Abstract
The Muzaffarpur-TMRC Health and Demographic Surveillance System (HDSS), established in 2007, was developed as an enlargement of the scope of a research collaboration on the project Visceral Leishmaniasis in Bihar, which had been ongoing since 2005. The HDSS is located in a visceral leishmaniasis (VL)-endemic area in the Muzaffarpur district of Bihar state in India. It is the only HDSS conducting research on VL, which is a vector-borne infectious disease transmitted by female phlebotomine sandflies and is fatal if left untreated. Currently the HDSS serves a population of over 105 000 in 66 villages. The HDSS collects data on vital events including pregnancies, births, deaths, migration and marriages, as well as other socio-economic indicators, at regular intervals. Incident VL cases are identified. The HDSS team is experienced in conducting both qualitative and quantitative studies, sample collection and rapid diagnostic tests in the field. In each village, volunteers connect the HDSS team with the community members. The Muzaffarpur-TMRC HDSS provides opportunities for studies on VL and other neglected tropical diseases (NTDs) and their interaction with demographic events such as migration. Queries related to research collaborations and data sharing can be sent to Dr Shyam Sundar at [drshyamsundar@hotmail.com].
Key Messages.
We have a comprehensive dataset of patients of visceral leishmaniasis and their clinical characteristics (including laboratory data) since 2002.
Poor-quality houses (damp floors and walls with cracks and crevices) and poor socio-economic conditions lead to higher indoor sandfly density (vector) and a higher visceral leishmaniasis caseload.
Asymptomatic serologically positive individuals are at higher risk of subsequent clinical manifestations of visceral leishmaniasis.
Village volunteers have encouraged greater participation of the community in the HDSS.
Why was the health and demographic surveillance system set up?
The Muzaffarpur-TMRC Health and Demographic Surveillance System (Muzaffarpur-TMRC HDSS) has been built on the work conducted by research projects on visceral leishmaniasis (VL) in Muzaffarpur district, Bihar (India), since 2005. Visceral leishmaniasis (VL)—commonly known as kala-azar—is a vector-borne infectious disease transmitted by female phlebotomine sandflies and the disease is fatal if left untreated.1 Common symptoms of VL are prolonged fever of more than 2 weeks with enlarged spleen and non-response to anti-malaria drugs. Relapse or recurrence is common in treated cases even after months. The estimated worldwide annual incidence of VL is 200 000–400 000 cases, two-thirds of which occur in the Indian subcontinent.2 VL is known as the disease of the poorest of the poor.3 Bihar state of India appears at the bottom of the socio-economic rankings in India and it is one of the regions in the world with the highest VL caseload. Bihar contributes to more than 90% of the national cases and 50% of the VL caseload in the Indian subcontinent.4 The government of India has set the elimination target to reduce the annual VL incidence rates to less than one new case per 10 000 population by the year 2015.
The Muzaffarpur-TMRC HDSS was formally set up in 2007 in the framework of the project Visceral Leishmaniasis in Bihar, funded by the National Institute of Health (NIH), USA, under its Tropical Medicine Research Centers (TMRC) grants. This project aimed at studying epidemiological, immunological and genetic determinants of visceral leishmaniasis in endemic areas. As part of the project we brought the populations living in 50 villages of Muzaffarpur district into the framework of the HDSS and monitored them from 2008 to 2012. This area, covering 85 000 people, was expanded later in 2012 to include 20 000 more people living in 16 additional villages which are now part of the Muzaffarpur-TMRC HDSS as well. The objective of creating this HDSS is to develop an efficient platform for research on control of VL as well as other NTDs and health-relevant issues in rural Bihar.
The Muzaffarpur-TMRC HDSS is the first demographic platform in a VL-endemic area and it is the only HDSS conducting research on VL. The main project in this HDSS, funded by NIH, aims at understanding the epidemiology and immune-pathogenesis of VL, as the basis for the development of improved control strategies through vector control, immunotherapeutic intervention and vaccines. This project studies factors that influence the prevalence, distribution and pathogenesis of VL as follows: (i) determinants of disease progression and role of latent infection in transmission; (ii) studies of the sandfly vector; (iii) immune regulation in VL; and (iv) molecular and cellular action of human leukocyte antigen (HLA) class II molecules, the major genetic risk factors for VL. In the HDSS we also record data on other neglected diseases prevalent in rural Bihar (e.g. lymphatic filariasis, helminthic infections) and evaluate their association with VL.
Where is the HDSS area?
The HDSS is located in Muzaffarpur district in Bihar (26.07oN, 85.45oE, area 3175 km2). Average temperatures vary from 32°C in April–May to 14°C in December– January. Rainfall is also variable, with a rainy season from June to September. People are mainly engaged in agricultural and related activities. Rice, wheat and litchis are the main crops. Muzaffarpur is endemic for VL and it has observed three major epidemics in 1978, 1992 and 2007.5 Between 1990 and 2008, over 70 000 cases were reported by the routine surveillance system of the district health services.6 In 2013, the annual VL incidence rate in the Muzaffarpur-TMRC HDSS area was 4.3 per 10 000 individuals, which is above the elimination target set by the Indian government.
Two yearly peaks of VL are observed in the months of April and July (Figure 1). These peaks are evident when we plot monthly reported cases from 2000 to 2008 along with the cases from 2000 to 2004 (lower annual average) and 2005 to 2008 (higher annual average). The majority of the people live in houses made of thatched and/or mud-plastered walls and earthen floors. Living together with animals in mixed dwellings is common (Figure 2). Together with the generally poor housing conditions, lack of sanitation, humid soils littered with organic waste and piles of animal dung, these mixed dwellings provide a perfect habitat for the vector of VL: Phlebotomus argentipes sandflies. The vector control programme relies on indoor residual spraying (IRS) with DDT. Our data show that the IRS coverage (i.e. number of houses sprayed over the total number of houses) in the Muzaffarpur-TMRC HDSS area has increased from 17% in 2008 to 80% in 2012 (Table 1).
Figure 1.
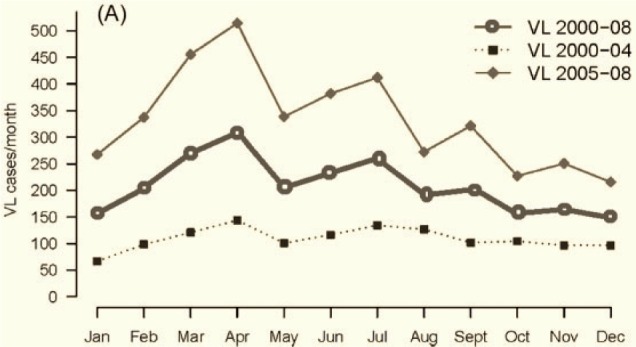
Annual VL peaks based on the data between 2000 and 2008.
Figure 2.
Living together with animals: a mixed dwelling.
Table 1.
Demographic characteristics of 50 original villages of Muzaffarpur-TMRC HDSS, Bihar, India, years 2008 and 2012
| Index results | 2008 | 2012 |
|---|---|---|
| Participants | 81 875 | 94 684 |
| Residents | 68 574 | 72 653 |
| Households | 13 397 | 14 444 |
| Population density | 1360/km2 | 1392/km2 |
| Population growth yearly | – | 3.13% |
| Female/male ratio | 936/1000 | 956/1000 |
| General dependency ratio (GDR) | 81 | 72 |
| Crude birth rate/1000 | 36.7 | 21.1 |
| Mobile coverage | – | 75% |
| IRS coverage | 17% | 80% |
| Net migration | – | −189/1000 |
| Literacy rate | 61% | 64% |
| Male literacy rate | – | 68.28% |
| Female literacy rate | – | 57.10% |
General dependency ratio (GDR) = (number of people aged 0–14 and those aged 65 and over) / number of people aged 15–64) x 100; IRS, indoor residual spraying.
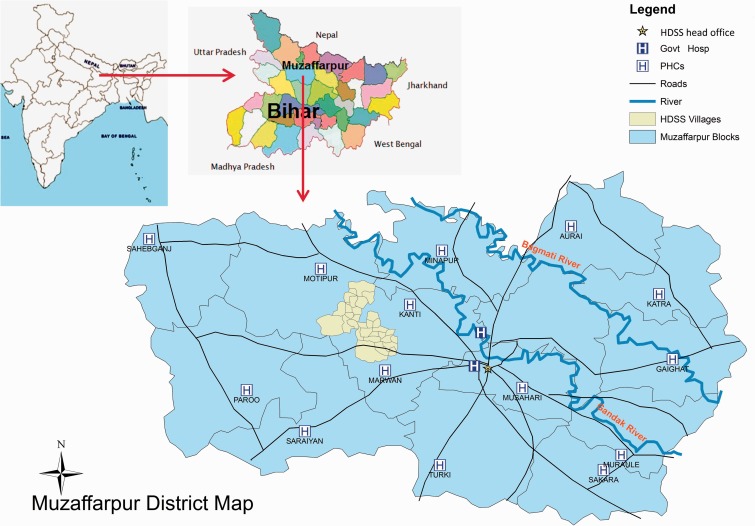
The Muzaffarpur district has 16 blocks (i.e. an administrative sub-division) with an average of 300 000 inhabitants per block and surface areas ranging from 139 km2 (Musahari) to 282 km2 (Paroo). There are 14 community health centres (CHCs)—one per block, plus one district hospital and one medical college reporting epidemiological surveillance data on several diseases to the district health office in Muzaffarpur town (Figure 3).
Figure 3.
Map of Muzaffarpur TMRC-HDSS (PHC: Primary health centre).
The Muzaffarpur-TMRC HDSS area is located 20 km from the district headquarters. It covers 66 villages out of which 50 villages are located in a geographically contiguous area of 68 km2 in the Kanti and Marwan blocks of Muzaffarpur (Figure 3). The perimeter of these 50 villages is 55 km. The remaining 16 villages are located in seven other blocks. In total the HDSS covers a population of about 105 000 (52% male and 48% female). The HDSS population has easy access to a 30-bed hospital located in Muzaffarpur city. The hospital is a centre of excellence in VL research and treatment. Free diagnosis and treatment are available to all VL patients. Staffing consists of three physicians and about 40 paramedical personnel.
Who is covered by the HDSS and how often have they been followed up?
We have conducted house-to-house surveys every year since 2008 and covered all the residents in each household. All communities in the HDSS are located in a rural area. Household is the lowest unit of survey. A household consists of all family members living in the same compound and sharing the same kitchen. A resident is an individual who lives, eats and sleeps in the house for at least 6 months in a year. The average family size is 6 and normally the oldest member of the household is regarded as the head of the household. At the start in 2008, we screened a population of 73 000 individuals living in the 13 674 households of 50 villages. These numbers were increased to 105 000 individuals in 16 284 households from 66 villages in 2012. In this HDSS, only 8.4% of the total households are headed by women; 13% of the families are joint family, i.e. family with grandparents, parents and children. The whole population consists of 70.3% Hindus and 29.7% Muslims. Indian communities are caste based and our HDSS population consists of upper castes (26.8%), backward classes (56.8%) and scheduled castes (16.4%).
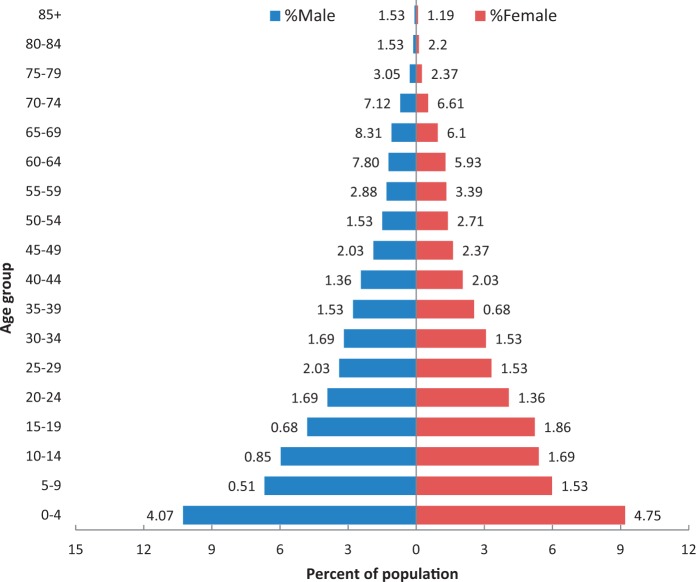
The population pyramid based on the data collected in the 2012 survey shows that the HDSS population matches the demographic profile of low- and middle-income countries. The wide base indicates high birth rates; yearly population growth is more than 3% (Table 1). Under 5-year-old children make up 18%, and only 7% of people are above 60 years of age. The mortality is high in the 0–4 age group; male mortality is higher than female mortality in the 60+ age group (Figure 4). The infant sex ratio is 0.899, i.e. 899 female children per 1000 male children; the overall sex ratio is 0.956. The data available do not allow us to identify the reason behind the higher proportion of boys among infants. The fact that the ratio is closer to one when considering the total population can be attributed to higher life expectancy among women and higher out- migration rates among the male population (overall 231 per 1000 for men vs 145 for women). Out-migration is mainly for job and business and also for higher education. The overall literacy rate is 64%, 68% for men vs 57% for women.
Figure 4.
Population pyramid Muzaffarpur TMRC-HDSS (2012).
We conduct update rounds every 2 months according to the World Health Organization (WHO) and INDEPTH guidelines. During those update rounds, we visit every single household and register demographic events such as births, deaths, marriages, immigration, emigration, pregnancies, miscarriages and stillbirths. Fieldworkers supported by a large network of key community informants (i.e. local leaders, health workers, village volunteers) record these vital events in the study area and maintain a pregnancy register. Similarly our field supervisors visit the community health centres regularly to collect more information (i.e. deliveries during the past 24 h). Fieldworkers and supervisors collect all data onto pre-coded questionnaires.
In our annual survey we record/update socio- economic variables in every household including the materials used to build the house, the number of constructions within the compound, number and types of animals and assets owned, the existence of a latrine and availability of electricity, clean drinking water and garbage disposal facilities. Finally, we also record the level of education of all residents and any mobile numbers. About 75% of households have at least one mobile phone.
What has been measured and how have the HDSS databases been constructed?
Informed consent and census
We invited the community leaders and all the heads of household to participate. We discussed with them the objectives of establishing a HDSS and its benefits to the community. We explained the activities, procedures, services to be provided and how could they support us. After their written informed consent, the initial household census was conducted between July and December 2008. Census and basic socio-economic indicators of the population were repeated in 2009, 2010, 2011, 2012 and 2013. With the consent of community leaders, we recruited at least one village volunteer in each village. Now 75 such volunteers are working as informants for four vital events: birth, death, marriage-in and marriage-out (Figure 5).
Figure 5.
Data collection rounds in Muzaffarpur TMRC-HDSS.
GIS mapping and meteorological data
We mapped the HDSS area (i.e. area boundaries and GPS coordinates of households) just after the baseline survey. We regularly update the map as soon as a new household enters into our database. All the important landmarks such as schools, hospitals, water-bodies, roads etc. have also been mapped. Data on rainfall, temperature and humidity are collected on a routine basis using a meteorological station. Site-specific meteorological data (temperature and humidity) are also collected at the household level for specific study purposes.
Verbal autopsies
We investigate the possible causes of deaths with the use of a verbal autopsy technique. Specially trained field supervisors carry out the verbal autopsies with a standard form.
Serological surveys
In 2009 we conducted the first serological survey to detect Leishmania donovani antibodies among 13 000 individuals of the 11 most endemic villages in the HDSS area. In this survey we collected blood samples using filter papers and tested for VL infection using two serological markers of infection: direct agglutination test (DAT) and rk39 ELISA. In 2010 we repeated the same procedure. Individuals positive for either of the tests in the second round, who were negative for both of the tests in the baseline serosurvey, were considered seroconvertors. These seroconvertors were visited again within 3 weeks to take extra blood samples. To determine the best serological marker (DAT, rk39, Quantiferon or qPCR) we collected 5 ml of blood from each of the seroconvertors. We also collected DNA samples for genetic studies. We also conducted the entire procedure in 16 newly added villages in 2011 and 2012. We are currently following up these seroconvertors every month for at least 2 years after the second survey. We record clinical progressors, verify them and provide treatment through our HDSS hospital (Figure 5).
Data management
Paper-based questionnaires are still used for all information collected. Each paper form receives a serial number. Once the questionnaires are entered into the electronic database they are stored based on the type of demographic event, ordered by serial number. We have adopted the double entry procedures for quality control. The researchers carry out weekly checks for incompleteness of information and inconsistencies. All data management procedures and databases are designed and pre-tested by the programmer in coordination with data manager. The consistency, confidentiality and quality of data are ensured by applying the standard data management processes.
Key findings and publications
We have been primarily conducting studies on visceral leishmaniasis. We showed that people living in poor housing conditions (e.g. thatched houses with earthen floors) and those belonging to lower socio-economic strata are at higher risk of acquiring the disease. We found no associations between VL and owning [odds ratio (OR) 0.97; 95% confidence interval (CI) 0.62–1.51) or keeping bovines inside the house (OR 0.88; 95% CI 0.37–2.08).7 We also observed that people from Mushahar caste are at higher risk of acquiring VL than other castes (OR 2.9; 95% CI 1.3–6.8).8 This could be because Mushahars are among the poorest of the poor and take more time to seek and obtain appropriate health care.9
April/May (summer), July/August (rainy season) and October/November (start of winter) have been identified as three peak sandfly density periods.10 In each of these seasons, we conducted two entomological surveys for 2 successive years between 2009 and 2011. In 500 households spread out over 50 villages, we installed Centers for Disease Control (CDC) light traps to collect sandflies. We also collected information regarding housing conditions and conditions of the immediate surroundings; that included information about main materials used in floor, walls and roof of the room in the house where the light trap was installed, dampness of the floor, penetration of daylight, presence of windows and cross-ventilation, presence of cooking stoves and presence of animals inside the house. Our data show that better housing conditions are associated with lower sandfly densities, independent of other socio-economic conditions (Table 2). We also questioned the role of other extra- domiciliary factors at play as the sandflies were most abundant in outdoor locations. Currently we are running a project to study the outdoor sandfly abundance and their breeding and biting behaviours.
Table 2.
Type of housing and socio-economic status associated with sandfly indoor density
| Factor | No. of households (HHs) | Median of total numbers of sandflies captured per HH |
|---|---|---|
| Socio-economic status | ||
| - Group 1 (poorest) | 101 | 35.0 |
| - Group 2 | 70 | 37.5 |
| - Group 3 | 98 | 24.0 |
| - Group 4 | 96 | 25.0 |
| - Group 5 (wealthiest) | 117 | 17.0 |
| Type of housing | ||
| - Thatched | 161 | 41.0 |
| - Brick, unplastered | 150 | 22.0 |
| - Brick, plastered | ||
| Earth floor | 113 | 18.0 |
| Cement floor | 76 | 17.0 |
In our serological surveys we were able to show that there is a strongly increased risk of progressing to disease among sero-positive individuals with high antibody titres.11 Agreement between two markers of infection, DAT and rk39 ELISA, was weak (kappa = 0.30) and these serological markers revert to negative, although infection is assumed to be permanent.12 Most clinical VL cases occur at younger ages, yet we observed a steady increase with age in the frequency of sero-positivity and seroconversion. These findings are important as these asymptomatically infected individuals could be infectious to the sandflies, a hypothesis still to be elucidated.
We also have been conducting studies on operational issues related to the management of VL in the district. In 2008 we found critical flaws in VL case management in the primary health care services in Bihar. We found that patients typically showed long delays in seeking health care. Moreover, 40% of patients were treated with antimonial drugs despite their higher resistance profile, and this instead of Miltefosine which is the recommended drug of choice. Treatment failure rates with antimonial drugs were also high.13 We recommended more active case detection strategies and involvement of village health worker networks in the VL elimination programme. We conducted a survey to assess treatment outcomes and concluded that a health service-based reporting system can be a cost-effective and feasible alternative.14 During the interviews with our study team, the village-level health workers expressed their willingness to be involved in the VL elimination programme and they were ready to pay regular visits to VL patients on their doorstep.15 In the Kanti block CHC where our HDSS is located, we installed and implemented a new tool to record diagnosis and treatment of all VL patients and to monitor their treatment outcome on a long-term basis (early and late treatment outcome monitoring).16 The district programme now uses a slightly adapted version of this tool.
Currently the TMRC project conducts studies on genetic factors related to VL and on the co-infection of lymphatic filariasis and geo-helminths with VL. Under the same framework, we will conduct qualitative research to assess the acceptability and perceived effectiveness of the IRS programme. Other studies include exposure to sandflies, using sandfly saliva antibody testing, and entomology studies for vector behaviour, breeding sites and patterns.
Strengths and weaknesses
We have a comprehensive dataset of VL patients along with their clinical characteristics and laboratory data at different points in time during and after treatment. These patients were treated in our hospital as participants in various studies conducted by the centre over the years or as general VL patients. Our oldest dataset goes back to year 2002. We have annually collected longitudinal data at individual and household level since 2008. During these years, we have been able to build a team experienced in data collection through interview and observations, sample collection (e.g. blood, DNA) and performance of rapid diagnostic tests in the field. Many of our staff are trained in qualitative data collection (e.g. focus group discussions).
We also have a good network of village volunteers. Village volunteers are permanent residents of the villages and create a link between the HDSS team and the community. Through their support, we have been able to build a good rapport with the community. This is reflected in a generally very high response rate which has been over 95% in different surveys. We have geo-referenced all the households in the HDSS, allowing rapid and easy location of individuals when required (e.g. VL cases). We have full capacity of data management and data analysis at the site.
On the other hand, we still use paper format to collect data—but we never compromise with the quality of our data. Electronic capturing of data (e.g. smartphones) would simplify the data collection activities, so we are planning to implement those new technologies in the future. Verbal autopsies are done systematically after the yearly household census. Ideally these should be done within 90 days of death.
Data sharing and collaboration
Being an associate member of the INDEPTH network, we are committed to sharing our data and findings with collaborators. Currently the data are provided in MS Excel files or in MS Access tables upon a specific request. Our HDSS is a study platform which is open to all, and interested collaborators and researchers may contact the authors for conducting their studies. Requests may be sent to Dr Shyam Sundar at [drshyamsundar@hotmail.com].
Funding
The National Institute of Health—National Institute of Allergic and Infectious Diseases provided TMRC grant no. 1P50AI074321 to establish this HDSS.
Acknowledgements
We acknowledge the work of our all team members, village volunteers and our collaborators. We would also like to acknowledge the great support that we receive from the community itself. We are thankful to the Kaladrug-R consortium for implementing new monitoring tools on treatment outcome in primary health care. We are grateful to INDEPTH for recognizing us as an associate member.
Conflict of interest: None declared.
References
- 1.Barnett PG, Singh SP, Bern C, et al. Virgin soil: the spread of visceral leishmaniasis into Uttar Pradesh, India. Am J Trop Med Hyg 2005;73:720–25 [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012;7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelaert M, Meheus F, Sanchez A, et al. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health 2009;14:639–44 [DOI] [PubMed] [Google Scholar]
- 4.Joshi A, Narain JP, Prasittisuk C, et al. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis 200;45:105–11. [PubMed] [Google Scholar]
- 5.Thakur CP. A new strategy for elimination of kala-azar from rural Bihar. Indian J Med Res 2007;126:447–51 [PubMed] [Google Scholar]
- 6.Malaviya P, Picado A, Singh SP, et al. Visceral Leishmaniasis in Muzaffarpur District, Bihar, India from 1990 to 2008. PLoS One 2011;6:e14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SP, Hasker E, Picado A, et al. Risk factors for visceral leishmaniasis in India: further evidence on the role of domestic animals. Trop Med In. Health 2010;15(Suppl 2):29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasker E, Singh SP, Malaviya P, et al. Visceral leishmaniasis in rural Bihar, India. Emerg Infect Dis 2012;18:1662–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FP, Picado A, Roddy P, Palma P. Low castes have poor access to visceral leishmaniasis treatment in Bihar, India. Trop Med Int Health 2012;17:666–73 [DOI] [PubMed] [Google Scholar]
- 10.Bhattarai NR, Auwera GV, Rijal S, et al. Domestic animals and epidemiology of Visceral Leishmaniasis, Nepal. Emerg Infect Dis 2010;16:231–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasker E, Malaviya P, Gidwani K, et al. Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. PLoS Negl Trop Dis 2014;8: e2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasker E, Kansal S, Malaviya P, et al. Latent infection with Leishmania donovani in highly endemic villages in Bihar, India. PLoS Negl Trop Dis 2013;7:e2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasker E, Singh SP, Malaviya P, et al. Management of visceral leishmaniasis in rural primary healthcare services in Bihar, India. Trop Med Int Health 2010;15(Suppl 2):55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malaviya P, Singh RP, Singh SP, et al. Monitoring drug effectiveness in kala-azar in Bihar, India: cost and feasibility of periodic random surveys versus a health service-based reporting system. Trop Med Int Health 2011; 16:1159–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malaviya P, Hasker E, Singh RP, et al. Village health workers in Bihar, India: an untapped resource in the struggle against kala-azar. Trop Med Int Health 2013;18:188–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostyn B, Malaviya P, Hasker E, et al. Retrospective quarterly cohort monitoring for patients with visceral leishmaniasis in the Indian subcontinent: outcomes of a pilot project. Trop Med Int Health 2013;18:725–33 [DOI] [PubMed] [Google Scholar]