMS) treatment of immature embryos. Kinmaze-gh2 and Kinmaze-WT were also cultivated during both years. Field experiments using these varieties and the gh2 mutant were conducted in 2010 and 2011. The growth conditions were nearly the same for both years. Here, we describe the 2010 cultivation method.
Seeds were sown in nursery boxes on April 27, 2010. Seedlings at the fourth-leaf stage were transplanted into a paddy field at the University Farm in Tokyo containing alluvial soil of the Tama River at a rate of three plants per hill on May 19, 2010. The planting density was 22.2 hills m−2, with a spacing of 15 cm × 30 cm. Manure was applied at a rate of approximately 20 t ha−1 before puddling, and compound fertilizer containing 14% each of N, P2O5 and K2O was applied as basal dressing at a rate of 50 kg each per hectare. The top dressing of N and K2O was applied at a rate of 3.0 kg each per hectare at 20 days before heading and at a rate of 20 kg each per hectare on the day of heading. The field was kept submerged throughout the course of the experiments. In 2010, the headings (50%) occurred on August 5 for Koshihikari, August 13 for Chugoku 117, September 2 for Leaf Star and August 31 for Kinmaze-WT and Kinmaze-gh2. In 2011, the headings occurred on August 3 for Koshihikari, August 10 for Chugoku 117, August 30 for Leaf Star and August 18 for Kinmaze-WT and Kinmaze-gh2.
The experiments were designed with three randomly arranged replicates (36 m2 per replicate) each year.
Construction of mapping populations and QTL analyses
The two mapping populations used for the QTL analysis of the gold hull trait were bred as follows: for the first population, Koshihikari was crossed with Chugoku 117 to produce F1 plants. The F1 plants were backcrossed with Koshihikari. The BC1F1 plants were self-pollinated six times to construct BILs using the single-seed descent method. We constructed 93 lines that were backcrossed with Koshihikari. To map the desired QTLs, we selected one BC4F1 plant that was heterozygous for the genomic region containing the putative QTL of interest but was homozygous for the other QTLs. Self-pollinated progeny (BC4F2 plants) from the BC4F1 plants were raised, and BC4F2 plants were selected that, when compared to the Koshihikari and Chugoku 117 reference genotypes, appeared to have undergone recombination in the vicinity of the putative QTL of interest. The self-pollinated progenies (BC4F4 lines) were used to determine the genotype at the QTL. For the second population, Koshihikari was crossed with Leaf Star, and the F1 plants were self-pollinated five times to construct RILs using the single-seed descent method. A total of 140 RILs were used for the QTL analysis.
Linkage maps of 102 SSR markers were constructed for the BILs derived from the Chugoku 117 and Koshihikari cross. The orders of and the genetic distances between the marker loci were calculated using the MAPMAKER/Exp 3.0 software29. For the RILs derived from the Leaf Star and Koshihikari cross, 140 single-nucleotide polymorphism (SNP) markers were used. QTL analyses were performed using the QTL Cartographer 2.5 software (http://statgen.ncsu.edu/qtlcart/WQTLCart.htm)30.
Enzyme assay
The CAD enzyme activity was assayed according to Zhang et al6. A total of 120 mg of culm tissues was milled to a fine powder in liquid N2 and was extracted with 800 μl of extraction buffer (100 mM Tris-HCl pH 7.5, 2% polyvinylpyrrolidone, 5 mM dithiothreitol, 2% polyethylene glycol 6000) at 4°C. The supernatants were decanted, and 10 μg of total soluble protein was used for the enzyme assays. The enzyme activities were measured using coniferyl alcohol and sinapyl alcohol as substrates. The production of hydroxycinnamaldehydes was monitored spectrophotometrically using a PowerWave HT microplate spectrophotometer (BioTek, Winooski, USA) set at 400 nm.
RNA isolation and expression analysis
Total RNA from the various organs was prepared by the phenol-SDS method and treated with DNase I. First-strand cDNA was synthesized from 1 μg of total RNA using the PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan). Quantitative real-time PCR was performed using the EXPRESS SYBR GreenER qPCR SuperMix, universal (Invitrogen, Carlsbad, USA), using a StepOnePlus instrument (Applied Biosystems, Foster City, USA) with the following cycling profile: 95°C for 2 min, 40 cycles at 95°C for 15 s, and 60°C for 60 s. The primer sets used for the real-time PCR are listed in Supplementary Table S3. Housekeeping genes were used as internal standards8,31.
Sequence analysis of OsCAD2
The OsCAD2 DNA sequences were determined for both strands using ABI PRISM dye-labeled terminators and the automated ABI 3100 sequence analyzer (Applied Biosystems). The primer sets used for sequencing are listed in Supplementary Table S4.
Measurements of culm strength
The physical parameters of the main culms, which were closely associated with lodging resistance, were used for the precise phenotyping. Eight main culms were sampled from each plot to determine the average leaf number, and the bending load at breaking was measured at a distance of 4 cm between two supporting points according to Ookawa et al.13 using the Tensilon RTG-1210 universal testing machine (A&D, Tokyo, Japan). The Tensilon is a useful tool for precisely evaluating lodging resistance traits in rice. The physical parameters were calculated as follows:
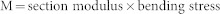 |
where M is the bending moment of the basal internode at breaking (gf · cm); and
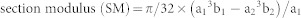 |
where a1 is the outer diameter of the minor axis in an oval cross-section, b1 is the outer diameter of the major axis in an oval cross-section, a2 is the inner diameter of the minor axis in an oval cross-section, and b2 is the inner diameter of the major axis in an oval cross-section.
BS is a mechanical parameter for lodging resistance that is influenced by the chemical composition of the culm, such as its cellulose and lignin concentrations.
Determinations of cell wall compositions
After the bending tests, the hand-cut internodes were collected, dried in an oven at 80°C until their weights were constant and powdered with a ball mill (TissueLyser, Qiagen, Hilden, Germany). The powdered samples were extracted with 80% ethanol three times at 80°C for 1 h and washed with hexane to remove any residual oil. The residues were hydrolyzed with α-amylase (Roche, Mannheim, Germany) and amyloglucosidase (Roche) at 37°C for 16 h to remove storage starch and were then analyzed for their holocellulose, hemicellulose and lignin concentrations.
Holocellulose concentrations were measured according to the sodium chlorite method of Wise et al32. A total of 150 mg of each residual sample was delignified with three additions of sodium chlorite and glacial acetic acid at 80°C. The residues were washed on glass filters, dried in an 80°C oven and weighed. Holocellulose concentrations were calculated by subtracting the weight of the delignified material from the weight of the starting material.
Hemicellulose was measured according to Schädel et al33. For each residual sample, 50 mg was dissolved in a neutral detergent solution (18 mM sodium tetraborate decahydrate, 66 mM ethylenediaminetetraacetic acid, 10.4 mM sodium dodecyl sulfate, 32 mM dibasic sodium phosphate and 1% (v/v) triethylene glycol), boiled for 1 h, washed with acetone and dried in an oven at 80°C. This ‘total cell wall fraction’ was dissolved in an acidic detergent solution (1 M H2SO4, 55 mM hexadecyl trimethylammonium bromide) and boiled for 1 h to hydrolyze the hemicellulose. After centrifugation, the residual ‘cellulose and lignin fraction’ was dried and weighed, and the hemicellulose concentration was calculated by subtracting the ‘cellulose and lignin fraction’ from the ‘total cell wall fraction’. The cellulose concentration was calculated by subtracting the hemicellulose concentration from the holocellulose concentration.
The lignin concentration was directly measured according to the high-throughput determination of thioglycolic acid lignin (TGAL) method of Suzuki et al34. A total of 20 mg of each residual sample was transferred to a microcentrifuge tube, and 1 ml of 3 M HCl and 0.1 ml of thioglycolic acid were added. The samples were heated at 80°C for 3 h. After centrifugation, the pellets were washed with distilled water and resuspended in 1 ml of 1 M NaOH. After centrifugation, 1 ml of supernatant was acidified with 200 μl of concentrated HCl. After chilling at 4°C for 4 h, the samples were centrifuged, and the pellets were dissolved in 1 ml of 1 M NaOH. These TGAL solutions were diluted 50-fold with 1 M NaOH, 200 μl of each diluted TGAL solution was transferred to a 96-well glass microplate and absorbances were read at 280 nm using the PowerWave HT microplate reader (BioTek).
Observations of transverse sections of basal internodes
The fifth internodes were sampled at 20 days after heading and fixed in FAA. To observe the lignified cell walls, the tissue samples were stained with 0.05% crystal violet35. Transverse sections of the internodes were visualized using the BX-53 fluorescence digital microscope (Olympus, Tokyo, Japan), and the thickness of the cortical fiber tissues was measured using the CellSens digital image processing software (Olympus). For the histochemical staining of the lignin, the sections were stained with a phloroglucinol solution. For the calcofluor staining, the sections were treated with 0.05% calcofluor white (Becton, Dickinson and Company, Maryland, USA) and were observed by UV excitation. The cortical fiber cell development was observed using a TM3000 scanning electron microscope (Hitachi High Technologies, Tokyo, Japan).
Statistical analysis
The statistical comparisons of multiple datasets were conducted using Dunnett's multiple comparison test and Tukey's significant difference test.
Author Contributions
T.O., T.E., T.T., T.Y., T.U., H.K. and M.V. performed the phenotyping and genotyping of gold hull trait using RILs and BILs for QTL analysis. T.O., T.Y. and M.M. performed to the sequencing of OsCAD2. K.I., C.S., S.N. and R.F. performed the morphological observations using microscope. K.I., T.O., S.A. and M.K. analyzed the enzyme activity and the gene expression of CAD. K.T. and T.M. contributed to the practice of field experiments. T.O., K.I., M.M., S.T. and T.H. wrote the paper.
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics-based for Agricultural Improvement, RBS2003).
References
- Chen F. & Dixon R. A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotech. 25, 759–761 (2007). [DOI] [PubMed] [Google Scholar]
- Reddy M. S. S. et al. Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). PNAS 46, 16573–16578 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen L. R. Brown midrib in maize and its linkage relations. J. Am. Soc. Agron. 23, 549–557 (1931). [Google Scholar]
- Li X., Weng J. K. & Chapple C. Improvement of biomass through lignin modification. Plant J. 54, 569–581 (2008a). [DOI] [PubMed] [Google Scholar]
- Oliver A. L., Pedersen J. F., Grant R. J. & Klopfenstein T. J. Comparative effects of the sorghum bmr-6 and bmr-12 genes: Forage sorghum yield and quality. Crop Sci. 45, 2234–2239 (2005). [Google Scholar]
- Zhang K. et al. GOLD HULL AND INTERNODE2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol. 140, 972–983 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias C. M. & Chow E. K. Structure of the cinnamyl-alcohol dehydrogenase gene family in rice and promoter activity of a member associated with lignifications. Planta 220, 678–688 (2005). [DOI] [PubMed] [Google Scholar]
- Li X. et al. FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol. Biol. 69, 685–697 (2008). [DOI] [PubMed] [Google Scholar]
- Hirano K. et al. OsCAD2 is major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep. 31, 91–101 (2012). [DOI] [PubMed] [Google Scholar]
- Ma Q. H. The expression of cafferic acid 3-O-methyltransferase in two wheat genotypes differing in lodging resistance. J. Exp. Bot. 60, 2763–2771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. H. Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J. Exp. Bot. 61, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. S., Colbert T. R. & Bauman L. F. Effect of brown-midrib-3 mutant in maize (Zea mays L.) on stalk strength. Z. Pflanzenzücht. 79, 310–314 (1977). [Google Scholar]
- Ookawa T. et al. Biomass production and lodging resistance in ‘Leaf Star’, a new long-culm rice forage cultivar. Plant Prod. Sci. 13, 58–66 (2010). [Google Scholar]
- Morinaka Y. et al. Impact of cinnamyl alcohol dehydrogenase (CAD) deficiency on mechanical rigidity of rice plant and enzymatic saccharification of rice straw. Breed Sci. 13 (Extra1), 87 (2011). [Google Scholar]
- Koshiba T. et al. CAD2 deficiency causes both brown midrib and gold hull and internode phenotypes in Oryza sativa L. cv. Nipponbare. Plant Biotech. 30, 365–373 (2013). [Google Scholar]
- Tsuruta S., Ebita M., Kobayashi M., Akashi R. & Kawamura O. Structure and expression profile of the cinnamyl alcohol dehydrogenase gene and its association with lignification in the sorghum (Sorghum bicolor (L.) Moench) bmr-6 mutant. Breed. Sci. 60, 314–323 (2010). [Google Scholar]
- Chen W. et al. Transposon insertion in a cinnamyl alcohol dehydrogenase gene is responsible for a brown midrib1 mutation in maize. Plant Mol. Biol. 80, 289–297 (2012). [DOI] [PubMed] [Google Scholar]
- Vanholme R., Morreel K., Ralph J. & Boerjan W. Lignin engineering. Curr. Opin. Plant Biol. 11, 278–285 (2008). [DOI] [PubMed] [Google Scholar]
- Ookawa T. & Ishihara K. Varietal difference of the cell wall components affecting the bending stress of the culm in relation to the lodging resistance in paddy rice. Jpn. J. Crop Sci. 62, 378–384 (1993). [Google Scholar]
- Tanaka K. et al. Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol. 133, 73–83 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambavaram M. M., Krishnan A., Trijatmiko K. R. & Pereira A. Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice. Plant Physiol, 155, 916–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. J. et al. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotech. 17, 808–812 (1999). [DOI] [PubMed] [Google Scholar]
- Park J. Y. et al. DiSC (direct saccharification of culms) process for bioethanol production from rice straw. Bioresour. Technol. 102, 6502–6507 (2011). [DOI] [PubMed] [Google Scholar]
- Matsuda T., Kawahara H. & Chonan N. Histological studies on breaking resistance of lower internodes in rice culm. IV The role of each tissue of internode and leaf sheath in breaking resistance. Jpn. J. Crop Sci. 52, 355–361 (1983). [Google Scholar]
- Kawahara H., Chonan N. & Wada K. Studies on morphogenesis in rice plants. III. Interelation of the growth among leaves, panicle and internodes, and a histological observation on the meristem of culm. Proc. Crop Sci. Soc. Jpn. 36, 372–383 (1968). [Google Scholar]
- Aohara T., Kotake T., Kaneko Y., Takatsuji H. & Tsumuraya Y. Rice BRITTLE CULM5 (BRITTLE NODE) is involved in secondary cell wall formation in the sclerenchyma tissue of nodes. Plant Cell Physiol. 50, 1886–1897 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li S., Qian Q., Zeng D. & Zhang M. BC10, a DUF266-containing and Golgi-located type II membrane protein, is required for cell-wall biosynthesis in rice (Oryza sativa L.). Plant J. 57, 446–462 (2009). [DOI] [PubMed] [Google Scholar]
- Ookawa T. et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat. commun. 1, 132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S. et al. Mapmaker: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1, 174–181 (1987). [DOI] [PubMed] [Google Scholar]
- Basten C. J., Weir B. S. & Zeng Z. B. QTL cartographer, version 1.17. A Reference Manual and Tutorial for QTL Mapping. (North Carolina State University, Raleigh, 2005). [Google Scholar]
- Jain M., Nijhawan A., Tyagi A. K. & Khurana J. P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 345, 646–651 (2006). [DOI] [PubMed] [Google Scholar]
- Wise L. E., Murphy M. & D'Addieco A. A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and studies on the hemicelluloses. Paper Trade J. 122, 35–43 (1946). [Google Scholar]
- Schädel C., Blöchl A., Richter A. & Hoch G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol. Biochem. 48, 1–8 (2010). [DOI] [PubMed] [Google Scholar]
- Suzuki S. et al. High throughput determination of thioglycolic acid lignin from rice. Plant Biotech. 26, 337–340 (2009). [Google Scholar]
- Cutler F. Practical micotechnique. In: Cutler, F., Botha, T. & Stevenson, D. (eds.) Plant Anatomy: An Applied Approach. 170–194 (Blackwell publishing, Oxford, 2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information