The evidence for a causal association between tobacco consumption and mortality presented in the Mendelian randomization (MR) study by Rode and colleagues1 is not unexpected. Nevertheless, it is the first time that the smoking-mortality relationship has been demonstrated using these causal analysis methods, although studies of monozygotic twins discordant for smoking have arrived at similar conclusions.2 This is an important proof of principle of the MR technique for investigating the causal effects of smoking. However, this finding also raises a potentially important methodological issue for future MR studies using this variant.
The genetic variant used in this analysis, rs1051730, is a single nucleotide polymorphism (SNP) in perfect correlation with a variant in the CHRNA5 gene (rs16969968) that leads to an amino acid change (D398N) in the nicotinic receptor alpha-5 subunit protein. This is by far the strongest genetic determinant of smoking behaviour identified in genome-wide association studies to date.3 This variant is robustly associated with smoking heaviness among smokers and shows evidence in some populations of associations with smoking cessation.4,5 However, associations with smoking initiation (i.e. being an ever rather than a never smoker) have not been clearly established.6
The lack of evidence for an association of this variant with ever smoking has been a useful feature of the smoking MR analyses conducted to date. As the variant only associates with smoking heaviness among smokers, never smokers can be used as a control group to test the assumption of no pleiotropy. The lack of association between rs1051730 and mortality in never smokers in the analyses by Rode and colleagues is good evidence that these effects are due to tobacco consumption, and not to a pleiotropic effect of the gene.1
However, this in turn raises an interesting possibility. We would expect that higher rates of mortality among smokers carrying the smoking-increasing allele of this variant1 would lead to an association between genotype and likelihood of being an ever smoker in older age. As smokers with the smoking-increasing allele are less likely to survive into old age, the smoking increasing allele will in turn become less prevalent among smokers as the age of the population increases. Some support for this was found by Rode and colleagues in the Copenhagen General Population Study, where there was some evidence that the smoking increasing allele of rs1051730 was weakly associated with lower age in ever smokers.1 Furthermore, a negative association between the smoking-increasing allele and being an ever smoker, could potentially mask associations between the variant and smoking initiation in samples with a wide age range.
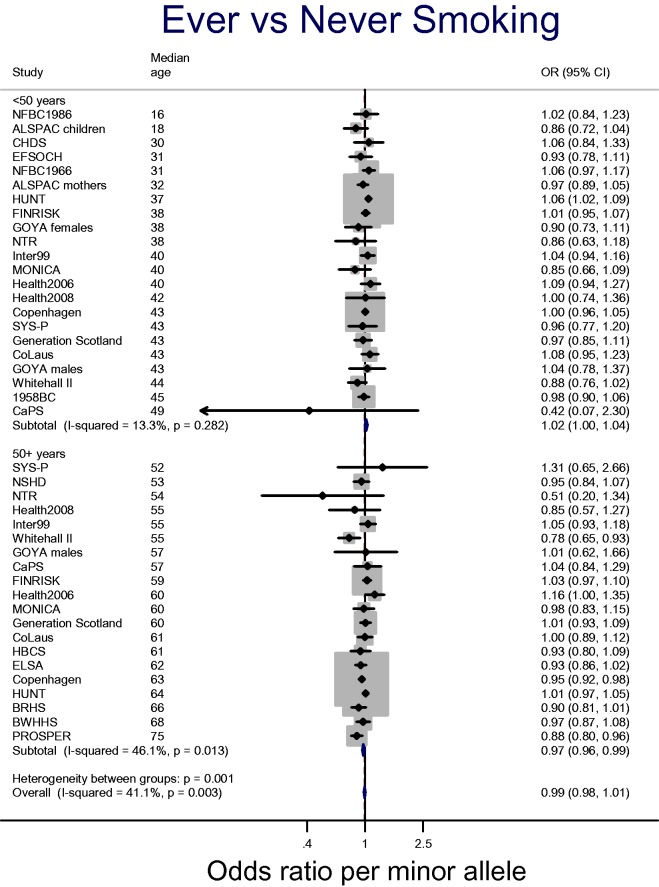
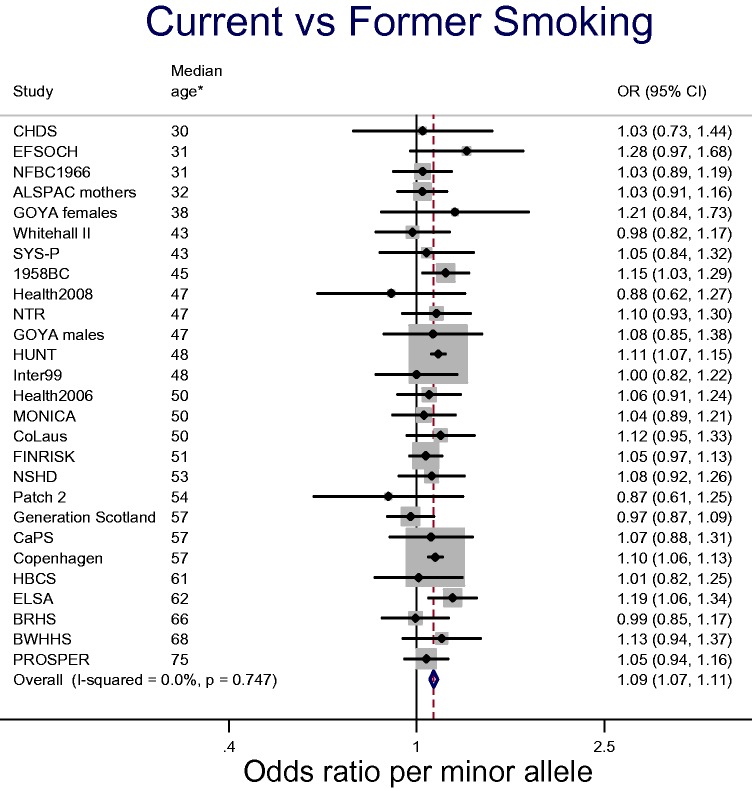
It is not currently clear to what extent this attrition by genotype impacts on associations with ever smoking. Figure 1 shows data from the Copenhagen General Population Study combined with data from the consortium for Causal Analysis Research in Tobacco and Alcohol (CARTA: http://www.bris.ac.uk/expsych/research/brain/targ/research/collaborations/carta), a collaboration of over 30 studies established to conduct MR analyses of the health and socioeconomic effects of smoking. We stratified analyses within each study by age (<50 years, ≥50 years), based on data suggesting that the effects of smoking on mortality are strongest after the age of 50 years.7 There is suggestive evidence that the smoking increasing allele may be positively associated with smoking initiation (i.e. ever vs never) in the under-50 age group, but negatively associated with smoking initiation in the 50 and over age group (P-value for heterogeneity between age groups = 0.001). It is important to note that these data are not conclusive, and more in-depth analyses of this possibility are required. For example, the crude age stratification we have employed may not be the most appropriate. Family studies may also be useful for investigating selection effects due to mortality, as genotypes of missing parents may be inferred from offspring genotypes. Within the CARTA and Copenhagen General Population Study samples, there is also clear evidence for an effect of rs16969968-rs1051730 genotype on smoking cessation, with the minor allele associated with an 9% increase in the odds of being a current rather than a former smoker [95% confidence interval (CI): 7% to 11%] (see Figure 2).
Figure 1.
Association between rs16969968-rs1051730 and smoking initiation in the CARTA consortium and the Copenhagen General Population Study.
Figure 2.
Association between rs16969968-rs1051730 and smoking cessation in the CARTA consortium and the Copenhagen General Population Study. *Median age is median age of total sample (including never smokers).
Associations between genotype and smoking status have potentially important implications for future MR studies of the causal effects of smoking. This is because stratification on a common effect of two variables can induce a phenomenon known as collider bias.8 If smoking status is a common effect of both genotype and either the outcome of interest or confounders of the association between smoking status and the outcome of interest, this can lead to a statistical association between genotype and outcome measure in the absence of a true causal effect.9 Researchers will therefore need to consider associations between rs16969968-rs1051730 and smoking initiation and cessation when conducting MR analyses. The association between this variant and smoking cessation may be less problematic if we are interested in the difference between ever and never smokers. One approach to overcome the problem of collider bias, which has been used in MR analyses of alcohol, is to stratify analyses by an exogenous variable, sex, rather than by drinking status.10 This can only be done in samples where prevalence of alcohol consumption is strongly patterned by sex (e.g. in East Asian populations where alcohol consumption is very low among females11). This may not be possible for smoking MR due to the low allele frequencies of rs16969968-rs1051730 in many non-European populations.
MR is a potentially very useful tool for unravelling the causal effects of tobacco use and the pathways through which these operate; there are still many health outcomes which are strongly associated with smoking, but for which causal evidence is lacking. It is important that future MR studies of tobacco use take into account issues such as collider bias and other selection biases related to this variant, such as misreporting of smoking status, to prevent the reintroduction of confounding into analyses designed to minimize this problem.
Acknowledgements
We are grateful to the CARTA studies for providing data for these analyses. Acknowledgements for each of the CARTA studies can be found at [http://www.bris.ac.uk/expsych/research/brain/targ/re search/collaborations/carta]. We would also like to thank Rode and colleagues for providing genotype counts from the Copenhagen General Population Study to contribute to this commentary.
Funding
Amy Taylor and Marcus Munafò are members of the UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. This work was supported by the Wellcome Trust (grant number 086684) and the Medical Research Council (grant numbers MR/J01351X/1, G0800612, G0802736, MC_UU_12013/1-9).
Conflict of interest: None declared.
References
- 1.Rode L, Bojesen SE, Weischer M, Nordestgaard BG. High tobacco consumption is causally associated with increased all-cause mortality in a general population sample of 55,568 individuals, but not with short telomeres: A Mendelian randomization study. Int J Epidemiol 30 June 2014. Epub ahead of print. PMID: 24906368 [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol 2002;156:985–93 [DOI] [PubMed] [Google Scholar]
- 3.Loukola A, Hallfors J, Korhonen T, Kaprio J. Genetics and smoking. Curr Addict Rep 2014;1:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freathy RM, Ring SM, Shields B, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet 2009;18:2922–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics 2013;23:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware JJ, van den Bree M, Munafò MR. from men to mice: chrna5/chrna3, smoking behavior and disease. Nicotine Tob Res 2012;14:1291–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004;328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol 2010;39:417–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glymour MM, Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol 2012;175: 332–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr 2011;6:27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Davey Smith G, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med 2008;5:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]