Abstract
Background: Age at natural menopause (ANM) is considered a marker of biological ageing and is increasingly recognized as a sentinel for chronic disease risk in later life. Socioeconomic position (SEP) and lifestyle factors are thought to be associated with ANM.
Methods: We performed a systematic review and meta-analyses to determine the overall mean ANM, and the effect of SEP and lifestyle factors on ANM by calculating the weighted mean difference (WMD) and pooling adjusted hazard ratios. We explored heterogeneity using meta-regression and also included unpublished findings from the Australian Longitudinal Study on Women’s Health.
Results: We identified 46 studies across 24 countries. Mean ANM was 48.8 years [95% confidence interval (CI): 48.3, 49.2], with between-study heterogeneity partly explained by geographical region. ANM was lowest among African, Latin American, Asian and Middle Eastern countries and highest in Europe and Australia, followed by the USA. Education was associated with later ANM (WMD middle vs low education 0.30, 95% CI: 0.10, 0.51; high vs low education 0.64, 95% CI 0.26, 1.02). A similar dose-response relationship was also observed for occupation. Smoking was associated with a 1-year reduction of ANM (WMD: -0.91, 95% CI: –1.34, –0.48). Being overweight and moderate/high physical activity were modestly associated with later ANM, but findings were less conclusive.
Conclusions: ANM varies across populations, partly due to differences across geographical regions. SEP and some lifestyle factors are associated with ANM, but further research is needed to examine the impact of the associations between risk factors and ANM on future health outcomes.
Keywords: Menopause, smoking, body mass index, physical activity, socioeconomic factors, systematic review, meta-analysis
Key Messages.
Among 46 community-based populations across six continents, differences in age at natural menopause were partly explained by geographical region.
Lower education and occupation levels were associated with earlier age at natural menopause.
Smoking was associated with earlier age at natural menopause by almost a year.
Associations between physical activity and body mass index and age at natural menopause remain inconclusive.
To better understand the effect of socioeconomic and lifestyle factors on ANM and subsequent health for postmenopausal women, pooling of individual level data from prospective cohort studies of different populations is needed.
Introduction
Menopause onset marks the end of a women’s reproductive stage in life and the start of a time of permanently lowered estrogen exposure that is increasingly recognized as having significant health implications. Earlier age at onset of natural menopause (ANM) has been shown to be associated with reduced risk of breast cancer,1 ovarian cancer2 and, by contrast, with increased risk of cardiovascular disease,3 atherosclerosis,4 stroke5 and osteoporosis.6 Overall, all-cause mortality has been found to be reduced by 2% with each increasing year of ANM.7,8
ANM appears to vary across different regions, countries and ethnic groups.9,10 This may be due to genetic variation,11 but may also reflect differences in socioeconomic position (SEP) and environmental, lifestyle, reproductive or early childhood factors. SEP and lifestyle factors that may affect timing of menopause include education, occupation, income, smoking, physical activity and body mass index (BMI). Of these, smoking has been consistently recognized to have an association with earlier menopause.12–14 However, far fewer studies have examined the effect of other socioeconomic and lifestyle factors on ANM, with inconsistent findings being reported.15,16 There are therefore a number of gaps in our understanding of preventable factors that may influence ANM. To our knowledge, and with the exception of smoking, no review has sought to synthesize the effect of SEP and lifestyle factors on ANM by means of meta-analysis. We therefore carried out a systematic review of existing literature to investigate the international variability and the socioeconomic and lifestyle determinants of ANM. We performed a series of meta-analyses that: (i) summarize the mean ANM across studies; and (ii) examine the associations between SEP and lifestyle factors and ANM, using meta-regression to explore between-study heterogeneity. The analyses also incorporate unpublished data on ANM and associated risk factors from mid-age women in the Australian Longitudinal Study on Women’s Health (ALSWH), a large population-based study with prospective data on SEP and lifestyle factors.
Methods
The Australian Longitudinal Study on Women’s Health
We included additional unpublished results from ALSWH, which is a prospective population-based study of factors affecting the health and well-being of three cohorts of Australian women born in 1973–78, 1946–51, and 1921–26. Women were randomly selected from the national Medicare health insurance database, which includes all Australian citizens and permanent residents. Since 1996, surveys have been administered to each cohort every 2–3 years. Further details of the recruitment methods and response have been described elsewhere.17,18 Informed consent was obtained from all participants at each survey, with ethical approval obtained from the Human Research Ethics Committees of the University of Newcastle and the University of Queensland.
Our meta-analysis includes data from women in the 1946–51 cohort. In 1996, 13 715 women aged 45–50 years participated in the baseline survey and were followed up until 2010, when they were aged 59–64 years. Using data from all six surveys, we included 7575 women in our analyses, after excluding women with: surgical menopause (n = 4301); unclassifiable menopause status (n = 1675); or missing ANM (n = 135); or who were pregnant (n = 29). We included a total of 4519 women in survival analysis described below, after additionally excluding women who were postmenopausal at baseline (n = 1665) or with missing covariate data (n = 1391). Menopause status was determined through responses to survey questions on hysterectomy, oophorectomy, hormone therapy (HT) and menstrual pattern.19 Women were classified as having surgical menopause if they reported hysterectomy, oophorectomy or both. Women were defined as: premenopausal if they had menstruated in the past 3 months and reported no change in menstrual frequency in the past 12 months; perimenopausal if they reported changes in menstrual frequency or 3 to 11 months of amenorrhoea; and naturally postmenopausal if they reported amenorrhoea for 12 consecutive months or more.20 If women reported use of HT or oral contraception before reaching postmenopause, their menopause status was defined as unclassifiable. Based on answers to the question: ‘If you have reached menopause, at what age did your periods completely stop?’, age at menopause was defined as the age at the final menstrual period. If data on ANM were missing or incongruous with the derived menopause status, age at menopause was imputed by using the age at midpoint of the surveys between which menopausal transition occurred, minus 1 year (because women were already free of menstrual periods for at least 12 months when their status was defined as postmenopausal).19
SEP and lifestyle factors included in the analyses were based on responses given in the baseline survey, with the exception of physical activity, information on which was used from survey 2. To maximize similarity with other studies included in the meta-analysis, variables were classified into comparable categories: education as low (no formal qualifications), middle (higher school certificate/diploma) and high (university or higher degree); occupation as low (no paid work or manual worker), middle (trade, administrative, sales and service) and high (manager, professional); smoking status as non-smoker, ex-smoker and current smoker as well as dichotomized into non- or ex-smoker and current smoker. Physical activity was categorized as sedentary/low (0 to <600 metabolic equivalent (MET)/min/week) and moderate/high (≥600 MET/min/week). BMI was computed as self-reported weight (kg)/height (m2) and categorized as underweight (<18.5 kg/m2), healthy weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) or obese (≥30 kg/m2).21
Survival analysis of ALSWH data
Cox proportional hazards regression analysis was used to obtain hazard ratios (HRs) for the associations between each risk factor and ANM in ALSWH. Survival time for each participant was defined as the time in years between age at baseline and reported ANM. All women reached menopause during the study. Multivariable models were adjusted for all other explanatory variables and age (years), number of times given birth (never, once, twice, three times, four or more), age at menarche (years), duration of oral contraceptive use (never, ≤10 years, >10 years), duration of use of HT (never, ≤4 years, >4 years), and country of birth (Australia, other English-speaking, Europe, Asia, other). These analyses were performed using SAS version 9.3.
Literature review
Search strategy and selection criteria
To identify relevant primary studies, we performed a systematic review of existing published literature by searching six databases from earliest date available up to December 2012: MEDLINE, EMBASE, PubMed, Science Direct and Web of Science. The Cochrane Library was searched to identify additional review articles. The search strategy consisted of a combination of search terms relating to menopause and country or ethnicity. We also included terms for six socioeconomic and lifestyle factors of interest (education, occupation, income, smoking, physical activity and BMI), to identify studies that reported menopause in the context of these factors. The search was restricted to studies published in English (see Supplementary data for full search strategy, available at IJE online). We also perused the reference lists of relevant articles to identify any additional studies not identified by our search strategy.
J.R. screened titles and abstracts of identified articles, and J.R. or D.S. and G.M. reviewed the full text of potentially relevant articles. Disagreements about the eligibility of a study or differences between the two sets of extracted information were resolved through discussion between authors. Our inclusion criteria were: community-based studies; studies reporting on ANM and/or the association between any of education, occupation, income, smoking, physical activity and BMI, and ANM. Studies were excluded if they: focused on early menopause due to causes other than natural menopause (e.g. hysterectomy, bilateral oophorectomy); were highly selective (e.g. clinical study populations); or had fewer than 100 participants.
Data extraction
J.R. or D.S. and G.M. independently extracted the following information from included articles: first author; baseline study year; baseline age range; study design; years of follow-up; study country; ethnicity; study population; definition of natural menopause; exclusion criteria for study population; and relevant data. Data on ANM were generally presented in one of two ways. Where presented as a continuous variable, we extracted the overall mean ANM with standard error (SE) and/or standard deviation (SD) or confidence interval (CI), and where reported, the mean or median by SEP and/or lifestyle factor. Where a median was reported, it was extracted along with the range and interquartile range. Where data on the effect of SEP and lifestyle on ANM were reported, we extracted the relevant adjusted effect estimates (e.g. HR, risk ratio etc.). If studies reported adjusted effect estimates but used a different reference category compared with the most commonly used reference category, the authors were contacted and requested to re-analyse their data in line with other studies.22–28 A total of four authors responded and could provide additional results that were included in meta-analyses.
Preparation of data for meta-analysis
Where the overall mean ANM was not reported in a study but was reported for subgroups of the study population (e.g. stratified by smoking status), a weighted mean was calculated as an estimate of the overall mean. Where the SE was not reported, it was calculated from the SD or the CI, if provided.
Studies that measured the risk factors in any categorical manner were included in the meta-analysis. In order to compare and pool the results of studies in the meta-analyses, the original categories for variables were harmonized to maximize the available data. Variables were re-classified as: low, middle and high level for education and occupation; none to low and moderate to high level for physical activity; and <20, 20–25, 25–30 and ≥30 kg/m2 for BMI. Smoking status was categorized as non-smoker, ex-smoker and current smoker and also dichotomized into non- or ex-smoker and ever- or current smoker.
Meta-analysis
Meta-analysis and meta-regression were performed using Stata version 12.0.29 We combined studies that reported on mean ANM to obtain a pooled mean with 95% CI, using the mean and SE from each study. For the association between risk factors and ANM, we used the mean age and SD to obtain study-specific mean differences with 95% CIs, and the overall pooled weighted mean difference (WMD). Where possible, we also combined adjusted HRs to obtain summary estimates for the associations between each risk factor and ANM. We pooled HRs since this was the most commonly reported measure of effect, with few studies reporting other effect estimates.
We assessed heterogeneity between studies using the chi2 (Cochrane Q) and I2 statistics. Where substantial heterogeneity was observed in fixed effects models, we obtained summary estimates using random effects models. In the meta-analyses of mean ANM and of the unadjusted effect of risk factors on ANM, we investigated sources of heterogeneity using subgroup analysis and, where at least 10 studies were included, performed meta-regression. We also created funnel plots where more than five studies were included, as one method of assessing likelihood of publication bias resulting from non-publication of small studies that observed smaller or no associations.
Meta-regression
We used random effects univariable and multivariable meta-regression to investigate the role of a priori study characteristics in explaining observed heterogeneity in ANM using the ‘metareg’ function in Stata.30 These characteristics included: the region from which the study population was selected (Middle East, Asia, Latin America, USA, Europe, Australia and Africa); the study design (prospective or not); whether the study population was aged younger than or equal to 65 years or older than 65 years (i.e. age when they were asked to report ANM); and the definition of menopause [World Health Organization (WHO) definition or other definition/no definition reported]. Natural menopause is defined by the WHO as the permanent cessation of menstruation resulting from the loss of ovarian follicular activity, occurring after 12 consecutive months of amenorrhoea, for which there is no other obvious pathological or physiological cause.31 We obtained univariable and multivariable coefficients with 95% confidence intervals (CIs) and associated P-values for the association between each characteristic and ANM. Characteristics with a P-value of <0.2 were then included in the multivariable model. The P-value for region in the unadjusted model and the P-values for all characteristics in the multivariable model were adjusted for multiple testing.30
Similarly, we explored the heterogeneity in the effect of smoking and education on ANM using meta-regression to investigate the role of study characteristics described above. However, we dichotomized region into ‘economically more developed regions’(USA, Europe, Australia) vs ‘other regions’ (Middle East, Asia, Latin American, Africa), since we had too few studies to explore the effect of region as classified above.
This manuscript was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.32
Results
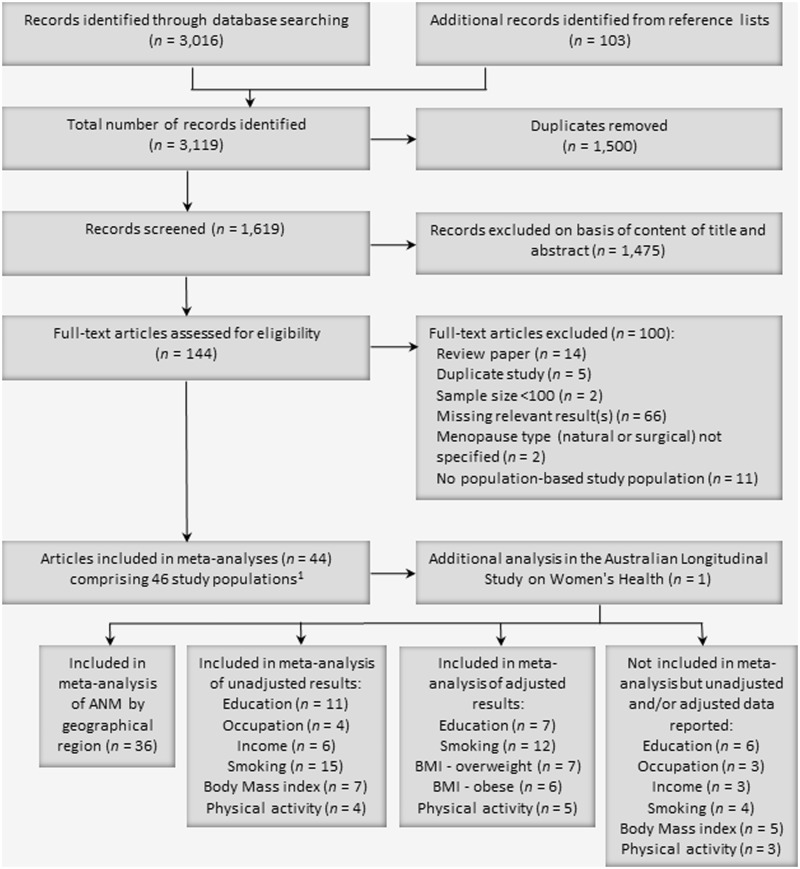
The numbers of identified and included studies are summarized in Figure 1. The search initially identified 3119 articles. After excluding duplicate records, the titles and abstracts of 1619 articles were screened, with 1475 identified as not relevant. We therefore reviewed the full text of 144 articles, 44 of which met the inclusion criteria.22–24,26–28,33–70 Two studies each contained two separate study populations.26,55 After incorporating additional results from ALSWH, the review encompassed a total of 46 study populations. Of these, the meta-analyses for ANM included: 36 studies for overall ANM by region; 16 for risk factors for ANM; and 13 for adjusted hazard ratios of the effect of risk factors on ANM.
Figure 1.
Flow diagram of selection process and inclusion of studies for meta-analysis.
1Studies by Dravta et al. (2009) and Ku et al. (2004) each included two study populations.
Study characteristics
Table 1 presents characteristics of the 46 studies that were part of at least one of the three sets of meta-analyses. Of the identified studies, 36 were cross-sectional, one was retrospective and 9 were prospective cohort studies. Study populations were drawn from a total of 24 countries. Since detailed information on ethnicity was presented in only a few studies, we grouped studies according to the geographical region from which the study population was derived: Africa; Asia; Australia; Latin America; Middle East; Europe; and the USA. Most studies defined ANM according to the WHO definition (n = 30),31 but some used a different definition (n = 9) or did not specify a definition (n = 7). All of the 46 studies either excluded or censored women with surgical menopause from their analysis.
Table 1.
Description of studies included in meta-analysis
| First author | Baseline study year | Baseline age range | Years of follow-up | Na | Study country | Study population | Menopause definitionb | HRT users excluded | Surgical menopause excluded | Included in meta-analysis of unadjusted data on risk factors | Included in meta-analysis of adjusted data on risk factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort studies | |||||||||||
| Akahoshi33 | 1958–1959 | 40–41 | 30 | 493 | Japan | Survivors of the atomic bombings in Nagasaki | 12 months | n/r | Yes | BMI | No data reported |
| Do44 | 1980–1982 | 17–88 | 16 | 5593 | Australia | Twins selected from the Australian twin registry | 12 months | Censored | Yes | No | Education, smoking |
| Dorjgochoo45 | 1997–2000 | 40–70 | 16 | 33054 | China | Selected from 7 urban communities in Shanghai | 12 months | Yes | Yes | Education, occupation, income, smoking, physical activity | Data not in suitable format |
| Dravta (a)d,26 | 1998–2002 | 20–44 | n/r | 3281c | Europe | Selected from at least 3 areas in each of the 9 participating countries or regions | 12 months | Yes | Yes | No data reported | Smoking, BMI, physical activity |
| Dravta (b)d,26 | 2002 | 18–60 | n/r | 1524c | Switzer- land | Random sample recruited from 8 areas using population registries | 12 months | Yes | Yes | No data reported | Smoking, BMI, physical activity |
| Hong52 | 1985 | ≥55 | 16 | 2658 | Korea | Kangwha Country residents (selection method not reported) | n/r | n/r | Yes | No data reported | No data reported |
| Nagel22 | 1994–1998 | 35–65 | 6 | 1009 | Germany | Selected in Heidelberg and surrounding communities from general population registries | 12 months | n/r | Yes | No data reported | Smoking, BMI, physical activity |
| Palmer27 | 1995 | 35–55 | 4 | 1323 | USA | Subscribers to Essence magazine, members of selected Black professional organizations, and friends and relatives of early respondents | Age last period | Censored | Censored | No data reported | Smoking, BMI, physical activity |
| Van Noord65 | 1975–1977 | 53–64 | 9 | 4686 | Nether- lands | From population-based cancer screening project conducted in Utrecht city | 12 months | Yes | Yes | No data reported | No data reported |
| ALSWHd | 1996 | 45–50 | 14 | 7575 | Australia | Randomly selected from the national Medicare health insurance database, which includes all Australian citizens and permanent residents | Age last period | No | Yes | Education, occupation, smoking, BMI, physical activity | Education, smoking, BMI, physical activity |
| Retrospective cohort study | |||||||||||
| Cooper42 | 1990–1991 | 69–81 | n/a | 543 | USA | College-educated women in Minnesota (selection method not reported) | Age last period | n/r | Yes | Smoking | No data reported |
| Cross-sectional studies | |||||||||||
| Ansari34 | 2008 | n/r | n/a | 500 | Iran | Randomly selected from 38 health centres in Zahedan city | n/r | n/r | Yes | No data reported | No data reported |
| Ashrafi35 | 2004–2005 | >35 | n/a | 2462 | Tehran | Selected using randomized cluster sampling in each of the 22 metropolitan districts of Tehran | n/r | n/r | Yes | Smoking | No data reported |
| Ayatollahi36 | 2000 | n/r | n/a | 948 | Iran | Living in Shiraz for at least 1 year, selected using cluster sampling | 12 months | n/r | Yes | Education, occupation, income, smoking, BMI, physical activity | No data reported |
| Bener37 | 1996–1997 | ≥40 | n/a | 742 | UAE | Attending primary health clinics for preventive health care and members of women’s associations in Al Ain City, Abu Dhabi, Dubai and Sharjah Emirates | 12 months | n/r | Yes | Education, BMI | No data reported |
| Beser38 | 1990 | 40–54 | n/a | 1076 | Turkey | Selected using cluster-stratified and random sampling in Trabzon city | 12 months | n/r | Yes | Education, BMI | No data reported |
| Blumel39 | n/r | 40–59 | n/a | 384 | Latin America | Sample of women from 49 healthcare centres in 47 cities in 15 Latin American countries | 12 months | Yes | Yes | No | No data reported |
| Chang40 | 1991 | 40–60 | n/a | 124 | Taiwan | Chinese women randomly sampled from a community in Taipei | 12 months | Yes | Yes | No data reported | No data reported |
| Chmara41 | 1998–2002 | 35–69 | n/a | 2117 | Poland | Selected from participants in several studies conducted in 2 regions | n/r | Yes | Yes | Smoking | No data reported |
| Cramer69 | 1989–1992 | 45–54 | n/a | 8657 | USA | Selected from town census lists from 10 suburbs in eastern Massachusetts | Age last period | n/r | Yes | Smoking | Smoking |
| Delavar43 | n/r | 45–63 | n/a | 1397 | Iran | Selected from 60 clusters identified according to the latest census of Babol city | 12 months | n/r | Yes | Education, smoking, BMI | Data not in suitable format |
| Dvronyk46 | n/r | >40 | n/a | 248 | USA | Randomly selected from existing studies | 12 months | n/r | Yes | Smoking | No data reported |
| Fallahzadeh47 | 2006 | 40–70 | n/a | 346 | Iran | Living in Yazd city for at least 1 year, selected using a cluster sampling | 12 months | n/r | Yes | Education, income, smoking, BMI | Education, income, smoking |
| Fleming 48 | 1988–1991 | ≥25 | n/a | 1825 | USA | Representative sample of the US civilian population participating in the National Health and Nutrition Examination Survey | 12 months | n/r | Yes | Occupation, smoking | Data not in suitable format |
| Garrido-Latorre49 | 1990–1993 | n/r | n/a | 472 | Mexico | Control population of a case-control study on gynaecological cancer, drawn from the National Health Surveys System, Mexico City | 12 months | n/r | Yes | Education, smoking | No data reported |
| Gold28 | 1995–1997 | 40–55 | n/r | 14620c | USA | Multi-ethnic population residing near 1 of the 7 clinic sites, selected using community-based sampling from lists of the population at 5 sites and using random digit dialling combined with ‘snowball' approach at 2 sites | 12 months | Censored | Censored | No data reported | Education, smoking |
| Hagstad50 | 1979 | 37–66 | n/a | 490 | Sweden | Randomly selected from census registry in Goteborg | 6 months | n/r | Yes | No data reported | No data reported |
| Harris51 | 1967 | n/r | 36 | 1311 | USA | Women re-entering the workforce and balancing the roles of homemakers, mothers, and labour force participants | n/r | n/r | Yes | Income | No data reported |
| Kaczmarek53 | 2000–2004 | 35–65 | n/a | 7183c | Poland | Randomly selected from sites of all residence categories | 12 months | Censored | Censored | No data reported | Education, smoking, |
| Kriplani54 | 2001–2002 | mid-aged and elderly | n/a | 201 | India | Accompanying other patients to an outpatient clinic in northern India | 12 months | n/r | Yes | No data reported | Data not in suitable format |
| Ku (a)55 | 1998–1999 | >35 | n/a | 961 | Korea | Living in Korea, randomly recruited from community-based social groups | 12 months | n/r | Yes | Education, smoking | Data not in suitable format |
| Ku (b)55 | 1998–1999 | >35 | n/a | 1011 | China | Korean second- or third-generation emigrants to China, randomly recruited from community-based social groups | 12 months | n/r | Yes | Education, smoking | Data not in suitable format |
| Kwawukume56 | 1991–1992 | 40–56 | n/a | 123 | Ghana | Women from Akosombo District were interviewed at churches, markets, houses and common gathering places for women (selection method not reported) | 12 months | n/r | Yes | No data reported | No data reported |
| Lawlor57 | 1999–2001 | 60–79 | n/a | 3513 | UK | Randomly selected from general practitioner lists in 23 British towns | Age last period | Yes | Yes | No data reported | No data reported |
| Li24 | 2010–2011 | 40–65 | n/a | 6070 | China | ‘Systematic’ sampling of women who attend annual health screening in Jiangsu Province of China, with sample size in each city proportional to city population size | 12 months | n/r | Yes | No data reported | Education, smoking, BMI |
| Loh58 | 2001 | 45–60 | n/a | 656 | Singapore | Random sample from the database of all female citizens of Singapore | 12 months | n/r | Yes | No data reported | No data reported |
| Luoto68 | 1989 | 45–64 | n/a | 1713 | Finland | Random sample from the Finnish Population Registry | Age last period | n/r | Yes | No | Education |
| Magursky59 | 1967 | 38–58 | n/a | 6877 | Czecho- slovakia | From Martin district (selection method not reported) | n/r | n/r | Yes | No | No data reported |
| McKnight60 | 2003–2007 | ≥45 | n/a | 10440 | USA | Selected from a commercially available nationwide list including telephone numbers | 12 months | n/r | Yes | Education, income, smoking, physical activity | Data not in suitable format |
| Morris67 | 2003–2011 | 40–98 | n/r | 21511 | UK | Initial recruits were registered supporters of the Breakthrough Breast Cancer charity (4.2%) and women who referred themselves to the study (24.6%) living in the UK. Participants could nominate other women to join the study (71.2%) | Age last period | Yes | Yes | No | Smoking, BMI, physical activity |
| Nagata70 | 1992 | 45–55 | 1445 | Japan | Female residents in Takayama | Age last period | n/r | Yes | No data reported | Data not in suitable format | |
| Okonofua61 | n/r | 44–87 | n/a | 563 | Nigeria | Random sample from Oyo State in Nigeria | n/r | n/r | Yes | Smoking | No data reported |
| Olaolurun62 | 2006–2007 | 40–60 | n/a | 489 | Nigeria | Residents of Ibadan, Oyo State in Nigeria selected using cluster sampling | 12 months | n/r | Yes | Smoking | Data not in suitable format |
| Ortiz66 | 2000–2001 | 40–59 | n/a | 324 | Puerto Rico | Health fair visitors in 22 municipalities | 12 months | Yes | Censored | No data reported | No data reported |
| Ozdemir63 | 2001 | 50–65 | n/a | 262 | Turkey | Random selection from primary health centres in Ankara | Age last period | n/r | Yes | No data reported | No reported |
| Sievert64 | 1999–2000 | 40–70 | n/a | 451 | Mexico | Recruited from public parks, on the streets outside their homes, in open markets, in small shops and in front of large public buildings in the capital city of Puebla | 12 months | Yes | Yes | No data reported | Data not in suitable format |
| Yasui23 | 2001–2007 | ≥40 | n/a | 5602 | Japan | Registered nurses, licensed practical nurses, public health nurses, and/or midwives (The Japan Nurses’ Health Study) | 12 months | n/r | Yes | No data reported | BMI |
ALSWH, Australian Longitudinal Study on Women’s Health; BMI, body mass index; f/u, follow-up, HRT, hormone replacement therapy; n/a, not applicable; n/r, not reported; UAE, United Arab Emirates; UK, United Kingdom; USA, United States of America.
aN for number of women with reported age at natural menopause.
bWhen reported in months: minimal length of time without menstruation.
cN includes pre- and postmenopausal women included in analysis.
dThe combined results from the two cohorts included in the Dravta article were included in the meta-analysis of adjusted data.
eAdditional unpublished results obtained from the Australian Longitudinal Study on Women's Health (ALSWH).
For each SEP measure or lifestyle factor, up to seven studies reported data on unadjusted and/or adjusted associations with ANM, but were not included in the analyses because: they used a different referent group in their analysis compared with the majority of studies; they reported estimates other than HRs; or it was not possible to calculate a mean and standard deviation (Figure 1).
Covariates used in studies included in meta-analyses of adjusted effect estimates are presented in Table 2. The majority of studies adjusted for education, parity, age at menarche, BMI and smoking.
Table 2.
Overview of covariates used in multivariate analysis in studies included in meta-analyses of adjusted hazard ratios for socioeconomic and lifestyle factors and ANM
 |
aAdditional unpublished results obtained from the Australian Longitudinal Study on Women's Health (ALSWH).
International variability in age of natural menopause
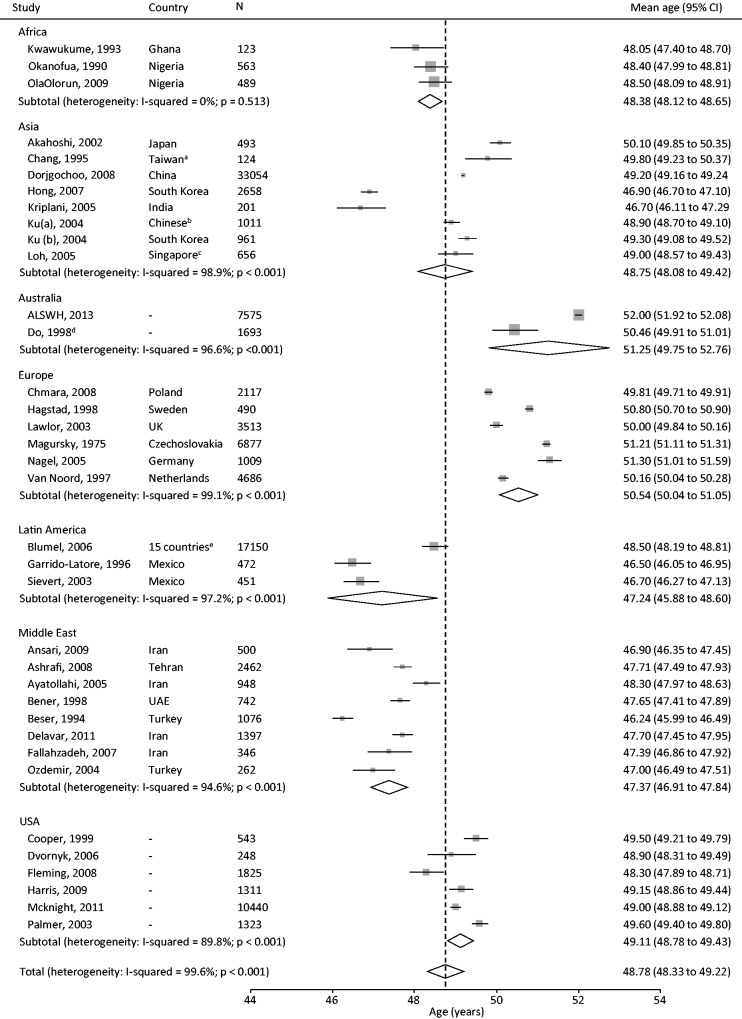
The meta-analysis of ANM included 36 studies, which gave an overall summary mean ANM of 48.78 (95% CI: 48.33, 49.22). However, there was substantial heterogeneity between studies (I2 = 99.6%), with mean age ranging from 46 to 52 years (Figure 2). Sub-group analysis by geographical region demonstrated that ANM was generally lower among African, Latin American, Asian and Middle Eastern countries, and was highest in Europe and Australia, followed by the USA. However, despite this overall pattern there remained substantial heterogeneity between countries within each region (Figure 2). Meta-regression analysis indicated that region was statistically associated with mean ANM, and explained 68.5% of the observed heterogeneity. Study design and inclusion of women aged over 65 years were also associated with ANM, but contributed to only a small proportion of the observed heterogeneity. Together, region, study design and age of study population explained 71.1% of the between-study variance (Table 3).
Figure 2.
Meta-analysis of mean age at natural menopause, stratified by geographical region (using random effects). CI, confidence interval.
aChinese women in Taiwan; bsecond- or third-generation emigrants from Korea to China; cChinese Malay and Indian women in Singapore; dmean and standard error were calculated from the median and range using recommended formula;86 eStudy included centres from North, Central and South America (Argentina; Bolivia; Brazil; Chile; Colombia; Costa Rica; Cuba; Dominican Republic; Ecuador; Honduras; Mexico; Panama; Paraguay; Peru; Uruguay. Dotted line represents the overall summary mean age.
Table 3.
Univariable and multivariate meta-regression analysis of the effect of potential sources of heterogeneity on menopausal age
| Characteristic | Meta-regression coefficient (95% CI) | P-valuea | % variance explained |
|---|---|---|---|
| Univariable meta-regression | |||
| Regionb | |||
| Australia | 0.73 (–0.68 to 2.14) | 0.717 | 68.5 |
| Asia | –1.80 (–2.72 to –0.87) | 0.001 | |
| Africa | –2.23 (–3.45 to –0.99) | 0.003 | |
| Latin American | –3.30 (–4.51 to –2.08) | <0.001 | |
| Middle East | –3.18 (–4.10 to –2.25) | <0.001 | |
| USA | –1.46 (–2.45 to –0.47) | 0.015 | |
| Joint effect of region | <0.001 | ||
| Prospective study design | –1.46 (–2.52 to –0.41) | 0.008 | 16.7 |
| Study population did not include women aged >65 years | –0.75 (–1.77 to 0.27) | 0.146 | 3.4 |
| Ideal definition of menopause used | 0.61 (–0.42 to 1.64) | 0.237 | 1.4 |
| Multivariate meta-regressionc | |||
| Regionb | 71.1 | ||
| Australia | 0.77 (–0.69 to 2.22) | 0.845 | |
| Asia | –1.62 (–2.53 to –0.72) | 0.010 | |
| Africa | –2.35 (–3.58 to –1.12) | 0.005 | |
| Latin American | –3.22 (–4.42 to –2.02) | <0.001 | |
| Middle East | –3.12 (–4.05 to –2.20) | <0.001 | |
| USA | –1.25 (–2.22 to –0.28) | 0.075 | |
| Joint effect of region | <0.001 | ||
| Prospective study design | 0.08 (–0.74 to 0.90) | 1.000 | |
| Study population did not include women aged >65 years | –0.65 (–1.29 to –0.01) | 0.232 | |
aP-value for region adjusted for multiplicity in the univariate analysis; P-value for region, age and study design adjusted for multiplicity in the multivariate model.
bComparing each region against Europe (which had the largest number of studies).
cOnly those characteristics with a P-value of <0.2 in the univariate models were included in the multivariate model.
Socioeconomic position
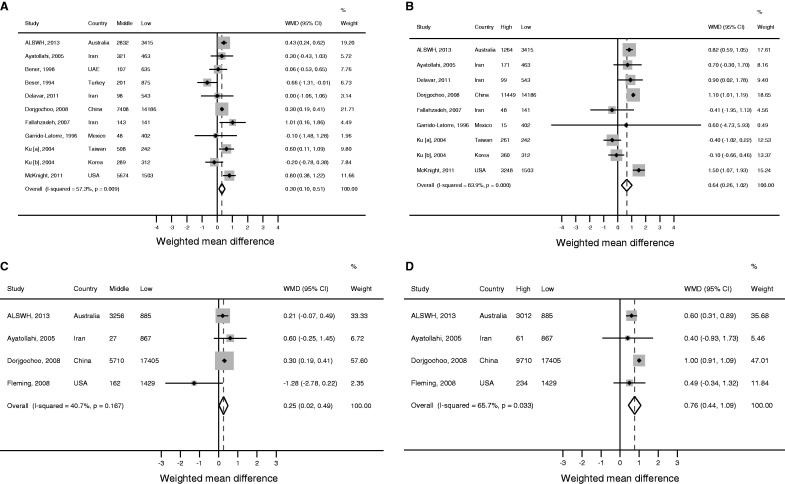
The meta-analysis for the effect of education on ANM comprised 11 study populations including ALSWH,37,38,43,45,47,49,55,60,71 all of which were included in the analysis of middle vs low education level and nine of which were included in the analysis of high vs low education level. Onset of menopause occurred one-third of a year later in women with a middle education level compared with a low education level [WMD (random effects) 0.30, 95% CI: 0.10, 0.51; Figure 3a], and two-thirds of a year later in women with a high education level [WMD (random effects) 0.64, 95% CI: 0.26, 1.02; Figure 3b]. However, there was moderate to high heterogeneity between studies included in each of these analyses (I2 = 57.3% and 87.9%, respectively). Two of the included studies were from ‘economically more developed regions’, whereas the remaining studies were from ‘other regions’. Sub-group analyses revealed that the effect of education appeared to be stronger in ‘economically more developed regions’ than ‘other regions’. Meta-regression of the results for mid-level education was consistent with this, revealing that 19.9% of the between-study variance was explained by region. None of the between-study variance appeared to be explained by differences in study design, age of population or definition of menopause.
Figure 3.
Meta-analysisa of the unadjusted effect of (a) middle education level, (b) high education level, (c) middle occupation level and (d) high occupation level on age of natural menopause.
aRandom effects estimates. Third and fourth columns in the figures give the number of women classified as having a middle, high or low education level, and middle, high or low occupation level, as appropriate.
Six studies24,28,44,47,53,68 that did not provide sufficient data to be included in the meta-analysis of unadjusted WMD but reported adjusted HRs were pooled with results from ALSWH. Both middle and high education levels remained associated with a later ANM compared with low education level (pooled HR 0.93, 95% CI: 0.87, 0.98, and 0.81, 95% CI: 0.74, 0.89, respectively), although there was moderate heterogeneity between studies for the effect of high education (Table 4).
Table 4.
Summary estimates for meta-analysis of adjusted hazard ratios for the effect of smoking, body mass index, physical activity and education on age of menopause onset
| Characteristic | Number of studies | Pooled hazard ratio (95% CI) | Heterogeneity (I2 [%]; P-valuea) |
|---|---|---|---|
| Educationb | |||
| Low | 1.00 (ref) | - | |
| Middle | 7 | 0.93 (0.87 to 0.98) | 58.4; 0.025 |
| High | 7 | 0.81 (0.74 to 0.89) | 48.6; 0.070 |
| Cigarette smoking | |||
| Non/ex-smoker | 1.00 (ref) | - | |
| Current smoker | 12 | 1.32 (1.25 to 1.40) | 70.7; <0.001 |
| Body mass indexc | |||
| Normal | 1.00 (ref) | - | |
| Overweight | 7 | 0.93 (0.91 to 0.95) | 1.3; 0.414 |
| Obese | 6 | 0.92 (0.85 to 0.99) | 78.9; <0.001 |
| Physical activity | |||
| Low/none | 1.00 (ref) | - | |
| Moderate/high | 5 | 0.97 (0.95 to 1.04) | 65.6; 0.02 |
aP-value of chi2 test for heterogeneity.
bSeparate meta-analyses were performed, pooling data on middle education level vs low, and high education level vs low.
cSeparate meta-analyses were performed, pooling data on overweight vs normal, and obese vs normal.
The meta-analysis of occupation and ANM comprised ALSWH plus three published studies.45,48,71 Occupation had an effect comparable to education, with ANM being higher in women with a middle occupation level compared with a low occupation level [WMD (random effects) 0.25, 95% CI: 0.02, 0.49; Figure 3c], and higher again in women with a high occupation level [WMD (random effects) 0.76, 95% CI: 0.44, 1.09; Figure 3d]. However, moderate heterogeneity existed between studies in each analysis (I2 = 40.7% and 65.7%, respectively).
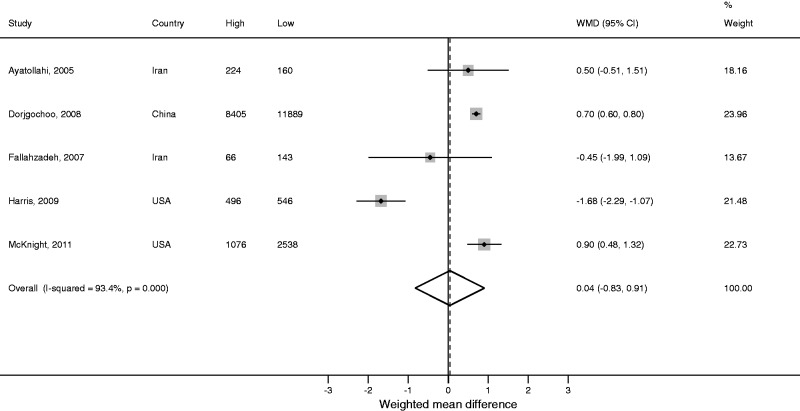
Five studies reported ANM by level of income.45,47,51,60,71 A pooled analysis of these studies revealed no effect of income on ANM (WMD 0.04, 95% CI: −0.83, 0.91), but there was substantial heterogeneity between studies (I2 = 93.4%; Figure 4).
Figure 4.
Meta-analysisa of the unadjusted effect of income on age of menopause.
aRandom effects estimate.
Smoking
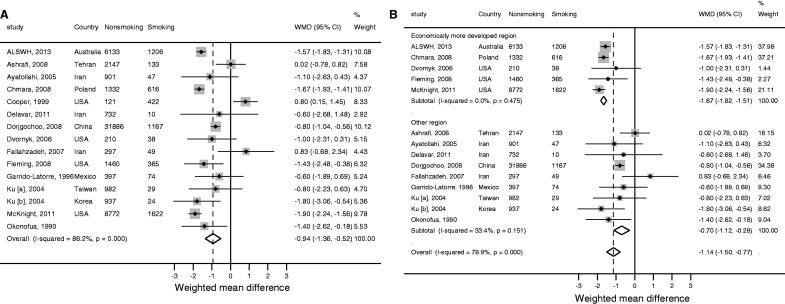
The meta-analysis of smoking comprised 15 study populations, including ALSWH.35,36,41–43,45–49,55,60,61 Overall, smoking was associated with having an earlier mean ANM by almost a year (WMD -0.94, 95% CI: -1.36, -0.52; Figure 5a). Substantial heterogeneity was found between studies (I2 = 86.2%), which remained after excluding one outlying study where the estimate was in the opposite direction42 (I2 = 78.9%). A subgroup analysis revealed that the heterogeneity was explained by region (Figure 5b), with the effect of smoking greater in ‘economically more developed regions’ (WMD -1.67, 95% CI: -1.82, -1.51; heterogeneity: I2 = 0%) than in ‘other regions’ (WMD -0.70, 95% CI: -1.12, -0.28). Meta-regression similarly demonstrated that region was associated with the effect of smoking on mean ANM (coefficient for ‘economically more developed regions’ vs ‘other regions’ -0.91, 95% CI: -1.25, -0.57; P < 0.001), explaining 99.6% of the observed between-study variance.
Figure 5.
Meta-analysisa of (a) the unadjusted effect of smoking on age of menopause and (b) the unadjusted effect of smoking on age of natural menopause, stratified by whether countries were ‘economically more developed regions’ or ‘other regions’.b WMD, weighted mean difference; CI, confidence interval.
aRandom effects estimates; bthe meta-analysis stratified by region excludes one outlying study (Cooper et al.), since the direction of the effect estimate for smoking was clearly contrary to all other studies, and the exclusion of which removed all heterogeneity among ‘economically more developed regions’. The third and fourth columns in each figure give the number of women who were classified as smokers and non-smokers in each study.
One of these 14 studies,47 plus 11 study populations including ALSWH,22–24,26–28,44,53,67,69 reported adjusted HRs for the effect of smoking on ANM. Meta-analysis indicated that smokers were at a 33% increased risk of becoming postmenopausal at a given age compared with non-smokers (pooled HR 1.33, 95% CI: 1.26, 1.40), with substantial heterogeneity between studies (Table 4). Thus, smoking remains associated with an earlier ANM after adjusting for potential confounding factors.
ANM in ex-smokers was rarely reported across studies. Meta-analysis of unadjusted data from three studies, including ALSWH, revealed no difference in ANM among ex-smokers compared with non-smokers. Similarly, pooled adjusted effect estimates revealed no association between ex-smoking and ANM.
Body mass index
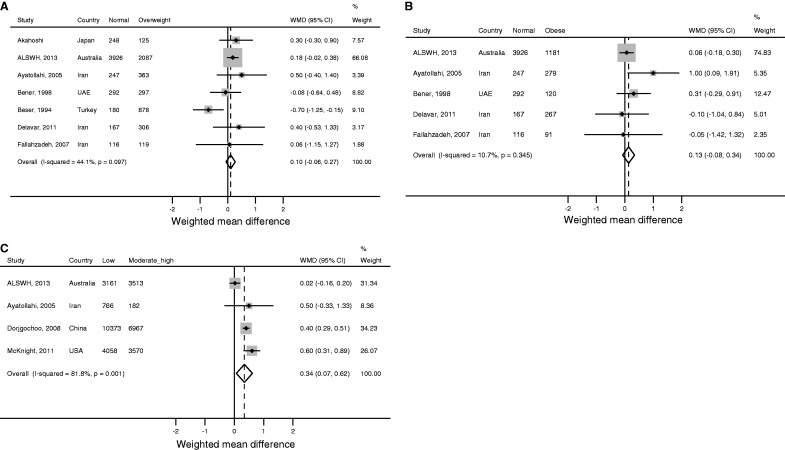
The meta-analysis of the effect of being overweight included ALSWH plus six published studies,33,36–38,43,47 five of which were also included in the analysis of obesity. Overall, no clear association was found between being overweight and ANM [WMD (random effects]) 0.05, 95% CI: -0.25, 0.35; Figure 6a], although there was moderate heterogeneity between studies (I2 = 44.1%). Similarly, there was no clear association between obesity and ANM, although there was a trend for obesity being associated with a later ANM [WMD (fixed effects) 0.13, 95% CI: −0.08, 0.34; Figure 6b], with little heterogeneity between studies (I2 = 10.7%).
Figure 6.
Meta-analysis of the unadjusted effect of (a) overweighta (b) obesityb and (c) moderate/high physical activitya on age of natural menopause. WMD, weighted mean difference; CI, confidence interval.
aRandom effects estimate; bfixed effects estimate. Third and fourth columns in the figures give the number of women classified as being normal weight, overweight or obese, and moderately to highly physically active or physically inactive (low or no physical activity), as appropriate.
Six studies22–24,26,27,67reported adjusted HRs, which we pooled with the results from our analysis of ALSWH. The summary estimate indicated that being overweight was associated with a decreased risk of menopause (i.e. menopause occurred later in overweight women) by about 7% (pooled HR 0.93, 95% CI: 0.91, 0.95). A similar effect was seen for obesity (pooled HR 0.92, 95% CI: 0.85, 0.99), although there was substantial heterogeneity between studies (I2 = 78.9%) (Table 4).
Physical activity
The meta-analysis of physical activity and ANM included three published studies45,60,71 plus ALSWH. Onset of menopause occurred about one-third of a year earlier among women who were physically inactive compared with those who were moderately or highly physically active [WMD (fixed effects) -0.34, 95% CI: -0.62, -0.07; Figure 6c]. There was however substantial heterogeneity between studies (I2 = 81.8%), which appeared to be explained by differences between the results for ALSWH and the other three studies, since the exclusion of ALSWH removed all heterogeneity.
When the results from ALSWH were pooled with an additional four studies22,26,27,67 that reported adjusted HRs there was no effect of physical activity on ANM (Table 4).
Examination of funnel plots for the effect of smoking, education and BMI revealed no suggestion of publication bias through non-publication of small or null associations among small study populations (Supplementary Figure 1, available as Supplementary data at IJE online).
In general, the findings from studies that reported data on associations between SEP and lifestyle factors and ANM that could not be included in the analyses were in keeping with the overall results obtained from the meta-analyses described above (Supplementary Tables 1, 3 and 4, available as Supplementary data at IJE online).
Discussion
This is the first systematic review and meta-analysis to investigate regional variability in ANM, and the effect of SEP and a range of lifestyle factors on ANM. Among 44 community-based studies across six continents, with additional unpublished findings from ALSWH, we found that ANM was generally lower among African, Latin American, Asian and Middle Eastern countries and highest in Europe and Australia, followed by the USA. In terms of SEP, the estimates from meta-analyses for education and occupation level both demonstrated a dose response for later ANM, with increasing education or occupation level associated with increasing ANM. Smoking was associated with having ANM almost 1 year earlier. Moderate or high physical activity was also associated with earlier menopause when unadjusted data were included in the meta-analysis, but this attenuated to give no evidence of effect when adjusted data were included. Meta-analysis of adjusted estimates also suggested that being overweight was marginally associated with slightly later ANM, whereas no association was found with obesity.
Timing of menopause is an indicator of ovarian function and ageing, and therefore critical for women’s health. Both early and late ANM have been shown to be associated with adverse health outcomes in postmenopause women, highlighting the importance of identification of factors across the life course that can affect ANM. Based on our findings, socioeconomic and lifestyle factors assessed in mid life are associated with timing of ANM, and may thereby influence future disease risk.
Although genetic and racial factors have been proposed to be an important determinant of ANM,9,11 other factors could also explain the international variability of ANM. The pattern of regional differences evident for ANM in this study, however, support the role of SEP and lifestyle factors rather than just genetic factors, with an ‘economically more developed region’/‘other region’ dichotomy tending to distinguish ANM across regions. Moreover, the two studies from ‘other regions’ nations with the highest ANM were from Japan33 and Taiwan,40 which arguably also have a relatively long record of being high-income and economically advanced nations.
To our knowledge, this is the first meta-analysis of the available evidence on SEP and ANM. It highlights the role of SEP in delaying ANM, with both higher education and occupation levels associated with later onset of menopause. Authors of a previous systematic review on education level and ANM concluded that there was no unequivocal evidence for an association between education and ANM.16 However, results from our meta-analysis demonstrate an overall clear dose-response relationship between lower education and earlier ANM, even among studies that had adjusted for confounding factors. In addition, the similar dose response that is evident for occupation in our meta-analysis complements these findings on education and provides further support for the role of SEP in influencing ANM. The mechanism underlying this association remains unclear, but may be at least partially explained by lifestyle factors such as smoking and diet. Even though most studies adjusted for lifestyle factors smoking and BMI, no adjustments were made for dietary factors. Also, a birth cohort study found that lower childhood, but not adulthood, social class was associated with earlier ANM.72 Adverse childhood experiences may include household crowding, father’s occupation, no hot water supply in the house, shared bedroom and no car access.73 Childhood experiences may explain some of the observed association between education and occupation and ANM.
Our finding that current smoking is a risk factor for earlier ANM concurs with the conclusions drawn from previous reviews.13,14 The overall magnitude of effect of smoking on ANM in our meta-analysis is similar to that found in an earlier meta-analysis. The latter included six studies, compared with the 14 studies included in our review.12 However, the previous meta-analysis mostly identified studies performed in the USA, included some non-population-based studies and, after excluding one study, found no heterogeneity between studies. In contrast, our review was more comprehensive in identifying additional relevant studies from a range of countries. Sub-group and meta-regression analyses also suggested that observed heterogeneity is due to differences in results from ‘economically more developed regions’ vs ‘other regions’. It remains unclear whether this is a true difference in the effect of smoking, or is due to differences in study methodology or measurement error. In terms of biological mechanisms, smoking has been associated with hormonal production and metabolism, including expression of CYP1A2 genotype and decreased serum estrogen levels,74 increased concentrations of 2-hydroxyestrogen,75 and increased quantity of androgens,76 all contributing towards an anti-estrogen effect resulting in earlier natural menopause. This proposed biological mechanism, whereby smoking may influence hormone levels in a way which is reversible upon cessation of smoking, is consistent with studies that have identified ex-smokers as having ANM close to non-smokers.26,42,45,53,67 In keeping with this, we found no difference in ANM when comparing ex-smokers with non-smokers, although data on ex-smoking was rarely reported, with most studies comparing current vs never smokers. This finding is inconsistent with the notion of cumulative and irreversible damage to ovarian follicles due to exposure to polycyclic aromatic hydrocarbons77,78 and requires further investigation.
In comparison with smoking, BMI categories in relation to ANM have been less frequently studied. Our review suggests that being overweight or obese may be associated with a very slightly later ANM. However, published data are limited and findings from studies are mixed. Overweight and obese women have higher circulating level of estrogen coupled with low levels of sex hormone-binding globulin which may result in delayed ANM.79 Studies looking at weight change, which has been suggested to create hormonal imbalances resulting in decreased rate of follicular atresia,79 found a positive correlation between weight change and timing of natural menopause.45,80,81 Evidence on physical activity and ANM is also scarce. Vigorous activity has been shown to restrict ovarian function by decreasing serum estrogens and increasing sex hormone-binding globulin, which could lead to earlier menopause.82 Overall, a modest association of moderate to high physical activity with earlier ANM was observed in unadjusted, but not adjusted, meta-analysis. These inconclusive findings for overweight/obesity and physical activity may reflect the inconsistent results from included studies, the weakness of the association or the lack of power due to limited number of studies that could be included in meta-analyses. Given these limited and mixed findings, further research on how BMI and physical activity influence ANM is needed.
Heterogeneity in mean ANM was moderate to substantial, based on estimates of I2, for many of the meta-analyses. However, in keeping with the recommendations that heterogeneity between studies does not preclude synthesis in a meta-analysis, and that sources of heterogeneity should be investigated,83,84 we explored heterogeneity using sub-group analysis and meta-regression based on a priori study characteristics.
Despite restricting our inclusion criteria to identify more methodologically robust studies, many of the studies included in this systematic review had a number of limitations. Most studies were based on cross-sectional data, which are more prone to recall errors as accuracy of reported age may vary according to the duration from the time of ANM to the time of the study. For women with natural menopause, it has been found previously that 70% recalled their ANM correctly within 1 year,85 whereas older women tend to report later ANM.44 Responses for SEP and lifestyle factors obtained in cross-sectional studies may also be misclassified as they can reflect the participants’ status at the time of the study, particularly an issue for studies with older women, rather than at or prior to menopause. The process of study selection for the review also revealed the use of different definitions for ANM. Some studies have reported ANM as age at last menstrual period, whereas others use 12 months since last menstrual period according to the WHO definition. However, the meta-regression analysis undertaken as part of this review did not identify the inclusion of women over the age of 65 years or the definition of ANM as a source of heterogeneity with respect to mean ANM across studies. Study design, in terms of prospective vs cross-sectional, did however account for a modest proportion of the observed heterogeneity. The definitions of and strata within risk factors differed across studies, rendering them sometimes difficult to compare and potentially leading to issues of misclassification. Finally, studies varied with regard to factors adjusted for in the multivariable analysis, and others were unable to adjust for important confounding factors at all as the relevant data were not collected. These limitations are reflected in the number of studies available for inclusion on the meta-analyses, relative to the total number of relevant studies identified.
Since we completed our literature search, a multiethnic longitudinal study from the USA was published on modifiable factors and ANM.86 Gold et al. found no evidence of racial/ethnic variation in ANM after adjusting for socioeconomic, lifestyle and health variables. Higher education level, higher weight at baseline, not smoking and having less physical activity were all associated with later ANM.86 These results are consistent with our findings and therefore inclusion of this study in our review is unlikely to have changed our results or conclusions. Furthermore, we examined selected risk factors in relation with ANM to maintain the feasibility of the review within the limits of our available resources. Other potential determinants of ANM may also include diet, alcohol intake and anthropometric measures such as waist and hip circumference. Also, we did not seek to identify studies on diet and ANM in this review, since the differences in how diet is measured would have impeded any sensible pooling of study results. A careful examination of how diet impacts on ANM will benefit from a pooled analysis of individual patient data where similar dietary measurements were used.
Conclusion and future research
In summary, our systematic review and series of meta-analyses indicate that there is a dose-response association between lower SEP and later menopause, and confirms the association of smoking with earlier menopause. By contrast, the impacts of BMI and physical activity remain inconclusive. Findings also highlighted that geographical region in part explained the variation in mean ANM evident across studies.
Future research needs to establish a deeper understanding of the effect of socioeconomic position and biological and lifestyle factors on ANM. To achieve this, research utilizing large prospective cohort studies and pooling of individual-level data from studies that use similar methods but are drawn from different populations is needed. Future research also needs to determine the impact of ANM variability on subsequent health for postmenopause women and estimate the long-term clinical and public health relevance of the observed effects of risk factors on ANM.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The Australian Longitudinal Study on Women’s Health, which was conceived and developed by groups of interdisciplinary researchers at the Universities of Newcastle and Queensland, is funded by the Australian Government Department of Health. Caroline A Jackson is sponsored by the Australian National Health and Medical Research Council Centre for Research Excellence in Women’s Health (APP100986) and Gita D Mishra is supported by an Australian Research Council Future Fellowship (FT120100812).
Conflict of interest: None declared.
Supplementary Material
References
- 1.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev 1993;15:36. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi S, La Vecchia C, Booth M, et al. Pooled analysis of 3 European case-control studies of ovarian cancer: II. Age at menarche and at menopause. Int J Cancer 1991;49:57–60 [DOI] [PubMed] [Google Scholar]
- 3.Atsma F, Bartelink M-LEL, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 2006;13:265–79 [DOI] [PubMed] [Google Scholar]
- 4.Joakimsen O, Bønaa KH, Stensland-Bugge E, Jacobsen BK. Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis: The Tromsø Study. J Clin Epidemiol 2000;53:525–30 [DOI] [PubMed] [Google Scholar]
- 5.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: The Framingham Heart Study. Stroke 2009;40:1044–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–71 [DOI] [PubMed] [Google Scholar]
- 7.Ossewaarde ME, Bots ML, Verbeek ALM, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–62 [DOI] [PubMed] [Google Scholar]
- 8.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–97 [DOI] [PubMed] [Google Scholar]
- 9.Thomas F, Renaud F, Benefice E, De Meeus T, Guegan JF. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol 2001;73:271–90 [DOI] [PubMed] [Google Scholar]
- 10.Morabia A, Costanza MC, ; World Health Organization Collaborative Study N. International variability in ages at menarche, first livebirth, and menopause. Am J Epidemiol 1998;148:1195–205 [DOI] [PubMed] [Google Scholar]
- 11.Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab 2005;90:3427–30 [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Tan L, Yang F, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause 2012;19:126–32 [DOI] [PubMed] [Google Scholar]
- 13.Parente RC, Faerstein E, Celeste RK, Werneck GL. The relationship between smoking and age at the menopause: A systematic review. Maturitas 2008;61:287–98 [DOI] [PubMed] [Google Scholar]
- 14.Midgette AS, Baron JA. Cigarette smoking and risk of natural menopause. Epidemiology 1990;1:474–80 [DOI] [PubMed] [Google Scholar]
- 15.Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3–9 [DOI] [PubMed] [Google Scholar]
- 16.Canavez FS, Werneck GL, Parente RCM, Celeste RK, Faerstein E. The association between educational level and age at the menopause: a systematic review. Arch Gynecol Obstet 2011;283:83–90 [DOI] [PubMed] [Google Scholar]
- 17.Brown WJ, Bryson L, Byles JE, et al. Women's Health Australia: recruitment for a national longitudinal cohort study. Women Health 1999;28:23–40 [DOI] [PubMed] [Google Scholar]
- 18.Lee C, Dobson AJ, Brown WJ, et al. Cohort profile: the Australian longitudinal study on women's health. Int J Epidemiol 2005;34:987–91 [DOI] [PubMed] [Google Scholar]
- 19.Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause 2009;16: 1021–29 [DOI] [PubMed] [Google Scholar]
- 20.Guthrie J, Dennerstein L, Dudley E. Weight gain and the menopause: a 5-year prospective study. Climacteric 1999;2:205–11 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO, 2000 [PubMed] [Google Scholar]
- 22.Nagel G, Altenburg H-P, Nieters A, Boffetta P, Linseisen J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005;52:337–47 [DOI] [PubMed] [Google Scholar]
- 23.Yasui T, Hayashi K, Mizunuma H, et al. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas 2012;72:249–55 [DOI] [PubMed] [Google Scholar]
- 24.Li L, Wu J, Pu D, et al. Factors associated with the age of natural menopause and menopausal symptoms in Chinese women. Maturitas 2012;73:354–60 [DOI] [PubMed] [Google Scholar]
- 25.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the multiethnic cohort study. Am J Epidemiol 2008;167:1287–94 [DOI] [PubMed] [Google Scholar]
- 26.Dratva J, Real FG, Schindler C, et al. Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–94 [DOI] [PubMed] [Google Scholar]
- 27.Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health 2003;93:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–74 [DOI] [PubMed] [Google Scholar]
- 29.Sterne JAC. Meta-analysis in Stata: An Updated Collection from the Stata Journal. College Station, TX: Stata Press, 2009 [Google Scholar]
- 30.Harbord RM, Higgins JP. Meta-regression in Stata. Stata J 2008;8:493–519 [Google Scholar]
- 31.World Health Organization (WHO). Research on the Menopause in the 1990s. Geneva: World Health Organization, 1996 [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:W65–94 [DOI] [PubMed] [Google Scholar]
- 33.Akahoshi M, Soda M, Nakashima E, et al. The effects of body mass index on age at menopause. Int J Obes Relat Metab Disord 2002;26:961–68 [DOI] [PubMed] [Google Scholar]
- 34.Ansari H, Rakhshani F, Vahedi MM, Mostafapour FK. Evaluation of socio-economic factors related to the natural menopause age in Zahedan, Southeastern Iran. Maturitas 2009;63:S67 [Google Scholar]
- 35.Ashrafi M, Ashtiani SK, Malekzadeh F, Amirchaghmaghi E, Kashfi F, Eshrati B. Factors associated with age at natural menopause in Iranian women living in Tehran. Int J Gynaecol Obstet 2008;102:175–76 [DOI] [PubMed] [Google Scholar]
- 36.Ayatollahi SMT, Ghaem H, Ayatollahi SAR. Sociodemographic factors and age at natural menopause in Shiraz, Islamic Republic of Iran. East Mediterr Health J 2005;11:146–54 [PubMed] [Google Scholar]
- 37.Bener A, Rizk DE, Ezimokhai M, Hassan M, Micallef R, Sawaya M. Consanguinity and the age of menopause in the United Arab Emirates. Int J Gynaecol Obstet 1998;60:155–60 [DOI] [PubMed] [Google Scholar]
- 38.Beser E, Aydemir V, Bozkaya H. Body mass index and age at natural menopause. Gynecol Obstet Invest 1994;37:40–42 [DOI] [PubMed] [Google Scholar]
- 39.Blumel JE, Chedraui P, Calle A, et al. Age at menopause in Latin America. Menopause 2006;13:706–12 [DOI] [PubMed] [Google Scholar]
- 40.Chang C, Chow SN, Hu Y. Age of menopause of Chinese women in Taiwan. Int J Gynaecol Obstet 1995;49:191–92 [DOI] [PubMed] [Google Scholar]
- 41.Chmara-Pawlinska R, Szwed A. Cigarette smoking and the age of natural menopause in women in Poland. Przegl Lek 2004;61:1003–05 [PubMed] [Google Scholar]
- 42.Cooper GS, Sandler DP, Bohlig M. Active and passive smoking and the occurrence of natural menopause. Epidemiology 1999;10:771–73 [PubMed] [Google Scholar]
- 43.Delavar MA, Hajiahmadi M. Age at menopause and measuring symptoms at midlife in a community in Babol, Iran. Menopause 2011;18:1213–18 [DOI] [PubMed] [Google Scholar]
- 44.Do KA, Treloar SA, Pandeya N, et al. Predictive factors of age at menopause in a large Australian twin study. Hum Biol 1998;70:1073–91 [PubMed] [Google Scholar]
- 45.Dorjgochoo T, Kallianpur A, Gao YT, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008;15:924–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dvornyk V, Long J-R, Liu P-Y, et al. Predictive factors for age at menopause in Caucasian females. Maturitas 2006;54:19–26 [DOI] [PubMed] [Google Scholar]
- 47.Fallahzadeh H. Age at natural menopause in Yazd, Islamic republic of Iran. Menopause 2007;14:900–04 [DOI] [PubMed] [Google Scholar]
- 48.Fleming LE, Levis S, LeBlanc WG, et al. Earlier age at menopause, work, and tobacco smoke exposure. Menopause 2008;15:1103–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrido-Latorre F, Lazcano-Ponce EC, López-Carrillo L, Hernández-Avila M. Age of natural menopause among women in Mexico city. Int J Gynaecol Obstet 1996;53:159–66 [DOI] [PubMed] [Google Scholar]
- 50.Hagstad A. Gynecology and sexuality in middle-aged women. Women Health 1988;13:57–80 [DOI] [PubMed] [Google Scholar]
- 51.Harris AES, Bernstein RM, Kuhle BX. Predicting age at onset of menopause: Testing the “adaptive onset” hypothesis. Maturitas 2009;64:193–95 [DOI] [PubMed] [Google Scholar]
- 52.Hong JS, Yi S-W, Kang HC, et al. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas 2007;56:411–19 [DOI] [PubMed] [Google Scholar]
- 53.Kaczmarek M. The timing of natural menopause in Poland and associated factors. Maturitas 2007;57:139–53 [DOI] [PubMed] [Google Scholar]
- 54.Kriplani A, Banerjee K. An overview of age of onset of menopause in northern India. Maturitas 2005;52:199–204 [DOI] [PubMed] [Google Scholar]
- 55.Ku SY, Kang JW, Kim H, et al. Regional differences in age at menopause between Korean-Korean and Korean-Chinese. Menopause 2004;11:569–74 [DOI] [PubMed] [Google Scholar]
- 56.Kwawukume E, Ghosh T, Wilson J. Menopausal age of Ghanaian women. Int J Gynaecol Obstet 1993;40:151–55 [DOI] [PubMed] [Google Scholar]
- 57.Lawlor DA, Ebrahim S, Davey Smith G. The association of socio-economic position across the life course and age at menopause: the British Women's Heart and Health Study. Int J Gynaecol Obstet 2003;110:1078–87 [PubMed] [Google Scholar]
- 58.Loh F-H, Khin L-W, Saw S-M, Lee JJM, Gu K. The age of menopause and the menopause transition in a multiracial population: a nation-wide Singapore study. Maturitas 2005;52:169–80 [DOI] [PubMed] [Google Scholar]
- 59.Magursky V, Mesko M, Sokolik L. Age at the menopause and onset of the climacteric in women of Martin District, Czechoslovkia. Int J Fertil 1975;20:17–23 [PubMed] [Google Scholar]
- 60.McKnight KK, Wellons MF, Sites CK, et al. Racial and regional differences in age at menopause in the United States: findings from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Am J Obstet Gynecol 2011;205:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okonofua F, Lawal A, Bamgbose J. Features of menopause and menopausal age in Nigerian women. Int J Gynaecol Obstet 1990;31:341–45 [DOI] [PubMed] [Google Scholar]
- 62.OlaOlorun F, Lawoyin T. Age at menopause and factors associated with attainment of menopause in an urban community in Ibadan, Nigeria. Climacteric 2009;12:352–63 [DOI] [PubMed] [Google Scholar]
- 63.Özdemir O, Çöl M. The age at menopause and associated factors at the health center area in Ankara, Turkey. Maturitas 2004;49:211–19 [DOI] [PubMed] [Google Scholar]
- 64.Sievert LL, Hautaniemi SI. Age at menopause in Puebla, Mexico. Hum Biol 2003;75:205–26 [DOI] [PubMed] [Google Scholar]
- 65.van Noord PAH, Dubas JS, Dorland M, Boersma H, Velde ET. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril 1997;68:95–102 [DOI] [PubMed] [Google Scholar]
- 66.Ortiz AP, Harlow SD, Sowers M, Nan B, Romaguera J. Age at natural menopause and factors associated with menopause state among Puerto Rican women aged 40-59 years, living in Puerto Rico. Menopause 2006;13:116–24 [DOI] [PubMed] [Google Scholar]
- 67.Morris DH, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: analyses from the breakthrough generations study. Am J Epidemiol 2012;175:998–1005 [DOI] [PubMed] [Google Scholar]
- 68.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol 1994;139:64–76 [DOI] [PubMed] [Google Scholar]
- 69.Cramer DW, Harlow BL, Xu H, Fraer C, Barbieri R. Cross-sectional and case-controlled analyses of the association between smoking and early menopause. Maturitas 1995;22:79–87 [DOI] [PubMed] [Google Scholar]
- 70.Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Association of diet and other lifestyle with onset of menopause in Japanese women. Maturitas 1998;29:105–13 [DOI] [PubMed] [Google Scholar]
- 71.Ayatollahi SMT, Ghaem H, Ayatollahi SAR. Determinants of age at natural menopause in Shiraz. Iran J Med Sci 2002;27:131–33 [Google Scholar]
- 72.Hardy R, Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. BJOG 2005;112:346–54 [DOI] [PubMed] [Google Scholar]
- 73.Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Women Health 2009;5:175–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meek MD, Finch GL. Diluted mainstream cigarette smoke condensates activate estrogen receptor and aryl hydrocarbon receptor-mediated gene transcription. Environ Res 1999;80: 9–17 [DOI] [PubMed] [Google Scholar]
- 75.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 1986;315:1305–09 [DOI] [PubMed] [Google Scholar]
- 76.Bancroft J, Cawood EHH. Androgens and the menopause; a study of 40–60-year-old women. Clin Endocrinol 1996; 45:577–87 [DOI] [PubMed] [Google Scholar]
- 77.Essenberg J, Fagan L, Malerstein A. Chronic poisoning of the ovaries and tests of albino rats and mice by nicotine and cigarette smoke. West J Surg Obstet Gynecol 1951;59:27–32 [PubMed] [Google Scholar]
- 78.Mattison D, Thorgeirsson S. Smoking and industrial pollution, and their effects on menopause and ovarian cancer. Lancet 1978;311:187–88 [DOI] [PubMed] [Google Scholar]
- 79.Leidy LE. Timing of menopause in relation to body size and weight change. Hum Biol 1996;68:967–82 [PubMed] [Google Scholar]
- 80.Aydin ZD. Determinants of age at natural menopause in the Isparta Menopause and Health Study: premenopausal body mass index gain rate and episodic weight loss. Menopause 2010;17:494–505 [DOI] [PubMed] [Google Scholar]
- 81.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol 1997;145:124–33 [DOI] [PubMed] [Google Scholar]
- 82.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res 2004;64:2923–28 [DOI] [PubMed] [Google Scholar]
- 83.Higgins JP. Heterogeneity in meta-analysis should be expected and appropriately quantified (Commentary). Int J Epidemiol 2008;37:1158–60 [DOI] [PubMed] [Google Scholar]
- 84.Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ 2008;336:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–23 [DOI] [PubMed] [Google Scholar]
- 86.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.